Key Points
Question
Can novel cardiovascular biomarkers aid physicians in the early discrimination of type 2 myocardial infarction (T2MI) from type 1 myocardial infarction (T1MI)?
Findings
In this international, multicenter diagnostic study, biomarkers quantifying endothelial dysfunction, microvascular dysfunction, and/or hemodynamic stress were higher in individuals with T2MI vs T1MI.
Meaning
In this analysis, biomarkers quantifying endothelial dysfunction, microvascular dysfunction, and/or hemodynamic stress provided modest discrimination in the early, noninvasive diagnosis of T2MI vs T1MI; clinical parameters may remain the only reliable means for the identification of patients with T2MI.
This international, multicenter prospective diagnostic study tests whether novel cardiovascular biomarkers quantifying different pathophysiological pathways involved in type 2 and/or type 1 myocardial infarction may aid physicians in the rapid discrimination of these 2 types of myocardial infarction.
Abstract
Importance
Rapid and accurate noninvasive discrimination of type 2 myocardial infarction (T2MI), which is because of a supply-demand mismatch, from type 1 myocardial infarction (T1MI), which arises via plaque rupture, is essential, because treatment differs substantially. Unfortunately, this is a major unmet clinical need, because even high-sensitivity cardiac troponin (hs-cTn) measurement provides only modest accuracy.
Objective
To test the hypothesis that novel cardiovascular biomarkers quantifying different pathophysiological pathways involved in T2MI and/or T1MI may aid physicians in the rapid discrimination of T2MI vs T1MI.
Design, Setting, and Participants
This international, multicenter prospective diagnostic study was conducted in 12 emergency departments in 5 countries (Switzerland, Spain, Italy, Poland, and the Czech Republic) with patients presenting with acute chest discomfort to the emergency departments. The study quantified the discrimination of hs-cTn T, hs-cTn I, and 17 novel cardiovascular biomarkers measured in subsets of consecutively enrolled patients against a reference standard (final diagnosis), centrally adjudicated by 2 independent cardiologists according to the fourth universal definition of MI, using all information, including cardiac imaging and serial measurements of hs-cTnT or hs-cTnI.
Results
Among 5887 patients, 1106 (18.8%) had an adjudicated final diagnosis of MI; of these, 860 patients (77.8%) had T1MI, and 246 patients (22.2%) had T2MI. Patients with T2MI vs those with T1MI had lower concentrations of biomarkers quantifying cardiomyocyte injury hs-cTnT (median [interquartile range (IQR)], 30 (17-55) ng/L vs 58 (28-150) ng/L), hs-cTnI (median [IQR], 23 [10-83] ng/L vs 115 [28-576] ng/L; P < .001), and cardiac myosin-binding protein C (at presentation: median [IQR], 76 [38-189] ng/L vs 257 [75-876] ng/L; P < .001) but higher concentrations of biomarkers quantifying endothelial dysfunction, microvascular dysfunction, and/or hemodynamic stress (median [IQR] values: C-terminal proendothelin 1, 97 [75-134] pmol/L vs 68 [55-91] pmol/L; midregional proadrenomedullin, 0.97 [0.67-1.51] pmol/L vs 0.72 [0.53-0.99] pmol/L; midregional pro–A-type natriuretic peptide, 378 [207-491] pmol/L vs 152 [90-247] pmol/L; and growth differentiation factor 15, 2.26 [1.44-4.35] vs 1.56 [1.02-2.19] ng/L; all P < .001). Discrimination for these biomarkers, as quantified by the area under the receiver operating characteristics curve, was modest (hs-cTnT, 0.67 [95% CI, 0.64-0.71]; hs-cTn I, 0.71 [95% CI, 0.67-0.74]; cardiac myosin-binding protein C, 0.67 [95% CI, 0.61-0.73]; C-terminal proendothelin 1, 0.73 [95% CI, 0.63-0.83]; midregional proadrenomedullin, 0.66 [95% CI, 0.60-0.73]; midregional pro–A-type natriuretic peptide, 0.77 [95% CI, 0.68-0.87]; and growth differentiation factor 15, 0.68 [95% CI, 0.58-0.79]).
Conclusions and Relevance
In this study, biomarkers quantifying myocardial injury, endothelial dysfunction, microvascular dysfunction, and/or hemodynamic stress provided modest discrimination in early, noninvasive diagnosis of T2MI.
Introduction
Myocardial infarction (MI) is one of the most common causes of death worldwide.1,2 Two different pathophysiological mechanisms underlie spontaneously occurring MI: a supply-demand mismatch because of impaired systemic hemodynamics, including hypotension, hypertension, tachycardia, or hypoxemia (type 2 MI [T2MI]); and coronary atherothrombosis triggered by plaque rupture and plaque erosion, with resulting intraluminal thrombosis (type 1 MI [T1MI]).1,2,3,4 Because treatments differ substantially,1,5,6 the early and accurate discrimination of T2MI is a major yet largely unmet clinical need.3,7 Unfortunately, established biomarkers of cardiomyocyte injury, including high-sensitivity cardiac troponin (hs-cTn) T and I levels, have only modest diagnostic discrimination.8 We hypothesized that novel cardiovascular biomarkers quantifying different pathophysiological pathways involved in T2MI and/or T1MI, including endothelial dysfunction, microvascular dysfunction, and/or hemodynamic stress, may aid physicians to rapidly discriminate T2MI from T1MI.
Methods
Patient Population
Advantageous Predictors of Acute Coronary Syndrome Evaluation (APACE) is an ongoing international, multicenter prospective diagnostic study including 12 centers in 5 countries (Switzerland, Spain, Italy, Poland, and the Czech Republic) designed to contribute to improving the management of patients with MI (ClinicalTrials.gov identifier: NCT00470587).9,10,11 Adult patients presenting to the emergency department with acute chest discomfort with an onset or peak within the last 12 hours were recruited. The study was carried out according to the principles of the Declaration of Helsinki and approved by the local ethics committees. Written informed consent was obtained from all patients.
While recruitment was independent of kidney function at presentation, patients with end-stage kidney failure who were receiving long-term dialysis were excluded. For this analysis, patients were also excluded if (1) they presented with ST-elevation myocardial infarction, because T2MI rarely presents this way, or (2) the final diagnosis remained unclear after adjudication in patients with elevated hs-cTn concentrations that possibly indicated MI.
The authors designed the studies, gathered, and analyzed the data according to the Standards for Reporting of Diagnostic Accuracy Studies (STARD) guidelines12 vouched for the data and analysis, wrote the manuscript, and decided to publish. The assays were donated by the manufacturers (QuickSens heart-type fatty acid–binding protein assay [8sens.biognostic GmbH]; AxSym and Architect assays for B-type natriuretic peptide, myeloperoxidase, and hs-cTnI [Abbott Laboratories]; Elecsys pro–B-type natriuretic peptide, hs-cTnT, placental growth factor, pregnancy-associated plasma protein-A, and growth differentiation factor [GDF] 15 assays [Roche Diagnostics]; Maxisorp plates apolipoprotein A-1 IgG autoantibodies AB assay [Nunc]; CVDefine anti-phosphorylcholine IgM assay [Athera Biotechnologies]; cardiac myosin-binding protein C [cMyC] assay [Millipore Sigma]; midregional pro–A-type natriuretic peptide [MR-proANP] LIA assay, midregional proadrenomedullin and C-terminal proendothelin 1[CT-proET-1] assay, and Brahms LUMItest copeptin assay [Brahms AG]), who had no role in the design of the study, data analysis, manuscript preparation, or decision to submit for publication.
