Key Points
Questions
Do family genetic risk scores (FGRSs) clarify the genetic relationship between major affective and psychotic disorders, and does genetic risk affect clinical features?
Findings
In this national Swedish cohort study of 4.1 million individuals, FGRSs produced clear separations between major affective and psychotic disorders and were associated with early age at onset and high rates of recurrence. The FGRS for schizophrenia clearly distinguished the psychotic and nonpsychotic forms of major depression and bipolar illness.
Meaning
These findings provide validation, from a genetic perspective, for these major diagnostic categories and extend prior observations of the association of FGRS with age at onset, recurrence, psychotic subtypes, and diagnostic conversions.
This national cohort study assesses whether family genetic risk scores, calculated for the entire Swedish population, can elucidate the genetic association between major affective and psychotic disorders and clarify the effect of genetic risk on important clinical features of disease.
Abstract
Importance
Family and genetic approaches have traditionally been used to evaluate our diagnostic concepts. Using a novel method, the family genetic risk score (FGRS), can we validate the genetic architecture of major affective and psychotic disorders in a national Swedish sample?
Objective
To determine whether FGRSs, calculated for the entire Swedish population, can elucidate the genetic relationship between major affective and psychotic disorders and clarify the association of genetic risk with important clinical features of disease.
Design, Setting, and Participants
This cohort study included the native Swedish population born from January 1, 1950, through December 31, 1995, and followed up through December 31, 2017. Data were collected from Swedish population-based primary care, specialist, and hospital registers, including age at first registration for a psychiatric diagnosis and number of registrations for major depression, bipolar disorder, and schizophrenia. Data were analyzed from October 15, 2020, to February 2, 2021.
Exposures
FGRSs for major depression, bipolar disorder, and schizophrenia calculated from morbidity risks for disorders in first- through fifth-degree relatives, controlling for cohabitation.
Main Outcomes and Measures
Diagnoses of major depression, bipolar disorder, schizophrenia, schizoaffective disorder, and other nonaffective psychoses (ONAPs), age at registration, and number of registrations for major depression, bipolar disorder, and schizophrenia. Diagnostic conversion of major depression to bipolar disorder and ONAPs to schizophrenia was assessed by Cox proportional hazards regression models.
Results
The cohort included 4 129 002 individuals (51.4% male) with a mean (SD) age at follow-up of 45.5 (13.4) years. Mean FGRSs for major depression, bipolar disorder, and schizophrenia produced distinct patterns for major depression, bipolar disorder, schizophrenia, schizoaffective disorder, and ONAPs with large separations between disorders. In major depression, bipolar disorder, and schizophrenia, high FGRSs were associated with early age at onset and high rates of recurrence: a high mean FGRS for bipolar disorder was associated with early age at onset (younger than 25 years, 0.11; 95% CI, 0.11-0.12) and higher recurrence (8 or more registrations, 0.11; 95% CI, 0.11-0.12) in major depression. The schizophrenia FGRS was separately associated with psychotic and nonpsychotic forms of major depression (0.10; 95% CI, 0.06-0.14 vs 0.03; 95% CI, 0.02-0.03) and bipolar disorder (0.22; 95% CI, 0.16-0.28 vs 0.11; 95% CI, 0.09-0.12). The bipolar disorder and schizophrenia FGRSs were associated with conversion from major depression to bipolar disorder (eg, hazard ratio, 1.70 [95% CI, 1.63-1.78] for high vs low bipolar FGRS) and ONAP to schizophrenia (eg, hazard ratio, 1.38 [95% CI, 1.27-1.51] for high vs low schizophrenia FGRS).
Conclusions and Relevance
In this Swedish cohort study, the FGRSs for major depression, bipolar disorder, and schizophrenia for the Swedish population clearly separated major affective and psychotic disorders from each other in a larger and more representative patient sample than previously possible. These findings provide possible validation, from a genetic perspective, for these major diagnostic categories. These results replicated and extended prior observations on more limited samples of the association of FGRS with age at onset, recurrence, psychotic subtypes, and diagnostic conversions.
Introduction
The interrelationship and validity of major psychiatric diagnoses have been evaluated by several different genetic strategies.1,2,3 Family studies have established the separation of bipolar disorder and major depression with clear differences in rates of these affective disorders in the relatives of probands with bipolar disorder and major depression.4,5 Adoption studies have provided empirical evidence for the schizophrenia spectrum personality disorders.6,7 Multivariate twin studies have supported the division of common psychiatric disorders into internalizing and externalizing subgroups.8 Aggregated common variants from genome-wide association studies have examined the genetic interrelationships between pairs9 and groups10 of psychiatric disorders. Such strategies have also been used for more focused questions, such as the association between genetic risk and early age at onset, recurrence, and diagnostic changes over time.11,12,13
We add to this long empirical tradition by using a novel method of assessing genetic risk: the family genetic risk score (FGRS). We calculated the FGRSs for major depression, bipolar disorder, and schizophrenia from the rates of illness in first- through fifth-degree relatives in the Swedish population and examined these risk scores in population-based cohorts of individuals with 5 major affective and psychotic disorders: major depression, bipolar disorder, schizophrenia, schizoaffective disorder, and other nonaffective psychoses (ONAPs). We herein address the following 4 questions. First, how well do the patterns of FGRSs for major depression, bipolar disorder, and schizophrenia discriminate these 5 disorders? Second, are the FGRSs for major depression, bipolar disorder, and schizophrenia associated with age at first registration for the psychiatric diagnosis and the number of registrations for each disorder? Third, can FGRS patterns clearly distinguish psychotic and nonpsychotic forms of major depression and bipolar disorder? Fourth, among individuals with a first diagnosis of major depression, is the FGRS for bipolar disorder associated with conversion to bipolar disorder, and among those with a diagnosis of ONAP, is the FGRS for schizophrenia associated with the future development of schizophrenia?
