Abstract
Coronavirus disease 2019 (COVID-19) is a viral infection which can cause a variety of respiratory, gastrointestinal, and vascular symptoms. The acute illness phase generally lasts no more than 2–3 weeks. However, there is increasing evidence that a proportion of COVID-19 patients experience a prolonged convalescence and continue to have symptoms lasting several months after the initial infection. A variety of chronic symptoms have been reported including fatigue, dyspnea, myalgia, exercise intolerance, sleep disturbances, difficulty concentrating, anxiety, fever, headache, malaise, and vertigo. These symptoms are similar to those seen in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), a chronic multi-system illness characterized by profound fatigue, sleep disturbances, neurocognitive changes, orthostatic intolerance, and post-exertional malaise. ME/CFS symptoms are exacerbated by exercise or stress and occur in the absence of any significant clinical or laboratory findings. The pathology of ME/CFS is not known: it is thought to be multifactorial, resulting from the dysregulation of multiple systems in response to a particular trigger. Although not exclusively considered a post-infectious entity, ME/CFS has been associated with several infectious agents including Epstein–Barr Virus, Q fever, influenza, and other coronaviruses. There are important similarities between post-acute COVID-19 symptoms and ME/CFS. However, there is currently insufficient evidence to establish COVID-19 as an infectious trigger for ME/CFS. Further research is required to determine the natural history of this condition, as well as to define risk factors, prevalence, and possible interventional strategies.
Keywords: chronic fatigue syndrome, COVID-19, human coronavirus, myalgic encephalomyelitis, post-infectious fatigue, review
Introduction
Coronavirus disease 2019 (COVID-19) is a viral illness caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 The acute illness phase has been well characterized: symptoms can include a variety of respiratory, neurologic, gastrointestinal, and vascular manifestations that generally last no more than 2–3 weeks.2 However, some COVID-19 patients experience a prolonged convalescence phase and continue to have symptoms for several months after the initial infection.1 Data from narrative patient experiences after COVID-19 infection and early observational studies suggest a syndrome similar to myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), a chronic multi-system illness that has been associated with other infections.3,4 The term “long COVID” has been used to describe this entity by many researchers. However, an agreed-upon case definition does not yet exist.5 In this article, we will use “post-acute COVID-19 symptoms” to describe symptoms lasting longer than 3 weeks. We aim to review similarities and differences between ME/CFS and post-acute COVID-19 symptoms, potential mechanisms of pathogenesis, and management strategies.
ME/CFS
Epidemiology and diagnostic criteria
ME/CFS is a complex chronic multi-system illness associated with a variety of constitutional and neurocognitive symptoms. It has a prevalence of 0.17–0.89% in the general population and occurs more frequently in women.6 Many other predisposing factors such as age, pre-existing psychiatric conditions, socio-economic status, and activity level have been inconsistently associated with increased risk of developing ME/CFS.7 Although the pathogenesis is not well understood, many cases of ME/CFS are thought to be triggered by infection.8 For example, a large retrospective study of 837 patients found symptoms of acute infection (fever, upper respiratory tract infection, flu-like illness, or gastroenteritis) preceded ME/CFS symptom onset in 77% of patients.9 This is in agreement with rates of preceding infectious symptoms seen in other studies.10,11 However, the true extent to which infections contribute to the development of ME/CFS remains undefined.8,12,13 A higher rate of stressful life events has also been observed in the 3 months prior to onset of ME/CFS, while physical stressors such as severe injury or surgery have not been associated with ME/CFS.10,11 In many cases no particular trigger can be identified.8,10
The characterization of ME/CFS remains controversial. The pathogenesis of the disease is poorly understood and there are no specific diagnostic physical signs or biomarkers. As a result there is no universally agreed-upon definition of ME/CFS. Twenty-five different diagnostic criteria have been proposed so far.8,12,14–18
The ME/CFS research community has commonly used the revised Center for Disease Control (CDC) criteria defined by Fukuda et al. in 1994.8,16,17 This case definition has been criticized as being non-specific by focusing only on fatigue as the key symptom. Epidemiologic studies have found up to a five-fold higher prevalence of ME/CFS using these CDC criteria compared with the more recent and stringent International Consensus Criteria (ICC) or Canadian Consensus Criteria (CCC), as summarized in Table 1.7,15–19
Table 1.
Three commonly used diagnostic criteria for ME/CFS.
| 1994 CDC Criteria (Fukuda et al.)16 | 2006 Canadian Consensus Criteria19 | 2015 Institute of Medicine Criteria13 |
|---|---|---|
| Required symptoms: | Required symptoms: | Required symptoms: |
| Persistent or relapsing chronic fatigue | Persistent or relapsing chronic fatigue | Persistent or relapsing chronic fatigue |
| Lasting 6 months | Lasting 6 months | Lasting 6 months |
| New or definite onset | New or definite onset | New or definite onset |
| Not due to ongoing exertion | Not due to ongoing exertion | Not due to ongoing exertion |
| Not alleviated by rest | Substantial reduction in daily activities | Not alleviated by rest |
| Substantial reduction in daily activities | Not due to other medical condition | Substantial reduction in daily activities |
| Not due to other medical condition | Post-exertional malaise or fatigue | Not due to other medical condition |
| Additional symptoms: | Worsening symptoms after exertion | Post-exertional malaise or fatigue |
| (Four or more concurrently present) | Inappropriately low physical and mental stamina | Worsening symptoms after exertion |
| Impaired concentration or memory | Pathologically slow recovery >24 h | Mentally and physically drained following minimal exertion |
| Sore throat | Sleep dysfunction | Failure to reproduce results on exercise tests 24 h apart |
| Tender cervical/axillary lymphadenopathy | Unrefreshing sleep | Sleep dysfunction |
| Muscle pain | Circadian rhythm disturbance | Unrefreshing sleep |
| Pain in several joints | Pain – widespread or migratory | Circadian rhythm disturbance |
| New headaches | Headaches, joint, or muscle pain | Additional Symptoms: |
| Unrefreshing sleep | Neurologic or cognitive manifestations | (One symptom category) |
| Malaise after exertion | Confusion or disorientation | Cognitive impairment |
| Impaired concentration or memory | Confusion or disorientation | |
| Information processing difficulty | Impaired concentration or memory | |
| Perceptual or sensory disturbances | Information processing difficulty | |
| Ataxia, weakness, or fasciculation | Altered executive function/attention | |
| Overload phenomena (sensory or emotional) | Impaired psychomotor function | |
| Additional symptoms: | Orthostatic intolerance | |
| (One symptom from two or more categories) | Light-headedness, imbalance, or fainting with postural change | |
| Autonomic manifestations | Delayed postural symptoms | |
| Orthostatic intolerance | Abnormal blood pressure/tachycardia on postural testing | |
| Irritable bowel or bladder dysfunction | ||
| Palpitations or exertional dyspnea | ||
| Neuroendocrine manifestations | ||
| Loss of thermostatic ability | ||
| Intolerance of extremes of temperature | ||
| Immune manifestations | ||
| Tender lymph nodes | ||
| Sore throat/flu-like symptoms |
The most recent Institute of Medicine diagnostic criteria published in 2015 characterize ME/CFS as a spectrum of five core symptoms: fatigue, post-exertional malaise, cognitive changes (impaired memory, concentration, information processing), sleep disturbance (unrefreshing sleep, circadian rhythm reversal), and orthostatic intolerance.13 Post-exertional malaise in particular is considered an important feature of ME/CFS that distinguishes it from other chronic illnesses such as fibromyalgia, somatic depression, or primary sleep disorders.7,18 A wide variety of secondary symptoms such as pain, sensorimotor abnormalities, arthralgias, gastrointestinal symptoms (nausea, bloating, irritable bowel), urinary symptoms (frequency, urgency), sore throat, and lymphadenopathy (cervical and/or axillary) are included in some criteria but not required for diagnosis.8,13–16,20,21 Symptoms must not be relieved by rest and must persist for more than 6 months in the absence of any significant clinical or laboratory findings.8,13
Post-infectious ME/CFS
Clusters of illnesses resembling ME/CFS have been observed throughout the 20th century following institutional or epidemic infectious outbreaks.12,22–25 Symptom patterns following these outbreaks include chronic fatigue, lethargy, malaise, sleep disturbance, and poor concentration, often exacerbated by physical exertion or stress.23,25 Although diagnostic criteria did not exist at the time, this spectrum of symptoms is highly suggestive of post-infectious ME/CFS.25,26 As both ME/CFS case definitions and diagnostic methods in microbiology evolved over time, a clearer link between infection and ME/CFS has emerged.
