Abstract
Background:
Oropharyngeal squamous cell carcinoma (OPSCC) epidemiology has not been examined previously in the nationwide Veterans Affairs (VA) population.
Methods:
Joinpoint regression analysis was applied to OPSCC cases identified from VA administrative data from 2000 to 2012.
Results:
We identified 12 125 OPSCC cases (incidence: 12.2 of 100 000 persons). OPSCC incidence declined between 2000 and 2006 (annual percent change [APC] = −4.27, P < .05), then increased between 2006 and 2012 (APC = 7.02, P < .05). Significant incidence increases occurred among white (APC = 7.19, P < .05) and African American (APC = 4.87, P < .05) Veterans and across all age cohorts. The percentage of never-smokers increased from 8% in 2000 to 15.7% in 2012 (P < .001), and 2-year overall survival improved from 31.2% (95% confidence interval (CI) [30-33.4]) to 55.7% (95% CI [54.4-57.1]).
Conclusions:
OPSCC incidence is increasing across all racial and age cohorts in the VA population. Smoking rates remain high among Veterans with OPSCC and gains in survival lag those reported in the general population.
Keywords: human papilloma virus, joinpoint, oropharyngeal cancer, survival, Veterans Affairs
1. INTRODUCTION
Over the last two decades, the epidemiology of head and neck cancer has changed significantly. While the overall incidence of head and neck cancer has declined along with a decrease in smoking prevalence, there has been a recent increase in the incidence of oropharyngeal squamous cell carcinoma (OPSCC) associated with human papillomavirus (HPV) infection.1–4 Head and neck cancer is traditionally associated with heavy smoking and alcohol consumption, yet it is estimated that approximately 60% to 70% of new OPSCC cases are attributable to HPV.5,6 In 2015, OPSCC overtook cervical cancer as the most common HPV-associated in the United States.7
The Veterans Health Administration (VHA) represents the largest integrated health care system in the United States.8 The demographic characteristics of VHA patients differ from the general population; the Veteran population is significantly older9 and with higher exposure to tobacco10,11 compared to the general population. Several recent single-institutional series have examined the changing nature of OPSCC in the VHA population, demonstrating an increase in HPV-positive OPSCC cases.12,13 The degree to which these trends are generalizable to the broader Veteran population remains unknown. To date, no population-level analysis of OPSCC epidemiology in Veterans has been conducted. The purpose of this study was to use the national VHA administrative database to (a) measure the incidence of OPSCC in the national VHA population and (b) measure the number of OPSCC cases potentially attributable to tobacco exposure. We hypothesized that OPSCC incidence has increased among Veterans but without the dramatic concomitant reduction in tobacco exposure noted in the general population.
2. METHODS
2.1. Veterans Affairs population
After approval from the Institutional Review Board (IRB) for Human Subjects Research at Baylor College of Medicine and the Michael E. DeBakey VA Medical Center (MEDVAMC), a retrospective cohort study of all Veterans diagnosed with OPSCC between 2000 and 2012 at VA hospitals nationwide was conducted using VA administrative databases. Given the retrospective nature of the study waiver of consent was requested and approved by the IRB. The VA Inpatient and Outpatient Medical SAS Datasets were used to identify patients with OPSCC using International Classification of Diseases, 9th edition (ICD-9) codes. ICD-9 codes for OPSCC include: 141.0 (malignant neoplasm of base of tongue, 141.6 (malignant neoplasm of lingual tonsil), 146 (malignant neoplasm of oropharynx), 146.0 (malignant neoplasm of tonsil), 146.1 (malignant neoplasm of tonsillar fossa), 146.2 (malignant neoplasm of tonsillar pillars), 146.3 (malignant neoplasm of vallecula epiglottica), 146.4 (malignant neoplasm of anterior aspect of epiglottis), 146.5 (malignant neoplasm of junctional region of oropharynx), 146.6 (malignant neoplasm of the lateral wall of oropharynx), 146.7 (malignant neoplasm of posterior wall of oropharynx), 146.8 (malignant neoplasm of other specified sites of oropharynx), and 146.9 (malignant neoplasm of oropharynx, unspecified site). 145.3 (malignant neoplasm of soft palate) and 145.5 (malignant neoplasm of uvula) were not included. Individual subjects with at least two hospital or clinic visits in which OPSCC or ICD-9 codes were recorded within 6 months were included in this study. Incident cases of OPSCC were calculated as a proportion of the total number of unique patients who had at least one visit to a VA hospital or clinic each year.
