Abstract
Preeclampsia is a pregnancy disorder that is primarily associated with maternal and neonatal or fetal morbidity and mortality. The discovery of dysregulated microRNAs (miRs) and their roles in preeclampsia has provided new insight into the mechanisms involved in pregnancy-related disorders. In the present study, quantitative PCR demonstrated that the expression levels of miR-524-5p were lower in patients with preeclampsia than those in normal pregnant women. Cell Counting Kit-8 and Transwell assays indicated that overexpression of miR-524-5p promoted the proliferation and invasion of HTR-8/SVneo cells, whereas inhibition of miR-524-5p suppressed HTR-8/SVneo cell proliferation and invasion. Furthermore, NUMB endocytic adaptor protein (NUMB), a negative regulator of the Notch signaling pathway and a target gene of miR-524-5p, limited the effects of miR-524-5p on HTR-8/SVneo cell invasion and migration. The present study demonstrated that miR-524-5p regulated the proliferation and invasion of HTR-8/SVneo cells at least partly by targeting NUMB to regulate the Notch signaling pathway.
Keywords: preeclampsia, proliferation, invasion, microRNA, NUMB, Notch
Introduction
Preeclampsia is a common serious disorder of obstetrics and is a hypertensive complication that occurs after the 20th week of gestation, characterized by new-onset hypertension (1). The primary cause of maternal mortality in preeclampsia has not yet been elucidated. At present, it is hypothesized that placental maternal surface spiral arterial remodeling disorder and insufficient extravillous trophoblast invasion are key factors leading to preeclampsia (2,3).
MicroRNAs (miRNAs/miRs) are a type of small non-coding RNAs that have been reported to be post-transcriptional gene regulators and tumor suppressors (4,5). Previous studies have investigated the role of miR-524-5p in numerous types of cancer. For example, Chen et al (6) demonstrated that high/low miR-524-5p expression is associated with improved/worse survival rate and pathological grade of patients with glioma, and Liu et al (7) detected lower levels of miR-524-5p expression in human melanoma, whereas overexpression of miR-524-5p effectively inhibited melanoma cell proliferation and migration. Furthermore, Liu et al (7) demonstrated that tumors overexpressing miR-524-5p were significantly smaller than those in negative control (NC) mice. Additionally, a previous study has demonstrated that miR-524-5p expression is downregulated in preeclampsia (8). However, the role of miR-524-5p in preeclampsia has not been fully elucidated.
NUMB endocytic adaptor protein (NUMB), a cell fate determinant, serves an important role in asymmetric cell division (9). NUMB is a key negative regulator of the Notch signaling pathway (10) and is involved in numerous physiological processes, such as differentiation/proliferation balance, apoptosis regulation, cell migration and tissue regeneration (11–13). To the best of our knowledge, NUMB expression in placental tissues with extremely active growth and differentiation has not yet been identified. Therefore, the present study investigated the potential roles of miR-524-5p and NUMB in trophoblast proliferation and invasion.
Materials and methods
Patients
Patients at Hainan Provincial People's Hospital (Haikou, China) were enrolled in the present study between September 2017 and January 2019. A total of 40 patients with preeclampsia and 40 healthy pregnant women were admitted in the present study. The risk factors for the development of pre-eclampsia were recorded according to a previous study (14). According to the American College of Obstetricians and Gynecologists practice bulletin for diagnosis and management of preeclampsia and eclampsia, severe preeclampsia was defined as either sustained systolic blood pressure ≥160 mmHg and/or diastolic blood pressure ≥110 mmHg (measured twice, ≥6 h apart) or severe proteinuria (>5 g/24 h specimen or ≥3 g/l in ≥2 random samples collected 4 h apart) (15). Normal systolic blood pressure is <140 mmHg and diastolic blood pressure is <110 mmHg. Age, BMI and smoking history were also recorded but were not used as exclusion criteria. Patients with non-severe preeclampsia according to the criterion listed in the International Society for the Study of Hypertension in Pregnancy were excluded. Clinical information was recorded up to the expected date of delivery and the placenta was obtained for follow-up study. The present study was approved by the Ethics Committee of Hainan General Hospital and all patients provided written informed consent.
Cell culture
The human HTR-8/SVneo trophoblast cell line was purchased from Shanghai Enzyme Research Biotechnology Co., Ltd. HTR-8/SVneo cells were seeded in a 10-cm cell culture dish with DMEM medium (Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin at 37°C in a humidified atmosphere with 5% CO2.
miR inhibitor and mimic transfection
miR-524-5p inhibitor, mimic and negative control (NC) oligonucleotides (20 µM; Guangzhou RiboBio Co., Ltd.) were transfected into cells using 5 µl Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) in 6-well plates at 37°C for 24 h. The cells were divided into four groups: Scrambled Inhibitor NC (5′-GAGAAAGUGCUUCGGUUUUUUG-3′), miR-524-5p inhibitor (5′-GAGAAAGUGCUUCCCUUUGUAG-3′), scrambled mimic NC (5′-CAAAAAACCGAAGCACUUUCUC-3′) and miR-524-5p mimic (5′-CUACAAAGGGAAGCACUUUCUC-3′).
