Abstract
Background
Cardiac manifestations in COVID-19 are multifactorial and are associated with increased mortality. The clinical utility and prognostic value of echocardiography in COVID-19 inpatients is not clearly defined. We aim to identify echocardiographic parameters that are associated with 30-day clinical outcomes secondary to COVID-19 hospitalization.
Methods
This retrospective cohort study was conducted in a large tertiary hospital in New York City during the COVID-19 pandemic. It included 214 adult inpatients with a laboratory-confirmed diagnosis of COVID-19 by reverse transcriptase polymerase chain reaction assay (RT-PCR) for SARS-CoV-2 on nasopharyngeal swab and had a transthoracic echocardiogram performed during the index hospitalization. Primary outcome was 30-day all-cause inpatient mortality. Secondary outcomes were 30-day utilization of mechanical ventilator support, vasopressors, or renal replacement therapy.
Results
Mild right ventricular systolic dysfunction (odds ratio (OR): 3.51, 95% confidence interval (CI): 1.63–7.57, p = 0.001), moderate to severe right ventricular systolic dysfunction (OR: 7.30, 95% CI: 2.20–24.25, p = 0.001), pulmonary hypertension (OR: 5.39, 95% CI: 1.96–14.86, p = 0.001), and moderate to severe tricuspid regurgitation (OR: 3.92, 95% CI: 1.71–9.03, p = 0.001) were each associated with increased odds of 30-day all-cause inpatient mortality. Pulmonary hypertension and moderate to severe right ventricular dysfunction were each associated with increased odds of 30-day utilization of mechanical ventilator support and vasopressors.
Conclusions
Right ventricular dysfunction, pulmonary hypertension, and moderate to severe tricuspid regurgitation were associated with increased odds for 30-day inpatient mortality. This study highlights the importance of echocardiography and its clinical utility and prognostic value for evaluating hospitalized COVID-19 patients.
Keywords: COVID-19, echocardiography, right ventricular dysfunction, pulmonary hypertension
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified in December 2019 involving a cluster of pneumonia in the province of Wuhan, China.1 New York City was disproportionately affected by Coronavirus Disease 2019 (COVID-19) and became the epicenter for the pandemic in the United States of America. Within New York City, there were greater than 230,000 cases and 19,000 confirmed deaths following a positive COVID-19 laboratory test.2
Cardiac manifestations from COVID-19 include acute cardiac injury, arrhythmias, acute coronary syndromes, pericarditis, and myocarditis, and they are clinically relevant as they result in increased mortality.3,4 Other risk factors for mortality include older age, male sex, and medical history of hypertension, diabetes, cardiovascular disease, and chronic kidney disease, which are all associated with increased mortality risk from COVID-19. Moreover, elevated cardiac troponin, C-reactive protein, and elevated D-dimer are associated with reduced survival.5
Reports on cardiac imaging of COVID-19 patients are emerging. One study showed that abnormal right ventricular longitudinal strain (RVLS) was an independent predictor of higher mortality in 120 COVID-19 patients.6 A study of 110 COVID-19 patients found that right ventricular (RV) enlargement was present in 31%, and this was associated with increased mortality.7 Another study involving hospitalized non-intensive care unit (non-ICU) patients showed that pulmonary hypertension was associated with a higher rate of in-hospital mortality.8
As cardiac manifestations result in increased mortality in COVID-19, echocardiography may assist in the early implementation of therapeutic interventions based on imaging findings. Our primary aim is to identify echocardiographic variables that are associated with 30-day all-cause mortality in hospitalized COVID-19 patients. Our secondary aims are to identify echocardiographic parameters associated with mechanical ventilation use, vasopressors, and renal replacement therapy in hospitalized COVID-19 patients. Identification of these echocardiographic findings may augment the evaluation and management of COVID-19 patients.
Methods
Study design and population
This was a retrospective cohort analysis of 214 consecutive adult patients (ages ≥18 years old) admitted to the inpatient setting at Maimonides Medical Center with a diagnosis of COVID-19 between 1 March 2020 and 7 May 2020. This tertiary care hospital treats a diverse patient population that includes those of different race/ethnicities. Inclusion criteria were laboratory positive reverse-transcriptase polymerase chain reaction (RT-PCR) assay for SARS-CoV-2 in a respiratory tract sample and a transthoracic echocardiogram study performed during the index inpatient hospitalization. Five patients were excluded due to having non-diagnostic echocardiograms. Only the initial echocardiography study during the index hospitalization was included in the analysis. Ethical approval was obtained prior to the study from our Medical Center Institutional Review Board.
Clinical data
Baseline patient demographic characteristics included age (years), sex (male/female), race/ethnicity (White, Black, Asian, Hispanic, Other), body mass index (BMI obese), and smoking status (no/yes). Medical history variables included coronary artery disease (CAD), hypertension, diabetes mellitus, chronic obstructive pulmonary disease (COPD), heart failure, atrial fibrillation, end-stage renal disease (ESRD), and cancer, all measured as no/yes. Laboratory markers including peak creatinine, peak cardiac troponin I, lymphocyte count, d-dimer, C-reactive protein (CRP), and ferritin were reviewed. The treatments included the use of hydroxychloroquine, tocilizumab, and remdesevir.