Although the FGRS, like the polygenic risk score (PRS), attempts to measure aggregate genetic risk, these scores are fundamentally different measures. The FGRS is based on rates of phenotypes—herein psychiatric disorders—in relatives with corrections for age, sex, year of birth, area of residence, and association with cohabitation. The PRS is based on a sum of DNA sequence variants that differ in genome-wide association studies of cases and controls. Although the PRS, which is based on thousands of variants, has a classical Gaussian distribution, the FGRS, as seen in eFigures 4 to 6 in the Supplement, has skewed distributions that are more pronounced for rarer disorders.
Methods
For this cohort study, we collected information on individuals from Swedish population-based registers with national coverage linking each person’s unique personal identification number, which was replaced with a serial number by Statistics Sweden to preserve confidentiality (eMethods 1 in the Supplement). We secured ethical approval for this study from the regional ethical review board in Lund, Sweden, which did not require participant consent for the use of these data. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Our database consisted of all individuals born in Sweden to Swedish-born parents from January 1, 1950, through December 31, 1995. Follow-up ended on December 31, 2017, and data were analyzed from October 15, 2020, to February 2, 2021. In the database, we included registrations for major depression, bipolar disorder, schizophrenia, schizoaffective disorder, and ONAPs using codes from International Classification of Diseases, Eighth Revision, International Classification of Diseases, Ninth Revision, and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) from primary care, specialist, and hospital registries (eTable 1 in the Supplement). In the analyses, we used a hierarchy based on the number and timing of diagnoses in the registers so that an individual could only be considered as registered with a diagnosis of major depression, bipolar disorder, schizophrenia, schizoaffective disorder, or ONAP in our analyses (eFigure 1 and eTables 2 and 3 in the Supplement). For individuals with major depression and bipolar disorder, we further included psychotic and nonpsychotic forms (eTable 1 in the Supplement). We also included an individual FGRS for major depression, bipolar disorder, and schizophrenia based on a mean of 32.2 first-, second-, third-, fourth-, and fifth-degree relatives of the proband. Briefly (eFigure 3 in the Supplement), we first calculated the morbid risk for the phenotype based on age at first registration. Thereafter, we transformed the binary trait into an underlying liability distribution and calculated the mean z score for relatives with and without the trait. For first-degree relatives, we also multiplied the z score with a factor that controlled for the association with cohabitation (see eTable 6 in the Supplement). Within each type of relative, we then had 2 components: the sum of the z scores and the total weighted number of relatives. These 2 components were further weighted by their genetic resemblance to the proband. For each proband, we summed the 2 components across all groups of relatives and used the quotient, which was then multiplied by a shrinkage factor to take into account the number of relatives of the proband. To make the FGRS comparable across traits, we standardized the FGRS, based on year of birth and county of residence, into a z score with a mean of 0 and SD of 1. We explore the robustness of our FGRS to changes in methodology in eTable 7 and eFigures 7 and 8 in the Supplement. Sensitivity analyses for diagnostic hierarchy and FGRS are found in eMethods 2 and 3 in the Supplement.
For question 1 (patterns of FGRS for major depression, bipolar disorder, and schizophrenia), we calculated the mean FGRS with corresponding 95% CIs for individuals with major depression, bipolar disorder, schizophrenia, schizoaffective disorder, and ONAPs. For the first part of question 2 (FGRS and age at onset), we categorized age at first registration as a proxy for age at onset (for major depression, bipolar disorder, and schizophrenia) into 5 equal-sized groups. We calculated the least-squares means for the 5 different groups. For individuals registered with major depression and bipolar disorder, we investigated the effects of the FGRS for each disorder separately. For individuals with schizophrenia, we investigated the bipolar disorder and schizophrenia FGRSs separately.
For the second part of question 2 (FGRS and number of registrations), we categorized the number of registrations as a proxy for recurrence (for major depression, bipolar disorder, and schizophrenia) into quintiles and calculated the least-squares means for the 5 different groups. For individuals registered with major depression and bipolar disorder, we investigated the FGRS for each disorder separately. For individuals with schizophrenia, we investigated the bipolar disorder and schizophrenia FGRSs separately.