Infectious mononucleosis caused by the Epstein–Barr Virus (EBV) is the infection most consistently associated with the development of ME/CFS.13 A prospective study of 301 adolescents diagnosed with acute EBV infection by positive Monospot found that 13% of participants met 1994 CDC criteria for ME/CFS 6 months later, and 4% had still not recovered after 24 months.27 This is in agreement with previous reports of EBV-associated chronic fatigue in adults.28,29 Similar rates of post-infectious fatigue were reported following Q Fever or Ross River Virus infection (12% at 6 months by 1994 CDC criteria), and about 20% following West Nile Virus infection and other glandular fevers.23,28,30,31
There is evidence suggesting that a wider array of viral and bacterial illness can also be associated with increased risk of developing ME/CFS.28,32 For example, in a prospective cohort study of 618 patients diagnosed with a non-specific viral infection by their primary care provider 12.9% met criteria for chronic fatigue (using an independently validated fatigue scale) at 6 months.32 In a longitudinal study following patients with acute EBV, Ross River Virus, Q fever, or serologically unconfirmed febrile illness, the prevalence and severity of chronic fatigue, functional impairment, and neurocognitive disturbance post-infection was the same regardless of specific infectious trigger.28
ME/CFS and viral epidemics
Following the 1918 influenza pandemic, up to 40% of survivors remained chronically unwell with a variety of symptoms including fatigue, lethargy, and difficulty concentrating which were exacerbated by physical exertion.23,25 More recently, a population health registry surveillance study in Norway identified an increased incidence of ME/CFS diagnosis after the 2009 H1N1 pandemic.33 Survivors of recent coronavirus outbreaks, including severe acute respiratory syndrome (SARS) in 2002 and Middle East respiratory syndrome (MERS) in 2012, reported multiple persistent symptoms including fatigue, widespread pain, unrefreshing sleep, post-exertional malaise, and changes to cognition.34–38 One study of 233 SARS survivors found that 27.1% met criteria for ME/CFS (as defined by 1994 CDC criteria) at 41 months post-infection.36 A meta-analysis of post-infectious symptoms in MERS and SARS found that 19.3% of patients experienced ongoing fatigue up to 39 months after infection.35
In addition to persistent fatigue, psychiatric and neurocognitive complications following influenza and coronavirus epidemics have been observed.22,31,39 For example, first-time hospitalizations for psychiatric disorders increased by a factor of 7.2 for several years after the 1918 pandemic.22 More recently, a study of 37 patients with H1N1 influenza acute respiratory distress syndrome found high rates of anxiety (50%), depression (28%), and post-traumatic stress disorder (PTSD) (41%) after 1 year.40 Survivors of H7N9 influenza reported persistently reduced mental health scores on 36-item short form survey after 24 months.41 A meta-analysis of long-term symptoms in SARS and MERS survivors found a high prevalence of depression (14.9%), anxiety (14.8%), and PTSD (32.2%) compared with population rates of approximately 7%.35,42,43 One study found that the prevalence of comorbid psychiatric conditions was significantly higher in patients with post-SARS ME/CFS, but found no association with initial illness severity, other medical comorbidities, age, or gender.36 In contrast, pre-existing psychiatric conditions are not consistently associated with EBV-associated ME/CFS.31 The higher rates of both ME/CFS and psychiatric diagnoses observed in SARS survivors may reflect the role of stressful life events as an independent risk factors for developing ME/CFS.10,11,36
The existing evidence suggests a temporal relationship between viral epidemics and chronic post-infectious symptoms that are consistent with the criteria for ME/CFS.
Proposed mechanisms of post-infectious ME/CFS
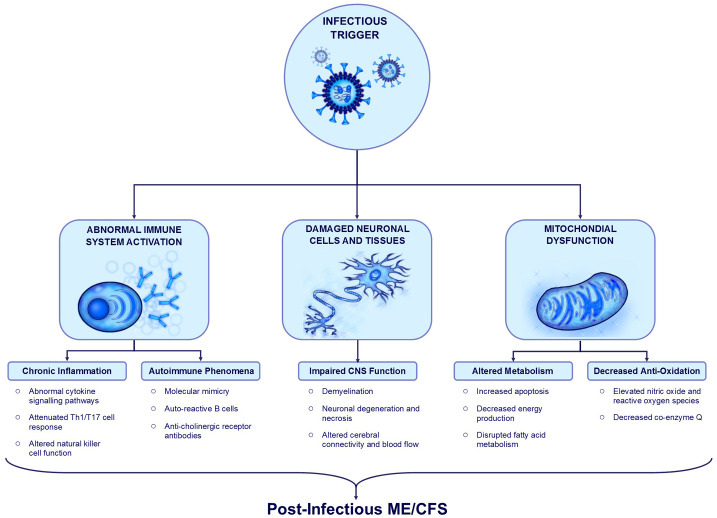
Although post-infectious ME/CFS has been associated with a variety of different pathogens, the incidence and disease manifestations are similar regardless of inciting pathogen.28,32,44 Symptoms persist long after clearance of the initial infection and occur in the absence of any significant abnormality detectable with diagnostic testing.8,13 This has resulted in a “hit-and-run” hypothesis, which suggests that susceptible individuals experience persistent dysregulation of immune, neurologic, and metabolic pathways following exposure to an infectious trigger (Figure 1).44 Multiple organ systems and signaling pathways have been investigated in both human and animal models. However, findings are not consistent between studies. The mechanism of post-infectious ME/CFS remains poorly understood, and is likely multifactorial.