Transthoracic echocardiogram
All echocardiogram examinations were performed by sonographers on a Philips Healthcare (Andover, MA) iE33 or CX50 ultrasound system. All echocardiographic images and data were independently analyzed and reviewed by board certified echocardiographers based on current American Society of Echocardiography (ASE) guidelines. These findings were compared with the original clinical reports and any discrepancies were adjudicated by a Level III echocardiographer.
Left ventricular systolic function was graded by visual estimation on two-dimensional (2D) images with the incorporation of endocardial border excursion, myocardial wall thickening, and fractional shortening by 2D linear measurements when available. Left ventricular ejection fraction (LVEF) greater than or equal to 51% was considered normal, LVEF 41%–50% mildly reduced, LVEF 30%–40% moderately reduced, and LVEF less than 30% was categorized as severely reduced. Left ventricular end-diastolic dimension was measured in the parasternal long-axis views obtained perpendicular to the long axis at the level of the mitral valve leaflet tips.9
RV size was measured in the apical 4 chamber or RV focused view and considered enlarged if in end-diastole the basal or mid-level diameter exceeded 41 mm or 35 mm, respectively. RV systolic function was assessed qualitatively on 2D images with incorporated visual recognition and qualitative measures and graded as normal, mildly reduced, or moderate to severely reduced based on free wall inward movement, shortening of the RV in the long axis secondary to contraction of the longitudinal fibers, and RV free wall traction.6 RV systolic excursion velocity (S′) was measured at the lateral annulus of the tricuspid valve by pulsed wave tissue Doppler interrogation.10
Pulmonary arterial systolic pressure (PASP) was determined by assessing peak tricuspid regurgitation (TR) velocity with continuous wave Doppler and right atrial pressure (RAP) based on inferior vena cava (IVC) size. PASP was calculated by using the simplified Bernoulli’s equation [4 (TR)2 + RAP]. In non-mechanically ventilated patients, RAP was assumed to be 3 mmHg with IVC size less than 2.1 cm and respiratory variation greater than 50%, 15 mmHg if IVC was greater than 2.1 cm with respiratory variation less than 50%, and otherwise considered 8 mmHg if it did not meet either criteria. The presence of pulmonary hypertension was defined by a PASP greater than 35 mmHg. In the setting of an incomplete TR jet velocity or indeterminate RAP, a TR velocity greater than 2.8 m/s was also considered to be positive for the presence of pulmonary hypertension.
Valvular regurgitation for aortic, mitral, tricuspid, and pulmonary valves was classified by severity based on qualitative assessment via color Doppler and continuous wave Doppler when available. Vena contracta width, flow convergence, jet area, and spectral wave density were used as supplemental measures when available to determine valvular regurgitation severity.11
LV diastolic function was defined by the incorporation of pulsed wave Doppler interrogation between the mitral leaflet tips to determine the peak modal velocity in early diastole (E-wave) and late diastole (A-wave) at the leading edge of the spectral waveform for E/A ratio, tissue Doppler interrogation of the lateral and septal basal regions for e’ velocity, average E/e’ ratio, TR velocity, and left atrial volume index (LAVI) as per the ASE guidelines.12 Left atrial volumes (LAV) were measured in the apical views at the end of LV systole using the biplane disk summation technique with incorporation of body surface area (BSA) to obtain the LAVI.
Study outcomes
The primary outcome was 30-day all-cause inpatient mortality. Secondary outcomes were utilization of mechanical ventilator support, intravenous vasopressors, or renal replacement therapy within 30 days during the index hospitalization. All-cause mortality was defined by death due to any etiology within 30 days during hospitalization for COVID-19. Mechanical ventilator support was defined by respiratory failure and the need for endotracheal intubation. Utilization of intravenous vasopressors was defined by the use of any vasoactive agents including intravenous norepinephrine, phenylephrine, vasopressin, dopamine, or epinephrine. Renal replacement therapy was defined by kidney failure requiring hemodialysis or continuous renal replacement therapy. All patients were evaluated for an outcome event within 30 days of the admission date to the hospital. Patient outcomes that did not achieve 30-day criteria were excluded from the analysis. The final follow-up date was 24 May 2020.
Statistical analysis
Descriptive statistics of mean and standard deviation were used to describe the continuous variables. Frequency and percentage were used to describe the categorical variables. Multivariable binary logistic regression analysis for the outcome variables was conducted for each of the 13 echocardiographic predictor variables while simultaneously adjusting for age, sex, coronary artery disease, hypertension, diabetes mellitus, and chronic obstructive pulmonary disease. All significant binary logistic regression multivariable analyses had the Hosmer and Lemenshow goodness-of-fit test and the C-statistic reported. All p-values were two-tailed. Alpha level for significance was p < 0.05. IBM SPSS Statistics Version 26 was used for all analyses (IBM, 2019).
Results
Table 1 describes the sample characteristics. Mean age of the sample was 66.5 (SD = 16.51). There were 63.1% male and 48.6% of a minority race/ethnicity origin. The sample had multiple co-morbidities with 68.2% of the cohort with hypertension, 36.4% with diabetes mellitus, 24.8% with coronary artery disease, and 12.1% with history of COPD. All mean laboratory values including troponin-I, creatinine, lymphocyte count, D-dimer, CRP, and ferritin were elevated and above normal laboratory range limits. With regard to treatments, nearly 75% of the sample received hydroxychloroquine treatment.
Table 1.