Clinical Assessment
All patients underwent clinical assessment that included a standardized and detailed medical history, vital signs, a physical examination, a 12-lead electrocardiogram (ECG), continuous ECG rhythm monitoring, pulse oximetry, standard blood tests, and chest radiography if indicated. Cardiac troponin levels, including hs-cTn levels in some centers, were measured at presentation and serially thereafter if clinically indicated. Treatment of patients was left to the discretion of the attending physician. The estimated glomerular filtration rate was determined using the Chronic Kidney Disease Epidemiology Collaboration formula.13
Investigational Biomarkers
Our objective was to assess the diagnostic discrimination of 17 individual cardiovascular biomarkers quantifying different pathophysiological pathways involved in T2MI and/or T1MI in subsets of consecutively enrolled patients to avoid selection bias. Measurements were performed in batches in a central laboratory from blood samples obtained at emergency department presentation and serial sampling thereafter. For logistic reasons, including remaining sample volume, not all biomarkers could be measured from the same subsets of patients. The Elecsys hs-cTnT and hs-cTnI values were measured as part of routine clinical care and/or from study blood samples.
The following pathophysiological pathways were evaluated: cardiomyocyte injury using alternative signals to hs-cTnT or hs-cTnI levels, including heart-type fatty acid–binding protein, cMyC, and creatine kinase–myocardial band measurements; endothelial dysfunction using CT-proET-1 and midregional proadrenomedullin levels; hemodynamic cardiac stress using B-type natriuretic peptide, N-terminal pro–B-type natriuretic peptide, and MR-proANP levels; endogenous stress using copeptin and glucose levels; plaque instability and angiogenesis using myeloperoxidase, soluble vascular endothelial growth factor receptor 1 or soluble soluble FMS-like tyrosine kinase-1, placental growth factor, pregnancy-associated plasma protein-A, autoantibodies to apolipoprotein A-1, and antibodies to phosphorylcholine levels; inflammation using C-reactive protein levels and leukocyte counts; and the combination of inflammation, hemodynamic stress, and vascular aging using GDF 15 measurements. Detailed information regarding individual immunoassay characteristics is shown in the eAppendix in Supplement 1.
Reference Standard: Adjudicated Final Diagnosis
Two independent cardiologists (in variable pairs, including T.N., J.B., M.R.G., L.K., R.T., C.M., 1 nonbyline author [K.W.], and nonauthors) reviewed all available medical records, including the patient history, physical examination, vital signs in the ambulance and within the first hours in the emergency department, results of laboratory testing, radiologic testing, ECG, echocardiography, cardiac exercise stress testing, lesion severity, and morphology on coronary angiography, pertaining to the patient from the time of emergency department presentation to 90-day follow up. In situations of disagreement about the diagnosis, cases were reviewed and adjudicated in conjunction with a third cardiologist (C.M.). Adjudication of the final diagnosis was performed centrally in the core laboratory (University Hospital Basel, Basel, Switzerland) and included 2 sets of serial cTn or hs-cTn measurements: serial cTn or hs-cTn measurements obtained as part of routine clinical care locally (with different cTn or hs-cTn assays) and serial measurements of hs-cTnT from study blood draws performed centrally in the core laboratory to take advantage of the higher sensitivity and higher overall diagnostic accuracy offered by hs-cTnT.14
Myocardial infarction was defined and hs-cTnT concentrations were interpreted as recommended in current guidelines.1 In brief, MI was diagnosed when there was evidence of myocardial necrosis in association with a clinical setting consistent with myocardial ischemia. Myocardial necrosis was diagnosed by at least 1 hs-cTnT value greater than the 99th percentile, together with a clinically significant rise and/or fall. Absolute changes in hs-cTnT were used to determine clinically significant changes based on the diagnostic superiority of absolute over relative changes.15 Based on studies of the biological variation of cTnT,16,17 as well as data from previous cohort studies on chest pain,18,19 a significant absolute change was defined as a rise or fall of at least 0.01 ng/mL within 6 hours or 0.006 ng/mL within 3 hours (to convert to micrograms per liter, multiply by 1.0). All other patients were classified in the categories of unstable angina, noncardiac chest pain, cardiac but noncoronary disease (eg, myocarditis, takotsubo cardiomyopathy, heart failure), and symptoms of unknown origin with normal levels of cardiac troponin.
Definition of T1MI and T2MI
Both T1MI and T2MI were defined according to the fourth universal definition of MI.1 In addition to the evidence of myocardial necrosis in a clinical setting consistent with acute myocardial ischemia, T1MI was defined as spontaneous MI associated with a primary atherothrombotic coronary event, such as a plaque erosion or rupture, intraluminal coronary thrombus, or distal microembolization. Meanwhile, T2MI was defined as secondary to an oxygen supply-demand mismatch. Conditions reflecting an imbalance between myocardial oxygen supply and demand, including bradyarrhythmias or tachyarrhythmias, hypoxemia, hypotension, hypertension, severe anemia, coronary artery spasm, coronary dissection, or coronary embolism. Underlying coronary artery disease was possible but not required for the diagnosis of T2MI.1 To qualify for T2MI, the same dynamic changes in cTn level were required as for T1MI. As recommended, the documentation of a clear trigger was essential for the diagnosis of T2MI.1 Also, coronary angiography was not mandatory for a diagnosis of T1MI, which limited the possible effect of selection bias because of clinical referral to coronary angiography.1
Follow-up
After hospital discharge, patients were contacted by telephone interview or written form after 3, 12, and 24 months of follow-up. In the case of reported clinical events, in particular cardiovascular events, after presentation to the emergency department, details were reviewed by discussion with the patients and traced by establishing contact with the respective family physician or treating institution. Information regarding death was obtained from the national registry on mortality, hospital’s diagnosis registry, or family physician’s records.
