Abstract
Among nucleos(t)ide analogue therapies for hepatitis B virus (HBV) treatment, entecavir (ETV) and tenofovir disoproxil fumarate (TDF)/tenofovir alafenamide are associated with the lowest rate of drug resistance. ETV is a drug requiring at least three substitutions in the reverse transcriptase (RT) domain to develop resistance, which is a rare occasion in treatment-naïve patients. However, pre-existing or acquired single mutations in the RT domain could lead to a virological breakthrough, after viral suppression. The present case report describes a 58-year-old female patient with hepatitis B virus (HBV) and high viral load who started HBV treatment with ETV. After 85 weeks of treatment, HBV-DNA declined to 0 IU/ml and remained undetectable for 3 years. However, after that period of time, the HBV-DNA rebounded, followed by the rise of liver enzymes (aspartate aminotransferase and alanine transaminase). Only the substitution M204I was detected in the HBV polymerase region. The patient was then switched to TDF treatment, achieving normalization of the liver enzymes and a decline in HBV-DNA levels. The present case report suggests that nucleoside-naïve patients should be cautiously monitored for resistance, even more than biochemically (transaminases, bilirubin) and virologically (HBV-DNA), even if complete HBV suppression is achieved.
Keywords: HBV, HBV resistance, entecavir therapy
Introduction
At present, it is estimated that 240 million people globally are chronically infected with hepatitis B virus (HBV) (1,2). In addition, patients with chronic HBV infection are at increased risk of developing a progressive liver disease, including fibrosis, cirrhosis or hepatocellular carcinoma (HCC) (3).
Antiviral therapies are used for prevention of clinical and histological progression of the disease, which is achieved through long-term suppression of HBV replication. Among these therapies, the nucleos(t)ide analogues (NAs) are the treatment of choice (3). However, the efficacy of antiviral therapy for chronic hepatitis B virus (HBV) is impaired by emerging viral resistance (4,5). The selection of antiviral-resistant mutations depends on viral factors, such as the lack of a proofreading mechanisms during reverse transcription, which creates a large pool of HBV quasispecies that are better able to replicate due to their increased resistance levels (6,7). In addition, the available replication space in either the cytoplasm or nucleus, and the structural flexibility of viral enzymes, alongside host and drug factors including pretreatment serum HBV-DNA level, quickness of viral suppression, duration of treatment, compliance and prior exposure to NA therapies, are other major factors involved in the development of resistance (6,7).
Among the approved NA therapies for HBV, lamivudine (LVD), adefovir-dipivoxil and telbivudine (LdT) are associated with the highest rate of drug resistance in NA-naïve patients, as these offer a lower barrier to resistance exerting modest antiviral activity that incompletely suppresses viral replication providing the greatest opportunity for selecting drug-resistant virus, whereas entecavir (ETV) and tenofovir (either TAF or TDF) are associated with the lowest rate of drug resistance (5,6). Resistance to nucleoside/nucleotide antivirals arises through specific mutations in the HBV polymerase (pol) reverse transcriptase (RT) domain (5). The first cases of ETV resistance (ETVr) were observed in patients receiving ETV after failure of LVD. In fact, resistance to ETV appears to occur through a two-hit mechanism with primary LVD resistance (LVDr) substitutions (M204V/I with/without rtL180M) followed by amino acid substitutions at the rtI169, rtT184, rtS202 or rtM250 sites (5,6).
The requirement for three substitutions for ETVr, combined with the potent suppression of HBV replication, results in a high genetic barrier (due to a complete suppression of viral replication) to ETV virological breakthrough in patients infected with wild-type virus (8,9). Hence, ETV is a potent NA against HBV, and emergence of drug resistance is rare in NA-naïve patients (10-12). However, cases of ETVr, which developed in nucleoside-naïve patients, have been described in the literature (5,8,11-13). The present report describes a NA-naïve patient with chronic HBV, who experienced ETVr and viral rebound during ETV treatment, when there was only one mutation present in the RT domain.
Case report
A 58-year-old Italian female patient underwent a check-up in January 2014 and was found to be seropositive for hepatitis B virus surface antigen (HBsAg) with elevated liver enzymes (glutamic oxaloacetic transaminase, 100 IU/ml (Normal level 15-45 IU/ml); glutamic pyruvic transaminase, 180 IU/ml (normal level 15-45 IU/ml)). HBeAb was positive and serum HBV-DNA was 2.7x107 IU/ml (measured by reverse transcription-PCR; normal level, 0 IT/ml). Additional baseline characteristics of the patient are presented in Table I.