Sample characteristics of the 214 COVID-19 patients.
| Variables | Mean (SD) or frequency (%) |
|---|---|
| Demographics | |
| Age (years), mean | 66.5 (16.51) |
| Sex (male) | 135 (63.1) |
| Race/Ethnicity | |
| White | 110 (51.4) |
| Black | 23 (10.7) |
| Asian | 30 (14.0) |
| Other | 23 (10.7) |
| Hispanic | 28 (13.1) |
| BMI (obese) (n = 170) | 69 (32.2) |
| Smoking (yes) (n = 125) | 28 (13.1) |
| Medical history | |
| Coronary artery disease (yes) | 53 (24.8) |
| Hypertension (yes) | 146 (68.2) |
| Diabetes mellitus (yes) | 78 (36.4) |
| COPD (yes) | 26 (12.1) |
| Heart failure (yes) | 18 (8.4) |
| Atrial fibrillation (yes) | 43 (20.1) |
| ESRD (yes) (n = 213) | 9 (4.2) |
| Cancer (yes) (n = 213) | 23 (10.7) |
| Laboratory values-mean (SD) | |
| Creatinine (mg/dl) | 3.2 (2.96) |
| Troponin I (ng/ml) (n = 202) | 2.9 (11.02) |
| Lymphocyte count (%) (n = 213) | 13.7 (9.87) |
| D-dimer (DDU ng/ml) (n = 161) | 1574.6 (1355.90) |
| CRP (mg/dl) (n = 206) | 22.3 (13.68) |
| Ferritin (ng/ml) (n = 198) | 1528.2 (1920.60) |
| COVID-19 treatments | |
| Hydroxycholoroquine (yes) | 160 (74.8) |
| Tocilizumab (yes) | 38 (17.8) |
| Remdesivir (yes) | 24 (11.2) |
| 30-day outcomes | |
| All-cause mortality (yes) (n = 210) | 91 (42.5) |
| Renal replacement therapy (yes) (n = 211) | 61 (28.5) |
| Vasopressor (yes) | 116 (54.2) |
| Ventilator (yes) | 118 (55.1) |
Note: The median value for creatinine was 1.9 and for D-dimer was 927.0. SD: standard deviation; BMI: body mass index; CABG: coronary artery bypass grafting; COPD: chronic obstructive pulmonary disease; ESRD: end-stage renal disease; CRP: C-reactive protein; ICU: intensive care unit.
The median time from date of admission to performance of echocardiography study was three days. Table 2 describes the echocardiographic variables. RV enlargement was identified in greater than one-third (33.6%) with RV systolic dysfunction present in 28.5%. Additionally, the presence of pulmonary hypertension was identified in 35.0%. The mean value of RV S’ mean (13.1 cm/s) was within normal limits but not all patients were within normal limits. Left ventricular systolic dysfunction was identified in 26.6%. The mean left ventricular end-diastolic dimension size was normal. With regard to diastology, the highest prevalence was Grade I diastolic dysfunction. Among valvular regurgitation, the highest prevalence was tricuspid regurgitation, which was present in 53.3% and with 20.6% having moderate to severe tricuspid regurgitation. A pericardial effusion was seen in 10.3%.
Table 2.
Echocardiographic variables.
| Variables | Mean (SD) or frequency (%) |
|---|---|
| Left ventricular systolic function | |
| Normal | 157 (73.4) |
| Mildly reduced | 31 (14.5) |
| Moderately reduced | 12 (5.6) |
| Severely reduced | 14 (6.5) |
| Left ventricular EDD (mean) | 4.4 (0.69) |
| Right ventricular systolic function | |
| Normal | 150 (70.1) |
| Mildly reduced | 41 (19.2) |
| Moderate to severely reduced | 20 (9.3) |
| Indeterminate | 3 (1.4) |
| Right ventricular size | |
| Enlarged | 72 (33.6) |
| Indeterminate | 11 (5.1) |
| Right ventricular S′ [mean] | 13.1 (3.35) |
| Pulmonary hypertension | |
| Present | 75 (35.0) |
| Indeterminate | 107 (50.0) |
| Left ventricular diastolic function | |
| Normal | 35 (16.4) |
| Grade 1 diastolic dysfunction | 72 (33.6) |
| Grade 2 diastolic dysfunction | 12 (5.6) |
| Grade 3 diastolic dysfunction | 11 (5.1) |
| Unknown or indeterminate | 84 (39.3) |
| Mitral regurgitation | |
| None | 150 (70.1) |
| Mild | 43 (20.1) |
| Moderate to severe | 19 (8.9) |
| Indeterminate | 2 (0.9) |
| Aortic regurgitation | |
| None | 169 (79.0) |
| Mild | 27 (12.6) |
| Moderate to severe | 17 (7.9) |
| Indeterminate | 1 (0.5) |
| Tricuspid regurgitation | |
| None | 97 (45.3) |
| Mild | 70 (32.7) |
| Moderate to severe | 44 (20.6) |
| Indeterminate | 3 (1.4) |
| Pulmonic regurgitation | |
| None | 125 (58.4) |
| Mild | 38 (17.8) |
| Moderate to severe | 11 (5.1) |
| Indeterminate | 35 (16.4) |
| Left atrial volume index (mean) | 34.0 (19.39) |
| Pericardial effusion | |
| Present | 22 (10.3) |
| Indeterminate | 5 (2.3) |
EDD: end diastolic dimension.