For question 3 (differentiation between psychotic and nonpsychotic forms of major depression and bipolar disorder), we calculated the mean FGRS with corresponding 95% CIs for individuals with psychotic and nonpsychotic forms of major depression and bipolar disorder, the diagnoses of which are only available in ICD-10. For question 4 (conversion to bipolar disorder and schizophrenia among individuals with major depression and ONAPs, respectively), we first categorized the FGRS for bipolar disorder and schizophrenia into quintiles and then calculated the time to bipolar disorder or schizophrenia from the first registration of major depression or ONAPs. We used a Cox proportional hazards regression model to investigate the time to bipolar disorder or schizophrenia while controlling for age at registration for major depression or ONAPs and did not apply the diagnostic hierarchy. We also present 1 minus the survival curve at the mean age at registration. All statistical analyses were performed using SAS, version 9.4.14 We used a 2-sided t test to test for equality between the mean values of the different groups, with P < .05 indicating statistical significance.
Results
Sample Description
Our sample included 4 129 002 individuals (51.4% male and 48.6% female), with a mean age (SD) age of 45.5 (13.4) years at the close of ascertainment on December 31, 2017. Table 1 provides descriptive statistics for our diagnostic groups. Population prevalence ranged from 0.12% for schizoaffective disorder to 12.0% for major depression. We noted a female preponderance for major depression (63.3%), bipolar disorder (61.9%), and schizoaffective disorder (56.3%); a male preponderance for schizophrenia (66.3%) and ONAPs (55.7%); and higher proportions of patients with bipolar disorder (9.2%) than major depression (1.0%) with a diagnosis of a psychotic subtype.
Table 1. Descriptive Results and Mean FGRSs for Major Depression, Bipolar Disorder, and Schizophrenia.
| Diagnosis | Population prevalence, %a | Sample size, No. | % Maleb | Standardized mean FGRS (95% CI)c | ||
|---|---|---|---|---|---|---|
| Major depression | Bipolar disorder | Schizophrenia | ||||
| Major depression | ||||||
| All | 12.00 | 495 981 | 36.7 | +0.30 (0.29-0.30) | +0.08 (0.08-0.08) | +0.03 (0.03-0.03) |
| Without psychosis | 11.89 | 490 848 | 36.6 | +0.30 (0.29-0.30) | +0.08 (0.07-0.08) | +0.03 (0.02-0.03) |
| With psychosis | 0.12 | 5131 | 46.0 | +0.31 (0.28-0.35) | +0.19 (0.16-0.23) | +0.10 (0.06-0.14) |
| P valued | NA | NA | NA | .30 | <.001 | <.001 |
| Bipolar disorder | ||||||
| All | 0.87 | 36 049 | 38.1 | +0.34 (0.33-0.36) | +0.61 (0.59-0.63) | +0.12 (0.10-0.13) |
| Without psychosis | 0.79 | 32 747 | 37.6 | +0.36 (0.34-0.37) | +0.58 (0.56-0.61) | +0.11 (0.09-0.12) |
| With psychosis | 0.08 | 3302 | 42.6 | +0.23 (0.19-0.27) | +0.86 (0.78-0.94) | +0.22 (0.16-0.28) |
| P valued | NA | NA | NA | <.001 | <.001 | <.001 |
| ONAP | 0.57 | 23 701 | 55.7 | +0.18 (0.17-0.20) | +0.18 (0.16-0.20) | +0.27 (0.24-0.29) |
| Schizoaffective disorder | 0.12 | 5065 | 43.7 | +0.17 (0.14-0.20) | +0.43 (0.38-0.48) | +0.47 (0.40-0.53) |
| Schizophrenia | 0.32 | 13 123 | 66.3 | +0.04 (0.03-0.06) | +0.16 (0.13-0.18) | +0.76 (0.71-0.81) |
Abbreviations: FGRS, family genetic risk score; NA, not applicable; ONAP, other nonaffective psychosis.
For the entire study population (n = 4 129 002).
Using sample size as the denominator.
The FGRS is a standardized z score statistic representing the level of genetic risk so that a score of zero indicates an average level of risk. A positive score therefore indicates a genetic risk greater than that expected in the general population.
Indicates difference between those with and without psychosis, calculated using a 2-sided t test to test for equality between the mean values of the different groups.
FGRS Patterns and Discrimination Between Different Disorders
Figure 1 illustrates the associations found in this study among the mean major depression, bipolar disorder, and schizophrenia FGRSs expressed as a z score for our 5 diagnostic categories, the results of which are also depicted in Table 1. Figure 1A depicts the placement of the disorders by their mean bipolar disorder and schizophrenia FGRSs on the x- and y-axes. The disorders are widely separated from each other in this Figure. Major depression is close to the lower left corner with very modest FGRSs for bipolar disorder and schizophrenia. Schizophrenia appears in the upper left corner with a high mean schizophrenia FGRS and a much lower bipolar disorder FGRS. Bipolar disorder is in the lower right corner with a high bipolar disorder FGRS but a low schizophrenia FGRS. Schizoaffective disorder appears midway between schizophrenia and bipolar disorder, with similar elevations of schizophrenia and bipolar disorder FGRSs. Other nonaffective psychoses had a much lower schizophrenia FGRS than schizophrenia and a much lower bipolar disorder FGRS than bipolar disorder.