Figure 1.
Summary of post-infectious ME/CFS mechanisms.
Infective agents activate and alter immune system function leading to chronic inflammation, increased pro-inflammatory cytokine signaling, and abnormal function of multiple cell types including Th1, Th17, T-regulatory, and natural killer cells. Autoimmune mechanisms such as molecular mimicry and auto-reactive bystander cell activation can also be triggered during acute infection. Infective agents with neuro-invasive potential can cause inflammatory and ischemic damage to central nervous system cells and tissues, resulting in neuronal degeneration, demyelination, and subsequent functional impairment. Infective agents may also cause structural damage to mitochondria, leading to decreased energy production, altered metabolism, and reduced anti-oxidant function. These processes may underlie the symptoms reported in post-infectious ME/CFS.
Immune/inflammatory mechanisms
Post-infectious ME/CFS has often been purported as an inflammatory disorder in which an infectious pathogen triggers an abnormal systemic immune response that persists beyond clearance of the infection.8,44 Proposed mechanisms linking acute infection and chronic immune system dysregulation in the pathogenesis of ME/CFS include altered immune cell function, abnormal signaling pathways, chronic inflammation, and autoimmune phenomena.44–47
Lasting patterns of altered immune system function favoring a pro-inflammatory milieu following an acute infection have been demonstrated in animal models.45,46,48 For example, in mice, the infection of astrocytes and microglia with a murine coronavirus (MHV-A59) creates a persistent pro-inflammatory environment within the central nervous system (CNS) which is not seen following exposure to non-neurotropic virus or in uninfected controls.46 Increased levels of five pro-inflammatory cytokines (interleukin-12 p40, interleukin-6, interleukin-15, interleukin-1, and tumor necrosis factor-α) were detected in the brain during the acute infectious encephalitis phase and remained persistently elevated within the spinal cord at 30 days post-infection.46
Findings suggestive of systemic chronic inflammation and abnormal pro-inflammatory cytokine expression have also been observed in ME/CFS patients.49 For examples, many studies have suggested that dysregulation of cytokine networks may play a role in ME/CFS, including a recent meta-analysis which found altered levels of tumor necrosis factor α, transforming growth factor β, interleukin-2, and interleukin-4 compared with healthy controls.50 However, important differences in cytokine levels are highly inconsistent between studies: a separate meta-analysis analyzing 64 cytokines found no significant association with ME/CFS.51 There is some emerging evidence that cytokine signaling and network connections are more significant than circulating cytokine levels alone.47
Abnormal immune cell function may also be associated with ME/CFS. For example, an attenuated TH1/TH17 cell response has been described in ME/CFS, which is similar to the pattern seen in latent infections such as EBV.25,47 Altered natural killer and T-regulatory cell function has also been reported in ME/CFS.52 There may be a genetic basis for predisposition to chronic immune system dysregulation after an infectious trigger.53,54 However, there is no inflammatory biomarker, altered cellular function, or genetic polymorphism that is seen consistently across cases of ME/CFS.47,50,51
Tissue damage sustained during acute infection leading to activation of auto-reactive bystander cells and molecular mimicry have been proposed as potential autoimmune mechanisms.44,49 For example, in severe COVID-19 infections significantly increased levels of anti-nuclear antibodies and rheumatic factor have been detected, suggesting heightened activation of auto-reactive B cells.55 Antibodies against muscarinic and adrenergic receptors have been identified in ME/CFS and are thought to be associated with postural orthostatic symptoms.52 However, no specific B-cell phenotype or auto-antibody has been consistently linked with ME/CFS.56
In summary, the immune system appears to be impacted in post-infectious ME/CFS. However, the precise mechanism is unclear and likely involves multiple pathways.
Central nervous system involvement
Several core symptoms of ME/CFS (impaired cognition, sleep disturbance) as well as some secondary symptoms (sensory overload phenomena, motor symptoms) may reflect altered CNS function.
A meta-analysis of imaging findings in ME/CFS found a greater proportion of altered cerebral blood flow, structural cortical abnormalities, focal inflammation, and changes to functional connectivity compared with healthy controls.20 It is not clear whether any specific neurocognitive deficits occur as a result of these structural abnormalities. Likewise, the mechanism of these changes as they relate to infectious triggers is not known.
Many viruses, including some coronaviruses, are known to have neuro-invasive potential and can cause inflammatory damage to CNS tissue.57 For example, SARS-CoV1 isolated from human brain tissue and cerebrospinal fluid has been associated with edema, neuronal degeneration, demyelination, and necrosis in severe cases.45,48,58,59 Increased risk of cerebral ischemic and microvascular events has been reported in acute SARS, MERS, and COVID-19 infection.60,61 There is some evidence of a functional association between certain viral infections and chronic neurologic disease. For example, one specific strain of human coronavirus (HCoV-0C43) that is known to cause a febrile respiratory and gastrointestinal illness in humans was found to be significantly more prevalent in the CNS tissue of people with multiple sclerosis than in healthy controls.58 A sleep study in SARS survivors who were unable to return to work due to chronic symptoms found a high proportion of rapid eye movement and alpha electroencephalographic sleep anomalies commonly seen in ME/CFS patients, suggesting a common pathologic mechanism.37
Although a multitude of post-infectious changes to inflammatory, autoimmune, and cellular signaling mechanisms within the CNS have been identified, the role of each of these in the pathogenesis of post-infectious ME/CFS remains unclear.20,44,49,60 A causal relationship between acute infection, altered CNS structure and function, and post-infectious ME/CFS symptoms has not been clearly established.
Mitochondrial function and fatigue
Fatigue is a defining feature in both ME/CFS and primary mitochondrial disorders, which has led to a large body of research investigating the connection between mitochondrial function and ME/CFS.62
Alterations in mitochondrial structure, metabolism, and energy production within muscle tissues may be associated with the fatigue and post-exertional malaise seen in ME/CFS.44,62 One study examining muscle biopsy samples in a population of 50 people diagnosed with post-viral fatigue syndrome (by Holmes 1988 criteria) found mitochondrial degeneration, pleomorphic features, and significant structural abnormalities in 80% of cases, as compared with minor structural changes seen in only 52% of healthy controls.63
Mitochondrial enzymes involved in inflammatory and anti-oxidant pathways are of particular interest as drivers of orthostatic intolerance and post-exertional malaise due to their involvement in peripheral vasodilation and autonomic regulation of the cardiovascular system.64–67 A small prospective study of gene expression in five people with post-EBV ME/CFS found significant differences in several genes associated with mitochondrial fatty acid metabolism, oxidation, membrane function and apoptosis relative to Human Leukocyte Antigen-matched healthy controls.68 These findings are in keeping with other mitochondrial function studies in ME/CFS which have found alterations in enzyme levels associated with oxidation (nitrous oxide, radical oxygen species), fatty acid metabolism, and energy production.65,69 One of the most commonly studied is the anti-oxidant coenzyme Q10, which has been found to be lower in ME/CFS than in healthy controls in some studies.62,64
There is currently insufficient data to classify ME/CFS as a mitochondrial disorder or to link post-infectious ME/CFS with mitochondrial dysfunction. Most studies are either limited by small sample size, difficult to compare based on different diagnostic criteria and case definitions, or inconsistent in their results. A clear plausible pathway to explain lasting mitochondrial abnormalities after acute infections is also lacking: two systematic reviews on the role of mitochondrial function in ME/CFS found no significant agreement in structural, genetic, metabolic, or oxidative pathway abnormalities between studies.62,70
COVID-19 and ME/CFS
Observational studies have described persistent symptoms of acute COVID-19 as lasting at least 3 weeks from disease onset, with some patients reporting lingering symptoms for longer than 4 months.2,22,71–78 A variety of chronic symptoms, including fatigue, dyspnea, joint pain, myalgia, sleep disturbances, difficulty concentrating, memory problems, cough, anosmia, anxiety, headache, fever, and vertigo have been reported.74–78 Many narrative reports of post-acute COVID-19 patient experiences describe profound fatigue and cognitive changes that are exacerbated by physical activity or stress.3,79–81 Although these symptoms parallel those that are seen in post-infectious ME/CFS, data supporting COVID-19 as an infectious trigger for ME/CFS are limited.