Table I.
Baseline characteristics of the female patient diagnosed with hepatitis B virus in the present case report.
| Baseline characteristic | Normal range | Patient value |
|---|---|---|
| Fasting blood glucose, mg/dl | 70-99 | 105 |
| Creatinine, mg/dl | 0.8-1.3 | 0.8 |
| AST, IU/ml | <40 | 100 |
| ALT, IU/ml | <40 | 180 |
| GGT, UI/l | 6-29 | 78 |
| Bilirubin total, mg/dl | 0.3-1 | 0.4 |
| Bilirubin direct, mg/dl | 0.1-0.3 | 0.2 |
| Albumin, g/dl | 3.5-5 | 4.37 |
| Hb, g/dl | 12-16 | 14.5 |
| RBC, x106 cells/mm3 | 4.10-5.10 | 4.56 |
| WBC, x103 cells/mm3 | 4.5-11 | 5.2 |
| PLT, x103 cells/mm3 | 150-450 | 150 |
| INR | 0.8-1.2 | 1 |
| Prothrombin time, % | 80-100 | 96 |
| AFP, IU/ml | <7 | 4.2 |
| HBsAg | Negative | Positive |
| HBsAb | Negative | Negative |
| HBeAg | Negative | Negative |
| HBeAb | Negative | Positive |
| HBcAb | Negative | Positive |
| HCVAb | Negative | Negative |
| HIVAb | Negative | Negative |
| Anti-Δ | Negative | Negative |
AST, aspartate aminotransferase; ALT, alanine transaminase; GGT, λ-glutamyl transferase; Hc, hemoglobin; RBC, red blood cells; WBC, white blood cells; PLT, platelets; AFP, α-fetoprotein; HBsAb, hepatitis B virus surface antigen; HBeAg, hepatitis B virus envelope antigen; HBcAb, hepatitis B virus core antigen; HIVAb, Human Immunodeficiency Virus antibodies; NA, not applicable.
Liver ultrasound revealed hepatomegaly with steatosis pattern, and liver elastography showed a stiffness of 23.4 kPa (14) (normal value <5 kPa). However, there were no focal lesions. The patient refused to undergo liver biopsy; therefore, only clinical diagnosis of compensated cirrhosis was performed. The patient had a history of diabetes, hypertension, hypercholesterolemia and depressive disorder, and he was treated with amlodipine, bisoprolol, simvastatin, reboxetine, quinapril, metformin and insulin. During this period of treatments, the patient had not been diagnosed with HBV and was naïve to antiHBV drugs. The patient had undergone cholecystectomy 10 years prior to treat lithiasis. In addition, the patient had no history of alcohol consumption, smoking, drug abuse or blood transfusions, nor did they have unprotected sex or sex with multiple partners. Family history was unremarkable and no relatives had been found to be positive for HBV.
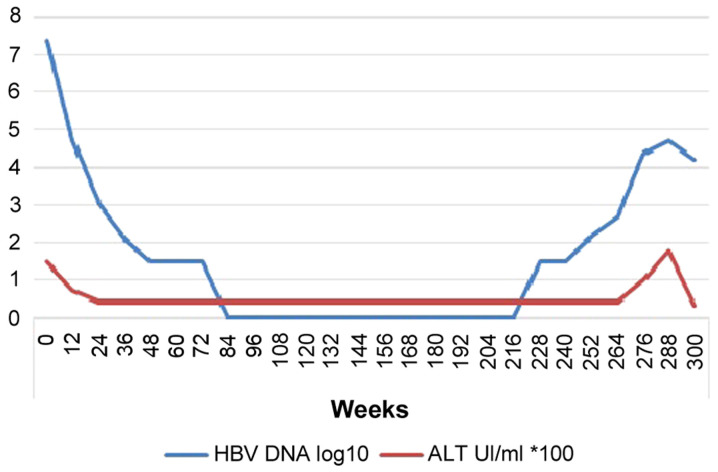
At that time of HBV diagnosis, treatment with ETV started at 0.5 mg/day; drug-drug interactions were negative (confirmed by Hepatic Drug Interactions Checker; University of Liverpool, Liverpool, UK; https://www.hep-druginteractions.org). After the start of ETV, serum HBV-DNA levels declined to a nadir point of 0 IU/ml at week 85 of ETV treatment. HBV-DNA remained undetectable for 3 years. However, from September 2018 (week 228), serum HBV-DNA started to rebound up to 52,500 IU/ml in September 2019 (week 288) (Fig. 1). At the first stages (week 228) of this rebound, liver enzymes were normal, but gradually started to increase along with HBV-DNA (Fig. 1). Other blood values (full blood count, renal function and coagulation) were normal and the patient denied any relevant symptoms, except for asthenia. Furthermore, the patient guaranteed complete therapeutic adherence and strongly denied the use of other non-prescribed medications. Ultrasonography analysis did not reveal focal lesions.