Smoking data for these patients were obtained from ICD-9 and CPT codes for tobacco-associated diseases using outpatient records, inpatient records, and from the VHA Corporate Data Warehouse (CDW) Health Factors database.14 This database is a national repository that contains clinical and administrative data from the VHA and its use in research has been previously validated.14,15 This administrative data source does not make it possible to separate current smokers from former smokers, nor to identify if and when smokers quit, therefore these patients were grouped as “ever-smokers.” Patients identifiable as “never have smoked” or “lifetime non-smoker” in this database were grouped as “never-smokers.”
2.2. Statistical methods
Age-standardized yearly incidence rates were calculated between 2000 and 2012. Incidence rates were age-adjusted based on the age distribution of the 2000 VA population. All incidence rates were expressed as the number of cases/100 000 persons.
Joinpoint regression analysis was used to determine annual percent change (APC) in incidence as well as to identify changes in OPSCC incidence trends over the study period. All joinpoint regression analyses were performed using statistical software from the United States National Cancer Institute Surveillance, Epidemiology, and End Results Program.16 This software identifies timepoints when a statistically significant change in a trend has occurred, known as a “joinpoint.” The maximum number of joinpoints was limited to 2, in accordance with Surveillance, Epidemiology, and End Results (SEER) recommendations given the number of years included in this study.17 To assess the number of joinpoints, slope in the trends, and their significance, the software uses a Monte Carlo based permutation test.18 APC was calculated assuming a Poisson distribution.18,19
The VHA Vital Status File was used to assess the time of death and overall survival (OS) for the subjects included in this study. This vital status file combines mortality data from multiple VHA and non-VHA data sources including the Beneficiary Identification Records Locator Subsystem (BIRLS) Death file, VHA Medicare Vital Status File, and the Social Security Administration (SSA) Death Master File. OS at 2 years was determined using the Kaplan-Meier method. In order to assess possible changes in survival during the study period, 2-year OS was determined for the time periods 2000-2006 and 2007-2012 and comparison between groups were made using log-rank statistics. Time periods were defined based on an initial joinpoint analysis showing a change in OPSCC incidence trend in 2006. All survival analyses were performed using SAS 9.3. (SAS Institute, Inc., Cary, North Carolina).
3. RESULTS
3.1. Patient demographics
A total of 12 125 patients with oropharyngeal cancer were identified from 10 889 840 individual patients receiving care within the VHA health care system between 2000 and 2012. Table 1 summarizes demographic characteristics for the study cohort. The average age of patients was 64 years and the vast majority of patients (99%) were males. Overall, 79.2% of patients were non-Hispanic white and 14.0% African American, 4.0% Hispanic, and 2.8% other/unknown race/ethnicity. Throughout the study period, we identified a statistically significant increase in the number of never-smokers among Veterans with OPSCC. The rate of never-smokers increased from 8.3% in 2000 to 15.5% in 2012 (P = .0003).
TABLE 1.
Demographic characteristics and joinpoint regression analysis of oropharyngeal cancer trends in the Veterans Health Administration, 2000-2012
| Variable | No. of subjects (%) | Time interval | APC |
|---|---|---|---|
| All subjects | 12 125 (100) | 2000-2006 | −4.27* |
| 2006-2012 | 7.02* | ||
| Age | |||
| 18-49 | 771 (6.4) | 2000-2012 | −3.94* |
| 50-69 | 9011 (74.3) | 2000-2006 | −1.42 |
| 2006-2012 | 7.13* | ||
| 70 and above | 2343 (19.3) | 2000-2004 | −11.29* |
| 2004-2012 | 4.77* | ||
| Racea | |||
| Non-Hispanic white | 9606 (79.2) | 2000-2006 | −2.17 |
| 2006-2012 | 7.19* | ||
| African American | 1689 (14) | 2000-2006 | −6.20* |
| 2006-2012 | 4.87* | ||
Abbreviation: APC, annual percent change.
Eight-hundred and thirty subjects (6.8%) that belong to the “Hispanic” and “other/unknown race/ethnicity” groups are not displayed in this table as the numbers are too small to perceive any significant APC or to detect any joinpoint.
