Abstract
Background.
Child abuse and other forms of adversity are associated with alterations in threat processing and emotion regulation brain circuits.
Objective.
The goal of the current investigation is to determine if the availability of positive social support can ameliorate the negative impact of adversity on these brain systems.
Participants and Setting.
Subjects included 55 children ages 7–16 (X=11.8, SD=2.0). Approximately one-third of the cohort had no significant history of adversity, one-third had a history of moderate adversity, and one-third had a history of severe adversity. Brain imaging was conducted at the University of Vermont using a 3.0 Tesla Philips scanner.
Methods.
The Emotional Go-NoGo task with fearful and calm facial stimuli was used to assess the neural correlates of threat processing and emotion regulation in children during functional magnetic resonance imaging (fMRI). Dimensional measures of anxiety, social supports, and children’s adverse experiences were also obtained.
Results.
A conjunction analysis was used to test if trauma-related brain activation in responding to fearful vs. calm targets was impacted by social support. This approach identified multiple activation foci, including a cluster extending from the left amygdala to several other key brain regions involved in emotion regulation, including the orbitofrontal cortex, anterior cingulate cortex (ACC), anterior insula, nucleus accumbens, and frontal pole (Family Wise Error (FWE) correction, p < 0.05).
Conclusions.
Greater social support may reduce the effect that adversity has on neural processing of threat stimuli, consistent with the protective role of positive supports in promoting resilience and recovery demonstrated in the literature.
Keywords: Child maltreatment, adverse childhood experiences, neuroimaging, threat processing, social support
1. Introduction
Globally, approximately 1 billion children ages 2–17 years will experience child maltreatment in a given year (Hillis, Mercy, Amobi, & Kress, 2016). Over the past two decades there has been a burgeoning of research on the effects of child maltreatment on brain development (Bick & Nelson, 2016; De Bellis et al., 1999; R. J. Herringa, 2017). A recent meta-analysis of 20 investigations that used various functional magnetic resonance imaging (fMRI) paradigms to study emotion processing in maltreated individuals concluded child abuse and neglect are associated with increased bilateral amygdala activation to emotional faces (Hein & Monk, 2017). The amygdala is a key brain structure known to be involved in threat processing and emotion regulation (LeDoux, 2007), with increases in amygdala activation to emotional stimuli observed in patients with Posttraumatic Stress Disorder (PTSD; (Garrett et al., 2012), Major Depressive Disorder (MDD; (Peluso et al., 2009), and adults maltreated as children without a history of psychiatric illness, suggesting this may be a vulnerability marker for mood and anxiety disorders (Dannlowski et al., 2012).
In our prior studies with maltreated children, positive social supports were found to decrease risk for the development of depressive disorders (Kaufman, 1991), minimize the likelihood of hypothalamic pituitary adrenal stress axis abnormalities (Kaufman, 1991), and significantly moderate the vulnerability conferred by high risk genes associated with psychopathology (Kaufman et al., 2006; Kaufman et al., 2004). Positive social supports are also known to decrease risk for the development of PTSD (Fletcher, Elklit, Shevlin, & Armou, 2017; Lowe, 2017), and the absence of social supports and exposure to ongoing psychosocial adversity are strong predictors of PTSD chronicity (Brewin, Andrews, & Valentine, 2000; Charuvastra & Cloitre, 2008; Pynoos, Steinberg, & Piacentini, 1999). Parental support in particular, is a potent factor in moderating the effects of trauma in children (Valentino, Berkowitz, & Stover, 2010).
1.1. Hypothesis
Given the protective role of social supports it was hypothesized that the availability of positive supports will ameliorate the negative impact of child aversity on the functioning of the amygdala and other brain regions involved in threat processing and emotion regulation. The fMRI Emotional Go-NoGo task (Hare et al., 2008) was administered to test this hypothesis. The Emotional Go-NoGo Task has been used extensively in the literature (Hein & Monk, 2017), and shown to effectively tap these neural systems in pediatric trauma (Hare et al., 2008; Malter Cohen et al., 2013) and adult trauma (Miller et al., 2015; Sadeh et al., 2015) populations. Given emerging findings on the negative impact of a broad range of stressful life experiences on the structure and function of key brain regions in the emotion and threat processing circuits (Bilek et al., 2019; Oshri et al., 2019), in the current investigation a total dimensional adversity score was derived for youth which included assessments of a number of intrafamilial and extrafamilial negative life experiences. It was hypothesized that greater adversity would be associated with increased amygdala activation in processing threat stimuli, and increased activation in this region would be associated with higher anxiety scores.
2. Method
2.1. Sample
Participants in this study were part of a larger investigation that examined risk and resilience in maltreated children (Kaufman, Montalvo-Ortiz, et al., 2018; Kaufman, Wymbs, et al., 2018; Orr et al., 2016). The imaging data reported in this manuscript were available on a sample of 55 right-handed children ages 7–16 (X=11.8, SD=2.0): 36 healthy control children with no history of referral to protective services and 19 maltreated children, 10 who received in-home child welfare services and 9 with a recent out-of-home placement. The children came from a wide range of different socioeconomic backgrounds (SES; Hollingshead X-3.46, SD-1.4, Range 1–5), but the groups did not differ in terms of Hollingshead SES scores (F=0.31, ns).
Maltreated children were recruited through collaboration with the State of Vermont child protective services, and healthy control children were recruited from the community via newspaper ads and flyers. The presence or absence of a maltreatment history was verified through child protective services records and child and parent report. Inclusion in the healthy control group required no reported or documented history of maltreatment and scores below established clinical thresholds on the Screen for Child Anxiety and Related Emotional Disorders (SCARED; (Birmaher, Khetarpal, et al., 1997) the Mood and Feelings Questionnaire (MFQ; (Angold et al., 1995) and the internalizing and externalizing scales of the Child Behavior Checklist (CBCL; (Achenbach & Rescorla, 2001).
Go-NoGo fMRI data were collected on an additional 38 children who were excluded from the final sample for poor performance on the Go-NoGo task (N=25, ≤ 5 correct responses in each non-target condition), or due to excessive motion (N=13, motion criteria for exclusion provided below). There were no differences between the children included and excluded in the final sample in terms of maltreatment status, sex, age, MFQ, SCARED, or CBCL data (p > .05, all comparisons).