Figure 1.
HBV-DNA and ALT levels trend during entecavir therapy. HBV, hepatitis B virus; ALT, alanine transaminase.
Amplification of the HBV pol region was performed for the analysis of HBV drug resistance (Sanger sequencing). ETVr-related substitution M204I was detected, but no other mutations were identified (Table II). The patient discontinued ETV therapy at week 296, and then received 245 mg/day of tenofovir disoproxil fumarate (TDF). Afterwards, HBV-DNA levels dropped to 15,000 IU/ml in December 2019 and liver enzymes were normalized. The patient is currently continuing a 3-month follow-up.
Table II.
Mutations assessed in the hepatitis B virus polymerase region of reverse transcriptase by Sanger sequencing (performed by Abbott Hepatitis B virus Sequencing assay).
| Mutation site | Mutation status |
|---|---|
| 80 | Absent |
| 84 | Absent |
| 85 | Absent |
| 169 | Absent |
| 173 | Absent |
| 180 | Absent |
| 181 | Absent |
| 184 | Absent |
| 194 | Absent |
| 202 | Absent |
| 204 | Present (substitution M204I) |
| 214 | Absent |
| 215 | Absent |
| 217 | Absent |
| 219 | Absent |
| 221 | Absent |
| 233 | Absent |
| 236 | Absent |
| 250 | Absent |
Methods
All data were collected from patient's clinical records, after informed consent. Biochemical and virological data were collected from blood venipuncture in the contest of routine blood sampling for clinical monitoring of patients treated with anti-HBV antivirals, as suggested by guidelines (3). Sanger sequencing (performed by Abbott HBV Sequencing assay) targets the polymerase (pol) region of the HBV viral genome and detects both HBV genotype and drug resistance.
Discussion
The treatment of chronic HBV infection has been markedly improved in the last decade, primarily due to the availability of oral NA antiviral agents, such as LdT, adefovir, LVD, ETV and tenofovir (15). The latest European Association for the Study of the Liver guidelines (2017) recommend the use of TDF (or the new formulation tenofovir alafenamide) or ETV in monotherapy as first line therapy, due to their current lack of HBV resistance (3). These agents are very efficient at inhibiting viral replication and are well tolerated by patients, appearing to be safe with no relevant or acute side effects, to the best of our knowledge and experience (3).
The major limitation of long-term antiviral therapy for chronic HBV is the development of drug resistance, shown initially as an increase in HBV-DNA level (virological breakthrough) and then as an increase of liver enzymes (biochemical breakthrough) (16). The mutations conferring resistance to NA can be classified as either single-base or multi-base mutations (17). To date, TDF is the only oral antiviral with no evidence of genotypical resistance development during monotherapy treatment in chronic HBV-naïve patients for up to 8 years (17), and with no detectable resistance mutations in treatment-experienced patients (15).
The substitution rtM204V/I in the tyrosine/methionine/aspartate/aspartate (YMDD) motif of the pol gene domain in HBV-DNA is the most common mutation linked to LVD resistance (18), which was observed at a rate of 14-32% after 1 year, and 60-70% after 5 years of LVD treatment (6). In patients infected with LVDr virus, the barrier to ETV is decreased as the virus already contains two of the three substitutions conferring resistance to ETV. These LVDr substitutions result in an 8-fold reduction in HBV susceptibility to ETV (6). Furthermore, data in the literature (18-22) show that, among primary drug resistance mutations, M204I/V is the most frequently encountered in treatment-naïve patients (23). In fact, a systematic review by Zhang et al (24) revealed that the global incidence of rtM204I/V/S is 4.85%.