Note: P < .05.
3.2. Joinpoint regression analysis
The mean OPSCC incidence for the entire time period was 12.2 cases of 100 000 persons, with a decrease in OPSCC incidence from 13.1 of 100 000 in 2000 to a low of 9.6 of 100 000 in 2006. This trend was followed by an increase in OPSCC incidence to a high of 15.6 of 100 000 in 2012. Joinpoint regression analysis was performed for the entire cohort, by age group, and by race (Table 1). Overall, a statistically significant decline in OPSCC incidence was noted between 2000 and 2006 (APC = −4.27, P < .05), followed by a statistically significant increase between 2006 and 2012 (APC = 7.02, P < .05) (Figure 1).
FIGURE 1.
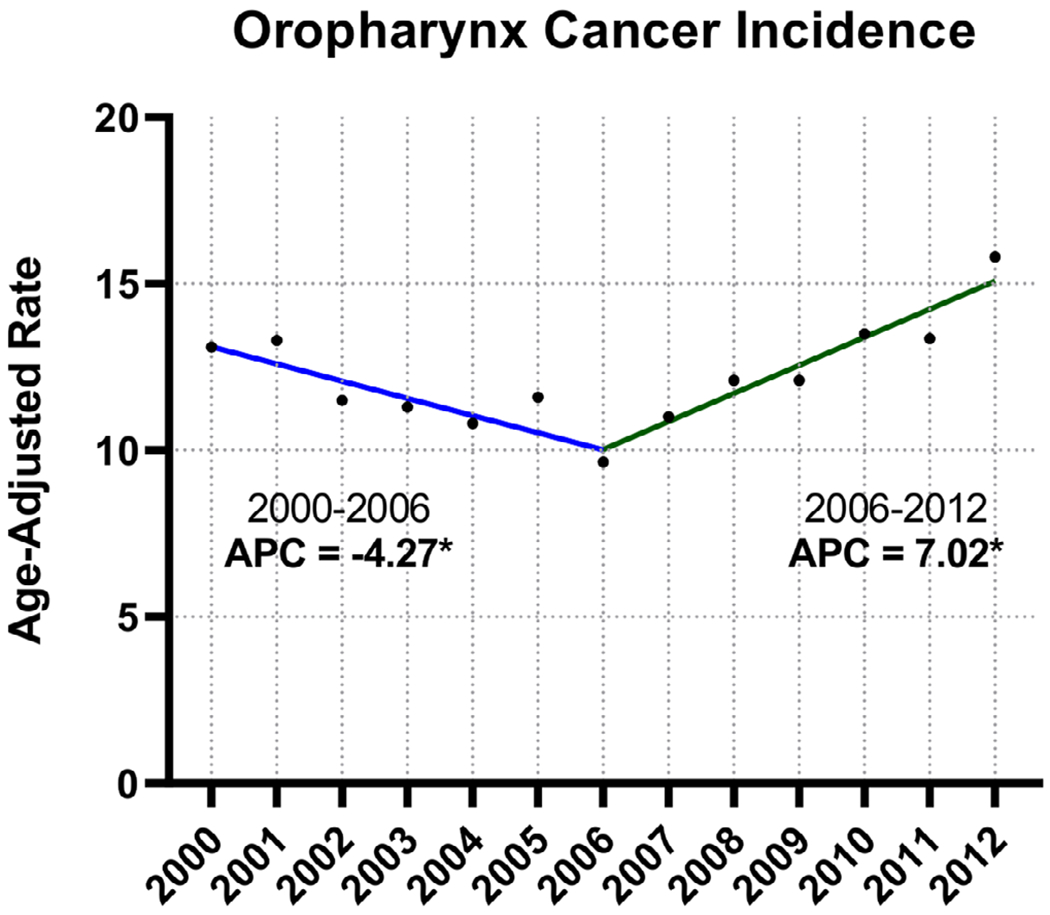
Joinpoint regression analysis, oropharyngeal cancer incidence in the Veterans Health Administration (VHA) population, 2000-2012. An asterisk following the annual percentage change (APC) value indicates statistical significance (P < .05) [Color figure can be viewed at wileyonlinelibrary.com]
OPSCC incidence trends by age group are found in Figure 2. OPSCC incidence in the 50 to 69 year age group decreased significantly between 2000 and 2006 (APC = −1.42, P < .05) and increased between 2006 and 2012 (APC = 7.13, P < .05). Similar trends were seen in the >70 age group, but with an earlier joinpoint and less pronounced changes.