2.2. Procedures
The present cross-sectional study was approved by the IRBs at the University of Vermont, Johns Hopkins, and Yale University. Prior to recruitment, an independent child advocate reviewed each case referred through protective services to determine that research participation was in the child’s best interest. The ‘best interest’ standard was set by the University of Vermont IRB, with only one child from the larger Risk and Resilience Study cohort excluded by the independent child advocate, with that child excluded due to his state of acute psychiatric instability. The child’s parent or legal guardian provided informed consent and each child provided assent for study participation. Birth parent assent for child participation for children in state custody was obtained when clinically appropriate (i.e., ongoing parent-child contact). Clinical measures were collected in 1:1 sessions with each child, and children received $20 for completion of the clinical questionnaires and $60 for completion of the imaging protocol.
2.3. Measures.
Anxiety.
Children completed the Screen for Child Anxiety Related Emotional Disorders (SCARED), a 41 item rating scale developed to assess anxiety symptoms in children and adolescents (Birmaher, Khetarpol, et al., 1997). The SCARED has exceptional psychometric properties and the internal consistency of the measure in the present study was excellent (Cronbach’s alpha=0.931).
Childhood Adversity.
As detailed elsewhere (Kaufman, Montalvo-Ortiz, et al., 2018; Kaufman, Wymbs, et al., 2018), multiple informants and data sources (i.e., parents, children, protective services records) were used to obtain a best estimate of each child’s adverse life experiences. The data from these various sources were integrated and rated using the Yale-Vermont Adversity in Childhood Scale (Y-VACS) scoring procedures (Holbrook et al., 2015). The Y-VACS assesses a range of intra-familial (i.e., physical abuse, sexual abuse, neglect, witnessing domestic violence, caregiver substance abuse, parental separation, caregiver incarceration) and extra-familial (i.e., community violence, bullying, natural disasters) adversities, and generates scores that consider severity and frequency of exposure. Y-VACS generates a total adversity score, intra-familial adversity score, and extra-familial adversity score. Y-VACS scores have high inter-rater reliability (Holbrook et al., 2015), and strong convergent (Kaufman, Montalvo-Ortiz, et al., 2018; Kaufman, Wymbs, et al., 2018) and predictive (Grasso, DiVietro, Beebe, M., & G., 2019) validity. The dimensional total adversity Y-VACS scores were used in the analyses in the present investigation (inter-rater reliability, α = .95, p < .001). No between group contrasts were conducted given the heterogeneity of adversity experiences and total Y-VACS scores among the community controls never referred to protective services (X=14.8, SD=9.2, Range=2–37), children recruited through the community with prior protective services referrals (X=24.4, SD=11.6, Range=5–38), and the children recruited through protective services with a recent out-of-home placement (X=33.8, SD=11.7, Range=25–47). While the community controls had no history of child abuse or neglect, 29% witnessed domestic violence, 23% had a parent who struggled with a substance use disorder, 17% had a parent who was incarcerated at some point during their childhood, 57% came from non-intact families, 6% had a parent with a significant mental health problem, and 11% of the youth reported severe histories of bullying experiences.
Social Supports.
The Arizona Social Support Interview Schedule (ASSIS) is an interview that was originally developed for adults and was revised for use with school-aged children (Barrera, 1980; Kaufman, 1991). During the interview, children are asked to name people they (i) talk to about personal things; (ii) count on to buy the things they need; (iii) share good news with; (iv) get together with to have fun; and (v) go to if they need advice. Sixty-six percent of the youth listed their biological mother as their top support, 9% listed their grandmother, 7% listed their father, 7% listed a friend, 6% listed a sibling, and 5% listed another adult as their top support. A continuous measure reflecting the total number of positive support categories listed for the child’s biological mother was the social support index examined in the current report. Exploratory analyses were done with the measure that included the number of positive categories the child listed for his or her top support, but as the analyses were not significant, they are not presented in this manuscript.
Emotional Go-NoGo Task.
The Emotional Go-NoGo Task presents participants with grayscale images of fearful and calm faces and participants are instructed to press a button (i.e., “Go”) when an image with the target facial expression appears on the screen, and withhold (i.e., “NoGo”) responding when the other non-target facial expression appears (Hare et al., 2008; Tottenham et al., 2011). This task requires participants to regulate the automatic avoidance response induced by threat stimuli and respond as quickly as possible when fearful faces are targets. Children completed two blocks (5min 30s each) of the Emotional Go-NoGo task, one in which fearful faces were targets (75%) to which they were told to respond as quickly as possible, and calm faces were non-targets to which they were told to withhold responding. In the other block, calm faces served as targets (75%) and fearful faces served as non-targets. Presentation of target and non-target stimuli was pseudorandomized within a run, and the order of fearful and calm target presentation across runs was balanced across participants. Face stimuli were presented for 500 ms, and the inter-trial interval varied between 2 and 14 s, with a mean interval of 5 s. In total, each run contained 48 trials, with 36 target and 12 non-target trials.
fMRI acquisition and preprocessing.
Children were scanned at the University of Vermont using a 3.0 Tesla Philips Achieva scanner (Philips Healthcare, Best, the Netherlands) with a 12-channel head coil. A total of 155 functional volumes (33 slices/volume) per run were collected using a gradient-recalled echo planar sequence along an oblique axial plane with anterior-posterior phase encoding (TR/TE = 2000/35 ms, flip angle = 90°, 3.5 mm slice thickness with no gap were obtained in the axial oblique plane, parallel to the AC-PC line using a FOV of 240 mm and a matrix size of 128 × 96). Field map correction for magnetic inhomogeneities was accomplished by acquiring images with offset TE at the end of the functional series.