Cell counting Kit-8 (CCK-8) assay
Cells were seeded into 6-well plates at a density of 1×104 cells/ml for 0, 24, 48 or 72 h. Cells were incubated with 10 µl CCK-8 solution (Beijing Solarbio Science & Technology Co., Ltd.) at 37°C for 2 h, and cell viability was measured. Absorbance values were measured using a microplate reader at a wavelength of 450 nm.
Bioinformatics analysis
The target genes of miR-524-5p were predicted using TargetScan (http://www.targetscan.org/mamm_31/) and microRNA.org databases (http://www.mirbase.org/) (16,17). Binding sites between miR-524-5p and the target genes were searched to identify potential interactions between them.
Luciferase reporter assay
A luciferase reporter assay was conducted to explore the association between miR-524-5p and NUMB, as described previously (18). HTR-/SVneo cells were divided into two groups, wild-type and mutant NUMB with 3′-untranslated region (UTR) reporters. HTR-8/SVneo cells at 80% confluence were co-transfected at 37°C for 24 h with wild-type or mutant NUMB 3′-UTR reporters (16 µg/ml; Generay Biotech Co., Ltd.) together with miR-524-5p mimic (50 pmol/ml) or NC using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). Subsequently, Dual-Luciferase Reporter Assay System (Promega Corporation) was performed according to the manufacturer's protocol. Cells were harvested and lysed for the assay 24 h after transfection. Renilla luciferase was used to normalize the data.
Transwell invasion assay
Transwell assays were carried out as described previously (19). Cells (2×104 cells/well) were cultured at 37°C for 24 h in 24-well plates with Transwell inserts precoated with Matrigel® (37°C for 30 min; BD Pharmingen; BD Biosciences). DMEM medium was added to the upper chamber, and DMEM containing 10% FBS in the lower chamber. Subsequently, cells were stained with 0.1% crystal violet (Sigma-Aldrich; Merck KGaA) at 20°C for 10 min and the number of cells that had invaded through the Matrigel membrane was counted using a light microscope (magnification, ×100; Leica Microsystems GmbH).
Reverse transcription-quantitative (RT-q)PCR analysis
Total RNA was extracted from cells using TRIzol® reagent and quantified using NanoDrop 2000c (both Thermo Fisher Scientific, Inc). For miR-524-5p detection, RNA was reverse transcribed to cDNA using the RevertAid First Strand cDNA kit (Thermo Fisher Scientific, Inc), according to the manufacturer's protocol. qPCR was performed in 96-well plates in the ABI Step-One plus Real-Time PCR system (Thermo Fisher Scientific, Inc.) using SYBR Green Master Mix (Takara Biotechnology Co., Ltd.). Expression levels of miR-524-5p were normalized to those of small nuclear RNA U6, while GAPDH was used as an endogenous control for NUMB. Primers were obtained from Invitrogen (Thermo Fisher Scientific, Inc.). The primer sequences were as follows: miR-524-5p forward, 5′-CTACAAAGGGAAGCACTTTTCTCAA-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′; U6 forward, 5′-CGCTTCGGCAGCACATATAC-3′ and reverse, 5′-CAGGGGCCATGCTAATCTT-3′; NUMB forward, 5′-AAGGCTTCTTTGGAAAAACTGG-3′ and reverse, 5′-CATGGCTCAACCTTTCACCT-3′; and GAPDH forward, 5′-TGTTCGTCATGGGTGTGAAC-3′ and reverse, 5′-ATGGCATGGACTGTGGTCAT-3′. Each well contained 1 µl template, 10 µl Master Mix, 0.5 µl forward primer (10 µM), 0.5 µl reverse primer (10 µM) and 8 µl diethyl pyrocarbonate H2O in a 20-µl reaction system. qPCR thermocycling conditions were as follows: Initial denaturation at 95°C for 2 min, followed by 40 cycles of denaturation at 95°C for 20 sec, annealing at 60°C for 45 sec and extension at 72°C for 30 sec. Finally, gene expression levels were calculated using the 2−ΔΔCq method (20).