FIGURE 2.
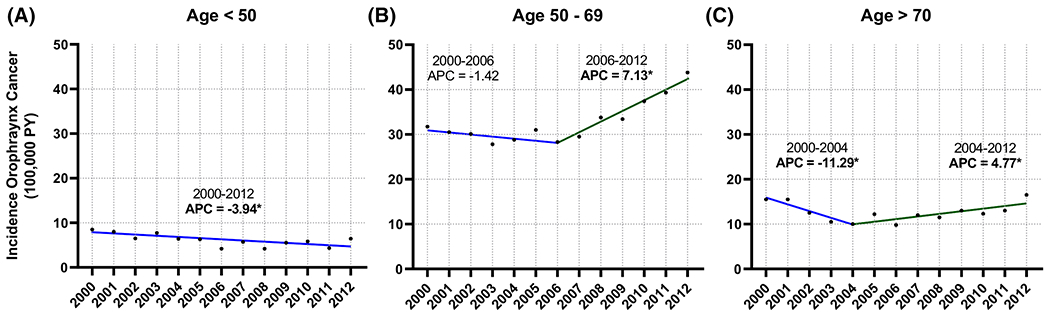
Joinpoint regression analysis, oropharyngeal cancer incidence in the Veterans Health Administration (VHA) population by age group, 2000-2012. A, Age <50; B, age 50 to 69; C, age >70. An asterisk following the annual percentage change (APC) value indicates statistical significance (P < .05) [Color figure can be viewed at wileyonlinelibrary.com]
We considered the impact of race on OPSCC incidence in the VHA (Figure 3). OPSCC incidence in both non-Hispanic whites (APC = −2.17, P < .05) and African Americans (APC = −6.20, P < .05) decreased significantly between 2000 and 2006. This was followed by a significant increase between 2006 and 2012 in both non-Hispanic whites (2006–2012, APC = 7.19, P < .05) and African American Veterans (2006-2012, APC = 4.87, P < .05).
FIGURE 3.
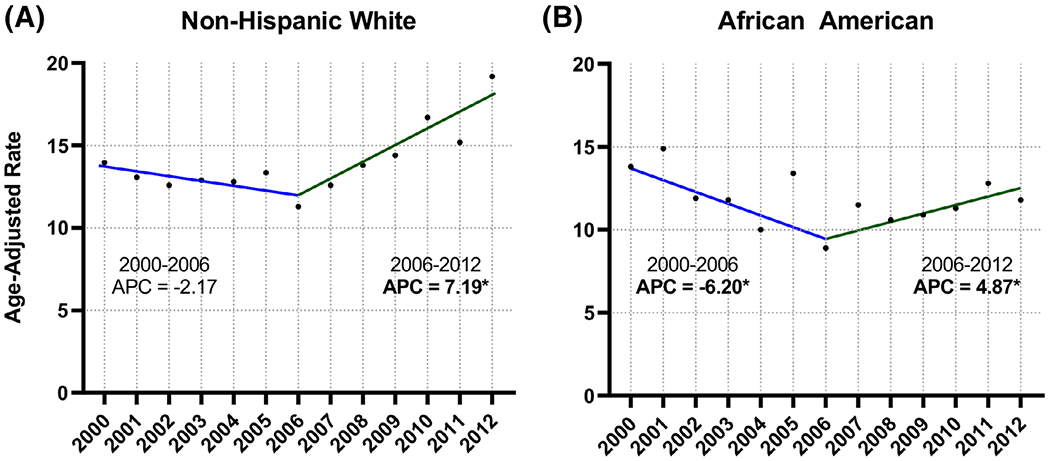
Joinpoint regression analysis, oropharyngeal cancer incidence in the Veterans Health Administration (VHA) population by race, 2000-2012. A, Non-Hispanic white; B, African Americans. An asterisk following the annual percentage change (APC) value indicates statistical significance (P < .05) [Color figure can be viewed at wileyonlinelibrary.com]
We then examined OS in the VHA OPSCC cohort over the study period. Two-year OS for the early (2000-2006) and the late (2007-2012) study periods was 31.2% (95% CI [30-33.4]) and 55.7% (95% CI [54.4-57.1]), respectively (log-rank P-value <.001) (Figure 4A). When we examined survival in 1 to 3 year time intervals throughout the study period, we note a more gradual improvement in survival between 2000 and 2012 (log rank P-value <.001) (Figure 4B). We also examined OS among ever-smokers vs never-smokers. Two-year OS was 45.2% (95% CI [44.1-46.4]) and 62.8% (95% CI [59.6-65.8]) in ever-smokers vs never-smokers, respectively (log-rank P-value <.001).