Figure 1. Association Among Mean Family Genetic Risk Scores (FGRS) for Major Depression, Bipolar Disorder, and Schizophrenia With Psychiatric Diagnoses.
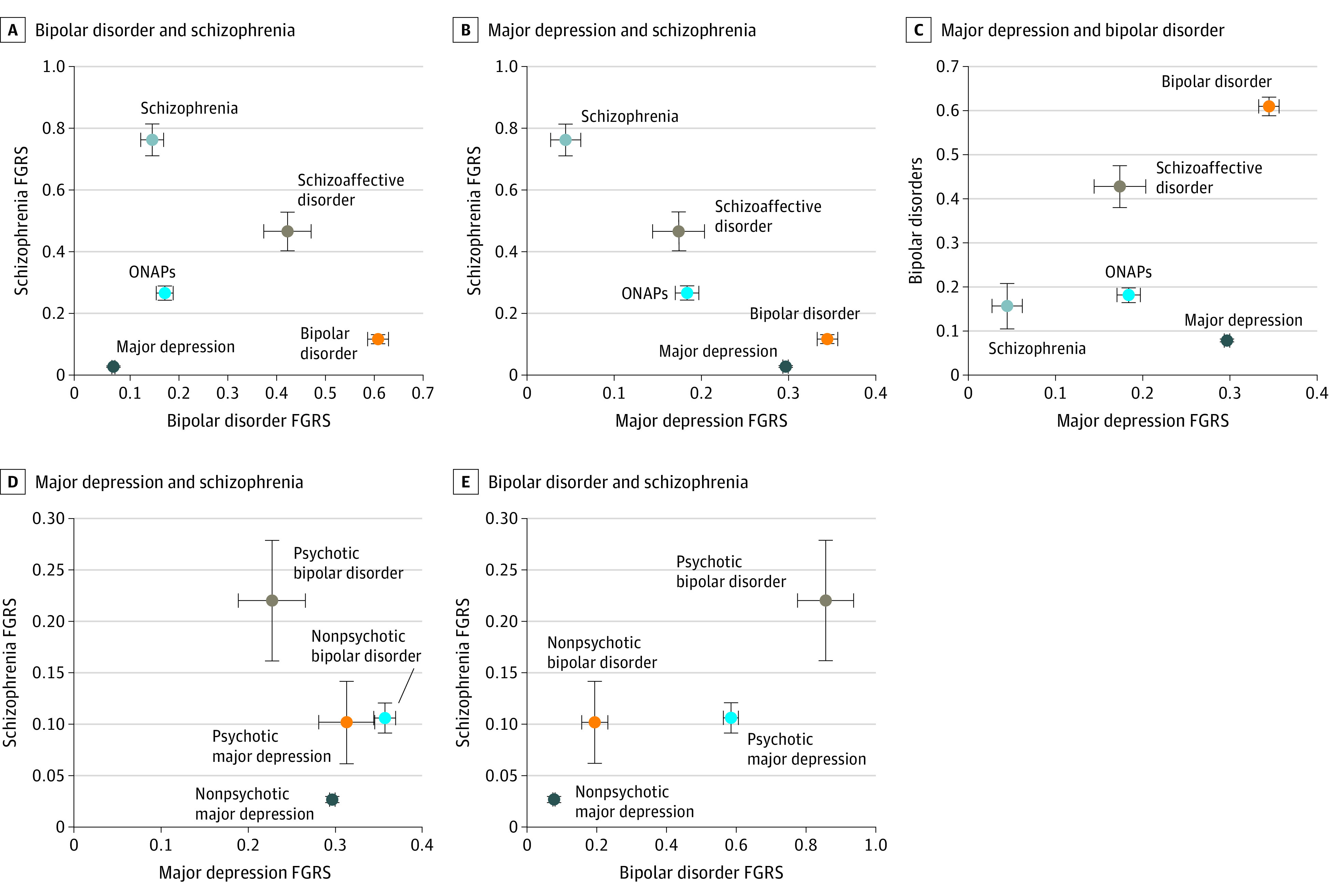
The mean standardized FGRSs and 95% CIs for bipolar disorder and schizophrenia (A), major depression and schizophrenia (B), and major depression and bipolar disorder (C) are shown in association with major depression, bipolar disorder, other nonaffective psychoses (ONAPs), schizoaffective disorder, and schizophrenia from national Swedish samples. For FGRSs in which 95% CIs are not visible, they are contained in the data point. The mean standardized FGRSs and 95% CIs for schizophrenia and major depression (D) and schizophrenia and bipolar disorder (E) are shown for psychotic and nonpsychotic major depression and psychotic and nonpsychotic bipolar disorder.
Figure 1B shows the disorders by their major depression and schizophrenia FGRSs on the x- and y-axes. Major depression and bipolar disorder are relatively close to one another in the right lower quadrant of the graph, with a high major depression FGRS and low schizophrenia FGRS. Schizophrenia is in the upper left quadrant with a low major depression FGRS. Other nonaffective psychoses and schizoaffective disorder had similar levels of major depression FGRS, with schizoaffective disorder having a higher schizophrenia FGRS.