Statistical Analysis
The data are expressed as medians and interquartile ranges (IQRs) for continuous variables and as numbers and percentages for categorical variables. All variables between T1MI and T2MI were compared by the Mann-Whitney U test for continuous variables or Pearson χ2 test for categorical variables. Receiver operating characteristic curves were constructed to assess the area under the curve (AUC) for the discrimination between T1MI and T2MI and between MI and no MI. To derive a diagnostic cutoff concentration with clear clinical consequences, a specificity of 90% for T1MI vs T2MI was selected. Because batch measurements of the different assays were always performed in consecutively enrolled patients, the main analyses were performed in all patients with concentrations available for any assay at any point to maximize the number of measurements available for analysis. Binary logistic regression analysis was used for evaluating the association between biomarker concentration levels (log base–10 transformed) and the presence of T2MI. Considering the total number of T2MI in our study and to avoid overfitting in the model, 2 multivariable models were built. Variable selection was based on relevant previous findings and clinical knowledge. The first model included sex, creatinine clearance, and previous MI (model 1) and was applied to all biomarkers. Model 2 included sex, creatinine clearance, previous MI, age, heart rate, systolic blood pressure, hypercholesterolemia, and body mass index and was applied only to the biomarkers with at least 80 T2MI events. Additionally, a sensitivity analysis was performed for biomarkers, with the highest performance in a subset of patients with all biomarkers measured.
All hypothesis testing was 2-tailed, and P values less than .05 were considered to indicate statistical significance. Statistical analyses were performed using SPSS Statistics for Windows version 25.0 (IBM), MedCalc for Windows version 16.8.4 (MedCalc Software), and Stata version 16.1 (Stata Corp). Data collection was performed from April 2006 to April 2020, and data analysis was performed from April 2020 to June 2020.
Results
Patients Characteristics
From April 2006 to April 2018, a total of 5887 patients were eligible for this analysis (median [IQR] age, 61 [49-74] years; 1977 women [32.6%]). In 1106 of the patients (18.8%), MI was the adjudicated final diagnosis, and of these, 246 patients (22.2%) had T2MI and 860 (77.8%) had T1MI.
Patients with T2MI were more frequently women (T2MI, 89 of 246 patients [36.2%]; T1MI, 225 of 860 patients [26.2%]; P = .002), more often had tachycardia (T2MI, 60 [24.4%]; T1MI, 22 [2.6%]; P < .001) and hypotension (T2MI, 7 [2.8%]; T1MI, 7 [0.8%]; P = .02), and had a lower frequency of previous MI (T2MI, 66 [26.8%]; T2MI, 295 [34.3%]; P = .03) (Table 1). Treatment differed markedly between T2MI and T1MI and included coronary revascularization by percutaneous coronary intervention or coronary artery bypass grafting in 2.8% in patients with T2MI (percutaneous coronary intervention, 6 [2.4%]; coronary artery bypass grafting, 1 [0.4%]) vs 72.8% in patients with T1MI (percutaneous coronary intervention, 551 [64.1%]; coronary artery bypass grafting, 75 [8.7%]; P < .001 for both procedures).
Table 1. Baseline Characteristics.
| Characteristic | Patients, No. (%) | P value | ||
|---|---|---|---|---|
| All | With myocardial infarction | |||
| Type 2 | Type 1 | |||
| Total | 5887 (100) | 246 (22.2) | 860 (77.8) | NA |
| Age, median (IQR), y | 61 (49-74) | 72 (60-80) | 70 (58-79) | .37 |
| Female | 1977 (33.6) | 89 (36.2) | 225 (26.2) | .002 |
| BMI, median (IQR) | 26 (24-30) | 26 (24-28) | 27 (24-30) | .04 |
| Risk factors | ||||
| Hypertension | 3502 (59.6) | 189 (76.8) | 663 (77.1) | .93 |
| Hypercholesterolemia | 2787 (47.4) | 130 (52.8) | 558 (64.9) | .001 |
| Diabetes | 1014 (17.3) | 64 (26.0) | 231 (26.9) | .79 |
| Current smoking | 1468 (25.0) | 45 (18.3) | 214 (24.9) | .03 |
| History of smoking | 2169 (36.9) | 97 (39.4) | 353 (41.0) | .65 |
| Positive family history | 775 (13.2) | 21 (8.5) | 146 (17.0) | .001 |
| Medical history | ||||
| Coronary artery disease | 1901 (32.3) | 101 (41.1) | 391 (45.5) | .22 |
| Previous myocardial infarction | 1359 (23.1) | 66 (26.8) | 295 (34.3) | .03 |
| Previous revascularization | 1597 (27.2) | 75 (30.5) | 319 (37.1) | .06 |
| Peripheral artery disease | 311 (5.3) | 25 (10.2) | 101 (11.7) | .49 |
| Previous stroke | 309 (5.3) | 20 (8.1) | 79 (9.2) | .61 |
| Biochemistry test results, median (IQR) | ||||
| Hemoglobin, g/dL | 14.3 (13.2-15.3) | 14.0 (12.5-15.2) | 14.2 (12.7-15.3) | .12 |
| Creatinine clearance, mL/min/1.73 m2 | 84 (68-100) | 69 (52-89) | 75 (59-94) | .001 |
| Vital signs, median (IQR) | ||||
| Heart rate, bpm | 76 (66-89) | 89 (73-119) | 76 (66-88) | <.001 |
| Bradycardiaa | 86 (1.5) | 5 (2.0) | 18 (2.1) | >.99 |
| Tachycardiab | 212 (3.6) | 60 (24.4) | 22 (2.6) | <.001 |
| Systolic blood pressure, mm Hg | 140 (125-156) | 134 (116-154) | 144 (128-161) | <.001 |
| Hypotensionc | 41 (0.7) | 7 (2.8) | 7 (0.8) | .02 |
| Hypertensiond | 1114 (19.0) | 50 (20.3) | 213 (24.8) | .13 |
| Oxygen saturation, % | 98 (97-99) | 98 (96-99) | 98 (96-99) | .82 |
| Medication at presentation | ||||
| Aspirin or thienopyridin | 2239 (38.1) | 109 (44.3) | 452 (52.6) | .02 |
| Angiotensin-converting enzyme or angiotensin II–receptor inhibitors | 2304 (39.2) | 132 (53.7) | 457 (53.1) | .89 |
| β-Blockers | 1974 (33.6) | 119 (48.4) | 352 (40.9) | .04 |
| Calcium antagonists | 878 (14.9) | 46 (18.7) | 175 (20.3) | .57 |
| Nitrates | 561 (9.5) | 27 (11.0) | 141 (16.4) | .04 |
| Statins | 2061 (35.1) | 108 (43.9) | 387 (45.0) | .76 |
| In-hospital procedures | ||||
| Coronary angiography | 1420 (24.2) | 67 (27.2) | 723 (84.1) | <.001 |
| Percutaneous coronary intervention | 798 (13.6) | 6 (2.4) | 551 (64.1) | <.001 |
| Coronary artery bypass graft | 118 (2.0) | 1 (0.4) | 75 (8.7) | <.001 |
| Ergometry, myocardial perfusion scan, positron emission scan, or stress echocardiography | 1227 (20.9) | 45 (18.3) | 139 (16.2) | .43 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range; NA, not applicable.
SI conversion factors: To convert hemoglobin to g/L, multiply by 10; creatinine clearance to mL/s/m2, multiply by 0.0167.
Less than 50 bpm.
More than 120 bpm.
Systolic blood pressure level less than 90 mm Hg.
Systolic blood pressure level greater than 160 mm Hg.