FIGURE 4.
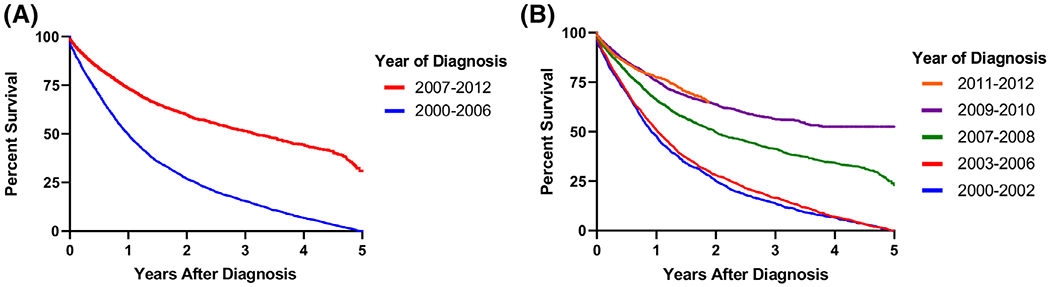
Oropharyngeal cancer survival in the Veterans Health Administration (VHA) population. A, Overall survival per group defined by joinpoint regression analysis: 2000-2006 and 2007-2012; B, overall survival per year of diagnosis: 2000-2012 [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
We are the first to examine the epidemiology of oropharyngeal cancer across the entire VHA in the modern era. Using VHA administrative data, we note a decline in OPSCC incidence between 2000 and 2006 that was followed by an incidence increase between 2007 and 2012. In addition to changes in OPSCC incidence within the VHA, we note a decline in tobacco use among OPSCC patients over the last 10 years, suggesting an alternative etiology for the recent increase in incidence. These trends underscore the dynamic nature of the patient population treated in the VHA and provide opportunities to explore the changing etiology of OPSCC among Veterans.
Shifts in OPSCC incidence within the VHA population differ temporally from trends in the general population. A SEER database analysis from 1975 to 2014 by Osazuwa-Peters et al demonstrated that OPSCC incidence increased in the overall population from 2000 to 2014.20 Among Veterans, we see a similar trend within the VHA occurring approximately 6 years later. Interestingly, the APC demonstrated in the VHA population is higher compared to the general population using the SEER database (7.02 vs 2.33). Although HPV data is not available in this administrative database study, several previous single-institutional series demonstrate an increase in HPV-positive OPSCC cases among Veterans over the same time period.12,21,22 At our own institution, we noted an increase in HPV-positive OPSCC from 41% (2000-2002) to 55% (2009-2011).12 These data, in combination with the system-wide increase in incidence and decrease in tobacco exposure, support our contention that the trends noted in the Veteran OPSCC population are likely associated with a rise in HPV-positive OPSCC. The reason for the delayed incidence increase in the VHA population is unclear. Given significantly higher rates of smoking in Veterans compared to the general population,10,11 we postulate that an early increase in HPV-positive OPSCC incidence may have been masked by the persistently elevated incidence of OPSCC cases attributable to smoking. Notwithstanding temporal changes in incidence, our data strongly suggest a growing burden of HPV-positive OPSCC in the VHA population that now parallels that in the general population.