There were 91 deaths (42.5%) that occurred within 30 days of the index hospitalization (Table 1). Table 3 shows multivariable logistic regression analyses for 30-day all-cause mortality. The presence of mildly reduced RV systolic function (OR: 3.51, 95% CI: 1.63–7.57, p = 0.001), moderate to severely reduced RV systolic function (OR: 7.30, 95% CI: 2.20–24.25, p = 0.001), pulmonary hypertension (OR: 5.39, 95% CI: 1.96–14.86, p = 0.001), and moderate to severe tricuspid regurgitation (OR: 3.92, 95% CI: 1.71–9.03, p = 0.001) were each significantly associated with higher odds for mortality.
Table 3.
Multivariable logistic regression analyses for 30-day all-cause inpatient mortality.
| Variables | Mortality OR (95% CI) | p-value | H C |
|---|---|---|---|
| Left ventricular systolic function | – | ||
| Normal | 1.00 | ||
| Mildly reduced | 2.11 (0.90, 4.94) | 0.09 | |
| Moderately reduced | 2.37 (0.63, 8.91) | 0.20 | |
| Severely reduced | 2.40 (0.71, 8.05) | 0.16 | |
| Left ventricular EDD | 1.21 (0.74, 1.95) | 0.45 | – |
| Right ventricular systolic function | 0.91 0.73 | ||
| Normal | 1.00 | ||
| Mildly decreased | 3.51 (1.63, 7.57) | 0.001 | |
| Moderate to severely reduced | 7.30 (2.20, 24.25) | 0.001 | |
| Right ventricular size (enlarged) | 1.76 (0.94, 3.28) | 0.08 | – |
| Right ventricular S′ | 0.89 (0.79, 1.004) | 0.06 | – |
| Pulmonary hypertension (present) | 5.39 (1.96, 14.86) | 0.001 | 0.17 0.74 |
| Left ventricular diastolic function | – | ||
| Normal | 1.00 | ||
| Grade 1 diastolic dysfunction | 0.62 (0.21, 1.81) | 0.38 | |
| Grade 2 diastolic dysfunction | 0.41 (0.08, 2.23) | 0.30 | |
| Grade 3 diastolic dysfunction | 1.04 (0.21, 5.15) | 0.97 | |
| Mitral regurgitation | – | ||
| None | 1.00 | ||
| Mild | 1.18 (0.56, 2.46) | 0.67 | |
| Moderate to severe | 1.30 (0.48, 3.56) | 0.61 | |
| Aortic regurgitation | – | ||
| None | 1.00 | ||
| Mild | 1.44 (0.59, 3.51) | 0.43 | |
| Moderate to severe | 1.26 (0.43, 3.65) | 0.67 | |
| Tricuspid regurgitation | 0.06 0.71 | ||
| None | 1.00 | ||
| Mild | 1.56 (0.79, 3.06) | 0.20 | |
| Moderate to severe | 3.92 (1.71, 9.03) | 0.001 | |
| Pulmonic regurgitation | – | ||
| None | 1.00 | ||
| Mild | 1.44 (0.64, 3.23) | 0.38 | |
| Moderate to severe | 0.82 (0.22, 3.15) | 0.78 | |
| Left atrial volume index | 1.01 (0.99, 1.02) | 0.60 | – |
| Pericardial effusion (present) | 1.32 (0.52, 3.40) | 0.56 | – |
Note: All multivariable analyses adjust for age, sex, coronary artery disease, hypertension, diabetes mellitus, and chronic obstructive pulmonary disease. Analyses classifying all missing data for pulmonary hypertension as absence of pulmonary hypertension also showed significantly increased odds for those with pulmonary hypertension (OR: 3.42, 95% CI: 1.79, 6.51, p < 0.001, H = 0.65, c = 0.71). OR: odds ratio; CI: confidence interval; EDD: end diastolic dimension; H: Hosmer and Lemenshow goodness-of-fit test; C: C statistic.
Most patients were critically ill as more than half of the cohort required mechanical ventilator support (55.1%) or vasopressor utilization (54.2%) within 30 days. Non-invasive ventilation of high-flow nasal cannula was utilized by 28.5% (n = 61) and 22.9% (n = 49) began with high-flow nasal cannula and then transitioned to mechanical ventilator support. Additionally, 28.5% of the patients required renal replacement therapy (Table 1). Table 4 shows multivariable logistic regression analyses for 30-day utilization of mechanical ventilator support, vasopressors, or renal replacement therapy. Moderate to severely reduced RV systolic function (OR: 3.49, 95% CI: 1.08–11.29, p = 0.04) and pulmonary hypertension (OR: 3.96, 95% CI: 1.33–11.75, p = 0.01) were each significantly associated with higher odds for ventilator use. Mildly reduced RV systolic function (OR: 2.26, 95% CI: 1.06–4.85, p = 0.04), moderate to severely reduced RV systolic function (OR: 3.69, 95% CI: 1.19–11.41, p = 0.02), and pulmonary hypertension (OR:4.88, 95% CI: 1.69–14.09, p = 0.003) were each significantly associated with higher odds for vasopressor use. Moderately reduced left ventricular systolic function (OR: 4.17, 95% CI: 1.12–15.53, p = 0.03), severely reduced left ventricular systolic function (OR: 3.44, 95% CI: 1.05–11.27, p = 0.04), moderate to severe tricuspid regurgitation (OR: 6.63, 95% CI: 2.58–17.02, p ≤ 0.001), mildly reduced RV systolic function (OR: 3.11, 95% CI: 1.43–6.74, p = 0.004), moderate to severely reduced RV systolic function (OR: 7.71, 95% CI: 2.56–23.22, p < 0.001), and enlarged RV size (OR: 3.11, 95% CI: 1.58–6.12, p = 0.001) were each significantly associated with higher odds for renal replacement therapy.