Concentrations of Cardiovascular Biomarkers
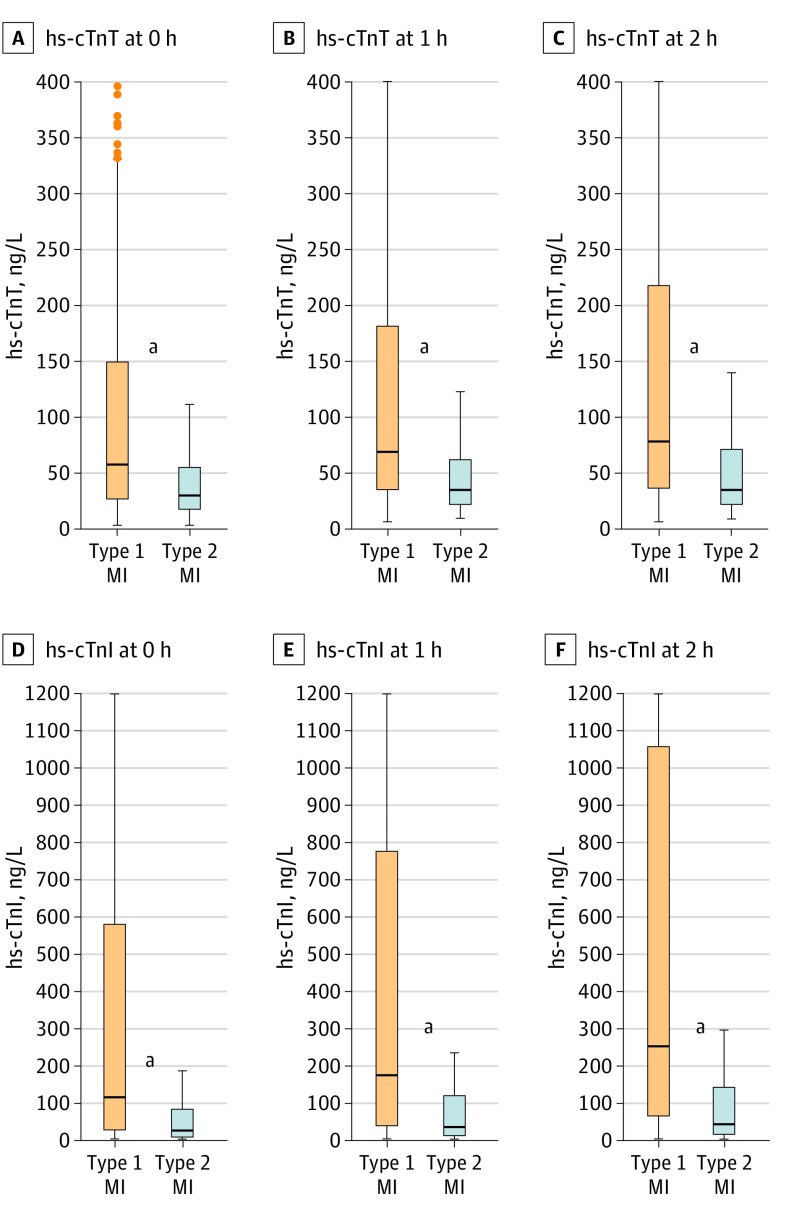
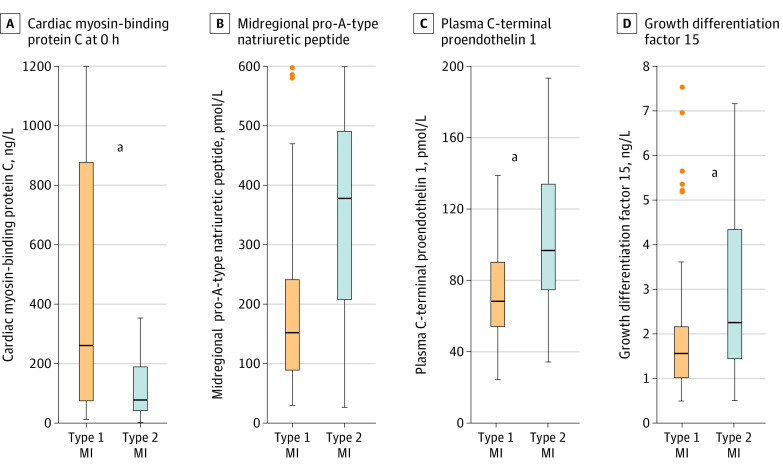
Concentrations of Hs-cTnT and hs-cTnI at presentation were significantly lower among patients with T2MI vs patients with T1MI (median [IQR] hs-cTnT, 30 (17-55) ng/L vs 58 (28-150) ng/L); hs-cTnI, 23 [10-83] ng/L vs 115 [28-576] ng/L; P < .001; Figure 1). Additionally, novel biomarkers quantifying cardiomyocyte injury, including cMyC (at presentation: median [IQR], 76 [38-189] ng/L vs 257 [75-876] ng/L; P < .001), heart-type fatty acid–binding protein (median [IQR], 4.57 [2.48-13.33] vs 6.96 [3.20-18.36] ng/mL), and other established markers, such as creatine kinase–myocardial band (median [IQR], 4.8 [3.3-6.5] vs 5.9 [4.3-11.6] ng/mL), were also significantly lower in patients with T2MI vs T1MI (Figure 2; eFigure in Supplement 1). In contrast, biomarkers quantifying endothelial dysfunction, microvascular dysfunction, and/or hemodynamic stress were higher in patients with T2MI (median [IQR] values: CT-proET-1, 97 [75-134] pmol/L vs 68 [55-91] pmol/L; midregional proadrenomedullin, 0.97 [0.67-1.51] pmol/L vs 0.72 [0.53-0.99] pmol/L; MR-proANP, 378 [207-491] pmol/L vs 152 [90-247] pmol/L; GDF 15, 2.26 [1.44-4.35] ng/L vs 1.56 [1.02-2.19] ng/L; all P < .001) (Figure 2). Most of the other cardiovascular biomarkers, including copeptin, glucose, C-reactive protein, and B-type natriuretic peptide, had comparable concentrations in patients with T2MI vs T1MI (eFigure in Supplement 1).
Figure 1. Boxplots for High-Sensitivity Cardiac Troponin T and I Concentrations.
Concentrations of high-sensitivity cardiac troponin T (measured in 851 patients with type 1 myocardial infarction [MI] and 246 patients with type 2 MI) and high-sensitivity cardiac troponin I (848 patients with type 1 MI and 241 patients with type 2 MI) at presentation, and 1 hour and 2 hours after presentation in patients with type 1 MI vs type 2 MI. Boxes represent medians and the interquartile ranges.
aStatistically significant differences between groups (P < .01).
Figure 2. Boxplots of 4 Biomarkers in Patients With Type 1 and Type 2 Myocardial Infarction (MI).