Preprocessing of functional images was carried out using SPM12 (Wellcome Trust Centre for Neuroimaging, London, United Kingdom). Separately for each functional run, scans were slice-time adjusted with respect to the first slice acquired during the TR as reference, and rigid body realignment parameters were estimated to adjust for displacement between volumes. Scans were then normalized to MNI-152 template space producing 3 mm isotropic voxels, and then spatially smoothed using a Gaussian filter with a kernel of 8 mm full-width at half-maximum. FSL Motion Outliers was then used to detect functional volumes that may have been corrupted by motion as defined by an outlier threshold as the upper limit used when creating boxplots (75th percentile + 1.5 times InterQuartile Range). These volumes were included as stick regressors in a confound matrix that also included the 6 degrees of freedom (DOF) realignment parameters, which were modeled as nuisance regressors in first-level analysis.
First-level estimation of the BOLD response for each participant was modeled using general linear model (GLM) analyses. An event-related approach modeled the BOLD response separately for correct fearful and calm targets, correct fearful and calm non-targets, and an additional regressor for any aberrant response. Because a formal analysis of error was not anticipated, aberrant trials were combined to include omission and commission errors and any target responses considered too slow (RT > 1000 ms) or too fast (RT < 200 ms). Events were convolved using the canonical hemodynamic response function and temporal and dispersion derivatives of modeled events. First-level condition images (fearful target, fearful non-target, calm target, calm non-target) were generated as the combined amplitude of the estimation of BOLD, in addition to temporal and dispersion derivatives. The following planned contrasts were then carried out: fear target > calm target, fear non-target > calm non-target. Analyses of NoGo trials were also examined, but as there were no significant findings, they are not presented. In addition, to confirm sensorimotor-related activation during targeted responding, the contrast Go > NoGo, collapsing over calm and fearful conditions was conducted and revealed the expected pattern of voxels for the execution of a button press response, showing cortical and subcortical sensorimotor regions with a lateralization to the contralateral left hemisphere, as expected as all the children in the study were right handed and responded using their right index finger. Participants were excluded from higher-level analyses for one of three reasons: (1) ≥ 10 functional volumes in a run with motion spikes ≥ 3 mm (framewise displacement, FD), (2) average FD of a run ≥ group mean FD + 3SD, or (3) for any given run, the lack of functional activation in motor cortex contralateral to their response hand for target trials.
2.4. Data Analyses
Effects of emotional expression on reaction time (RT) and accuracy, and their association with dimensional scales of trauma (Y-VACS) and anxiety (SCARED) were analyzed using paired samples t-tests and linear regression in MATLAB. Mixed-effects analyses were initially used to examine the main effects of the covariates of interest (Y-VACS total score, biological mother support) and non-interest (age, sex) on activation differences observed in the planned contrasts (fear target > calm target, fear non-target > calm non-target). A global conjunction analysis was then used to determine if trauma-related activation differences observed during the planned contrasts of the targets (fear target > calm target) covaried as a function of social support from the biological mother. A separate exploratory analysis was performed using the SCARED total score as a covariate, replacing Y-VACS total score in the model, because the SCARED and Y-VACS were highly correlated (r=0.49, p < .001). Significance was determined first by applying a cluster extent threshold of p < 0.005, a threshold commonly employed in the literature (Woo, Krishnan, & Wager, 2014), and then applying cluster-level family-wise error (FWE) correction, p < 0.05. As noted previously, additional exploratory analyses of the non-target trials (fear non-target > calm non-target) were also examined with covariates, but as there were no significant findings, they are not presented.
3. Results
3.1. Demographic and Clinical Measures
The demographic and clinical characteristics of the sample are depicted in Table 1. Group differences on the demographic and clinical measures were examined using ANOVAs and Chi-Square analyses. The groups did not differ on age, sex, race, or SES (p > .05, all comparisons). As expected, maltreated children had significantly higher SCARED scores than the healthy control children (F=19.5, p < .001), with 9 (47%) of the maltreated children above the clinical threshold on the SCARED. Maltreated children also had significantly greater scores on the Y-VACS intrafamilial adversity scale (F=25.4, p < .001), extrafamilial Y-VACS adversity scale (F=4.1, p < .05), and total adversity Y-VACS scale (F=25.9, p < .001). On the social support questionnaire, only 2 (5.6%) of the children in the comparison group listed their biological mother for two or fewer categories of positive support, 6 (16.7%) listed their mother for three categories, and 28 (77.7%) listed their mother for four or five categories of support. Among the maltreated children, 9 (47.4%) listed their biological mother for two or fewer categories of positive support, 2 (10.5%) listed their mother for three categories, and 8 (42.1%) listed their mother for four or five categories of positive support (X2 = 18.5, df=5, p < .002). As noted previously, adversity was analyzed dimensionally and no between group contrasts were conducted given the heterogeneity of adversity experiences and total Y-VACS scores among the children in both groups (see description of Y-VACS measure for details).
Table 1:
Demographic and Clinical Characteristics of the Sample (N=55)
| Maltreated Children N=19 | Control Children N=36 | Statistic p-value | |
|---|---|---|---|
| Age | 12.3 ± 2.1 | 11.4 ± 1.9 | F (1) = 1.85 p = ns |
| Sex (% Female / %Male) | 42% / 58% | 60% / 40% | X2=1.03 p = ns |
| Race (EA/AA or Biracial) | 90% / 10% | 85% / 15% | X2=2.5 p = ns |
| Hollingshead Socioeconomic Status | 3.17 ± 1.5 | 3.51 ± 1.4 | F (1) = 0.31 p = ns |
| SCARED Questionnaire | 30.7 ± 17.0 | 15.4 ± 6.1 | F (1) = 19.5 p < .001 |
| Y-VACS Intra-familial Adversity Score | 22.0 ± 9.9 | 9.6 ± 7.9 | F (1) = 25.4 p < .001 |
| Y-VACS Extra-familial Adversity Score | 6.8 ± 2.1 | 5.2 ± 3.1 | F (1) = 4.1 p < .05 |
| Y-VACS Total Adversity Score | 28.8 ± 10.5 | 14.8 ± 9.2 c | F (1) 25.9 p < .001 |
| High Social Support Rating for Mother | 42.1% | 77.7% | X2 = 18.5 p < .002 |
Abbreviations: EA=European American; AA=African American; Y-VACS=Yale-Vermont Adverse Childhood Experiences Scale; SCARED=Screen for Child Anxiety and Related Emotional Disorders scale.