The exact prevalence and expected duration of post-acute COVID-19 symptoms is under ongoing investigation. Some studies have reported at least one persistent symptom in 75% of post-COVID patients at follow-up ranging from 7 to 12 weeks later.82,83 A recent systematic review of 28 post-COVID-19 symptom studies found that fatigue, dyspnea, and anosmia were the most frequently reported symptoms lasting more than 3 weeks.84 However symptom duration, patient populations, and length of follow-up are highly variable between studies, with reported rates of full recovery between 13% and 86% at follow-up ranging from 30 to 186 days (Table 2).71–73,75,78,83,85–103
Table 2.
Post-acute COVID-19 symptom frequency.
| Author | Country | Population | Sample size | Follow-up (days) | Recovered at follow-p (%) | Most common symptoms (%) | Risk factors |
|---|---|---|---|---|---|---|---|
| Huang et al.71 | China | Inpatient | 1733 | 186 | 24 | Fatigue/myalgia (63)Sleep disturbance (26)Hair loss (22) | AgeFemale sexDisease severity |
| Nehme et al.89 | Switzerland | Inpatient | 669 | 43 | 68 | Fatigue (–)Dyspnea (–)Anosmia (–) | – |
| Khalaf et al.90 | Egypt | Mixed (51% Inpatient, 49% Outpatient) | 538 | 82 | 15 | Fatigue (59)Subjective fever (47)Diarrhea (24) | Disease severityHydroxychloroquine useAzithromycin useMultivitamin use |
| Xiong et al.86 | China | Inpatient | 538 | 97 | 50 | Fatigue (28)Diaphoresis (24)Post-exertion polypnea (21) | AgeFemale sexHospital length of stay |
| Chopra et al.91 | United States | Inpatient | 488 | 60 | – | Physical limitation (39)Exertional dyspnea (23)Anosmia (13) | – |
| Mohamed-Hussain et al.92 | Egypt | Mixed (76% Inpatient, 24% Outpatient) | 444 | 35 | 20 | – | AgeFemale sexDisease severitySeasonal flu vaccineSmoking historyAny medical comorbidity |
| Galal et al.93 | Egypt | Mixed (24% Inpatient, 76% Outpatient) | 430 | – | 14 | Myalgia (60)Arthralgia (57)Physical limitation (57) | Any medical comorbidityDisease severityInfluenza vaccination |
| Mandal et al.83 | England | Inpatient | 384 | 54 | 28 | Fatigue (67)Dypsnea (53)Cough (34)Sleep disturbance (61)Dyspnea (55) | – |
| Moradian et al.94 | Iran | Inpatient | 200 | 42 | 42 | Dyspnea (20)Weakness (19)Myalgia (18) | – |
| Jacobs et al.87 | United States | Inpatient | 183 | 35 | 27 | Fatigue (55)Myalgia (51)Dyspnea (45) | AgeFemale sex |
| Petersen et al.95 | Faroe Islands | Outpatient | 180 | 125 | 47 | Fatigue (–)Anosmia (–)Myalgia (–) | Age |
| Pilotto et al.88 | Italy | Inpatient | 165 | 97 | 50 | Fatigue (34)Memory loss (31)Sleep disturbance (30) | AgeDisease severity |
| Townsend et al.73 | Ireland | Mixed (48% inpatient, 52% outpatient) | 153 | 75 | 38 | Fatigue (48) | – |
| Carfi et al.72 | Italy | Inpatient | 143 | 60 | 13 | Fatigue (53)Dyspnea (43)Arthralgia (27) | – |
| Galvan-Tejada et al.96 | Mexico | Inpatient | 141 | 36 | 16 | Cough (25)Anosmia (24)Emesis (15) | – |
| Wang et al.78 | China | Inpatient | 131 | 30 | 86 | Cough (9)Dyspnea (2)Pharyngitis (2) | – |
| Garrigues et al.97 | France | Inpatient | 120 | 111 | – | Fatigue (55)Dyspnea (42)Memory loss (34) | – |
| Pellaud et al.85 | Switzerland | Inpatient | 116 | 30 | 37 | Fatigue (67)Respiratory symptoms (56)Anosmia (10) | – |
| Varghese et al.98 | Germany | Mixed (9% Inpatient, 91% Outpatient) | 116 | 66 | 79 | Fatigue (11)Dyspnea (6)Anosmia (5) | Reduced serum IgA |
| Arnold et al.82 | England | Inpatient | 110 | 90 | 26 | Fatigue (39)Dyspnea (39)Insomnia (24) | – |
| Halpin et al.75 | England | Inpatient | 100 | 48 | – | Fatigue (63)Dyspnea (50)Post-traumatic stress disorder symptom (31) | – |
| Darley et al.99 | Australia | Mixed (88% Inpatient, 12% Outpatient) | 78 | 69 | 60 | Fatigue (22)Dyspnea (19)Chest tightness (5) | – |
| Wong et al.100 | Canada | Inpatient | 78 | 90 | 24 | Reduced quality of life (51)Dyspnea (50)Cough (23) | – |
| Stavem et al.101 | Norway | Outpatient | 70 | 117 | 58 | Dyspnea (16)Anosmia (12)Dysgaeusia (10) | Medical comorbiditiesNumber of acute symptoms |
| Miyazato et al.102 | Japan | Inpatient | 63 | 120 | – | Dyspnea (11)Fatigue (10)Anosmia (10) | – |
| Zhao et al.103 | China | Inpatient | 55 | 64–93 | – | Gastrointestinal (31)Headache (18)Fatigue (16) | – |
An observational study investigating post-acute COVID-19 symptoms as defined by ME/CFS criteria does not yet exist. However, the high prevalence of persistent fatigue is very relevant to ME/CFS. A large prospective cohort study of 1733 patients admitted to hospital with COVID-19 found that 63% of them were still experiencing fatigue/myalgia at 6 months post-discharge.71 However, the presence of chronic fatigue alone is insufficient to diagnose ME/CFS. Future studies investigating other key features of ME/CFS, such as post-exertional malaise and neurocognitive changes, will be required to establish a diagnosis.