Concentrations of cardiac myosin-binding protein C (measured in 303 patients with type 1 MI and 105 patients with type 2 MI) at baseline (0 hours), midregional pro–A-type natriuretic peptide (measured in 97 patients with type 1 MI and 37 patients with type 2 MI), C-terminal proendothelin 1 measured in 97 patients with type 1 MI and 37 patients with type 2 MI), and growth differentiation factor 15 (measured in 91 patients with type 1 MI and 37 patients with type 2 MI) in patients with type 1 MI vs type 2 MI. Boxes represent medians and the interquartile ranges. The eFigure in Supplement 1 presents data on all biomarkers.
aStatistically significant differences between groups (P < .05).
Discrimination
The AUC to discriminate T2MI from T1MI was modest for hs-cTnT (0.67 [95% CI, 0.64-0.71]) and hs-cTnI (0.71 [95% CI, 0.67-0.74]) at presentation and slightly higher for subsequent points (eg, at 2 hours: hs-cTnT, 0.71 [95% CI, 0.66-0.75]; hs-cTnI, 0.74 [95% CI, 0.69-0.78]; Table 2). Similar results emerged for cMyC (AUC, 0.67 [95% CI, 0.61-0.73]; Table 2). Biomarkers quantifying endothelial dysfunction, microvascular dysfunction, and/or hemodynamic stress also had moderate discrimination (AUCs: CT-proET-1, 0.73 [95% CI, 0.63-0.83]; midregional proadrenomedullin, 0.66 [95% CI, 0.60-0.73]; MR-proANP, 0.77 [95% CI, 0.68-0.87]; GDF 15, 0.68 [95% CI, 0.58-0.79] (Table 2). Diagnostic performance of all biomarkers for differentiation of MI vs no MI are depicted in eTable 1 in Supplement 1. None of the biomarkers tested performed significantly better in discrimination of T2MI vs T1MI when compared with hs-cTnI (eTable 2 in Supplement 1). Multivariable regression analysis revealed that lower concentrations of hs-cTnT (odds ratio [OR], 0.314 [95% CI, 0.154-0.638]) or hs-cTnI (OR, 0.428 [95% CI, 0.280-0.655]), cMyC (OR, 0.317 [95% CI, 0.134-0.746]), creatine kinase–myocardial band (OR, 0.104 [95% CI, 0.019-0.568]), and heart-type fatty acid–binding protein (OR, 0.445 [95% CI, 0.218-0.908]), as well as higher concentrations of MR-proANP (OR, 12.1 [95% CI, 2.1-68.9]), CT-proET-1 (OR, 31.1 [95% CI, 2.078-464.6]), and midregional proadrenomedullin (OR, 35.2 [95% CI, 2.2-553.3]) remained independently associated with T2MI (Table 3). In a sensitivity analysis, the best performing biomarkers (AUC >0.65) were compared head to head in a subset of patients with all biomarkers measured. Performance of biomarkers did not vary significantly (eTable 3 in Supplement 1).
Table 2. Diagnostic Performance for Differentiation Between Type 2 Myocardial Infarction (T2MI) (n = 246) and Type 1 Myocardial Infarction (T1MI) (n = 860).
| Test | Patients, No. | Receiver operating area under the curve (95% CI) | Cutpoint for ≥90% specificity for T2MI | Specificity (95% CI) | Positive predictive value (95% CI) | Odds ratio for T2MI (95% CI) | Positive likelihood ratio for T2MI (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| With T2MI | With T1MI | |||||||
| Cardiomyocyte injury | ||||||||
| High-sensitivity cardiac troponin T | ||||||||
| 0 h | 246 | 851 | 0.67 (0.64-0.71) | <0.01558 ng/mL | 90.0 (87.8-91.9) | 36.6 (28.9-45.0) | 2.24 (1.53-3.29) | 1.99 (1.45-2.75) |
| 1 h | 215 | 680 | 0.70 (0.66-0.74) | <0.021 ng/mL | 90.2 (87.7-92.2) | 45.1 (36.5-53.9) | 3.15 (2.12-4.67) | 2.60 (1.88-3.58) |
| 2 h | 164 | 531 | 0.71 (0.66-0.75) | <0.023 ng/mL | 90.4 (87.6-92.6) | 45.2 (35.4-55.3) | 3.24 (2.06-5.09) | 2.67 (1.84-3.86) |
| High-sensitivity cardiac troponin I | ||||||||
| 0 h | 241 | 848 | 0.71 (0.67-0.74) | <0.0117 ng/mL | 90.1 (87.9-91.9) | 47.2 (39.6-54.9) | 4.11 (2.89-5.85) | 3.14 (2.38-4.14) |
| 1 h | 206 | 662 | 0.72 (0.68-0.76) | <0.019 ng/mL | 90.0 (87.5-92.1) | 51.1 (42.8-59.4) | 4.55 (3.10-6.68) | 3.36 (2.49-4.53) |
| 2 h | 160 | 510 | 0.74 (0.69-0.78) | <0.0244 ng/mL | 90.0 (87.1-92.3) | 53.2 (43.9-62.3) | 5.12 (3.32-7.88) | 3.63 (2.60-5.05) |
| Creatine kinase–myocardial band | 81 | 377 | 0.69 (0.63-0.75) | <3.2 ng/mL | 90.5 (87.1-93) | 34.5 (23.4-47.7) | 2.9 (1.57-5.36) | 2.46 (1.49-4.06) |
| Heart-type fatty acid–binding protein | 55 | 165 | 0.58 (0.49-0.67) | <2.14 ng/mL | 90.3 (84.8-93.9) | 40.7 (24.5-59.3) | 2.33 (1.02-5.31) | 2.06 (1.02-4.17) |