3.2. Go-NoGo Behavioral Data
Among the subjects included in the final cohort, there were no significant differences in RT or accuracy when responding to fearful and calm targets, and further, no significant differences in commission errors for non-target trials. A significant correlation (r = −0.29, p = 0.034) was observed between trauma (Y-VACS total score) and the difference in target RT (fear RT – calm RT), such that greater exposure to trauma was associated with slower RTs for fearful targets relative to calm targets. A similar relationship was observed between anxiety (SCARED total score) and the difference in RT between target conditions (R = −0.27, p = 0.049).
3.3. Imaging Results
Table 2 delineates the complete list of clusters of activation that were significant in the analyses. A test for the effect of emotion valence was conducted by examining if there was a difference between fearful and calm face conditions. Collapsing across target and non-target conditions, participants responded to fearful face cues with increased activation in an area corresponding to the left anterior insula as well as the right posterior cingulate and precuneus (FWE correction, p < 0.05).
Table 2:
Significant Clusters of Activation during Go-NoGo Task
| Fear (target, non-target) > Neutral (target, nontarget) | ||||||
|---|---|---|---|---|---|---|
| Region | Hemisphere | Peak t-stat | Voxels | MNI coordinates | ||
| x | y | z | ||||
| Posterior cingulate cortex/precuneus | R | 4.08 | 219 | 18 | −40 | 41 |
| Anterior insula/inferior frontal gyrus | L | 3.7 | 205 | −57 | 29 | 5 |
| Fear target > neutral target, trauma covariate | ||||||
| Region | Hemisphere | Peak t-stat | Voxels | MNI coordinates | ||
| x | y | z | ||||
| Orbital frontal cortex/anterior cingulate cortex | L | 4.05 | 331 | −21 | 32 | −10 |
| Fear target > neutral target, conjunction of trauma and biological support covariates | ||||||
| Region | Hemisphere | Peak t-stat | Voxels | MNI coordinates | ||
| x | y | z | ||||
| Anterior cingulate cortex/frontal pole | R | 2.89 | 246 | 15 | 47 | 11 |
| Amygdala/OFC, nucleus accumbens, anterior insula | L | 2.51 | 177 | −42 | 2 | −25 |
| Fear target > neutral target, conjunction of trauma and biological support covariates, left amygdala small volume correction (SVC) | ||||||
| Region | Hemisphere | Peak t-stat | Voxels | MNI coordinates | ||
| x | y | z | ||||
| Amygdala | L | 2.29 | 24 | −27 | 8 | −19 |
Significance was determined by first applying an uncorrected threshold of p < 0.005, and then applying cluster-level family-wise error (FWE) correction, p < 0.05.