As seen in previous coronavirus outbreaks, dyspnea is the other most common persistent symptom, reported in up to 56% of inpatients at follow-up ranging from 1 to 6 months.71,75,85 Dyspnea and exercise intolerance in the context of ME/CSF are mainly recognized as having a strong orthostatic component, a feature not clearly described in post-COVID-19 cases. Certain abnormalities on pulmonary testing have also been detected in this population: for example, studies in post-COVID-19 patients have demonstrated mild restrictive spirometry and imaging abnormalities in more than half of patients. However, these findings do not correlate well with initial disease severity or overall symptom burden.74,76,77 It is not clear in these studies whether dyspnea out of proportion to physical findings occurs in conjunction with postural symptoms such as tachycardia or hypotension, which would suggest an orthostatic component more in keeping with ME/CFS, or secondary to other factors such as deconditioning or post-viral lung injury.
An association between chronic symptoms and age, illness severity, and female gender was seen in some studies.71,86–88 Other proposed risk factors, including ethnicity, psychiatric condition, number of medical comorbidities, or obesity were not consistently associated with post-acute COVID19 symptoms.72,73,75,77,82 As seen in ME/CFS, there was no biomarker (complete blood count, lymphocyte count, neutrophil count, monocyte count, D-dimer, C-reactive protein, lactate dehydrogenase, interleukin-6, CD-25, liver function tests, or creatinine) differentiating patients who remained symptomatic from those who returned to baseline health.73,82,83
Management options
The approach to treating ME/CFS generally focuses on symptom management and minimizing unnecessary investigations.2 However, a thorough workup to rule out other organic cause for ME/CFS symptoms must be done prior to giving this diagnosis. In post-acute COVID-19 this includes outpatient pulmonary imaging for people with severe respiratory disease during acute illness, as well as screening and concurrent management for comorbid psychiatric illness.2,22,104
The National Institute for Health and Care Excellence guidelines on ME/CFS currently recommends graded exercise therapy and cognitive behavioral therapy.77 However, more recent evidence suggests that graded exercise therapy may accentuate post-exertional malaise in some patients.105–107 This effect has been demonstrated in patient narratives of post-acute COVID-19 symptoms, who describe even minimal physical exertion as exacerbating their symptoms and rendering them bedbound for several days.3,79–81 For this reason, some experts have cautioned against graded exercise therapy on the management of fatigue in post-acute COVID-19.108
Recent expert opinions on the management of post-acute COVID-19 in primary care recommend an approach based on conservative symptom relief strategies, referral to specialists for co-management of comorbidities, and a multidisciplinary approach to social, cultural, and financial support.2 However, further research will be required to determine the benefit of any specific treatment for this condition.
Discussion
The evidence for post-infectious ME/CFS following COVID-19 is not as strong as for other viruses such as EBV. Although persistent fatigue has been described extensively in post-acute COVID-19 symptom studies, no study has used ME/CSF criteria to characterize chronic fatigue in conjunction with other key symptoms and common disease manifestations.5,71,82,83 Another limitation is the degree of variability among different ME/CSF diagnostic criteria. Most post-infectious ME/CFS studies continue to use the 1994 CDC diagnostic criteria, which do not require the presence of other hallmark features of ME/CFS such as post-exertional malaise, cognitive changes, sleep disturbances, or orthostatic intolerance for diagnosis.7,8,16–19 This leads to difficulty interpreting the significance of individual chronic symptoms within the context of a post-infectious ME/CFS diagnosis. Diagnosis of post-infectious ME/CFS in COVID-19 patients is further limited by its emerging infection status, as a duration of follow-up of at least 6 months is required to make this diagnosis.
Some symptoms seen in post-acute COVID-19 may occur as a consequence of critical illness or as a side effect of treatments such as steroids. For example, dyspnea is seen in up to 36% of people diagnosed with ME/CFS and is considered part of the broader category of orthostatic intolerance, along with postural tachycardia and hypotension.109 However, the dyspnea reported in post-COVID studies is not clearly described as a manifestation of orthostatic intolerance and may in fact represent fibrosis following inflammatory lung injury.76–78 This theory would be supported by the presence of clinically detectable abnormalities on imaging and pulmonary function testing in post-acute COVID-19 patients.73,74 Similar findings can be seen in survivors of acute respiratory distress syndrome, suggesting an organic cause for dyspnea.110–112 Other complications of critical illness and acute respiratory distress syndrome such as loss of muscle mass, deconditioning, steroid-induced myopathy, and multi-organ failure are correlated with poorer long-term health outcomes, chronic fatigue, and decreased functional capacity.111 There is some overlap between these outcomes and symptoms of ME/CFS. However, it is important to note that multiple post-acute COVID-19 studies have found no association between illness severity, presence of chronic symptoms, and objective measures of respiratory function, suggesting an alternate mechanism of pathogenesis.72,73,75,77,82
The importance of the higher rate of psychiatric comorbidities seen following epidemic outbreaks is similarly unclear. This association is likely caused by external stressors rather than due to the infection itself.11,35,42 While specific psychiatric conditions have not been consistently associated with increased risk of post-infectious ME/CFS, other psychosocial factors such as stressful life events, persistent high levels of anxiety, and reduced community support may play a role.7,17,28,29 Evidence from prior viral epidemics suggests that this period of multiple stressful life events may be an independent risk factor for developing ME/CFS; it will be difficult to separate the impact of pandemic-associated stress from the impact of the infection itself in defining COVID-19 as a risk factor for ME/CFS.
Although the symptom patterns seen in post-acute COVID-19 are similar to those seen in ME/CFS, further investigation with longer periods of follow-up and clearly defined diagnostic criteria will be required to establish COVID-19 as an infectious trigger for ME/CFS.
Bottom line
Many post-acute COVID-19 symptoms resemble post-infectious ME/CFS
Acute disease severity does not clearly correlate with persistent symptoms
Long-term monitoring of post-acute COVID-19 symptoms and screening for common comorbid conditions is essential
Further research is required to establish COVID-19 as an infectious trigger for ME/CFS as well as to define risk factors, prevalence, natural history, and possible interventional strategies to treat this condition
Footnotes
Author contributions: SP conducted the literature review and wrote the first draft of the manuscript, including creating all figures and tables. SP, SJA, VC-M, and JC contributed equally to subsequent edits and revisions.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: This study does not require ethics approval as it does not contain any new human or animal trials: it is a review of previously published and anonymized data that is freely available in the public domain.
ORCID iD: Juthaporn Cowan  https://orcid.org/0000-0001-6648-5109
https://orcid.org/0000-0001-6648-5109
Contributor Information
Sonia Poenaru, Department of Medicine, The Ottawa Hospital, General Campus, 501 Smyth Road, Box 206, Ottawa, Ontario, K1H 8L6, Canada.