Western blotting
Proteins were extracted from cells using RIPA buffer (EMD Millipore) and quantified using a BCA kit (Beijing Solarbio Science & Technology Co., Ltd.). Target proteins (30 µg) were separated according to their mass via 10% SDS-PAGE, then transferred to a PVDF membrane. Membranes were incubated with primary antibodies against proliferating cell nuclear antigen (PCNA; 1:1,000; cat. no. ab92552), Ki67 (1:2,000; cat. no. ab92742), NUMB (1:1,000; cat. no. ab220362), Bcl-2 (1:500; cat. no. ab196495), Notch1 (1:1,000; cat. no. ab52627), cyclin D1 (1:500; cat. no. ab40754), CDK6 (1:2,000; cat. no. ab151247) and GAPDH (1:10,000; cat. no. ab181602) (all Abcam) at 4°C overnight, then incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:5,000; cat. no. A0208; Beyotime Institute of Biotechnology) at 25°C for 1 h. Bands were visualized using the ECL reagent (Merck KGaA) and density was measured via ImageJ software (v 2.1.4.7).
Statistical analysis
Data are presented as the mean ± standard deviation of three independent experiemnts. Statistical comparisons were performed using SPSS 17.0 software (SPSS, Inc.) Differences between two groups were compared using an unpaired Student's t-test. One-way ANOVA followed by Tukey's multiple comparison test was used for comparisons among three groups. Pearson's correlation analysis was used to examine the correlation between NUMB and miR-524-5p expression. The χ2 test was used to analyze the number of volunteers who smoked. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-524-5p is downregulated in preeclamptic placenta compared with in normal placenta
A total of 40 patients with preeclampsia and 40 normal pregnant females (controls) were enrolled in the present study. Clinical characteristics of patients and normal pregnant females were recorded, including age, BMI, smoking history and systolic/diastolic blood pressure (Table I). Maternal age, BMI and smoking history exhibited no significant difference between the two groups. Patients with preeclampsia had a significantly shorter gestational age and lower fetal birth weight, while systolic and diastolic blood pressure in patients with preeclampsia were significantly higher than in normal controls. qPCR demonstrated that expression levels of miR-524-5p were significantly lower in patients with preeclampsia than in normal controls (Fig. 1), which indicated that downregulation of miR-524-5p expression may be a biomarker of preeclampsia.
Table I.
Clinical information of patients with PE and normal pregnant females.
| Parameter | Control (n=40) | PE (n=40) | P-value |
|---|---|---|---|
| Maternal age at delivery, years | 29.53±2.90 | 30.43±3.15 | 0.1878 |
| Gestational age, weeks | 38.18±2.24 | 36.70±3.15 | <0.05 |
| Proteinuria, g/24 h | – | 4.57±1.40 | <0.001 |
| Systolic blood pressure, mmHg | 105.81±13.41 | 168.43±5.43 | <0.001 |
| Diastolic blood pressure, mmHg | 77.38±4.33 | 116.06±3.75 | <0.001 |
| Fetal birth weight, g | 3,556.88±309.88 | 2,689.85±428.90 | <0.001 |
| BMI | 23.42±3.28 | 23.01±3.66 | 0.594 |
| Number of individuals who smoked, n | 3.00 | 4.00 | 0.694 |
Data are expressed as the mean ± standard deviation, unless otherwise stated. Significance was calculated between control and PE groups. PE, preeclampsia.
Figure 1.
Relative miR-524-5p expression levels in controls and patients with preeclampsia. **P<0.001. miR, microRNA.
miR-524-5p regulates proliferation and invasion of human HTR-8/SVneo trophoblasts
In order to investigate the effect of miR-524-5p on the proliferative and invasive abilities of human HTR-8/SVneo trophoblasts, miR-524-5p mimic and inhibitor were transfected into cells. Expression levels of miR-524-5p were significantly higher in the miR-524-5p mimic group than in the mimic NC group (Fig. 2A) and significantly lower in the miR-524-5p inhibitor group than in the inhibitor NC group (Fig. 2B), which indicated that mimic and inhibitor had been transfected successfully. The CCK-8 assay demonstrated that cell viability of the miR-524-5p mimic group was significantly higher than that of the mimic NC group at 72 h (Fig. 2C), while cell viability of the miR-524-5p inhibitor group was significantly lower compared with the inhibitor NC group (Fig. 2D). Expression levels of PCNA and Ki67 were determined by western blotting to investigate the underlying mechanism of cell proliferation. Overexpression of miR-524-5p increased PCNA and Ki67 protein expression, while PCNA and Ki67 expression in the miR-524-5p inhibitor group was lower than in the inhibitor NC group, which demonstrated that inhibition of miR-524-5p inhibited cell proliferation (Fig. 2E).
Figure 2.