As depicted in Figure 1 and Table 2, when examining the effect of trauma on brain imaging parameters, elevated Y-VACS total trauma scores were associated with increased activation towards fearful vs. calm targets, with a significant cluster localized to the left orbital frontal cortex (OFC) and extending dorsally into the anterior cingulate cortex (ACC; FWE correction, p < .05). There were no main effects of support from the biological mother on activation patterns (p > .05, all contrasts). Results of the conjunction analysis using the dimensional measures of trauma and support from the biological mother to test if trauma-related brain activation changes in responding to fearful vs. calm targets were impacted by support from the biological mother are depicted in Figure 2. Multiple activation foci throughout cortical and subcortical regions emerged significant in this analysis, including a cluster predominantly localized to the right ACC and frontal pole, and an additional cluster extending to the left amygdala, nucleus accumbens, OFC, and anterior insula (FWE Correction, p < .05). Activation of the left amygdala was confirmed using small volume correction (left amygdala mask, Harvard-Oxford atlas). The results of the conjunction analysis show that heightened adversity and a lack of support similarly modulate the activation of brain regions sensitive to fearful faces, and suggest greater support from a biological mother may reduce the effect that adversity has on cortical processing of threat stimuli.
Figure 1: Effects of Y-VACS Trauma Scores on Brain Activation Fearful vs. Neutral Faces.
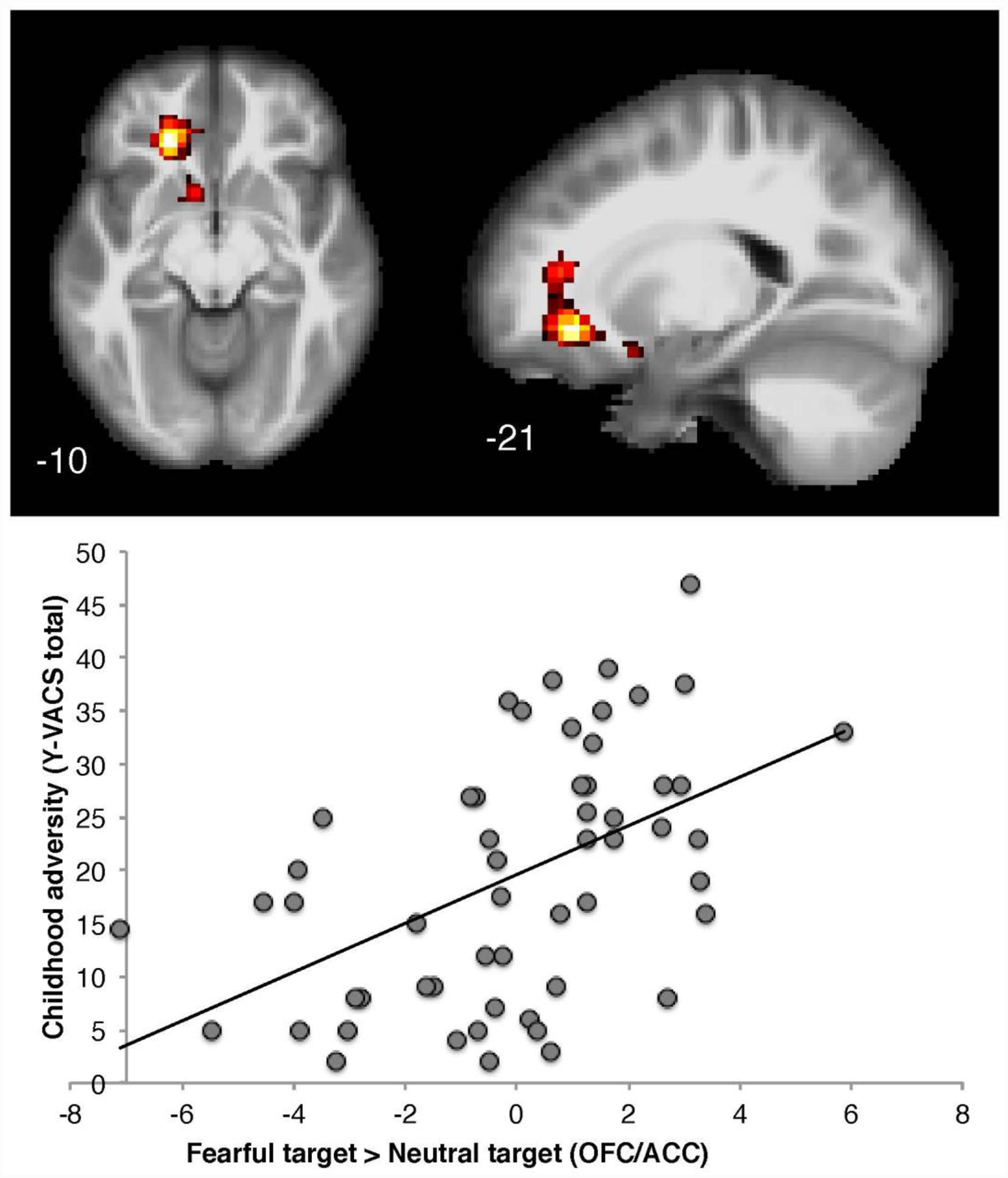
Elevated Y-VACS total trauma scores were associated with increased activation towards fearful vs. neutral target, with a significant cluster localized to the left orbital frontal cortex extending dorsally into the anterior cingulate cortex (FWE Correction, p < .05).
Figure 2: Effect of Birth Parent Support on Trauma-Related Cluster of Activation to Fearful vs. Neutral Faces.
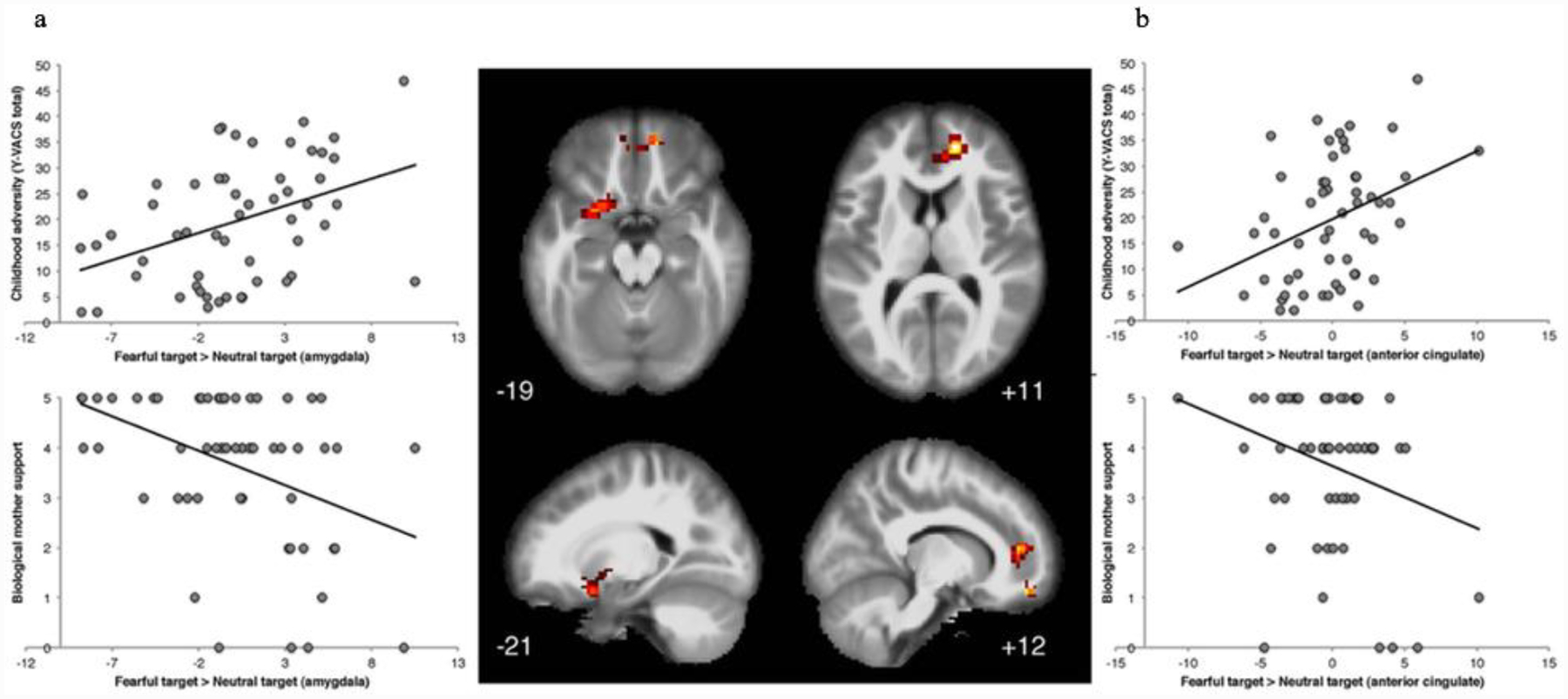
Results of the conjunction analysis using the dimensional measures of trauma and support from the biological mother to test if trauma-related activation changes in responding to fearful vs. neutral targets were impacted by support from the biological mother identified a cluster predominantly localized in the left amygdala extending to the nucleus accumbens, OFC, and anterior insula (a), and a second cluster in the right ACC and frontal pole (b).