Table 4.
Multivariable logistic regression for 30-day ventilator use, vasopressor use, and renal replacement therapy.
| Variables | VentilatorOR (95% CI) | p-value | H C | VasopressorOR (95% CI) | p-value | H C | Renal RTOR (95% CI) | p-value | H C |
|---|---|---|---|---|---|---|---|---|---|
| Left ventricular | – | – | 0.88 0.70 | ||||||
| systolic function | |||||||||
| Normal | 1.00 | 1.00 | 1.00 | ||||||
| Mildly reduced | 1.13 (0.47, 2.70) | 0.79 | 1.29 (0.56, 2.98) | 0.55 | 1.29 (0.51, 3.27) | 0.59 | |||
| Moderately reduced | 2.20 (0.57, 8.51) | 0.25 | 3.87 (0.93, 16.05) | 0.06 | 4.17 (1.12, 15.53) | 0.03 | |||
| Severely reduced | 0.78 (0.23, 2.68) | 0.70 | 1.27 (0.38, 4.26) | 0.70 | 3.44 (1.05, 11.27) | 0.04 | |||
| Left ventricular EDD | 0.98 (0.60, 1.61) | 0.95 | – | 1.06 (0.66, 1.70) | 0.81 | – | 1.08 (0.65, 1.78) | 0.77 | – |
| Right ventricular systolic function | 0.18 0.75 | 0.43 0.69 | 0.88 0.75 | ||||||
| Normal | 1.00 | 1.00 | 1.00 | ||||||
| Mildly reduced | 1.55 (0.71, 3.35) | 0.27 | 2.26 (1.06, 4.85) | 0.04 | 3.11 (1.43, 6.74) | 0.004 | |||
| Moderate to severely reduced | 3.49 (1.08, 11.29) | 0.04 | 3.69 (1.19, 11.41) | 0.02 | 7.71 (2.56, 23.22) | <0.001 | |||
| Right ventricular size (enlarged) | 1.50 (0.79, 2.88) | 0.22 | – | 1.80 (0.96, 3.37) | 0.07 | – | 3.11 (1.58, 6.12) | 0.001 | 0.38 0.73 |
| Right ventricular S′ | 1.07 (0.96, 1.19) | 0.25 | – | 1.05 (0.95, 1.17) | 0.33 | – | 0.89 (0.79, 1.01) | 0.07 | – |
| Pulmonary hypertension (present) | 3.96 (1.33, 11.75) | 0.01 | 0.29 0.83 | 4.88 (1.69, 14.09) | 0.003 | 0.02 0.81 | 2.48 (0.85, 7.24) | 0.10 | – |
| LV diastolic function | – | – | – | ||||||
| Normal | 1.00 | 1.00 | 1.00 | ||||||
| Grade 1 dysfunction | 1.12 (0.38, 3.30) | 0.83 | 0.89 (0.32, 2.52) | 0.83 | 1.02 (0.31, 3.30) | 0.98 | |||
| Grade 2 dysfunction | 0.67 (0.12, 3.66) | 0.64 | 0.47 (0.09, 2.48) | 0.47 | 1.58 (0.24, 10.34) | 0.63 | |||
| Grade 3 dysfunction | 0.51 (0.10, 2.53) | 0.41 | 0.62 (0.13, 2.92) | 0.54 | 2.14 (0.40, 11.48) | 0.37 | |||
| Mitral regurgitation | – | – | – | ||||||
| None | 1.00 | 1.00 | 1.00 | ||||||
| Mild | 0.53 (0.24, 1.15) | 0.11 | 0.68 (0.32, 1.42) | 0.30 | 0.69 (0.29, 1.65) | 0.40 | |||
| Moderate to severe | 0.39 (0.13, 1.19) | 0.10 | 0.67 (0.24, 1.87) | 0.45 | 1.01 (0.32, 3.14) | 0.99 | |||
| Aortic regurgitation | – | – | – | ||||||
| None | 1.00 | 1.00 | 1.00 | ||||||
| Mild | 0.70 (0.28, 1.76) | 0.45 | 0.67 (0.28, 1.64) | 0.38 | 0.70 (0.23, 2.09) | 0.52 | |||
| Moderate to severe | 0.41 (0.13, 1.27) | 0.12 | 1.05 (0.36, 3.09) | 0.93 | 0.69 (0.20, 2.37) | 0.56 | |||
| Tricuspid regurgitation | – | – | 0.64 0.74 | ||||||
| None | 1.00 | 1.00 | 1.00 | ||||||
| Mild | 1.28 (0.64, 2.57) | 0.49 | 1.64 (0.84, 3.21) | 0.15 | 1.95 (0.90, 4.22) | 0.09 | |||
| Moderate to severe | 1.16 (0.52, 2.63) | 0.72 | 1.66 (0.75, 3.68) | 0.21 | 6.63 (2.58, 17.02) | <0.001 | |||
| Pulmonic regurgitation | – | – | – | ||||||
| None | 1.00 | 1.00 | 1.00 | ||||||
| Mild | 0.49 (0.21, 1.13) | 0.09 | 0.58 (0.26, 1.30) | 0.19 | 1.67 (0.67, 4.15) | 0.27 | |||
| Moderate to severe | 0.68 (0.17, 2.69) | 0.59 | 0.66 (0.18, 2.49) | 0.54 | 2.98 (0.73, 12.16) | 0.13 | |||
| Left atrial volume index | 1.01 (0.99, 1.03) | 0.56 | – | 1.00 (0.98, 1.02) | 0.95 | – | 1.01 (0.99, 1.03) | 0.33 | – |
| Pericardial effusion (present) | 1.39 (0.52, 3.71) | 0.52 | – | 1.84 (0.71, 4.81) | 0.21 | – | 1.33 (0.48, 3.69) | 0.58 | – |