Low expression levels of miR-524-5p impede trophoblast cell proliferation and invasion. Relative miR-524-5p expression levels in HTR-8/SVneo cells following transfection with (A) miR-524-5p mimic and (B) inhibitor. Viability of HTR-8/SVneo cells transfected with (C) miR-524-5p mimic and (D) inhibitor for 0, 24, 48 and 72 h. (E) Western blot analysis of PCNA and Ki67 protein levels in HTR-8/SVneo cells transfected with miR-145-5p mimic or NC, and miR-145-5p inhibitor or NC. Transwell invasion assay was performed to assess the invasiveness of HTR-8/SVneo cells transfected with (F) miR-145-5p mimic or NC or (G) miR-145-5p inhibitor or NC. Scale bar, 100 µm. **P<0.01; miR, microRNA; PCNA, proliferating cell nuclear antigen; NC, negative control; OD, optical density.
In order to investigate the influence of miR-524-5p on the invasive ability of human HTR-8/SVneo trophoblasts, the degree of cell invasion was determined by Transwell invasion assay. Overexpression and inhibition of miR-524-5p in HTR-8/SVneo cells regulated cell invasion. After 24 h, the invasive ability of cells in the miR-524-5p mimic group was significantly increased compared with that in the mimic NC group (Fig. 2F), while the invasive ability of cells in the miR-524-5p inhibitor group was significantly decreased compared with that in the inhibitor NC group (Fig. 2G).
miR-524-5p regulates the expression levels of NUMB via binding to the 3′-UTR of NUMB mRNA
In order to determine the target genes of miR-524-5p, TargetScan and microRNA.org databases were used. Results from the databases indicated that NUMB was a candidate target gene regulated by miR-524-5p, and microRNA.org database was used to predict the binding sites (Fig. 3A).
Figure 3.
miR-524-5p regulates the expression levels of NUMB. (A) microRNA.org database was used to predict binding sites between miR-524-5p and NUMB. (B) Fluorescence intensity was measured using a dual luciferase assay. Overexpressed miR-524-5p bound to NUMB-WT and fluorescence intensity was weakened. NUMB-MUT exhibited no significant difference in fluorescence intensity. RT-qPCR was used to detect NUMB expression in the (C) inhibitor and (D) mimic groups. Compared with the inhibitor NC group, NUMB expression was significantly upregulated in the miR-524-5p inhibitor group, while compared with the mimic NC group, NUMB expression in the miR-524-5p mimic group was significantly downregulated. Western blotting was used to detect NUMB expression in the (E) inhibitor and (F) mimic groups. Compared with the inhibitor NC group, NUMB expression was significantly upregulated in the miR-524-5p inhibitor group, while it was significantly downregulated in the miR-524-5p mimic group compared with the mimic NC group. (G) RT-qPCR was used to detect the expression levels of NUMB in 40 preeclampsia tissues and Pearson's correlation coefficient was used to analyze the correlation between NUMB and miR-524-5p expression. There was a significant negative association between NUMB and miR-524-5p expression. **P<0.01. miR, microRNA; NUMB, NUMB endocytic adaptor protein; WT, wild-type; MUT, mutant; RT-q, reverse transcription-quantitative; NC, negative control; UTR, untranslated region; ns, not significant.
In order to determine whether inhibition of NUMB by miR-524-5p occurred via these predicted miR-524-5p binding sites, the binding site was mutated. The luciferase reporter assay indicated that transfection with the NUMB mutant 3′-UTR did not lead to a reduction in the relative luciferase activity in the presence of miR-524-5p, compared with miR NC (Fig. 3B). Subsequently, the expression levels of NUMB were determined following miR-524-5p mimic or inhibitor treatment. Inhibition of miR-524-5p increased the expression levels of NUMB mRNA and protein (Fig. 3C and E); conversely, when miR-524-5p was overexpressed, NUMB mRNA and protein expression levels were decreased (Fig. 3D and F). Additionally, Pearson's correlation analysis showed that NUMB exhibited a negative correlation with miR-524-5p (Fig. 3G), which suggested that the highly conserved sequence of the NUMB 3′-UTR may be the primary binding site of miR-524-5p, and further confirmed that miR-524-5p directly inhibited NUMB.