Figure 1C shows the disorders by their major depression and bipolar disorder FGRSs on the x- and y-axes. Schizophrenia is close to the lower left corner, with a low major depression FGRS and somewhat higher bipolar disorder FGRS. Bipolar disorder appears in the upper right corner, with high major depression and bipolar disorder FGRSs; major depression is in the lower right quadrant, with a high major depression FGRS but a low bipolar disorder FGRS. In this study, schizoaffective disorder and ONAPs were clearly differentiated from one another, with schizoaffective disorder having a higher bipolar disorder than major depression FGRS and ONAPs having similar risk scores.
Association of FGRS With Age at Onset and Recurrence
As seen in Figure 2A, a first registration for major depression before 25 years of age was associated with increased mean FGRSs for major depression and bipolar disorder, but little difference was seen in FRGSs between later ages at first registration. For bipolar disorder, first registration before 31 years of age was associated with a higher mean bipolar disorder FGRS but a lower mean major depression FGRS. Early age at registration for schizophrenia was associated with a higher mean schizophrenia FGRS, but the association with bipolar disorder FGRS was not significant.
Figure 2. Association of Family Genetic Risk Score (FGRS) for Major Depression, Bipolar Disorder, and Schizophrenia With Age at First Registration and Number of Registrations.
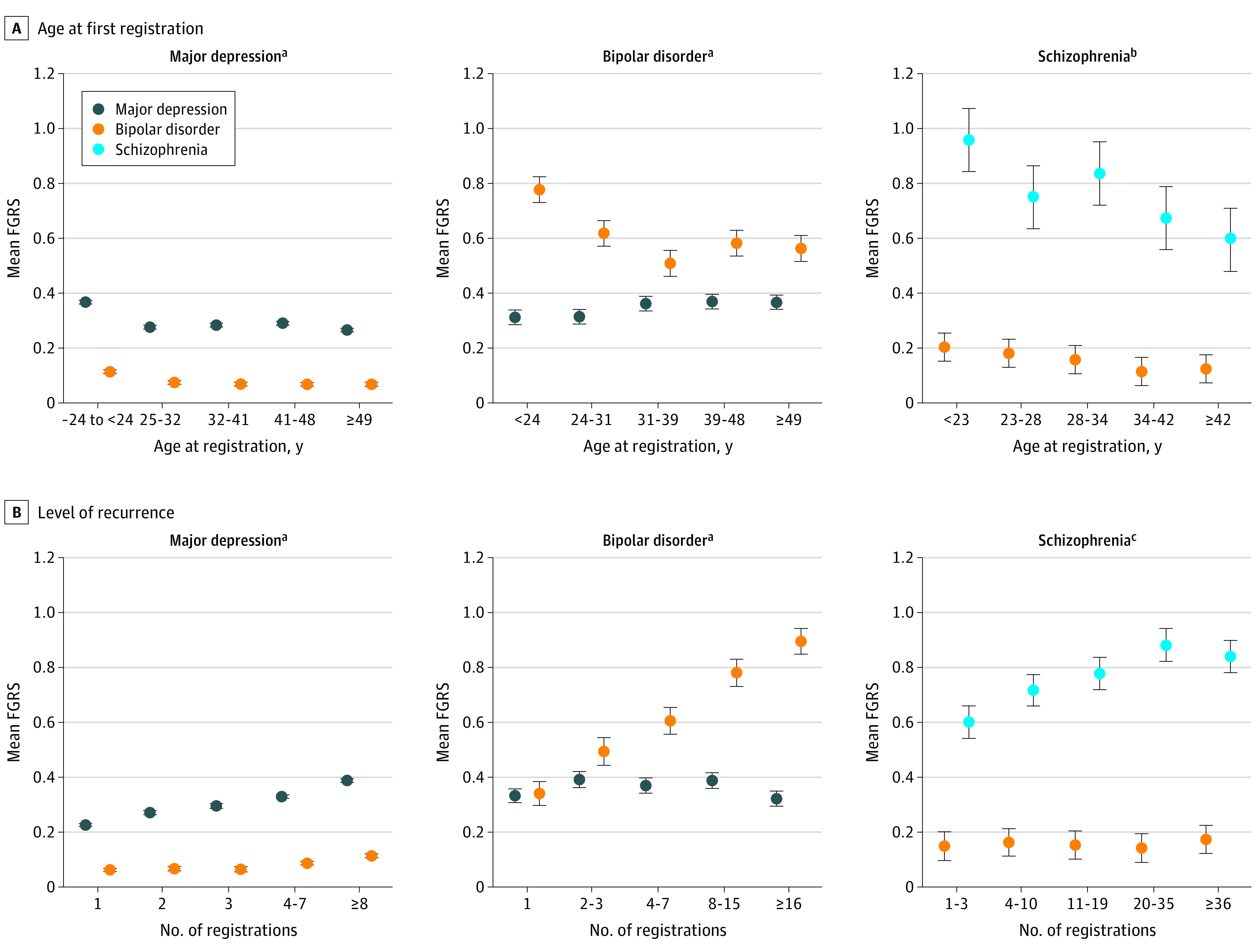
Mean standardized FGRS and 95% CI were estimated from a linear regression model controlling for year of birth.
aP for linear trend: major depression FGRS, <.001; bipolar disorder FGRS, <.001.
bP for linear trend: bipolar disorder FGRS, .09; schizophrenia FGRS, .01.
cP for linear trend: bipolar disorder FGRS, .93; schizophrenia FGRS, .08.