| Cardiac myosin-binding protein C | ||||||||
| 0 h | 105 | 303 | 0.67 (0.61-0.73) | <29.2 ng/L | 90.1 (86.2-93) | 34.8 (22.7-49.2) | 1.64 (0.86-3.12) | 1.54 (0.88-2.71) |
| 1 h | 93 | 246 | 0.70 (0.64-0.76) | <42.9 ng/L | 90.2 (85.9-93.4) | 48.9 (35.3-62.8) | 3.04 (1.63-5.68) | 2.53 (1.51-4.26) |
| 2 h | 68 | 196 | 0.71 (0.65-0.78) | <48.3 ng/L | 90.3 (85.4-93.7) | 42.4 (27.2-59.2) | 2.42 (1.15-5.09) | 2.12 (1.13-4) |
| Hemodynamic cardiac stress | ||||||||
| B-type natriuretic peptide | 115 | 402 | 0.52 (0.46-0.58) | >256.6 pg/mL | 90 (86.7-92.6) | 27.3 (17.3-40.2) | 1.36 (0.73-2.54) | 1.31 (0.75-2.29) |
| N-terminal pro–B-type natriuretic peptide | 50 | 140 | 0.59 (0.49-0.68) | >4639 ng/L | 90 (83.9-93.9) | 41.7 (24.5-61.2) | 2.25 (0.95-5.37) | 2 (0.95-4.21) |
| Midregional pro–A-type natriuretic peptide | 37 | 97 | 0.77 (0.68-0.87) | >434 ng/L | 90.7 (83.3-95) | 62.5 (42.7-78.8) | 6.67 (2.62-16.95) | 4.37 (2.1-9.11) |
| Endogenous stress | ||||||||
| Copeptin | ||||||||
| 0 h | 104 | 306 | 0.54 (0.47-0.61) | >76.3 pmol/L | 90.2 (86.3-93) | 28.6 (17.2-43.6) | 1.2 (0.6-2.42) | 1.18 (0.63-2.21) |
| 1 h | 87 | 231 | 0.55 (0.47-0.62) | >56.3 pmol/L | 90 (85.5-93.3) | 36.1 (22.5-52.4) | 1.59 (0.77-3.26) | 1.5 (0.8-2.83) |
| 2 h | 64 | 174 | 0.61 (0.53-0.69) | >40.8 pmol/L | 90.2 (84.9-93.8) | 46.9 (30.9-63.6) | 2.83 (1.33-6.01) | 2.4 (1.27-4.52) |
| Glucose | 241 | 832 | 0.52 (0.48-0.57) | >95 mg/dL | 90 (87.8-91.9) | 25.2 (18.1-34) | 1.19 (0.76-1.86) | 1.16 (0.78-1.74) |
| Plaque instability and angiogenesis | ||||||||
| Myeloperoxidase | 36 | 94 | 0.64 (0.55-0.74) | >964.4 pmol/L | 90.4 (82.8-94.9) | 35.7 (16.3-61.2) | 1.52 (0.5-4.71) | 1.45 (0.52-4.04) |
| Soluble vascular endothelial growth factor receptor | 36 | 96 | 0.61 (0.51-0.71) | >2954 ng/L | 90.6 (83.1-95) | 30.8 (12.7-57.6) | 1.21 (0.37-3.99) | 1.19 (0.39-3.61) |
| Placental growth factor | 24 | 65 | 0.59 (0.44-0.75) | >31.49 pg/mL | 90.8 (81.3-95.7) | 50 (25.4-74.6) | 3.28 (0.98-10.95) | 2.71 (0.97-7.59) |
| Pregnancy-associated plasma protein-A | 24 | 65 | 0.52 (0.38-0.66) | <89 mIU/L | 56.9 (44.8-68.2) | 28.2 (16.5-43.8) | 1.12 (0.44-2.83) | 1.06 (0.63-1.78) |
| Autoantibodies to apolipoprotein A-1 IgG OD | 55 | 163 | 0.51 (0.42-0.60) | <0.15 U/mL | 90.2 (84.7-93.9) | 30.4 (15.6-50.9) | 1.34 (0.53-3.37) | 1.3 (0.56-2.99) |
| Anti-phosphorylcholine IgM | 55 | 163 | 0.57 (0.48-0.67) | <21.32 U/mL | 90.2 (84.7-93.9) | 42.9 (26.5-60.9) | 2.56 (1.14-5.76) | 2.22 (1.12-4.4) |
| Endothelial dysfunction | ||||||||
| C-terminal proendothelin 1 | 37 | 97 | 0.73 (0.63-0.83) | >117 pmol/L | 90.7 (83.3-95) | 64 (44.5-79.8) | 7.45 (2.94-18.87) | 4.66 (2.26-9.61) |
| Midregional proadrenomedullina | 103 | 305 | 0.66 (0.60-0.73) | >1.34 nmol/L | 90.2 (86.3-93) | 53.1 (41.1 − 64.8) | 4.54 (2.6-7.86) | 3.36 (2.17-5.2) |
| Inflammation | ||||||||
| C-reactive protein | 204 | 669 | 0.51 (0.46-0.56) | >1.67 mg/dL | 90.1 (87.6-92.2) | 32.7 (24.2-42.4) | 1.7 (1.08-2.67) | 1.59 (1.07-2.35) |
| Leukocytes | 245 | 850 | 0.51 (0.46-0.55) | <5630 cells/μL | 90 (87.8-91.8) | 28.6 (21.2-37.3) | 1.45 (0.95-2.22) | 1.39 (0.96-2.01) |
| Combination of inflammation, hemodynamic stress, and vascular aging | ||||||||
| Growth differentiation factor 15b | 37 | 91 | 0.68 (0.58-0.79) | >3.52 ng/L | 90.1 (82.3-94.7) | 59.1 (38.7-76.7) | 4.94 (1.92-12.71) | 3.55 (1.66-7.59) |
SI conversion factors: To convert B-type natriuretic peptide to ng/L, multiply by 1.0; creatine kinase–myocardial band to μg/L, multiply by 1.0; C-reactive protein to mg/L, multiply by 10; high-sensitivity cardiac troponin T or I to μg/L, multiply by 1.0; leukocytes to cells × 109, multiply by 0.001.
The main pathophysiological triggers for the release of midregional proadrenomedullin are incompletely understood but seem to include microvascular and endothelial dysfunction.
For growth differentiation factor 15, if not mentioned otherwise, blood samples were collected immediately after presentation to the emergency department.
Table 3. Multivariable Regression Analysis for Prognostication of Type 2 Myocardial Infarction (T2MI).