miR-524-5p regulates proliferation and invasion of HTR-8/SVneo cells by targeting NUMB to regulate the Notch signaling pathway
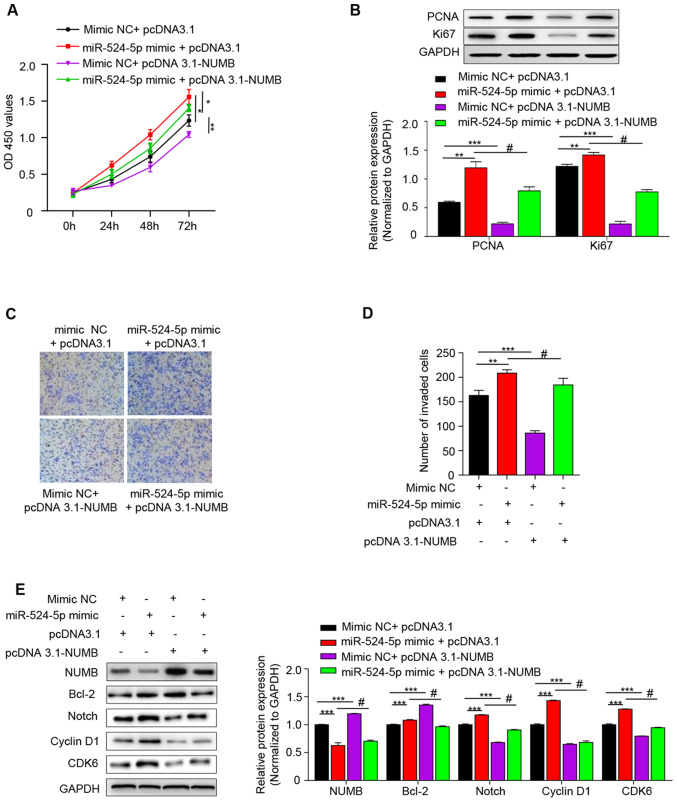
pcDNA3.1-NUMB and miR-524-5p mimic were transfected into human HTR-8/SVneo trophoblasts. Cells were divided into 4 groups, including mimic NC+ pcDNA 3.1, miR-524-5p mimic + pcDNA 3.1, mimic NC + pcDNA 3.1-NUMB and miR-524-5p mimic + pcDNA 3.1-NUMB. Cell viability was determined by CCK-8 assay, and the results revealed that cell viability was highest in the miR-524-5p mimic + pcDNA 3.1 group and lowest in mimic NC + pcDNA 3.1-NUMB, which indicated that activation of miR-524-5p may inhibit the expression levels of NUMB and cell viability was increased due to elevated miR-524-5p expression at 72 h (Fig. 4A).
Figure 4.
miR-524-5p regulates the Notch signaling pathway via NUMB. (A) Cell Counting Kit-8 was used to detect the proliferation ability of each group for 0, 24, 48 and 72 h. (B) Western blot analysis of PCNA and Ki67 protein expression. (C) Invasive ability was detected using Transwell assay (Scale bar, 100 µm) and (D) quantified. (E) Western blot was used to detect the expression levels of NUMB, Notch1, Bcl-2, Cyclin D1 and CDK6 in the Notch signaling pathway. *P<0.05; **P<0.01; ***P<0.001 vs. mimic NC+ pcDNA3.1 group; #P<0.001 vs. miR-524-5p mimic + pcDNA3.1 group. miR, microRNA; NUMB, NUMB endocytic adaptor protein; PCNA, proliferating cell nuclear antigen; NC, negative control; OD, optical density; EV, empty vector.
Apoptosis rate is significantly increased in the syncytiotrophoblast in preeclampsia (21). The effect of miR-524-5p and NUMB on cell proliferation was investigated. PCND and Ki67 are common proliferation markers in apoptosis (22), and therefore western blot analysis was performed to detect the expression levels of these two proteins. Expression levels of PCNA and Ki67 were highest in the miR-524-5p mimic + pcDNA 3.1 group and lowest in the mimic NC + pcDNA 3.1-NUMB group, which indicated that overexpression of miR-524-5p decreased NUMB and increased the cell proliferative ability (Fig. 4B). Additionally, the effect of miR-524-5p mimic on cell invasion was investigated. The results revealed that the miR-524-5p mimic + pcDNA3.1 group had the highest number of invading cells and the mimic NC + pcDNA3.1-NUMB group exhibited the lowest number. The number of invading cells in the miR-524-5p mimic + pcDNA3.1-NUMB was between the two aforementioned groups (Fig. 4C and D).
The present results suggested that miR-524-5p mimic inhibited NUMB expression and that high expression levels of NUMB inhibited cell proliferation and invasion. As the mechanism remains unknown, the present study investigated the specific mechanism by which miR-524-5p stimulates proliferation and invasion of HTR-8/SVneo cells by detecting the expression levels of signaling molecules associated with the Notch signaling pathway. Protein expression levels of Bcl-2, Notch, cyclin D1 and CDK6 were determined by western blotting. Protein expression level trends of Bcl-2, Notch, cyclin D1 and CDK6 in each group were downregulated when pcDNA 3.1-NUMB were transformed simultaneously with NC or mimic (Fig. 4E and S1). This indicated that miR-524-5p stimulated proliferation and invasion of HTR-8/SVneo cells by targeting NUMB to regulate the Notch signaling pathway.