Figure 2B presents for the association between levels of recurrence (ie, number of independent registrations) and FGRSs for major depression, bipolar disorder, and schizophrenia. The FGRS was associated with the number of diagnoses in the registries for major depression and especially bipolar disorder, with the association being present but weaker for schizophrenia. The mean FGRS for bipolar disorder had a positive association with recurrence risk in major depression but not schizophrenia. The major depression FGRS had no systematic association with the number of bipolar disorder episodes. The FGRSs and 95% CIs for the results depicted in Figure 2 are provided in Table 2.
Table 2. Familial Genetic Risk Scores (FGRSs) and 95% CIs for Associations Between Genetic Risk and Clinical Features.
| Variable | Affective disorder, standardized FGRS (95% CI) | ||
|---|---|---|---|
| Major depression | Bipolar disorder | Schizophrenia | |
| Age at first registration of bipolar disorder, ya | |||
| Major depression | |||
| ≤24 | 0.37 (0.36-0.37) | 0.11 (0.11-0.12) | NA |
| 25-32 | 0.28 (0.27-0.28) | 0.07 (0.07-0.08) | NA |
| 32-41 | 0.28 (0.28-0.29) | 0.07 (0.06-0.08) | NA |
| 41-49 | 0.29 (0.28-0.30) | 0.07 (0.06-0.07) | NA |
| ≥49 | 0.27 (0.26-0.27) | 0.07 (0.06-0.08) | NA |
| Bipolar disorder | |||
| ≤24 | 0.31 (0.29-0.34) | 0.78 (0.73-0.82) | NA |
| 24-31 | 0.31 (0.29-0.34) | 0.62 (0.57-0.66) | NA |
| 31-39 | 0.36 (0.34-0.39) | 0.51 (0.46-0.56) | NA |
| 39-48 | 0.37 (0.34-0.40) | 0.58 (0.54-0.63) | NA |
| ≥48 | 0.37 (0.34-0.39) | 0.56 (0.52-0.61) | NA |
| Schizophrenia | |||
| ≤23 | NA | 0.20 (0.15-0.25) | 0.96 (0.84-1.07) |
| 23-28 | NA | 0.18 (0.13-0.23) | 0.75 (0.63-0.86) |
| 28-34 | NA | 0.16 (0.11-0.21) | 0.84 (0.72-0.95) |
| 34-42 | NA | 0.11 (0.06-0.17) | 0.67 (0.56-0.79) |
| ≥42 | NA | 0.12 (0.07-0.18) | 0.59 (0.48-0.71) |
| Level of recurrence by affective disorder, No. of episodesb | |||
| Major depression | |||
| 1 | 0.23 (0.22-0.23) | 0.06 (0.06-0.07) | NA |
| 2 | 0.27 (0.26-0.28) | 0.07 (0.06-0.07) | NA |
| 3 | 0.30 (0.29-0.31) | 0.06 (0.05-0.07) | NA |
| 4-7 | 0.33 (0.32-0.34) | 0.09 (0.08-0.09) | NA |
| ≥8 | 0.39 (0.38-0.39) | 0.11 (0.11-0.12) | NA |
| Bipolar disorder | |||
| 1 | 0.33 (0.31-0.36) | 0.34 (0.30-0.38) | NA |
| 2-3 | 0.29 (0.36-0.42) | 0.49 (0.44-0.54) | NA |
| 4-7 | 0.37 (0.34-0.40) | 0.61 (0.56-0.65) | NA |
| 8-15 | 0.39 (0.36-0.42) | 0.78 (0.73-0.83) | NA |
| ≥16 | 0.32 (0.29-0.35) | 0.89 (0.85-0.94) | NA |
| Schizophrenia | |||
| 1-3 | NA | 0.15 (0.10-0.20) | 0.60 (0.54-0.66) |
| 4-10 | NA | 0.16 (0.11-0.21) | 0.72 (0.66-0.77) |
| 11-19 | NA | 0.15 (0.10-0.20) | 0.78 (0.72-0.84) |
| 20-35 | NA | 0.14 (0.09-0.19) | 0.88 (0.82-0.94) |
| ≥36 | NA | 0.17 (0.12-0.22) | 0.84 (0.78-0.90) |
Abbreviations: FGRS, family genetic risk score; NA, not applicable.
Depicted in Figure 2A.
Depicted in Figure 2B.