The findings from this study also provide more insight into the changing demographics of diverse OPSCC populations across the United States. Previous reports suggested that the HPV-positive OPSCC incidence increase was limited primarily to younger, non-Hispanic white males,23 while more recent data suggest that HPV-positive OPSCC incidence has increased in all racial/ethnic groups and age cohorts. Faraji et al recently noted rapidly rising prevalence of HPV-positive OPSCC in African Americans and Hispanics.24 A study of National Cancer Database of patients with OPSCC by Rettig et al noted an increase in the proportion of OPSCC cases associated with HPV across all age groups.25 Among patients >70 years of age, the proportion of HPV-positive cases is now 60%. They also demonstrate an increase in the age at diagnosis for patients with HPV-positive OPSCC over the 5 years.25 While we do not have HPV status for the cases in this study, the trends noted in this study further support these findings. Among Veterans, we demonstrate increased incidence in both non-Hispanic whites and African Americans and across multiple age groups. Although HPV-positive OPSCC is certainly most commonly encountered in non-Hispanic white patients, a high index of suspicion and appropriate HPV testing should be conducted on all Veterans seen with OPSCC.
Despite the increased incidence of OPSCC in the VHA, we do not see a parallel improvement in OS that would be expected given the suspected rise in HPV-positive cases. Even as HPV-positive OPSCC cases rise among Veterans, our data suggest that the majority of cases within the VHA remain strongly associated with tobacco use. The rate of never-smokers ranged from 8% to 15% per year, significantly lower compared to approximately 40% in recent OPSCC cohorts presented in the literature.5,6 In the recently released 2019 Survey of Veteran Enrollees’ Health and Use of Health Care, smoking prevalence among all Veterans was found to be unchanged compared to the previous year at 14.6% and more that 50% of current smokers were found to have made an unsuccessful quite attempt in the previous 12 months.26 As has been reported in other series, smokers with OPSCC have poor survival in the VHA cohort.12,21 Strikingly, OPSCC survival remained relatively poor even among never-smokers, with 2-year OS of 62.8% compared to 75% to 90% 5-year survival reported in other modern series.2,5,27,28 These findings may reflect the fact that the VHA population is significantly older9 and has higher rates of comorbidities compared to the general population.29 They also highlight the persistent heterogeneity of OPSCC, even as national research efforts are increasingly focused on treatment deintensification and excellent outcomes associated with HPV-positive OPSCC.27,30–32 A more complete, Veteran-specific analysis of the factors related with OPSCC outcomes (including race and smoking status) is required to provide more informed counseling and prognostication for this population.
This study has several limitations. The use of ICD-9 codes from VA administrative data is potentially subject to information bias, including the potential for misclassification. Although ICD-9 codes do not provide details on tumor histology, tumor stage, treatment, and outcomes, administrative data have been previously used to study other cancer sites.33,34 We conducted a validation of OPSCC ICD-9 codes on a sample of 200 cases from a single VHA tertiary center (Michael E. DeBakey VA Medical Center, MEDVAMC). We found a positive predictive value of 86%, which is similar to other administrative and claims databases.34–37 Given the large number of cases, the relatively small number of potential false positive OPSCC cases would not be sufficient to alter the trends noted in this study. We acknowledge that not all ICD-9 codes that represent the oropharynx were included; nevertheless, malignant neoplasms of the soft palate and uvula are rarely associated to HPV,38 represent the minority of OPSCC and cases have been steadily decreasing throughout the years.39 Therefore including these subsites would not add a significant impact or change in the noted epidemiologic trends. Another important limitation is the lack of data on HPV status among subjects in this cohort. While this administrative database study was not designed to determine the impact of HPV on OPSCC trends in the VHA population, our data built on two previous studies single-institutional VHA studies that demonstrate an increase in HPV-associated OPSCC. With respect of survival analysis, we recognize that our study was limited to OS only. However, OS remains an important metric for this study, especially given the significantly worse survival in the VHA OPSCC population compared to the civilian population.
This study provides important insight into the current and future burden of OPSCC in the VHA population. Taken together with previous work, our findings suggest that OPSCC incidence is increasing in the VHA population with persistently high rates of smoking and without the parallel improvement in OS. These findings underscore the need for Veteran-specific research on head and neck cancer epidemiology and outcomes.
ACKNOWLEDGMENTS
This study was supported by the Department of Veterans Affairs (VA) VISN 16 Pilot Project Grant (JPZ). This work was supported by the Seed Grant Program of the Michael E. DeBakey Veterans Affairs Medical Center (VCS) and a Career Development Award from the United States Department of Veterans Affairs (Clinical Science Research and Development- 1IK2CX001953). This work was supported by resources at the VA HSR&D Center for Innovations in Quality, Effectiveness, and Safety (#CIN 13-413) at the Michael E. DeBakey VA Medical Center, Houston, TX.