| Test | Patients, No. | Model 1a | Model 2b | |||
|---|---|---|---|---|---|---|
| With T2MI | With T1MI | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Cardiomyocyte injury | ||||||
| High-sensitivity cardiac troponin T | ||||||
| 0 h | 246 | 851 | 0.23 (0.16-0.33) | <.001 | 0.31 (0.15-0.64) | .001 |
| 1 h | 215 | 680 | 0.15 (0.10-0.23) | <.001 | 0.30 (0.13-0.67) | .003 |
| 2 h | 164 | 531 | 0.14 (0.09-0.24) | <.001 | 0.31 (0.13-0.76) | .01 |
| High-sensitivity cardiac troponin I | ||||||
| 0 h | 241 | 848 | 0.37 (0.29-0.45) | <.001 | 0.43 (0.28-0.66) | <.001 |
| 1 h | 206 | 662 | 0.32 (0.25-0.41) | <.001 | 0.46 (0.29-0.73) | .001 |
| 2 h | 160 | 510 | 0.31 (0.23-0.41) | <.001 | 0.50 (0.30-0.84) | .008 |
| Creatine kinase–myocardial band | 81 | 377 | 0.24 (0.13-0.47) | <.001 | 0.10 (0.02-0.57) | .009 |
| Heart-type fatty acid–binding protein | 55 | 165 | 0.45 (0.22-0.91) | .03 | NA | NA |
| Cardiac myosin-binding protein C | ||||||
| 0 h | 105 | 303 | 0.33 (0.22-0.49) | <.001 | 0.32 (0.13-0.75) | .009 |
| 1 h | 93 | 246 | 0.27 (0.18-0.43) | <.001 | 0.44 (0.19-1.06) | .07 |
| 2 h | 68 | 196 | 0.25 (0.15-0.42) | <.001 | NA | NA |
| Hemodynamic cardiac stress | ||||||
| B-type natriuretic peptide | 115 | 402 | 1.52 (0.70-3.28) | .29 | 1.23 (0.16-9.20) | .84 |
| N-terminal pro B-type natriuretic peptide | 50 | 140 | 0.97 (0.55-1.71) | .91 | NA | NA |
| Midregional pro–A-type natriuretic peptide | 37 | 97 | 12.12 (2.13-69.92) | .005 | NA | NA |
| Endogenous stress | ||||||
| Copeptin | ||||||
| 0 h | 104 | 306 | 0.90 (0.56-1.43) | .64 | 1.55 (0.60-3.96) | .36 |
| 1 h | 87 | 231 | 0.74 (0.43-1.28) | .29 | 1.18 (0.37-3.79) | .78 |
| 2 h | 64 | 174 | 1.21 (0.62-2.39) | .58 | NA | NA |
| Glucose | 241 | 832 | 1.05 (0.75-1.45) | .79 | 0.68 (0.33-1.39) | .28 |
| Plaque instability and angiogenesis | ||||||
| Myeloperoxidase | 36 | 94 | 1.54 (0.67-3.50) | .31 | NA | NA |
| Soluble vascular endothelial growth factor receptor | 36 | 96 | 1.00 (0.50-1.99) | .99 | NA | NA |
| Placental growth factor | 24 | 65 | 4.06 (0.07-227.75) | .50 | NA | NA |
| Pregnancy-associated plasma protein-A | 24 | 65 | 0.78 (0.18-3.37) | .74 | NA | NA |
| Autoantibodies to apolipoprotein A-1 IgG OD | 55 | 163 | 0.76 (0.31-1.83) | .54 | NA | NA |
| Antiphosphorylcholine IgM | 55 | 163 | 0.46 (0.16-1.31) | .15 | NA | NA |
| Endothelial dysfunction | ||||||
| C-terminal proendothelin 1 | 37 | 97 | 31.06 (2.08-464.61) | .01 | NA | NA |
| Midregional proadrenomedullin | 103 | 305 | 10.79 (2.45-47.47) | .002 | 35.19 (2.24-553.26) | .01 |
| Inflammation | ||||||
| C-reactive protein | 204 | 669 | 1.13 (0.87-1.47) | .35 | 1.15 (0.93-2.33) | .10 |
| Leukocytes | 245 | 850 | 0.96 (0.37-2.51) | .93 | 2.70 (0.51-14.35) | .25 |
| Combination of inflammation, hemodynamic stress, and vascular aging | ||||||
| Growth differentiation factor 15 | 37 | 91 | 4.76 (0.73-31.13) | .10 | NA | NA |
Abbreviations: NA, not applicable; T2MI, type 2 myocardial infarction.
Model 1 was adjusted for sex, creatinine clearance, and previous myocardial infarction.
Model 2 was adjusted for sex, creatinine clearance, previous myocardial infarction, age, heart rate, systolic blood pressure, hypercholesterolemia, and body mass index.
Discussion
This pilot study tested the hypothesis that novel cardiovascular biomarkers quantifying different pathophysiological pathways involved in T2MI may aid physicians to rapidly discriminate T2MI from T1MI. We report 4 major findings. First, most cardiovascular biomarkers evaluated had comparable concentrations in T2MI vs T1MI and were therefore not helpful in their discrimination. Second, 4 novel cardiovascular biomarkers were higher in T2MI vs T1MI and showed modest promise for the early discrimination of T2MI: MR-proANP, considered to quantify hemodynamic stress; CT-proET-1, considered to quantify endothelial dysfunction; midregional proadrenomedullin, considered to quantify microvascular and endothelial dysfunction; and GDF 15, considered to quantify hemodynamic stress, inflammation, and vascular aging. Third, cMyC concentrations, which may quantify cardiomyocyte injury even more accurately than hs-cTnT or hs-cTnI levels,20,21,22 were lower in T2MI vs T1MI and provided modest diagnostic accuracy, comparable with that provided by hs-cTnT and hs-cTnI. Fourth, while none of the tested cardiovascular biomarkers had significantly higher diagnostic discrimination, multivariable regression analysis suggested a possible additive value of MR-proANP to clinical variables.
These findings extend and corroborate previous studies evaluating mainly hs-cTnT or hs-cTnI for this indication,8,23,24,25 which have documented lower hs-cTnI concentrations in T2MI, but with a large overlap and a resulting modest AUC of 0.63 to 0.66. Given the suggestive findings observed for MR-proANP, future studies are warranted to develop diagnostic models combining routinely available information such as hs-cTnT or hs-cTnI, medical history, and the 12-lead ECG with selected biomarkers. Until these tools are derived and externally validated, however, most patients will still require coronary angiography and/or noninvasive functional or anatomic testing to achieve a high level of diagnostic discrimination. It is important to highlight that the adjudication of T2MI and T1MI can be challenging and should strictly adhere to the current universal definition of MI. It is important to emphasize that the mechanisms of cardiomyocyte injury in patients with myocarditis, takutsubo cardiomyopathy, pulmonary embolism, or heart failure are usually multifactorial and these patients should not be included in the T2MI category.
Limitations
Some limitations warrant consideration when interpreting the findings of this study. First, this study was conducted in patients presenting with symptoms suggestive of acute MI to the ED. We cannot comment on the early discrimination of T2MI in other clinical settings, including the perioperative setting and in patients with critical illness. Second, although we used a very stringent method to adjudicate T1MI and T2MI, including central assessment by experienced cardiologists reviewing cardiac imaging and serial measurements of hs-cTn, a small number of patients may have been misclassified. Limitations include the fact that not all patients underwent coronary angiography and that some of the shortcomings intrinsic to coronary angiography, such as the difficulty in correctly identifying rupture, fissure, erosion or dissection of plaque and intracoronary thrombus. Third, the number of patients in whom all (or most) novel biomarkers could be measured was underpowered for full comparison of diagnostic performance and too small to allow assessment of biomarker combinations. Positive predictive value has been calculated for each biomarker in the respective subset of consecutively enrolled patients and therefore does not allow direct comparison. Fourth, because patients receiving chronic hemodialysis were excluded, the generalizability to these patients remains unknown.
Conclusions
In conclusion, cardiovascular biomarkers provided modest diagnostic performance in the early discrimination of T2MI from T1MI. Clinical parameters may remain the only means for reliable identification of patients with T2MI.
eAppendix. Blood sampling and laboratory methods
eReferences.
eTable 1. Diagnostic performance for differentiation between AMI (n = 1106) and no AMI (n=4781)
eTable 2. Direct comparison of hs-cTnI with other biomarkers
eTable 3. Sensitivity analysis
eFigure. Boxplots for other cardiac biomarkers
Nonauthor Collaborators. APACE Investigators.