Note: All multivariable analyses adjust for age, sex, coronary artery disease, hypertension, diabetes mellitus, and chronic obstructive pulmonary disease. Analyses classifying all missing data for pulmonary hypertension as absence of pulmonary hypertension showed significantly increased odds for those with pulmonary hypertension for ventilator (OR: 2.30, 95% CI: 1.17, 4.52, p = 0.02, H = 0.19, c = 0.75), vasopressor (OR: 2.58, 95% CI: 1.34, 4.97, p = 0.01, H = 0.99, c = 0.70), renal replacement therapy (OR: 3.10, 95% CI: 1.55, 6.20, p = 0.001). OR: odds ratio; CI: confidence interval; RT: replacement therapy; EDD: end diastolic dimension; H: Hosmer and Lemenshow goodness-of-fit test; C: C statistic.
Discussion
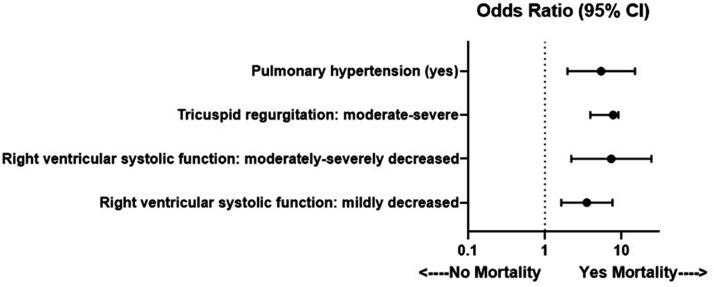
We found RV systolic dysfunction regardless of severity had increased odds for 30-day all-cause mortality. In addition, the risk was proportional to the degree of RV dysfunction with moderate to severe RV systolic dysfunction resulting in higher odds than mild RV dysfunction. For secondary outcomes, any degree of RV systolic dysfunction was associated with increased odds for utilization of vasopressors and renal replacement therapy. Pulmonary hypertension was also associated with adverse 30-day clinical outcomes of increased odds for all-cause inpatient mortality, need for mechanical ventilation, and vasopressor utilization. Moderate to severe tricuspid regurgitation was associated with increased odds for 30-day all-cause mortality and renal replacement therapy (see Fig. 1).
Fig. 1.
Echocardiographic variables associated with 30-day all-cause inpatient mortality. Forest plot of echocardiographic variables associated with 30-day all-cause inpatient mortality. All multivariable analyses adjust for age, sex, coronary artery disease, hypertension, diabetes mellitus, and chronic obstructive pulmonary disease. CI: confidence interval.
Acute right heart failure or systolic dysfunction can occur due to increased RV afterload or decreased RV contractility.13 COVID-19 is often associated with chest CT scans findings of bilateral multilobar ground-glass opacifications14 and associated hypoxic respiratory failure, which can increase RV afterload. In addition, COVID-19 may predispose patients to having venous thrombotic events, including pulmonary embolism, which may further increase RV afterload.15 RV dysfunction compromises cardiac output, and the shifting of the interventricular septum towards the left can compromise LV diastolic filling and reduce systemic output.16 This reduced cardiac output may help explain the increased odds for vasopressors. This may also contribute to renal hypoperfusion. In addition, as RV dysfunction progresses, there is usually a rise in central venous pressure and subsequent rise in renal venous pressure which may reduce GFR.17 This may explain why we found that any degree of RV systolic dysfunction was associated with an increased odds for both vasopressor support and renal replacement therapy. Additionally, approximately one-third of our cohort had RV enlargement. We found that RV enlargement was independently associated with renal replacement therapy. A previous study of 110 COVID-19 patients with RV enlargement reported an association with increased mortality.7 We did not show an association between RV enlargement and mortality with our larger sample size. This suggests that further studies are needed before one can conclude that RV enlargement is associated with increased mortality.