Discussion
In recent years, numerous studies have continued to investigate preeclampsia (1,23), but the exact cause has not yet been elucidated. There is still no ideal animal model that replicates all pathological conditions of preeclampsia that are produced. Preeclampsia is hypothesized to be associated with insufficient cell invasion and endothelial cell dysfunction (24). In the present study, patients with severe PE symptoms exhibited higher systolic and diastolic blood pressure and proteinuria levels, whereas in normal controls, proteinuria was not detected.
miRNAs serve roles in a number of diseases, such as sclerosis, lung cancer and neurodegenerative diseases like Alzheimer's disease (25). The discovery of dysregulated miRNAs and their gene-regulatory roles in placental development has provided a novel approach for elucidating the underlying mechanisms of pregnancy-specific diseases (26). In the present study, bioinformatics analysis was used to predict the target genes of a specific miRNA, revealing that miR-524-5p was expressed at lower levels in patients with preeclampsia compared with normal controls. miR-524-5p expression in patients with preeclampsia should continue to be monitored in future studies. The predicted target gene of miR-524-5p was NUMB (confirmed by luciferase reporter assay); expression levels of NUMB, a negative regulator of the Notch signaling pathway, were regulated by miR-524-5p (10). Notch is a type of receptor that mediates transmembrane communication and cell fate (27). Bcl-2 is a factor that regulates apoptosis in the intrinsic mitochondrial apoptosis pathway (28). Cyclin D1 is one member of the Cyclin-D family; it is commonly overexpressed and known to be a direct target of Jagged1-mediated Notch signaling in breast cancer (29). Inactive CDK6 kinase is reported to disrupt Notch-dependent survival and proliferation by altering the expression levels of Notch target gene (30).
NUMB facilitates the migration and invasion of trophoblastic cells (31). A number of mechanisms have been suggested to explain the dysregulation of NUMB in trophoblastic cells. NUMB serves a key role in asymmetric division (32), as well as in regulating cell recognition, differentiation, tissue renewal and stabilization of the differentiation environment (33). In addition, NUMB acts as an important negative regulator of the Notch signaling pathway (10). The present study demonstrated that miR-524-5p negatively regulated both mRNA and protein levels of NUMB. Viability of HTR-8/SVneo cells was decreased when miR-524-5p expression was inhibited, while cell viability was increased when miR-524-5p was activated using a miR mimic. Subsequently, the effect of miR-524-5p on the proliferative and invasive abilities of HTR-8/SVneo cells were investigated. While low expression levels of miR-524-5p significantly inhibited cell proliferation and migration compared with the control group, activation of miR-524-5p promoted cell proliferation and migration. Next, western blotting was performed to detected the association between miR-524-5p and NUMB, revealing that inhibition of miR-524-5p resulted in upregulation of NUMB. Finally, to investigate the mechanism underlying the miR-524-5p-mediated upregulation of cell proliferation and invasion, Notch signaling pathway-associated proteins were detected via western blotting, including Bcl-2, Notch, cyclin D1 and CDK6. Larger patient cohorts and animal studies are required to validate the results, as these were the two primary limitations of the present research.
In conclusion, the present results demonstrated that miR-524-5p regulated proliferation and invasion of HTR-8/SVneo cells by targeting NUMB to regulate the Notch signaling pathway.
Supplementary Material
Acknowledgements
Not applicable.
Glossary
Abbreviations
- NUMB
NUMB endocytic adaptor protein
- PCNA
proliferating cell nuclear antigen
Funding Statement
This study was supported by Provincial Natural Science Foundation of China Hainan (grant no. 819MS119).