References
- 1.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40(3):226. doi: 10.1093/eurheartj/ehy856 [DOI] [PubMed] [Google Scholar]
- 2.Collet J-P, Thiele H, Barbato E, et al. ; ESC Scientific Document Group . 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2020;ehaa575. doi: 10.1093/eurheartj/ehaa575 [DOI] [PubMed] [Google Scholar]
- 3.Vargas KG, Haller PM, Jäger B, et al. Variations on classification of main types of myocardial infarction: a systematic review and outcome meta-analysis. Clin Res Cardiol. 2019;108(7):749-762. doi: 10.1007/s00392-018-1403-3 [DOI] [PubMed] [Google Scholar]
- 4.Sandoval Y, Jaffe AS. Type 2 myocardial Infarction: JACC review topic of the week. J Am Coll Cardiol. 2019;73(14):1846-1860. doi: 10.1016/j.jacc.2019.02.018 [DOI] [PubMed] [Google Scholar]
- 5.McCarthy CP, Vaduganathan M, Januzzi JL Jr. Type 2 myocardial infarction-diagnosis, prognosis, and treatment. JAMA. 2018;320(5):433-434. doi: 10.1001/jama.2018.7125 [DOI] [PubMed] [Google Scholar]
- 6.DeFilippis AP, Chapman AR, Mills NL, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140(20):1661-1678. doi: 10.1161/CIRCULATIONAHA.119.040631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagele P. A simplified proposal to redefine acute myocardial infarction versus acute myocardial injury. Circulation. 2020;141(18):1431-1433. doi: 10.1161/CIRCULATIONAHA.119.044996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandoval Y, Thordsen SE, Smith SW, et al. Cardiac troponin changes to distinguish type 1 and type 2 myocardial infarction and 180-day mortality risk. Eur Heart J Acute Cardiovasc Care. 2014;3(4):317-325. doi: 10.1177/2048872614538411 [DOI] [PubMed] [Google Scholar]
- 9.Boeddinghaus J, Nestelberger T, Koechlin L, et al. ; APACE Investigators . Early diagnosis of myocardial infarction with point-of-care high-sensitivity cardiac troponin I. J Am Coll Cardiol. 2020;75(10):1111-1124. doi: 10.1016/j.jacc.2019.12.065 [DOI] [PubMed] [Google Scholar]
- 10.Nestelberger T, Boeddinghaus J, Badertscher P, et al. ; APACE Investigators . Effect of definition on incidence and prognosis of type 2 myocardial infarction. J Am Coll Cardiol. 2017;70(13):1558-1568. doi: 10.1016/j.jacc.2017.07.774 [DOI] [PubMed] [Google Scholar]
- 11.Schoepfer H, Nestelberger T, Boeddinghaus J, et al. ; APACE (Advantageous Predictors of Acute Coronary Syndrome Evaluation) Investigators . Effect of a proposed modification of the type 1 and type 2 myocardial infarction definition on incidence and prognosis. Circulation. 2020;142(21):2083-2085. doi: 10.1161/CIRCULATIONAHA.120.048920 [DOI] [PubMed] [Google Scholar]
- 12.Bossuyt PM, Reitsma JB, Bruns DE, et al. ; STARD Group . STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. doi: 10.1136/bmj.h5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Coresh J, Greene T, et al. ; Chronic Kidney Disease Epidemiology Collaboration . Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-254. doi: 10.7326/0003-4819-145-4-200608150-00004 [DOI] [PubMed] [Google Scholar]
- 14.Haaf P, Drexler B, Reichlin T, et al. High-sensitivity cardiac troponin in the distinction of acute myocardial infarction from acute cardiac noncoronary artery disease. Circulation. 2012;126(1):31-40. doi: 10.1161/CIRCULATIONAHA.112.100867 [DOI] [PubMed] [Google Scholar]
- 15.Reichlin T, Irfan A, Twerenbold R, et al. Utility of absolute and relative changes in cardiac troponin concentrations in the early diagnosis of acute myocardial infarction. Circulation. 2011;124(2):136-145. doi: 10.1161/CIRCULATIONAHA.111.023937 [DOI] [PubMed] [Google Scholar]
- 16.Vasile VC, Saenger AK, Kroning JM, Jaffe AS. Biological and analytical variability of a novel high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56(7):1086-1090. doi: 10.1373/clinchem.2009.140616 [DOI] [PubMed] [Google Scholar]
- 17.Wu AHB, Lu QA, Todd J, Moecks J, Wians F. Short- and long-term biological variation in cardiac troponin I measured with a high-sensitivity assay: implications for clinical practice. Clin Chem. 2009;55(1):52-58. doi: 10.1373/clinchem.2008.107391 [DOI] [PubMed] [Google Scholar]
- 18.Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361(9):868-877. doi: 10.1056/NEJMoa0903515 [DOI] [PubMed] [Google Scholar]
- 19.Hammarsten O, Fu MLX, Sigurjonsdottir R, et al. Troponin T percentiles from a random population sample, emergency room patients and patients with myocardial infarction. Clin Chem. 2012;58(3):628-637. doi: 10.1373/clinchem.2011.171496 [DOI] [PubMed] [Google Scholar]
- 20.Kaier TE, Twerenbold R, Puelacher C, et al. Direct comparison of cardiac myosin-binding protein c with cardiac troponins for the early diagnosis of acute myocardial infarction. Circulation. 2017;136(16):1495-1508. doi: 10.1161/CIRCULATIONAHA.117.028084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker JO, Tyther R, Liebetrau C, et al. Cardiac myosin-binding protein C: a potential early biomarker of myocardial injury. Basic Res Cardiol. 2015;110(3):23. doi: 10.1007/s00395-015-0478-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaier TE, Anand A, Shah ASV, Mills NL, Marber M. Temporal relationship between cardiac myosin-binding protein C and cardiac troponin I in type 1 myocardial infarction. Clin Chem. 2016;62(8):1153-1155. doi: 10.1373/clinchem.2016.257188 [DOI] [PubMed] [Google Scholar]
- 23.Neumann JT, Sörensen NA, Rübsamen N, et al. Discrimination of patients with type 2 myocardial infarction. Eur Heart J. 2017;38(47):3514-3520. doi: 10.1093/eurheartj/ehx457 [DOI] [PubMed] [Google Scholar]
- 24.Chapman AR, Adamson PD, Shah ASV, et al. ; High-STEACS Investigators . High-sensitivity cardiac troponin and the universal definition of myocardial infarction. Circulation. 2020;141(3):161-171. doi: 10.1161/CIRCULATIONAHA.119.042960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horiuchi Y, Wettersten N, Patel MP, et al. Biomarkers enhance discrimination and prognosis of type 2 myocardial infarction. Circulation. 2020;142(16):1532-1544. doi: 10.1161/CIRCULATIONAHA.120.046682 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Blood sampling and laboratory methods
eReferences.
eTable 1. Diagnostic performance for differentiation between AMI (n = 1106) and no AMI (n=4781)
eTable 2. Direct comparison of hs-cTnI with other biomarkers
eTable 3. Sensitivity analysis
eFigure. Boxplots for other cardiac biomarkers
Nonauthor Collaborators. APACE Investigators.