There is concern that patients with COVID-19 develop ARDS.18 RV systolic dysfunction is independently shown to be associated with worse outcomes in patients with ARDS.19 In our cohort, moderate to severe RV systolic dysfunction was associated with increased odds for mechanical ventilator support. In ARDS, the key reasons for RV dysfunction include elevated pulmonary vascular resistance secondary to imbalance between vasodilators and vasoconstrictors, hypoxic pulmonary vasoconstriction, endothelial injury, thrombosis, and possibly contractile impairment.20 There may be a role of inflammation as well in the development of RV dysfunction with IL-6 being associated with RV dysfunction.21 Higher IL-6 is associated with RV dysfunction independent of pulmonary vascular disease burden in pulmonary arterial hypertension. Thus, the inflammatory storm caused by COVID-19 may also contribute to RV dysfunction.22
Pulmonary hypertension was also associated with adverse 30-day clinical outcomes of increased odds for all-cause inpatient mortality, vasopressor utilization, and need for mechanical ventilator support. However, one of the limitations of PASP assessment on echocardiography is that non-physiologic TR jets (<0.8 m/s) and incomplete Doppler envelopes due to a lack of significant TR were excluded from the initial analysis unless the TR velocity was greater than 2.8 m/s. In addition, patients with unknown RAP in the setting of being mechanically ventilated were excluded in our primary analysis unless the TR velocity was greater than 2.8 m/s. We then performed an additional analysis by imputing that all patients who had an incomplete TR Doppler envelope or unknown RAP in the setting of TR velocity <2.8 m/s as having an absence of pulmonary hypertension. Our findings remained consistent with the original analysis as pulmonary hypertension continued to be associated with an increased odds for the adverse 30-day clinical outcomes.
Pulmonary hypertension has important prognostic value and is an independent predictor of mortality in cardiac conditions including chronic left heart failure23 and is associated with reduced functional status and worse outcomes in chronic lung disease.24 We suspect pulmonary hypertension in patients with COVID-19 is likely multifactorial and may be related to ventilation perfusion mismatch in the setting of significant hypoxic respiratory failure and acute respiratory distress syndrome, increased pulmonary vascular tone in the setting of inflammatory state, and presence of microthrombi or pulmonary embolism in the lungs. Recent autopsy studies with COVID-19 patients noted evidence of pulmonary microthrombi,18 which can lead to increased pulmonary artery systolic pressure and eventually RV failure. Further investigation is warranted in regard to the extent of pulmonary hypertension in COVID-19 patients and the etiology for such occurrences.
In a single-center, observational, cross-sectional study, involving hospitalized non-ICU patients, another study also showed that pulmonary hypertension was associated with a higher rate of in-hospital mortality. However, they did not establish RV dysfunction to be associated with these findings. They defined RV dysfunction by reduced tricuspid annular plane systolic excursion or RV S’.25 Mean RV S’ in our study was 13.1 cm/s, which was also within normal limits, and was not identified to be significantly associated with any of the 30-day clinical outcomes. While RV S’ is often used as a surrogate marker for RV systolic function, it is a reflection of contractility of the RV basal lateral segment10 and mostly reflects longitudinal myocardial contraction. It does not take into consideration radial and circumferential fiber shortening. As a result, it may not correlate with RV dysfunction involving the mid to apical or septal RV segments. Furthermore, RV dysfunction in the setting of pulmonary embolism often does not involve the basal segment.8 RV longitudinal strain from 2D speckle-tracking echocardiography is likely a more accurate estimate of RV systolic function. Abnormal RVLS has been shown to be an independent predictor of higher mortality in COVID-19 patients.6 Also, in a smaller cohort of 115 patients with COVID-19 pneumonia, elevated mean pulmonary arterial pressure and RV dysfunction were each associated with higher risk for in-hospital mortality.26 This finding for COVID-19 pneumonia is similar to our finding for COVID-19 patients of RV dysfunction associated with increased odds for mortality. We suggest careful monitoring of RV dysfunction in COVID-19 patients.
Moderate to severe tricuspid regurgitation was observed in approximately 20% of patients in this cohort and was associated with an increased odds for mortality and need for renal replacement therapy. In addition, RV enlargement was associated with an increased need for renal replacement therapy. Reduced RV stroke volume results in RV enlargement and subsequent increase in tricuspid regurgitation.13 We suggest that tricuspid regurgitation was functional in the setting of RV enlargement, RV dysfunction and pulmonary hypertension.
Moderate to severe LV systolic dysfunction was only associated with an increased need for renal replacement therapy. Left ventricular dysfunction can lead to the development of kidney injury and is classified as cardiorenal syndrome type 1.27 This is associated with adverse outcomes, including mortality.28 We did not identify any association with 30-day mortality, need for mechanical ventilation or vasopressor use. We suggest that our lack of significance may be due to small sample size. Diastolic dysfunction was also not statistically associated with any of the 30-day clinical outcomes. In addition, the highest prevalence was Grade I diastolic dysfunction with impaired LV relaxation but normal LV filling pressures, which is not unexpected considering that the mean age of our sample was approximately 66 years.
Limitations
There are several limitations. First, due to the COVID-19 pandemic and to ensure sonographer safety, our echocardiography laboratory had a modified, focused transthoracic echocardiogram protocol. For example, quantitative valvular stenosis was not assessed on the majority of studies. In addition, ultrasound contrast enhancing agents were not administered in the majority of patients to minimize acquisition time for the sonographers in the setting of COVID-19. Second, some patients were placed in the prone position due to advanced respiratory failure, and thus could not have an echocardiogram study performed. Third, this was a retrospective analysis with data obtained at a single center, and echocardiograms were only performed on patients that had requests placed by the primary providers. As a result, we cannot exclude selection bias. Fourth, RV longitudinal strain by 2D speckle tracking to objectively assess RV systolic function was not performed. Fifth, although we had many significant associations which suggests that for those associations that the study was adequately powered, it is possible that our non-significant associations were not adequately powered. Sixth, this is a cross-sectional study that can only report associations of variables. A clinical trial is necessary to determine if our predictors lead to and cause the outcomes. Seventh, obesity and tobacco use are known risk factors for adverse outcomes. However, due to unknown data in many patients, we could not adjust for these factors in our analyses. Eighth, we did not have the exact time the laboratory variables were obtained and included laboratory variables to describe the sample.