Funding
This study was supported by Provincial Natural Science Foundation of China Hainan (grant no. 819MS119).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LS conceptualized the study and gave final approval of the version to be published. LZ and JS designed and performed the experiments, and wrote and revised the manuscript. LW, RT, XC, DW and HC performed the experiments, analyzed the data and prepared figures and tables. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Hainan General Hospital. All patients provided written informed consent prior to participation in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Cheng D, Jiang S, Chen J, Li J, Ao L, Zhang Y. Upregulated long noncoding RNA Linc00261 in pre-eclampsia and its effect on trophoblast invasion and migration via regulating miR-558/TIMP4 signaling pathway. J Cell Biochem. 2019;120:13243–13253. doi: 10.1002/jcb.28598. [DOI] [PubMed] [Google Scholar]
- 2.Veerbeek JH, Brouwers L, Koster MP, Koenen SV, van Vliet EO, Nikkels PG, Franx A, van Rijn BB. Spiral artery remodeling and maternal cardiovascular risk: The spiral artery remodeling (SPAR) study. J Hypertens. 2016;34:1570–1577. doi: 10.1097/HJH.0000000000000964. [DOI] [PubMed] [Google Scholar]
- 3.Shyu MK, Chen CW, Lin NY, Liao WC, Chen CH, Lin CJ, Huang HC, Lee JJ, Huang MJ, Tseng GF, et al. MUC1 expression is elevated in severe preeclamptic placentas and suppresses trophoblast cell invasion via β1-integrin signaling. J Clin Endocrinol Metab. 2011;96:3759–3767. doi: 10.1210/jc.2011-1368. [DOI] [PubMed] [Google Scholar]
- 4.He J, Xiang D, Yin L. MicroRNA-708 inhibits the proliferation and invasion of osteosarcoma cells by directly targeting ZEB1. Mol Med Rep. 2019;19:3948–3954. doi: 10.3892/mmr.2019.10013. [DOI] [PubMed] [Google Scholar]
- 5.Bhagirath D, Yang TL, Tabatabai ZL, Shahryari V, Majid S, Dahiya R, Tanaka Y, Saini S. Role of a novel race-related tumor suppressor microRNA located in frequently deleted chromosomal locus 8p21 in prostate cancer progression. Carcinogenesis. 2019;40:633–642. doi: 10.1093/carcin/bgz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Zhang W, Yan W, Han L, Zhang K, Shi Z, Zhang J, Wang Y, Li Y, Yu S, et al. The putative tumor suppressor miR-524-5p directly targets Jagged-1 and Hes-1 in glioma. Carcinogenesis. 2012;33:2276–2282. doi: 10.1093/carcin/bgs261. [DOI] [PubMed] [Google Scholar]
- 7.Liu SM, Lu J, Lee HC, Chung FH, Ma N. miR-524-5p suppresses the growth of oncogenic BRAF melanoma by targeting BRAF and ERK2. Oncotarget. 2014;5:9444–9459. doi: 10.18632/oncotarget.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hromadnikova I, Kotlabova K, Ondrackova M, Pirkova P, Kestlerova A, Novotna V, Hympanova L, Krofta L. Expression profile of C19MC microRNAs in placental tissue in pregnancy-related complications. DNA Cell Biol. 2015;34:437–457. doi: 10.1089/dna.2014.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang XR, Sun J, Wang J, Lu YY. Advances in research on cell fate determinant Numb regulating liver cancer. Zhonghua Gan Zang Bing Za Zhi. 2018;26:714–717. doi: 10.3760/cma.j.issn.1007-3418.2018.09.019. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Shao X, Sun H, Liu K, Ding Z, Chen J, Fang L, Su W, Hong Y, Li H, Li H. NUMB negatively regulates the epithelial-mesenchymal transition of triple-negative breast cancer by antagonizing Notch signaling. Oncotarget. 2016;7:61036–61053. doi: 10.18632/oncotarget.11062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao C, Guo H, Li J, Myint T, Pittman W, Yang L, Zhong W, Schwartz RJ, Schwarz JJ, Singer HA, et al. Numb family proteins are essential for cardiac morphogenesis and progenitor differentiation. Development. 2014;141:281–295. doi: 10.1242/dev.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng H, Wang L, Su Q, Yi K, Du J, Wang Z. MiR-31-5p promotes the cell growth, migration and invasion of colorectal cancer cells by targeting NUMB. Biomed Pharmacother. 2019;109:208–216. doi: 10.1016/j.biopha.2018.10.048. [DOI] [PubMed] [Google Scholar]
- 13.George RM, Biressi S, Beres BJ, Rogers E, Mulia AK, Allen RE, Rawls A, Rando TA, Wilson-Rawls J. Numb-deficient satellite cells have regeneration and proliferation defects. Proc Natl Acad Sci USA. 2013;110:18549–18554. doi: 10.1073/pnas.1311628110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rebahi H, Elizabeth Still M, Faouzi Y, Rhassane El Adib A. Risk factors for eclampsia in pregnant women with preeclampsia and positive neurosensory signs. Turk J Obstet Gynecol. 2018;15:227–234. doi: 10.4274/tjod.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ACOG Committee on Obstetric Practice, corp-author. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American college of obstetricians and gynecologists. Int J Gynaecol Obstet. 2002;77:67–5. [PubMed] [Google Scholar]