As noted, a separate exploratory analysis was performed using the SCARED total score as a covariate, replacing Y-VACS total score in the conjunction analysis model, because the SCARED and Y-VACS were highly correlated (r=0.49, p < .001). A similar pattern of findings emerged in this analysis, with increased anxiety scores on the SCARED associated with increased activation in the left amygdala and right ACC (FWE Correction, p < .05).
4. Discussion
The results of the current study preliminary support the proposition that the availability of positive social support can ameliorate the negative impact of child maltreatment and other forms of adversity on the functioning of brain circuits involved in threat processing and emotion regulation. Consistent with prior reports, histories of greater adversity were associated with increased amygdala activation in response to threat stimuli (Hein & Monk, 2017), with the impact of adversity on amygdala activation in this investigation found to be reduced in youth with positive supports. Increased activation in response to threat stimuli was also observed in the anterior cingulate cortex, insula, nucleus accumbens, and the frontal pole, with the impact of adversity on activation in these brain regions similarly reduced in the presence of positive supports. These latter brain regions have been implicated in PTSD and other stress-related psychiatric disorders (Boccia et al., 2016; Goode; Karl et al., 2006; Sun, Haswell, Morey, & De Bellis; Tomoda; Zhu et al., 2017), with increased activation in the anterior cingulate cortex and insula in response to threat stimuli also reported in prior studies (Hein & Monk, 2017; Herringa, Phillips, Fournier, Kronhaus, & Germain, 2013).
In the current study, support from the biological mother was the measure that moderated the impact of trauma on brain activation during threat processing; in our prior studies it was the relationship with the child’s top support that was found to moderate the impact of adversity across the various outcomes examined (Kaufman, 1991; Kaufman et al., 2006; Kaufman et al., 2004). The salience of the relationship with the biological mother may be due to the lower proportion of children in out-of-home care and the higher proportion of youth living with their biological mothers in the current investigation compared to in our prior studies.
The current study has several limitations including, small sample size, heterogeneity in terms of child adversity experiences, the absence of developmental timing data for children’s trauma experiences, and the broad age range of the subjects. While prior investigations have reported developmental differences in neural activation during the Emotional Go-NoGo task across the age range of subjects included in the current investigation (Hare et al., 2008), age was not a significant covariate in the analyses in this study.
The research findings of the present report are also limited by the cross-sectional design of the current investigation. The results are, however, consistent with other reports. For example, the short-term benefit of social supports on brain systems processing threat stimuli have also been demonstrated in experimental studies that put subjects under the threat of shock during imaging, with and without a caring support holding their hand (Coan, 2017). The role of the positive attachments in diminishing the negative effects of child maltreatment on brain function and risk for psychopathology have also been demonstrated in the prospective longitudinal work of Dozier and colleagues, and their follow-up of children referred to child protective services due to concerns about child abuse and neglect who were randomized to one of two interventions: the Attachment Biobehavioral Catch-up (ABC) intervention or an education focused control intervention. When compared to children who received the control intervention, participation in the ABC intervention was associated with improvement in attachment, behavior, and stress system (i.e., cortisol) measures immediately post-treatment (Bernard, Dozier, Bick, & Gordon, 2015; Bernard et al., 2012), the maintenance of these outcomes at 3-year follow-up (Bernard et al., 2015), and adaptive behavior and healthy patterns of neural functioning examined with electroencephalography (EEG) in middle childhood (Bick, Palmwood, Zajac, Simons, & Dozier, 2019).
The clinical significance of the neuroimaging results reported in this study is supported by associations between brain activation in multiple of these regions and children’s anxiety ratings. The importance of amygdala function in promoting resilience and recovery also has independent support. In one prospective longitudinal study, increased network control of amygdala function at baseline was associated with decreased risk for internalizing problems at two-year follow-up within a cohort of maltreated children (Rodman, Jenness, Weissman, Pine, & McLaughlin, 2019). In another study that conducted functional brain imaging assessments before and after Trauma-Focused Cognitive Behavior Therapy (TF-CBT), the intervention with the strongest evidence base in treating PTSD and trauma-related symptomatology in children 3–17 years of age (Cohen & Mannarino, 2015), reduction in PTSD symptoms was associated with increased functional connectivity in the amygdala (Cisler et al., 2016). While it is unclear what elements of the intervention mediated the positive effects on brain imaging parameters, given research suggesting parental participation significantly enhances the beneficial impact of TF-CBT for traumatized children (Cohen & Mannarino, 2015), enhancing supportive parenting may be a common element that mediates positive outcomes across effective trauma-focused interventions (Hoover & Kaufman, 2017).
5. Conclusions
While there is a burgeoning literature on the negative effects of child maltreatment and other forms of early adversity on brain structure and function, this study adds to a growing body of research that confirms the negative effects associated with child adversity are not inevitable. Positive supports, together with evidence-based trauma-informed interventions, are key elements in promoting resilience and recovery.
Acknowledgments.
The authors thank the children and families who participated in this research, and the administration of the Vermont Department of Children and Families for their collaboration on this effort. Ms. Yoon Ji Moon is also acknowledged for her assistance conducing an updated literature review for the current manuscript.
Funding.
This work was supported by the Zanvyl and Isabelle Krieger Fund (JK), the NIH R01MH098073 (JK, JH), R01 MD011746-01 (JK); and the Biological Sciences Training Program through 5T32 MH14276 (JLMO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interests. None.