Conclusion
In patients with COVID-19, we found that any degree of RV systolic dysfunction, pulmonary hypertension, and moderate to severe tricuspid regurgitation were each associated with increased odds for 30-day all-cause inpatient mortality. This study highlights the importance of echocardiography and its clinical utility and prognostic value in the evaluation of hospitalized COVID-19 patients. Echocardiography in COVID-19 patients may help identify patients that may benefit from earlier implementation of therapeutic interventions such as antivirals, immunomodulators, anticoagulation, and/or convalescent plasma.
Footnotes
Author contributions: J. J. is the guarantor of the paper, taking responsibility for the content and integrity of the work. He had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. K. W., D. R., K. W. P., A. S., M. G., M. M., J. S. and J. J. were involved in the design and conception of the study. K. W., D. R., M. P. T., and J. J. were involved in the acquisition of the data. J. F., K. W., D. R., and J. J. were involved in the data analysis. All named authors were involved in the interpretation of the data and drafting or critical revision of the manuscript for intellectual content. All authors approved the final manuscript.
Conflict of interest: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: K. W. P. served on an advisory board for Actelion and receives grant funding from United Therapeutics.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: K. W. P. is funded by NIH K08 HL140100, the Cardiovascular Medical Research and Education Fund, a Lillehei Heart Institution Cardiovascular Seed Grant, and the United Therapeutics Jenesis Award.
ORCID iDs: Kurt W. Prins https://orcid.org/0000-0002-0364-6742
Jessen Jacob https://orcid.org/0000-0003-3475-2481
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 Data, www1.nyc.gov/site/doh/covid/covid-19-data.page (accessed 7 September 2020).
- 3.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020; 5: 802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020; 5: 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li LQ, Huang T, Wang YQ, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol 2020; 92: 577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Li H, Zhu S, et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC: Cardiovasc Imaging 2020; 13: 2287–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Argulian E, Sud K, Vogel B, et al. Right ventricular dilation in hospitalized patients with COVID-19 infection. JACC Cardiovasc Imaging 2020; 13: 2459–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McConnell MV, Solomon SD, Rayan ME, et al. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol 1996; 78: 469–473. [DOI] [PubMed] [Google Scholar]
- 9.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 16: 233–271. [DOI] [PubMed] [Google Scholar]
- 10.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–788. [DOI] [PubMed] [Google Scholar]
- 11.Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017; 30: 303–371. [DOI] [PubMed] [Google Scholar]
- 12.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29: 277–314. [DOI] [PubMed] [Google Scholar]
- 13.Konstam MA, Kiernan MS, Bernstein D, et al. Evaluation and management of right-sided heart failure: a scientific statement from the American Heart Association. Circulation 2018; 137: e578–e622. [DOI] [PubMed] [Google Scholar]
- 14.Salehi S, Abedi A, Balakrishnan S, et al. Coronavirus Disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol 2020; 215: 87–93. [DOI] [PubMed] [Google Scholar]
- 15.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol 2020; 75: 2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haddad F, Hunt SA, Rosenthal DN, et al. Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008; 117: 1436–1448. [DOI] [PubMed] [Google Scholar]
- 17.Damman K, van Deursen VM, Navis G, et al. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 2009; 53: 582–588. [DOI] [PubMed] [Google Scholar]
- 18.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020; 383: 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intens Care Med 2013; 39: 1725–1733. [DOI] [PubMed] [Google Scholar]
- 20.Price LC, Wort SJ, Finney SJ, et al. Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review. Crit Care 2010; 14: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dewachter C, Dewachter L, Rondelet B, et al. Activation of apoptotic pathways in experimental acute afterload-induced right ventricular failure. Crit Care Med 2010; 38: 1405–1413. [DOI] [PubMed] [Google Scholar]
- 22.Prins KW, Archer SL, Pritzker M, et al. Interleukin-6 is independently associated with right ventricular function in pulmonary arterial hypertension. J Heart Lung Transplant 2018; 37: 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bursi F, McNallan SM, Redfield MM, et al. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol 2012; 59: 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nathan SD, Barbera JA, Gaine SP, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J 2019; 53: 1801914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagnesi M, Baldetti L, Beneduce A, et al. Pulmonary hypertension and right ventricular involvement in hospitalized patients with COVID-19. Heart 2020; 106: 1324–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Andrea A, Scarafile R, Riegler L, et al. Right ventricular function and pulmonary pressures as independent predictors of survival in patients with COVID-19 pneumonia. JACC Cardiovasc Imaging 2020; 13: 2467–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ronco C. Cardiorenal syndromes: definition and classification. Contrib Nephrol 2010; 164: 33–38. [DOI] [PubMed] [Google Scholar]
- 28.Cowie MR, Komajda M, Murray-Thomas T, et al.; POSH Investigators. Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: results of the prospective outcomes study in heart failure (POSH). Eur Heart J 2006; 27: 1216–1222. [DOI] [PubMed] [Google Scholar]