FGRS and Differentiation of Psychotic and Nonpsychotic Forms of Major Depression and Bipolar Disorder
Figure 1D depicts the findings for placement of the psychotic and nonpsychotic forms of major depression and bipolar disorder as a function of their major depression and schizophrenia FGRSs on the x- and y-axes. Nonpsychotic and psychotic major depression had a similar major depression FGRS, but the psychotic form had higher schizophrenia FGRS. The findings for nonpsychotic and psychotic bipolar disorder were different in that psychotic bipolar disorder had a higher schizophrenia FGRS and a lower major depression FGRS.
Figure 1E, which presents the placement of the psychotic and nonpsychotic forms of major depression and bipolar disorder as a function of their bipolar disorder FGRS on the x-axis and schizophrenia FGRS on the y-axis, depicts different findings. For both bipolar disorder and major depression, the psychotic form differed from the nonpsychotic form in that it had both higher mean schizophrenia and higher mean bipolar disorder FGRSs. These differences were greater in cases of bipolar disorder than in cases of major depression. The FGRSs and 95% CIs for the results depicted in Figure 1D and E are provided in Table 2.
Association of FGRS Scores With Diagnostic Conversions in Major Depression and ONAPs
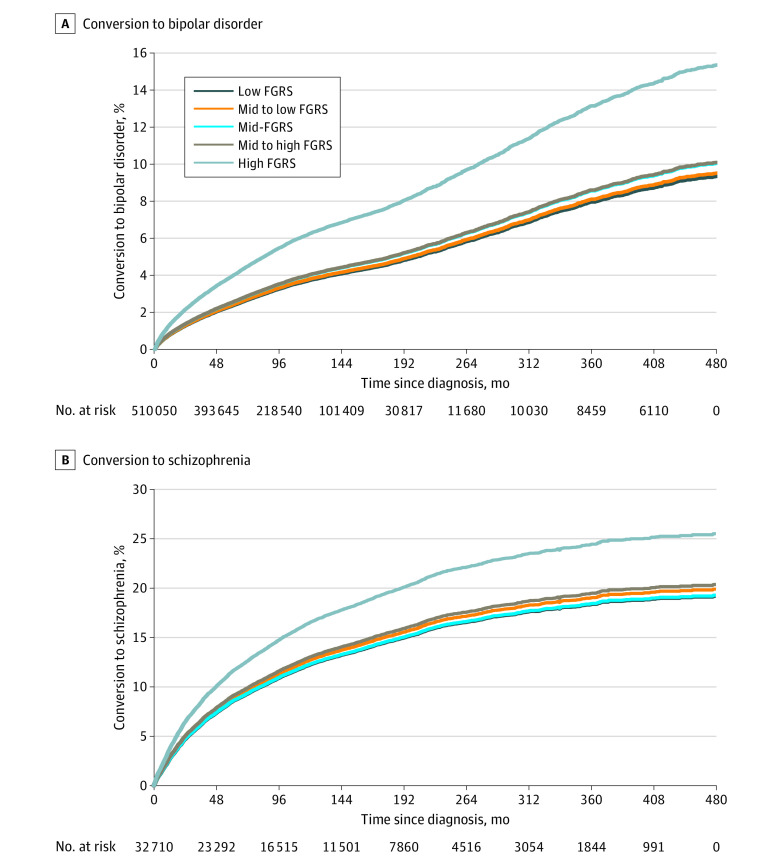
Finally, we assessed the association between FGRSs for bipolar disorder and schizophrenia with diagnostic conversions from major depression to bipolar disorder and ONAPs to schizophrenia. Figure 3A depicts an inverse survival curve for the proportion of individuals with an initial diagnosis of major depression who receive a subsequent diagnosis of bipolar disorder as a function of the quintile of their bipolar disorder FGRS. Small differences in risk are displayed across the lower 4 quintiles, whereas those individuals with a bipolar disorder FGRS in the highest quintile had a substantially increased risk of having their diagnosis converted to bipolar disorder. In Figure 3B, similar results are presented. The conversion to schizophrenia in those with an initial ONAP diagnosis was substantially higher in those in the highest quintile of schizophrenia FGRS.
Figure 3. Association of Family Genetic Risk Score (FGRS) With Diagnostic Conversions in Major Depression and Other Nonaffective Psychoses.
Results of an inverted survival curve applied to all cases, with no application of hierarchy. A, Conversion from a diagnosis of major depression to bipolar disorder by FGRS for bipolar disorder by quintiles. B, Conversion from a diagnosis of other nonaffective psychosis to schizophrenia by FGRS for schizophrenia by quintiles.
Discussion
We applied a new measure of aggregate genetic risk to a national sample of treated patients with major affective and psychotic disorders in Sweden. We thereby addressed 4 questions about the degree to which FGRSs for major depression, bipolar disorder, and schizophrenia were associated and may distinguish between major affective and psychotic disorders and their key clinical features.