Sara J. Abdallah, Clinical Epidemiology Program, The Ottawa Hospital Research Institute, Ottawa, Ontario, Canada
Vicente Corrales-Medina, Clinical Epidemiology Program, The Ottawa Hospital Research Institute, Ottawa, Ontario, Canada; Division of Infectious Diseases, Department of Medicine, University of Ottawa, Ottawa, Ontario, Canada.
Juthaporn Cowan, Clinical Epidemiology Program, The Ottawa Hospital Research Institute, Ottawa, Ontario, Canada; Division of Infectious Diseases, Department of Medicine, University of Ottawa, Ottawa, Ontario, Canada.
References
- 1. del Rio C, Collins LF, Malani P. Long-term health consequences of COVID-19. JAMA 2020; 324: 1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute covid-19 in primary care. BMJ 2020; 370: m3026. [DOI] [PubMed] [Google Scholar]
- 3. Garner P. Covid-19 at 14 weeks—phantom speed cameras, unknown limits, and harsh penalties. BMJ Opinion, https://blogs.bmj.com/bmj/2020/06/23/paul-garner-covid-19-at-14-weeks-phantom-speed-cameras-unknown-limits-and-harsh-penalties/2020. (2020, accessed 25 October 2020).
- 4. Ladds E, Rushforth A, Wieringa S, et al. Persistent symptoms after COVID-19: qualitative study of 114 “long COVID” patients and draft quality criteria for services. J Infect Dis. Epub ahead of print 14 October 2020. DOI: 10.1101/2020.10.13.20211854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Michelen M, Manoharan L, Elkheir N, et al. Characterising long-term covid-19: a rapid living systematic review. Glob Public Health. Epub ahead of print 9 December 2020. DOI: 10.1101/2020.12.08.20246025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lim EJ, Ahn YC, Jang ES, et al. Systematic review and meta-analysis of the prevalence of Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME). J Transl Med 2020; 18: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jason LA, Porter N, Brown M, et al. CFS: a review of epidemiology and natural history studies. Bull IACFS ME 2009; 17: 88–106. [PMC free article] [PubMed] [Google Scholar]
- 8. Prins JB, van der Meer JW, Bleijenberg G. Chronic fatigue syndrome. Lancet 2006; 367: 346–355. [DOI] [PubMed] [Google Scholar]
- 9. Naess H, Sundal E, Myhr K-M, et al. Postinfectious and chronic fatigue syndromes: clinical experience from a tertiary-referral centre in Norway. In Vivo 2010; 24: 185–188. [PubMed] [Google Scholar]
- 10. Hatcher S, House A. Life events, difficulties and dilemmas in the onset of chronic fatigue syndrome: a case–control study. Psychol Med 2003; 33: 1185–1192. [DOI] [PubMed] [Google Scholar]
- 11. Theorell T, Blomkvist V, Lindh G, et al. Critical life events, infections, and symptoms during the year preceding Chronic Fatigue Syndrome (CFS): an examination of CFS patients and subjects with a nonspecific life crisis. Psychosom Med 1999; 61: 304–310. [DOI] [PubMed] [Google Scholar]
- 12. Demitrack MA, Greden JF. Chronic fatigue syndrome: the need for an integrative approach. Biol Psychiatry 1991; 30: 747–752. [DOI] [PubMed] [Google Scholar]
- 13. Clayton EW. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: an IOM report on redefining an illness. JAMA 2015; 313: 1101–1102. [DOI] [PubMed] [Google Scholar]
- 14. Holmes GP. Chronic fatigue syndrome: a working case definition. Ann Intern Med 1988; 108: 387–389. [DOI] [PubMed] [Google Scholar]
- 15. Carruthers BM, van de Sande MI, De Meirleir KL, et al. Myalgic encephalomyelitis: International Consensus Criteria. J Intern Med 2011; 270: 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. 1994; 121: 953–959. [DOI] [PubMed] [Google Scholar]
- 17. Lim EJ, Son CG. Review of case definitions for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). J Transl Med 2020; 18: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jason LA, Kot B, Sunnquist M, et al. Chronic fatigue syndrome and myalgic encephalomyelitis: towards an empirical case definition. Health Psychol Behav Med 2015; 3: 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carruthers BM, Jain AK, De Meirleir KL, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: a clinical case definition and guidelines for medical practitioners (an overview of the Canadian consensus document). J Chronic Fatigue Syndr 2003; 11: 7–115. [Google Scholar]
- 20. Maksoud R, du Preez S, Eaton-Fitch N, et al. A systematic review of neurological impairments in myalgic encephalomyelitis/chronic fatigue syndrome using neuroimaging techniques. PLoS One 2020; 15: e0232475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown AA, Jason LA. Validating a measure of myalgic encephalomyelitis/chronic fatigue syndrome symptomatology. Fatigue 2014; 2: 132–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lyons D, Frampton M, Naqvi S, et al. Fallout from the COVID-19 pandemic – should we prepare for a tsunami of post viral depression? Ir J Psychol Med 2020; 37: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Briggs NC, Levine PH. A comparative review of systemic and neurological symptomatology in 12 outbreaks collectively described as chronic fatigue syndrome, epidemic neuromyasthenia, and myalgic encephalomyelitis. Clin Infect Dis 1994; 18(Suppl. 1): S32–S42. [DOI] [PubMed] [Google Scholar]
- 24. Levine PH, Dale JK, Benson-Grigg E, et al. A cluster of cases of chronic fatigue and chronic fatigue syndrome: clinical and immunologic studies. Clin Infect Dis 1996; 23: 408–409. [DOI] [PubMed] [Google Scholar]
- 25. Islam MF, Cotler J, Jason LA. Post-viral fatigue and COVID-19: lessons from past epidemics. Fatigue 2020; 8: 61–69. [Google Scholar]
- 26. Radusin M. The Spanish flu – part II: the second and third wave. Vojnosanit Pregl 2012; 69: 917–927. [PubMed] [Google Scholar]
- 27. Katz BZ, Shiraishi Y, Mears CJ, et al. Chronic fatigue syndrome after infectious mononucleosis in adolescents. Pediatrics 2009; 124: 189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hickie I, Davenport T, Wakefield D, et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ 2006; 333: 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buchwald DS, Rea TD, Katon WJ, et al. Acute infectious mononucleosis: characteristics of patients who report failure to recover. Am J Med 2000; 109: 531–537. [DOI] [PubMed] [Google Scholar]
- 30. Garcia MN, Hause AM, Walker CM, et al. Evaluation of prolonged fatigue post–West Nile virus infection and association of fatigue with elevated antiviral and proinflammatory cytokines. Viral Immunol 2014; 27: 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. White PD, Thomas JM, Amess J, et al. Incidence, risk and prognosis of acute and chronic fatigue syndromes and psychiatric disorders after glandular fever. Br J Psychiatry 1998; 173: 475–481. [DOI] [PubMed] [Google Scholar]
- 32. Cope H. Predictors of chronic ‘postviral’ fatigue. Lancet 1994; 344: 864–868. [DOI] [PubMed] [Google Scholar]
- 33. Magnus P, Gunnes N, Tveito K, et al. Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME) is associated with pandemic influenza infection, but not with an adjuvanted pandemic influenza vaccine. Vaccine 2015; 33: 6173–6177. [DOI] [PubMed] [Google Scholar]
- 34. Ahmed H, Patel K, Greenwood D, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med 2020; 52: jrm00063. [DOI] [PubMed] [Google Scholar]
- 35. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020; 7: 611–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lam MHB. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med 2009; 169: 2142–2147. [DOI] [PubMed] [Google Scholar]