References:
- Achenbach TM, & Rescorla LA (2001). Manual for ASEBA School-Age Forms & Profiles.. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Angold A, Costello EJ, Messer S, Pickles A, Winder F, & Silver D (1995). Development of a Short Questionnaire for use in Epidemiological Studies of Depression in Children and Adolescents. International Journal of Methods in Psychiatric Research., 5, 237–249. [Google Scholar]
- Barrera M (1980). A method for the assessment of social support networks in community survey research. Connections, 3, 8–13. [Google Scholar]
- Bernard K, Dozier M, Bick J, & Gordon MK (2015). Intervening to enhance cortisol regulation among children at risk for neglect: Results of a randomized clinical trial Developmental Psychopathology 27(3), 829–841. doi: 10.1017/S095457941400073X. Epub 095457941402014 Aug 095457941400026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Dozier M, Bick J, Lewis-Morrarty E, Lindhiem O, & Carlson E (2012). Enhancing attachment organization among maltreated children: results of a randomized clinical trial. Child Dev, 83(2), 623–636. doi: 10.1111/j.1467-8624.2011.01712.x. Epub 2012 Jan 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J, & Nelson CA (2016). Early adverse experiences and the developing brain. Neuropsychopharmacology, 41(1), 177–196. doi: 10.1038/npp.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J, Palmwood EN, Zajac L, Simons R, & Dozier M (2019). Early parenting intervention and adverse family environments affect neural function in middle childhood. Biological Psychiatry, 85(4), 326–335. doi: 10.1016/j.biopsych.2018.1009.1020. Epub 2018 Oct 1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilek E, Itz ML, Stossel G, Ma R, Berhe O, Clement L, … Tost H (2019). Deficient Amygdala Habituation to Threatening Stimuli in Borderline Personality Disorder Relates to Adverse Childhood Experiences. Biol Psychiatry, 19(19), 31447–31447. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, & Neer SM (1997). The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry, 36(4), 545–553. doi: 10.1097/00004583-199704000-00018 [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpol S, Brent D, Cully M, Balach L, Kaufman J, & McKenzie-Neer S (1997). The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale Construction and Psychometric Properties. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 545–553. [DOI] [PubMed] [Google Scholar]
- Boccia M, D’Amico S, Bianchini F, Marano A, Giannini AM, & Piccardi L (2016). Different neural modifications underpin PTSD after different traumatic events: An fMRI meta-analytic study. Brain Imaging Behav, 10(1), 226–237. doi: 10.1007/s11682-015-9387-3 [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, & Valentine JD (2000). Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol., 68(5), 748–766. doi: 10.1037/0022-006X.68.5.748 [DOI] [PubMed] [Google Scholar]
- Charuvastra A, & Cloitre M (2008). Social bonds and posttraumatic stress disorder. Annu Rev Psychol, 59, 301–328. doi: 10.1146/annurev.psych.58.110405.085650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Sigel BA, Steele JS, Smitherman S, Vanderzee K, Pemberton J, … Kilts CD (2016). Changes in functional connectivity of the amygdala during cognitive reappraisal predict symptom reduction during trauma-focused cognitive-behavioral therapy among adolescent girls with post-traumatic stress disorder. Psychol Med, 46(14), 3013–3023. doi: 10.1017/S0033291716001847. Epub 0033291716002016 Aug 0033291716001815 [DOI] [PubMed] [Google Scholar]
- Coan JA, Beckes L, Gonzalez MZ & Maresh EL, Brown CL & Hasselmo K. (2017). Relationship status and perceived support in the social regulation of neural responses to threat. Soc Cogn Affect Neurosci, 12(10), 1574–1583. doi: 10.1093/scan/nsx1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, & Mannarino AP (2015). Trauma-focused cognitive behavior therapy for traumatized children and families. Child Adolesc Psychiatr Clin N America, 24(3), 557–570. doi: 10.1016/j.chc.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, … Kugel H (2012). Limbic scars: Long-term consequences of childhood maltreatment revealed by functional and structural MRI. Biological Psychiatry, 71(4), 286–293. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, … Ryan ND (1999). Developmental traumatology. Part II: Brain development. Biol Psychiatry, 45(10), 1271–1284. doi: 10.1016/S0006-3223(99)00045-1 [DOI] [PubMed] [Google Scholar]
- Fletcher S, Elklit A, Shevlin M, & Armou C (2017). Predictors of PTSD treatment response trajectories in a sample of childhood sexual abuse survivors: The roles of social support, coping, and PTSD symptom clusters. Journal of Interpersonal Violence, 886260517741212. doi: 10.1177/0886260517741212 [DOI] [PubMed] [Google Scholar]
- Garrett AS, Carrion V, Kletter H, Karchemskiy A, Weems CF, & Reiss A (2012). Brain activation to facial expressions in youth with PTSD symptoms. Depress Anxiety, 29(5), 449–599. doi: 10.1002/da.21892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TM, S.. (2019). Common neurocircuitry mediating drug and fear relapse in preclinical models. Pharmacology, 236, 415–437. doi: 10.1007/s00213-018-5024-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso DJ, DiVietro S, Beebe R, M. C, & G. L (2019). Quantifying severity of maltreatment maltreatment, adversity, and trauma from child protective services case record files. Journal of Interpersonal Violence (0886260519847774). doi: 10.1177/0886260519847774 [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, & Casey BJ (2008). Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry, 63(10), 927–934. doi: 10.1016/j.biopsych.2008.1003.1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein TC, & Monk CS (2017). Research review: Neural response to threat in children, adolescents, and adults after child maltreatment - a quantitative meta-analysis. J Child Psychol Psychiatry, 58(3), 222–230. doi: 10.1111/jcpp.12651. Epub 12016 Oct 12625 [DOI] [PubMed] [Google Scholar]