Although the association between pre-existing RT mutations and advanced liver diseases has not been fully investigated, several types of HBV mutations in RT have previously been reported to be associated with the progression of liver diseases, such as cirrhosis and HCC (23). For example, Kim et al (25), by comparing frequencies and types of pre-existing RT mutations in treatment-naïve patients, found a significantly higher rate of RT mutations in patients with HCC compared with patients with chronic hepatitis, and also identified three mutations that induced NA resistance (rtL80I, rtN139K/T/H and rtM204I/V) and were significantly associated with the progression of HCC.
Genotype C infection, HBeAg-negative status, and low viral loads are significantly associated with higher frequencies and prevalence rates of pre-existing HBV RT mutations (23). Higher frequencies of pre-existing RT mutations were also generally associated, in addition to worse drug treatment outcomes, with liver disease progression, including HCC and cirrhosis. There were 8 mutations in the RT region, rtL80I, rtD134N, rtN139K/T/H, rtY141F, rtM204I/V, rtF221Y, rtI224V and rtM309K that were significantly associated with the progression of HCC in treatment-naïve patients (23).
In the present case report, despite the presence of only one RT substitution (rtM204I), the case showed both genotypical and clinical resistance to ETV, requiring a therapeutic switch to a higher barrier treatment, such as TDF. Analysis of HBV resistance was not performed prior to treatment in the present case, and thus, whether the baseline rtM204I substitution was present before the treatment is not known.
To the best of our knowledge, the present report is one of very few that describe emerging resistance to ETV in a NA-naïve patient after complete viral suppression. Furthermore, the presence of only the M204I substitution makes this case uncommon and unique. Despite the low rate of viral mutations during ETV treatment, NA-naïve patients should be cautiously monitored, even more so than biochemically (GOT and GPT) and virologically (HBV-DNA), for resistance even when complete HBV suppression is achieved.
Acknowledgements
The authors would like to thank Dr Pietro Leanza, Catania, Sicily, Italy for his help with English revisions.
Funding Statement
Funding: Not applicable.
Availability of data and materials
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
Authors' contributions
AM and FC contributed to study conception and design. MC, VM, AP, DS acquired the data. FB, FDA and EVR were involved in analysis and interpretation of data. GN, BMC and BC critically revised the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the patient for publication of this case report.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 2.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: New estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 3.EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Live. [DOI] [PubMed] [Google Scholar]
- 4.Baldick CJ, Eggers BJ, Fang J, Levine SM, Pokornowski KA, Rose RE, Yu CF, Tenney DJ, Colonno RJ. Hepatitis B virus quasispecies susceptibility to entecavir confirms the relationship between genotypic resistance and patient virologic response. J Hepatol. 2008;48:895–902. doi: 10.1016/j.jhep.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Doğan ÜB, Öztürk AB, Akın MS, Yalaki S, Sayan M. A case of entecavir resistance which is developed after complete viral suppression during entecavir treatment for nucleoside-naïve chronic hepatitis B. Turk J Gastroenterol. 2014;25 (Suppl 1):S206–S209. doi: 10.5152/tjg.2014.3605. [DOI] [PubMed] [Google Scholar]
- 6.Lok ASF, McMahon BJ. Chronic hepatitis B: Update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 7.Bartholomeusz A, Locarnini SA. Antiviral drug resistance: Clinical consequences and molecular aspects. Semin Liver Dis. 2006;26:162–170. doi: 10.1055/s-2006-939758. [DOI] [PubMed] [Google Scholar]
- 8.Zoulim F. Hepatitis B virus resistance to entecavir in nucleotide naïve patients: Does it exist? Hepatology. 2006;44:1404–1407. doi: 10.1002/hep.21451. [DOI] [PubMed] [Google Scholar]
- 9.Colonno RJ, Rose R, Baldick CJ, Levine S, Pokornowski K, Yu CF, Walsh A, Fang J, Hsu M, Mazzucco C, et al. Entecavir resistance is rare in nucleoside naïve patients with hepatitis B. Hepatology. 2006;44:1656–1665. doi: 10.1002/hep.21422. [DOI] [PubMed] [Google Scholar]