- 37. Moldofsky H, Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome: a case-controlled study. BMC Neurol 2011; 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tansey CM. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch Intern Med 2007; 167: 1312–1320. [DOI] [PubMed] [Google Scholar]
- 39. Bornand D, Toovey S, Jick SS, et al. The risk of new onset depression in association with influenza – a population-based observational study. Brain Behav Immun 2016; 53: 131–137. [DOI] [PubMed] [Google Scholar]
- 40. Luyt C-E, Combes A, Becquemin M-H, et al. Long-term outcomes of pandemic 2009 influenza A(H1N1)-associated severe ARDS. Chest 2012; 142: 583–592. [DOI] [PubMed] [Google Scholar]
- 41. Chen J, Wu J, Hao S, et al. Long term outcomes in survivors of epidemic Influenza A (H7N9) virus infection. Sci Rep 2017; 7: 17275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim H-C, Yoo S-Y, Lee B-H, et al. Psychiatric findings in suspected and confirmed Middle East respiratory syndrome patients quarantined in hospital: a retrospective chart analysis. Psychiatry Investig 2018; 15: 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mak IWC. Long-term psychiatric morbidities among SARS survivors. Gen Hosp Psychiatry 2009; 10: 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rasa S, Nora-Krukle Z, Henning N, et al. Chronic viral infections in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). J Transl Med 2018; 16: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun 2020; 87: 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li Y, Fu L, Gonzales DM, et al. Coronavirus neurovirulence correlates with the ability of the virus to induce proinflammatory cytokine signals from astrocytes and microglia. J Virol 2004; 78: 3398–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Broderick G, Fuite J, Kreitz A, et al. A formal analysis of cytokine networks in chronic fatigue syndrome. Brain Behav Immun 2010; 24: 1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Almqvist J, Granberg T, Tzortzakakis A, et al. Neurological manifestations of coronavirus infections – a systematic review. Ann Clin Transl Neurol 2020; 7: 2057–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun 2020; 87: 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Strawbridge R, Sartor M-L, Scott F, et al. Inflammatory proteins are altered in chronic fatigue syndrome—a systematic review and meta-analysis. Neurosci Biobehav Rev 2019; 107: 69–83. [DOI] [PubMed] [Google Scholar]
- 51. Corbitt M, Eaton-Fitch N, Staines D, et al. A systematic review of cytokines in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis/Systemic Exertion Intolerance Disease (CFS/ME/SEID). BMC Neurol 2019; 19: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Anderson G, Maes M. Mitochondria and immunity in chronic fatigue syndrome. Prog Neuropsychopharmacol Biol Psychiatry 2020; 103: 109976. [DOI] [PubMed] [Google Scholar]
- 53. Piraino B, Vollmer-Conna U, Lloyd AR. Genetic associations of fatigue and other symptom domains of the acute sickness response to infection. Brain Behav Immun 2012; 26: 552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vollmer-Conna U, Piraino BF, Cameron B, et al. Cytokine polymorphisms have a synergistic effect on severity of the acute sickness response to infection. Clin Infect Dis 2008; 47: 1418–1425. [DOI] [PubMed] [Google Scholar]
- 55. Woodruff MC, Ramonell RP, Lee FEH, et al. Clinically identifiable autoreactivity is common in severe SARS-CoV-2 infection. Infect Dis. Epub ahead of print 23 October 2020. DOI: 10.1101/2020.10.21.20216192. [DOI] [Google Scholar]
- 56. Mensah FKF, Bansal AS, Ford B, et al. Chronic fatigue syndrome and the immune system: where are we now? Clin Neurophysiol 2017; 47: 131–138. [DOI] [PubMed] [Google Scholar]
- 57. Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 2019; 12: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Arbour N, Day R, Newcombe J, et al. Neuroinvasion by human respiratory coronaviruses. J Virol 2000; 74: 8913–8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xu J, Zhong S, Liu J, et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis 2005; 41: 1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry 2020; 7: 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Clauw DJ, Häuser W, Cohen SP, et al. Considering the potential for an increase in chronic pain after the COVID-19 pandemic. Pain. Epub ahead of print 26 June 2020. DOI: 10.1097/j.pain.0000000000001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Filler K, Lyon D, Bennett J, et al. Association of mitochondrial dysfunction and fatigue: a review of the literature. BBA Clin 2014; 1: 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Behan WM, More IA, Behan PO. Mitochondrial abnormalities in the postviral fatigue syndrome. Acta Neuropathol 1991; 83: 61–65. [DOI] [PubMed] [Google Scholar]
- 64. Maes M, Twisk FNM. Why Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) may kill you: disorders in the Inflammatory and Oxidative and Nitrosative Stress (IO&NS) pathways may explain cardiovascular disorders in ME/CFS. Neuroendocrinol Lett 2009; 30: 677–693. [PubMed] [Google Scholar]
- 65. Broderick G, Craddock RC, Whistler T, et al. Identifying illness parameters in fatiguing syndromes using classical projection methods. Pharmacogenomics J 2006; 7: 407–419. [DOI] [PubMed] [Google Scholar]
- 66. Stevens S, Snell C, Stevens J, et al. Cardiopulmonary exercise test methodology for assessing exertion intolerance in myalgic encephalomyelitis/chronic fatigue syndrome. Front Pediatr 2018; 6: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nacul L, O’Boyle S, Palla L, et al. How Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) progresses: the natural history of ME/CFS. Front Neurol 2020; 11: 826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vernon SD, Whistler T, Cameron B, et al. Preliminary evidence of mitochondrial dysfunction associated with post-infective fatigue after acute infection with Epstein Barr virus. BMC Infect Dis 2006; 6: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kurup KK, Kurup PA. Isoprenoid pathway dysfunction in chronic fatigue syndrome. Acta Neuropsychiatrica 2003; 15: 266–273. [DOI] [PubMed] [Google Scholar]
- 70. Holden S, Maksoud R, Eaton-Fitch N, et al. A systematic review of mitochondrial abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome/systemic exertion intolerance disease. J Transl Med 2020; 18: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. Epub ahead of print 8 January 2020. DOI: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Carfi A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324: 603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. Infect Dis. Epub ahead of print 20 June 2020. DOI: 10.1101/2020.07.29.20164293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Huang Y, Tan C, Wu J, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Research Square. Epub ahead of print 16 June 2020. DOI: 10.21203/rs.3.rs-26415/v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. Epub ahead of print 30 July 2020. DOI: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 76. Frija-Masson J, Debray MP, Gilbert M, et al. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur Respir J 2020; 56: 2001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lv D, Chen X, Mao L, et al. Pulmonary function of patients with 2019 novel coronavirus induced pneumonia: a retrospective cohort study. Research Square. Epub ahead of print 27 April 2020. DOI: 10.21203/rs.3.rs-24303/v1. [DOI] [PubMed] [Google Scholar]