Funding information
Department of Veterans Affairs (VA) VISN 16 Pilot Project Grant (JPZ). Career Development Award from the United States Department of Veterans Affairs (Clinical Science Research and Development- 1IK2CX001953). VA HSR&D Center for Innovations in Quality, Effectiveness, and Safety (#CIN 13-413).
Footnotes
Section Editor: Benjamin Judson
REFERENCES
- 1.Hashibe M, Sturgis EM. Epidemiology of oral-cavity and oropharyngeal carcinomas: controlling a tobacco epidemic while a human papillomavirus epidemic emerges. Otolaryngol Clin North Am. 2013;46(4):507–520. [DOI] [PubMed] [Google Scholar]
- 2.Sturgis EM, Ang KK. The epidemic of HPV-associated oropharyngeal cancer is here: Is it time to change our treatment paradigms? J Natl Compr Canc Netw. 2011;9(6):665–673. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36):4550–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. [DOI] [PubMed] [Google Scholar]
- 7.Van Dyne EA, Henley SJ, Saraiya M, Thomas CC, Markowitz LE, Benard VB. Trends in human papillomavirus-associated cancers—United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67(33):918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.United States Department of Veterans Affairs. www.va.gov. Accessed June 8, 2020.
- 9.National Center for Veterans Analysis and Statistics. Profile of Veterans: 2011. Data from the American Community Survey. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2011.pdf. Published 2013. Accessed June 8, 2020.
- 10.Brown DW. Smoking prevalence among US veterans. J Gen Intern Med. 2010;25(2):147–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco product use among military veterans—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandulache VC, Hamblin J, Lai S, et al. Oropharyngeal squamous cell carcinoma in the veteran population: association with traditional carcinogen exposure and poor clinical outcomes. Head Neck. 2015;37(9):1246–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shay SG, Chang E, Lewis MS, Wang MB. Characteristics of human papillomavirus-associated head and neck cancers in a veteran population. JAMA Otolaryngol Head Neck Surg. 2015; 141(9):790–796. [DOI] [PubMed] [Google Scholar]
- 14.Golden SE, Hooker ER, Shull S, et al. Validity of Veterans Health Administration structured data to determine accurate smoking status. Health Informatics J. 2020;26(3):1507–1515. [DOI] [PubMed] [Google Scholar]
- 15.McGinnis KA, Brandt CA, Skanderson M, et al. Validating smoking data from the Veteran’s Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13(12):1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Cancer Institute Joinpoint Trend Analysis Software. www.surveillance.cancer.gov/joinpoint. Accessed June 8, 2020.
- 17.Joinpoint Trend Analysis Software Help System: Number of joinpoints. https://surveillance.cancer.gov/help/joinpoint/setting-parameters/method-and-parameters-tab/number-of-joinpoints. Accessed June 8, 2020.