- 10.Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, Wichroski MJ, Xu D, Yang J, Wilber RB, Colonno RJ. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years-of therapy. Hepatology. 2009;49:1503–1514. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki F, Akuta N, Suzuki Y, Yatsuji H, Sezaki H, Arase Y, Kawamura Y, Hosaka T, Kobayashi M, Ikeda K, et al. Selection of a virus strain resistant to entecavir in a nucleoside-naïve patient with hepatitis B of genotype H. J Clin Virol. 2007;39:149–152. doi: 10.1016/j.jcv.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Lee HW, Kim HJ, Hong SP, Cha BK, Chang HY, Choi CH, Do JH, Kim JG, Chang SK. Simultaneous emergence of entecavir resistance mutations in a nucleoside-naïve chronic hepatitis B patient. Intervirology. 2012;55:380–384. doi: 10.1159/000336561. [DOI] [PubMed] [Google Scholar]
- 13.Kobashi H, Fujioka S, Kawaguchi M, Kumada H, Yokosuka O, Hayashi N, Suzuki K, Okanoue T, Sata M, Tsubouchi H, et al. Two cases of development of entecavir resistance during entecavir treatment for nucleoside-naïve chronic hepatitis B. Hepatol Int. 2009;3:403–410. doi: 10.1007/s12072-008-9108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gherlan GS. Liver ultrasound elastography: More than staging the disease. World J Hepatol. 2015;7:1595–1600. doi: 10.4254/wjh.v7.i12.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho WH, Lee HJ, Bang KB, Kim SB, Song IH. Development of tenofovir disoproxil fumarate resistance after complete viral suppression in a patient with treatment-naïve chronic hepatitis B: A case report and review of the literature. World J Gastroenterol. 2018;24:1919–1924. doi: 10.3748/wjg.v24.i17.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hulgan T, Haas DW. Toward a pharmacogenetic understanding of nucleotide and nucleoside analogue toxicity. J Infect Dis. 2006;194:1471–1474. doi: 10.1086/508550. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Corsa AC, Buti M, Cathcart AL, Flaherty JF, Miller MD, Kitrinos KM, Marcellin P, Gane EJ. No detectable resistance to tenofovir disoproxil fumarate in HBeAg+ and HBeAg-patients with chronic hepatitis B after 8 years of treatment. J Viral Hepat. 2017;24:68–74. doi: 10.1111/jvh.12613. [DOI] [PubMed] [Google Scholar]
- 18.Lai CL, Chien RN, Leung NWY, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi S, Ide T, Sata M. Detection of YMDD motif mutations in some lamivudine-untreated asymptomatic hepatitis B virus carriers. J Hepatol. 2001;34:584–586. doi: 10.1016/s0168-8278(00)00023-4. [DOI] [PubMed] [Google Scholar]
- 20.Lee CZ, Lee HS, Huang GT, Yang PM, Sheu JC. Detection of YMDD mutation using mutant-specific primers in chronic hepatitis B patients before and after lamivudine treatment. World J Gastroenterol. 2006;12:5301–5355. doi: 10.3748/wjg.v12.i33.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tunçbilek S, Köse Ş, Elaldi A, Akman S. Lamivudine resistance in untreated chronic hepatitis B patients in Turkey. Turkish J Gastroenterol. 2008;19:99–103. [PubMed] [Google Scholar]
- 22.Huang CJ, Wu CF, Lan CY, Sung FY, Lin CL, Liu CJ, Liu HF, Yu MW. Impact of Genetic Heterogeneity in Polymerase of Hepatitis B virus on dynamics of viral load and hepatitis B progression. PLoS One. 2013;8(e70169) doi: 10.1371/journal.pone.0070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi YM, Lee SY, Kim BJ. Naturally occurring hepatitis B virus reverse transcriptase mutations related to potential antiviral drug resistance and liver disease progression. World J Gastroenterol. 2018;24:1708–1724. doi: 10.3748/wjg.v24.i16.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Q, Liao Y, Cai B, Li Y, Li L, Zhang J, An Y, Wang L. Incidence of natural resistance mutations in naïve chronic hepatitis B patients: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2015;30:252–261. doi: 10.1111/jgh.12831. [DOI] [PubMed] [Google Scholar]
- 25.Kim JE, Lee SY, Kim H, Kim KJ, Choe WH, Kim BJ. Naturally occurring mutations in the reverse transcriptase region of hepatitis B virus polymerase from treatment-naïve Korean patients infected with genotype C2. World J Gastroenterol. 2017;23:4222–4232. doi: 10.3748/wjg.v23.i23.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.