- Herringa RJ (2017). Trauma, PTSD and the developing brain. Curr Psychiatry Rep, 19(10), 69. doi: 10.1007/s11920-11017-10825-11923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Phillips ML, Fournier JC, Kronhaus DM, & Germain A (2013). Childhood and adult trauma both correlate with dorsal anterior cingulate activation to threat in combat veterans.. Psycholical Medicinen, 43(7), 1533–1542.. doi:doi: 10.1017/S0033291712002310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis S, Mercy J, Amobi A, & Kress H (2016). Global prevalence of past-year violence against children: A systematic review and minimum estimates. Pediatrics, 137(3), e20154079. doi: 10.1542/peds.2015-4079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook H, O’Loughlin K, Althoff R, Douglas-Palumberi H, Kaufman J, & Hudziak J (2015). The Yale-Vermont Adversity in Childhood Scale: A Quantitative Approach to Adversity Assessment. Paper presented at the American Academy of Child and Adolescent Psychiatry’s 61st Annual Meeting, San Diego, CA. [Google Scholar]
- Hoover D, & Kaufman J (2017). Posttraumatic Stress Disorder. In M. H. B. a. F. R. V. Andrés Martin (Ed.), Lewis’ Child and Adolescent Psychiatry: A Comprehensive Textbook, Fifth Edition. Philadelphi, PA: Wolters Kluwer. [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, & Werner A (2006). A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev, 30(7), 1004–1031. doi: 10.1016/j.neubiorev.2006.03.004 [DOI] [PubMed] [Google Scholar]
- Kaufman J (1991). Depressive disorders in maltreated children. J Am Acad Child Adolesc Psychiatry, 30(2), 257–265. doi: 10.1097/00004583-199103000-00014 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Montalvo-Ortiz JL, Holbrook H, O’Loughlin K, Orr C, Kearney C, … Hudziak H (2018). Adverse childhood experiences, epigenetic measures, and obesity in youth. J Pediatr, 202, 150–156.e153. doi: 10.1016/j.jpeds.2018.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Wymbs NF, Montalvo-Ortiz JL, Orr C, Albaugh MD, Althoff R, … Hudziak H (2018). Methylation in OTX2 and related genes, maltreatment, and depression in children. Neuropsychopharmacology, 43(11), 2204–2211. doi: 10.1038/s41386-018-0157-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, … Gelernter J (2006). Brain-Derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biological Psychiatry, 59, 673–680. doi: 10.1016/j.biopsych.2005.10.026 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal J, & Gelernter J (2004). Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci USA, 101(49), 17316–17321. doi: 10.1073/pnas.0404376101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J (2007). The amygdala. Curr Biol, 17(20), R868–874. doi: 10.1016/j.cub.2007.08.005 [DOI] [PubMed] [Google Scholar]
- Lowe SR, & Galea S. (2017). The mental health consequences of mass shootings. Trauma Violence Abuse, 18(1), 62–68. doi: 10.1177/1524838015591572. Epub 1524838015592015 Jun 1524838015591517 [DOI] [PubMed] [Google Scholar]
- Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, & Casey BJ (2013). Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc Natl Acad Sci U S A, 110(45), 18274–18278. doi: 10.11073/pnas.1310163110. Epub 1310162013 Oct 1310163121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, McTeague LM, Gyurak A, Patenaude B, Williams LM, Grieve SM, … Etkin A (2015). COGNITION-CHILDHOOD MALTREATMENT INTERACTIONS IN THE PREDICTION OF ANTIDEPRESSANT OUTCOMES IN MAJOR DEPRESSIVE DISORDER PATIENTS: RESULTS FROM THE iSPOT-D TRIAL. Depress Anxiety, 32(8), 594–604. doi: 10.1002/da.22368. Epub 22015 Apr 22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr C, Hudziak J, Albaugh M, Carlozzi B, Holbrook H, O’Loughlin K, … J. K (2016). Social Supports Moderate the Effects of Child Maltreatment on Neural Correlates of Threat Processing. Paper presented at the Annual Meeting of the Society of Biological Psychiatry, Atlanta, GA. [Google Scholar]
- Oshri A, Gray JC, Owens MM, Liu S, Duprey EB, Sweet LH, & MacKillop J (2019). Adverse Childhood Experiences and Amygdalar Reduction: High-Resolution Segmentation Reveals Associations With Subnuclei and Psychiatric Outcomes. Child Maltreat., 24(4), 400–410. doi: 10.1177/1077559519839491. Epub 1077559519832019 Apr 1077559519839428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso MA, Glahn DC, Matsuo K, Monkul ES, Najt P, Zamarripa F, … Soares JC (2009). Amygdala hyperactivation in untreated depressed individuals. Psychiatry Res, 173(2), 158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pynoos RS, Steinberg AM, & Piacentini JC (1999). A developmental psychopathology model of childhood traumatic stress and intersection with anxiety disorders. Biological Psychiatry, 46(11), 1542–1554. doi: 10.1016/S0006-3223(99)00262-0 [DOI] [PubMed] [Google Scholar]
- Rodman AM, Jenness JL, Weissman DG, Pine DS, & McLaughlin KA (2019). Neurological markers of resilience to depression following childhood maltreatment: The role of neural circuits supporting the cognitive control of emotion.. Biological Psychiatry, 86, 464–473. doi: 10.1016/j.biopsych.2019.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh N, Spielberg JM, Miller MW, Milberg WP, Salat DH, Amick MM, … McGlinchey RE (2015). Neurobiological indicators of disinhibition in posttraumatic stress disorder. Hum Brain Mapp., 36(8), 3076–3086. doi: 10.1002/hbm.22829. Epub 22015 May 22829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Haswell C, Morey R, & De Bellis M (2018). Brain structural covariance network centrality in maltreated youth with PTSD and in maltreated youth resilient to PTSD. Development and Psychopathology, 31(2), 557–571. doi: 10.1017/S0954579418000093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda A (2016). Preliminary evidence for impaired brain activity of neural reward processing in children and adolescents with reactive attachment disorder. Yakugaku Zasshi, 36(5), 711–714. doi: 10.1248/yakushi.15-00262-5 [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, & Casey BJ (2011). Elevated amygdala response to faces following early deprivation. Dev Sci, 14(2), 190–204. doi: 10.1111/j.1467-7687.2010.00971.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino K, Berkowitz S, & Stover CS (2010). Parenting behaviors and posttraumatic symptoms in relation to children’s symptomatology following a traumatic event. J Trauma Stress, 23(3), 403–407. doi: 10.1002/jts.20525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Krishnan A, & Wager TD (2014). Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. Neuroimage, 91, 412–419. doi: 10.1016/j.neuroimage.2013.1012.1058. Epub 2014 Jan 1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Helpman L, Papini S, Schneier F, Markowitz JC, Van Meter PE, … Neria Y (2017). Altered resting state functional connectivity of fear and reward circuitry in comorbid PTSD and major depression. Depression and Anxiety, 34(7), 641–650. doi: 10.1002/da.22594 [DOI] [PMC free article] [PubMed] [Google Scholar]