- 18.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. [DOI] [PubMed] [Google Scholar]
- 19.Cayuela A, Rodríguez-Domínguez S, López-Campos JL, Otero Candelera R, Rodriguez Matutes C. Joinpoint regression analysis of lung cancer mortality, Andalusia 1975-2000. Ann Oncol. 2004;15(5):793–796. [DOI] [PubMed] [Google Scholar]
- 20.Osazuwa-Peters N, Simpson MC, Massa ST, Adjei Boakye E, Antisdel JL, Varvares MA. 40-year incidence trends for oropharyngeal squamous cell carcinoma in the United States. Oral Oncol. 2017;74:90–97. [DOI] [PubMed] [Google Scholar]
- 21.Feinstein AJ, Shay SG, Chang E, Lewis MS, Wang MB. Treatment outcomes in veterans with HPV-positive head and neck cancer. Am J Otolaryngol. 2017;38(2):188–192. [DOI] [PubMed] [Google Scholar]
- 22.Vahabzadeh-Hagh AM, Rwigema JM, Nabili V, Wang MB, Lorentz WC. Predictors of prolongation in radiation treatment time in a veteran population treated with chemoradiation for oropharyngeal cancer. Acta Otolaryngol. 2018;138(1):80–84. [DOI] [PubMed] [Google Scholar]
- 23.Settle K, Posner MR, Schumaker LM, et al. Racial survival disparity in head and neck cancer results from low prevalence of human papillomavirus infection in black oropharyngeal cancer patients. Cancer Prev Res (Phila). 2009;2 (9):776–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faraji F, Rettig EM, Tsai HL, et al. The prevalence of human papillomavirus in oropharyngeal cancer is increasing regardless of sex or race, and the influence of sex and race on survival is modified by human papillomavirus tumor status. Cancer. 2019;125(5):761–769. [DOI] [PubMed] [Google Scholar]
- 25.Rettig EM, Zaidi M, Faraji F, et al. Oropharyngeal cancer is no longer a disease of younger patients and the prognostic advantage of human papillomavirus is attenuated among older patients: analysis of the national cancer database. Oral Oncol. 2018;83:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang ZJ, Dhanireddy P, Prince C, Larsen M, Schimpf M, Pearman G. 2019 Survey of Veteran enrollees’ health and use of health care—data findings report. https://www.va.gov/HEALTHPOLICYPLANNING/SOE2019/2019_Enrollee_Data_Findings_Report-March_2020_508_Compliant.pdf. Published 2020. Accessed June 8, 2020.
- 27.Quon H, Richmon JD. Treatment deintensification strategies for HPV-associated head and neck carcinomas. Otolaryngol Clin North Am. 2012;45(4):845–861. [DOI] [PubMed] [Google Scholar]
- 28.Richards L Human papillomavirus—a powerful predictor of survival in patients with oropharyngeal cancer. Nat Rev Clin Oncol. 2010;7(9):481. [DOI] [PubMed] [Google Scholar]
- 29.Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker?: a comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252–3257. [DOI] [PubMed] [Google Scholar]
- 30.Chera BS, Amdur RJ. Current status and future directions of treatment deintensification in human papilloma virus-associated oropharyngeal squamous cell carcinoma. Semin Radiat Oncol. 2018;28(1):27–34. [DOI] [PubMed] [Google Scholar]
- 31.Fundakowski CE, Lango M. Considerations in surgical versus non-surgical management of HPV positive oropharyngeal cancer. Cancers Head Neck. 2016;1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hargreaves S, Beasley M, Hurt C, Jones TM, Evans M. Deintensification of adjuvant treatment after transoral surgery in patients with human papillomavirus-positive oropharyngeal cancer: the conception of the PATHOS study and its develop-ment. Front Oncol. 2019;9:936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung KM, Hasan AG, Rees KS, Parker RG, Legorreta AP. Patients with newly diagnosed carcinoma of the breast: validation of a claim-based identification algorithm. J Clin Epidemiol. 1999;52(1):57–64. [DOI] [PubMed] [Google Scholar]
- 34.Cooper GS, Yuan Z, Stange KC, Dennis LK, Amini SB, Rimm AA. The sensitivity of Medicare claims data for case ascertainment of six common cancers. Med Care. 1999;37(5): 436–444. [DOI] [PubMed] [Google Scholar]
- 35.McClish DK, Penberthy L, Whittemore M, et al. Ability of Medicare claims data and cancer registries to identify cancer cases and treatment. Am J Epidemiol. 1997;145(3):227–233. [DOI] [PubMed] [Google Scholar]
- 36.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27(3):274–282. [DOI] [PubMed] [Google Scholar]
- 37.Kramer JR, Giordano TP, Souchek J, Richardson P, Hwang LY, El-Serag HB. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005;100(1):56–63. [DOI] [PubMed] [Google Scholar]
- 38.Gelwan E, Malm I- J, Khararjian A, Fakhry C, Bishop JA, Westra WH. Nonuniform distribution of high-risk human papillomavirus in squamous cell carcinomas of the oropharynx: rethinking the anatomic boundaries of oral and oropharyngeal carcinoma from an oncologic HPV perspective. Am J Surg Pathol. 2017;41(12):1722–1728. [DOI] [PubMed] [Google Scholar]
- 39.Ellington TD, Henley SJ, Senkomago V, et al. Trends in incidence of cancers of the oral cavity and pharynx—United States 2007-2016. MMWR Morb Mortal Wkly Rep. 2020;69:433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]