- 16.Zhao X, Ren Y, Cui N, Wang X, Cui Y. Identification of key microRNAs and their targets in exosomes of pancreatic cancer using bioinformatics analysis. Medicine (Baltimore) 2018;97:e12632. doi: 10.1097/MD.0000000000012632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen K, Chen H, Zhang K, Sun S, Mo J, Lu J, Qian Z, Yang H. MicroRNA profiling and bioinformatics analyses reveal the potential roles of microRNAs in chordoma. Oncol Lett. 2017;14:5533–5539. doi: 10.3892/ol.2017.6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan J, Yang L, Liu C, Yan Z. MicroRNA-26a targets MAPK6 to inhibit smooth muscle cell proliferation and vein graft neointimal hyperplasia. Sci Rep. 2017;7:46602. doi: 10.1038/srep46602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song X, Li C, Li J, Liu L, Meng L, Ding H, Long W. The long noncoding RNA uc.294 is upregulated in early-onset pre-eclampsia and inhibits proliferation, invasion of trophoblast cells (HTR-8/SVneo) J Cell Physiol. 2019;234:11001–11008. doi: 10.1002/jcp.27916. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002;186:158–166. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- 22.Iatropoulos MJ, Williams GM. Proliferation markers. Exp Toxicol Pathol. 1996;48:175–181. doi: 10.1016/S0940-2993(96)80039-X. [DOI] [PubMed] [Google Scholar]
- 23.Benkő Z, Chaveeva P, de Paco Matallana C, Zingler E, Wright A, Wright D, Nicolaides KH. Validation of competing-risks model in screening for pre-eclampsia in twin pregnancy by maternal factors. Ultrasound Obstet Gynecol. 2019;53:649–654. doi: 10.1002/uog.20265. [DOI] [PubMed] [Google Scholar]
- 24.Haram K, Bjørge L, Guttu K. Pathophysiology and clinical manifestations in pre-eclampsia. Tidsskr Nor Laegeforen. 2000;120:1426–1431. (In Norwegian) [PubMed] [Google Scholar]
- 25.Hewel C, Kaiser J, Wierczeiko A, Linke J, Reinhardt C, Endres K, Gerber S. Common miRNA patterns of Alzheimer's disease and parkinson's disease and their putative impact on commensal gut microbiota. Front Neurosci. 2019;13:113. doi: 10.3389/fnins.2019.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang W, Song WY, Xie Y, Hu LL, Hou XM, Wang R, Gao Y, Zhang JN, Zhang L, Li WW, et al. miR-181a-5p suppresses invasion and migration of HTR-8/SVneo cells by directly targeting IGF2BP2. Cell Death Dis. 2018;9:16. doi: 10.1038/s41419-017-0045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi S, Lei Y, Zhao L, Mu YL, Li M, Zhao X, Chen ZJ, Zhang H. Melatonin inhibits 17β-estradiol-induced migration, invasion and epithelial-mesenchymal transition in normal and endometriotic endometrial epithelial cells. Reprod Biol Endocrinol. 2018;16:62. doi: 10.1186/s12958-018-0375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frenzel A, Grespi F, Chmelewskij W, Villunger A. Bcl2 family proteins in carcinogenesis and the treatment of cancer. Apoptosis. 2009;14:584–596. doi: 10.1007/s10495-008-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reedijk M, Reedijk M, Reedijk M, Cohen B, Shimizu M, Ng N, Bukhman Y, Pan J, Dering J. Cyclin D1 is a direct target of JAG-mediated Notch signaling in breast cancer. Cancer Res. 2009;69(Suppl 24):S2150. doi: 10.1007/s10549-009-0621-9. [DOI] [PubMed] [Google Scholar]
- 30.Hu MG, Deshpande A, Schlichting N, Hinds EA, Mao C, Dose M, Hu GF, Van Etten RA, Gounari F, Hinds PW. CDK6 kinase activity is required for thymocyte development. Blood. 2011;117:6120–6131. doi: 10.1182/blood-2010-08-300517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Zhou T, Sun Z, Ye T, Zhou S, Li J, Liu Y, Kong L, Tang J, Liu D, Xing HR. Zeb1 regulates the symmetric division of mouse lewis lung carcinoma stem cells through Numb mediated by miR-31. Int J Biol Sci. 2018;14:1399–1410. doi: 10.7150/ijbs.27446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu YC, Lee KS, Song Y, Gehrke S, Lu B. The bantam microRNA acts through Numb to exert cell growth control and feedback regulation of Notch in tumor-forming stem cells in the Drosophila brain. PLoS Genet. 2017;13:e1006785. doi: 10.1371/journal.pgen.1006785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tosoni D, Zecchini S, Coazzoli M, Colaluca I, Mazzarol G, Rubio A, Caccia M, Villa E, Zilian O, Di Fiore PP, Pece S. The Numb/p53 circuitry couples replicative self-renewal and tumor suppression in mammary epithelial cells. J Cell Biol. 2015;211:845–862. doi: 10.1083/jcb.201505037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.