- 78. Wang X, Xu H, Jiang H, et al. Clinical features and outcomes of discharged coronavirus disease 2019 patients: a prospective cohort study. QJM Int J Med 2020; 113: 657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Perrin R, Riste L, Hann M, et al. Into the looking glass: post-viral syndrome post COVID-19. Medl Hypotheses 2020; 144: 110055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Peel M. What can we tell patients with prolonged Covid-19? BMJ 2020; 370: m2923. [DOI] [PubMed] [Google Scholar]
- 81. Salisbury H. Helen Salisbury: when will we be well again? BMJ 2020; 369: m2490. [DOI] [PubMed] [Google Scholar]
- 82. Arnold DT, Hamilton FW, Milne A, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. Epub ahead of print 3 December 2020. DOI: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mandal S, Barnett J, Brill SE, et al. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. Epub ahead of print 10 November 2020. DOI: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ayoubkhani D, Khunti K, Nafilyan V, et al. Epidemiology of post-COVID syndrome following hospitalisation with coronavirus: a retrospective cohort study. Epidemiology. Epub ahead of print 15 January 2021. DOI: 10.1101/2021.01.15.21249885. [DOI] [Google Scholar]
- 85. Pellaud C, Grandmaison G, Pham Huu Thien HP, et al. Characteristics, comorbidities, 30-day outcome and in-hospital mortality of patients hospitalised with COVID-19 in a Swiss area – a retrospective cohort study. Swiss Med Wkly. Epub ahead of print 14 July 2020. DOI: 10.4414/smw.2020.20314. [DOI] [PubMed] [Google Scholar]
- 86. Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect 2021; 27: 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jacobs LG, Gourna-Paleoudis E, Lesky-Di Bari D, et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS One 2020; 15: e0243882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pilotto A, Cristillo V, Piccinelli SC, et al. COVID-19 severity impacts on long-term neurological manifestation after hospitalisation. Neurology. Epub ahead of print 2 January 2021. DOI: 10.1101/2020.12.27.20248903. [DOI] [Google Scholar]
- 89. Nehme M, Braillard O, Alcoba G, et al. COVID-19 symptoms: longitudinal evolution and persistence in outpatient settings. Ann Intern Med. Epub ahead of print 8 December 2020. DOI: 10.7326/M20-5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Khalaf M, Bazeed SE, Abdel-Gawad M, et al. Prevalence and predictors of persistent symptoms after clearance of SARS-CoV-2 infection: a report from Egypt. SSRN J. Epub ahead of print 3 December 2020. DOI: 10.2139/ssrn.3727954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chopra V, Flanders SA, O’Malley M, et al. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. Epub ahead of print 11 November 2020. DOI: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mohamed-Hussein A, Galal I, Saad M, et al. Post-COVID-19 functional status: relation to age, smoking, hospitalization and comorbidities. Respir Med. Epub ahead of print 1 September 2020. DOI: 10.1101/2020.08.26.20182618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Galal I, Hussein AAM, Amin MT, et al. Determinants of persistent post COVID-19 symptoms: value of a novel COVID-19 symptoms score. Respir Med. Epub ahead of print 12 November 2020. DOI: 10.1101/2020.11.11.20230052. [DOI] [Google Scholar]
- 94. Moradian ST, Parandeh A, Khalili R, et al. Delayed symptoms in patients recovered from COVID-19. Iran J Public Health 2020; 49: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Petersen MS, Kristiansen MF, Hanusson KD, et al. Long COVID in the Faroe Islands - a longitudinal study among non-hospitalized patients. Clin Infect Dis. Epub ahead of print 30 November 2020. DOI: 10.1093/cid/ciaa1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Galván-Tejada CE, Herrera-García CF, Godina-González S, et al. Persistence of COVID-19 symptoms after recovery in Mexican population. Int J Environ Res Public Health 2020; 17: 9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect 2020; 81: e4–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Varghese J, Sandmann S, Vollenberg R, et al. Follow up of COVID-19 features in recovered adults without comorbidities– persistent symptoms and lab abnormalities. Research Square. Epub ahead of print 30 November 2020. DOI: 10.21203/rs.3.rs-116030/v1. [DOI] [Google Scholar]
- 99. Darley DR, Dore GJ, Cysique L, et al. High rate of persistent symptoms up to 4 months after community and hospital-managed SARS-CoV-2 infection. J Med Aust. Epub ahead of print 3 March 2021. DOI: 10.5694/mja2.50963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wong AW, Shah AS, Johnston JC, et al. Patient-reported outcome measures after COVID-19: a prospective cohort study. Eur Respir J 2020; 56: 2003276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Stavem K, Ghanima W, Olsen MK, et al. Persistent symptoms 1.5–6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax. Epub ahead of print 3 December 2020. DOI: 10.1136/thoraxjnl-2020-216377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Miyazato Y, Morioka S, Tsuzuki S, et al. Prolonged and late-onset symptoms of coronavirus disease 2019. Open Forum Infect Dis 2020; 7: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhao Y-M, Shang Y-M, Song W-B, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020; 25: 100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. George PM, Barratt SL, Condliffe R, et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax 2020; 75: 1009–1016. [DOI] [PubMed] [Google Scholar]
- 105. Larun L, Brurberg KG, Odgaard-Jensen J, et al. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev 2019; 10: CD003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kim DY, Lee JS, Park SY, et al. Systematic review of randomized controlled trials for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME). J Transl Med 2020; 18: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kindlon T. and Irish ME/CFS Association. Reporting of harms associated with graded exercise therapy and cognitive behavioural therapy in myalgic encephalomyelitis/chronic fatigue syndrome. Bull IACFS ME 2011; 19: 59–111. [Google Scholar]
- 108. Torjesen I. NICE cautions against using graded exercise therapy for patients recovering from Covid-19. BMJ 2020; 370: m2912. [DOI] [PubMed] [Google Scholar]
- 109. Johnston S, Staines D, Marshall-Gradisnik S. Epidemiological characteristics of chronic fatigue syndrome/myalgic encephalomyelitis in Australian patients. Clin Epidemiol 2016; 8: 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ngai JC, Ko FW, Ng SS, et al. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology 2010; 15: 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Herridge MS, Matte-Martyn A, Diaz-Granados N, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 2003; 348: 683–693. [DOI] [PubMed] [Google Scholar]
- 112. Cheung AM, Tansey CM, Tomlinson G, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med 2006; 174: 538–544. [DOI] [PubMed] [Google Scholar]