First, FGRSs for major depression, bipolar disorder, and schizophrenia robustly separated major depression, bipolar disorder, schizophrenia, schizoaffective disorder, and ONAPs from each other. We found a clearer separation of the genetic risk for schizophrenia and bipolar disorder than that seen in a large-scale twin and nontwin sibling analysis in Sweden15 or in molecular genetic studies.10,16 To address the stability of this finding, we examined the association between schizophrenia and bipolar disorder separately in male and female individuals and obtained a similar finding in both sexes (eFigure 7 in the Supplement). Our results for schizoaffective disorder—with an FGRS pattern midway between those of bipolar disorder and schizophrenia—are consistent with findings from the Iowa 50017 and Roscommon family18 studies and molecular genetic investigations.19
Second, for major depression, bipolar disorder, and schizophrenia, high FGRSs were associated with early age at onset. As shown in 1 prior study,20 high bipolar disorder PRS was significantly associated with early age of onset and recurrence rates for major depression. Our results are consistent with several family and twin studies showing an association between familial and/or genetic risk for major depression and early age at onset,21,22,23 although this was not replicated by a PRS analysis.24 A prior study of major depression in Swedish twins found, as we did, that the association of age at onset with genetic risk for major depression disappears in later-onset cases.23 Family study data also show an inverse association of familial risk and age at onset in bipolar disorder,21,25 although this finding was not replicated using molecular genetic methods.26 The association between age at onset and genetic risk has been less clear in schizophrenia, with 1 molecular genetic study showing a clear association13 but family studies showing more negative results.27,28,29 Consistent with our results, a study using measures of polygenic heritability30 found stronger genetic effects in cases of schizophrenia with high vs low levels of recurrence.
We found particularly robust associations between FGRS and recurrence in major depression, bipolar disorder, and schizophrenia. These results are consistent with prior evidence that recurrence in major depression is an index of familial risk31,32,33 and that elevated molecular genetic risk for schizophrenia is associated with recurrence.30 Bipolar disorder FGRS also is associated with recurrence in major depression, although we have been unable to find prior studies confirming this association.33
Third, consistent with family studies reporting higher rates of schizophrenia in relatives of patients with psychotic vs nonpsychotic affective disorder17,34,35 and molecular genetic studies reporting similar patterns with PRS,19,36,37 we found clear separations in the pattern of FGRS in psychotic vs nonpsychotic major depression and bipolar disorder. We also observed findings that to our knowledge have not been observed previously. Although psychotic and nonpsychotic forms of major depression and bipolar disorder had a similar major depression FGRS, the psychotic forms of both disorders were associated with an elevated bipolar disorder FGRS.
Fourth, congruent with evidence using both family and molecular designs,11,38,39,40,41,42 individuals with major depression at high genetic risk for bipolar disorder had a considerably elevated rate of conversion to bipolar disorder. We could usefully compare our specific results with those obtained recently using the PRS from a Danish national sample.11 Using similar controls, the authors reported a hazard ratio for a standardized bipolar disorder PRS of 1.11 (95% CI, 1.03-1.21) for the association of major depression conversion with bipolar disorder,11 statistically consistent with our results. In our sample, a high schizophrenia FGRS was associated with diagnostic conversion from ONAPs to schizophrenia. The finding most similar to this of which we are aware emerged from the Suffolk County Mental Health Project, where a schizophrenia PRS was associated with diagnostic shifts, over time, from affective psychotic to nonaffective psychotic disorders.12
Although most of our results were consistent with prior expectations, one was not. We expected the genetic profile of ONAPs to closely resemble that of schizophrenia. This was not the case (Figure 1A and B). Compared with schizophrenia (which we defined narrowly, excluding latent, simple, acute, and schizoaffective forms), ONAPs had a much lower schizophrenia FGRS. These findings call into question the validity, from a genetic perspective, of constructs such as nonaffective psychoses or broadly defined schizophrenia that would typically include individuals with a schizophrenia diagnosis and a substantial proportion of those defined herein as having ONAPs.
Limitations
These findings should be viewed in the context of 4 potential methodological limitations. First, we cannot rule out that clinicians knew the family history of their patients and that knowledge influenced their diagnoses. Second, the validity of FGRS depends on the quality of diagnoses in the Swedish national registries, which for hospital diagnoses of schizophrenia and bipolar disorder have been well supported.43,44,45 The validity of major depression diagnoses is supported by its prevalence, sex ratio, sibling and twin correlations, and associations with known psychosocial risk factors.46,47 Third, we applied algorithms to establish diagnostic hierarchies for our cases. We outline these in eFigure 1 and in eTables 2 and 3 in the Supplement, showing that changes in the hierarchies (eTables 4 and 5 in the Supplement) or their complete elimination (eFigure 2 in the Supplement) produced only modest alterations in results. Fourth, given the novelty of our FGRS method, we explored its stability across sex, time, and space in eFigures 7 and 8 in the Supplement. We examined 21 comparisons of FRGS in major depression, bipolar disorder, and schizophrenia in the 2 sexes, in the older and younger halves of our cohort (1950-1972 vs 1972-1995), and in southern and northern Sweden. Three of these comparisons were significant at a chance corrected P < .002, and for all of these comparisons, the observed differences were quantitatively modest.
Conclusions
By assessing an individual’s genetic risk based on rates of disorder in their immediate and extended relatives and controlling for the association with cohabitation, the FGRSs for major depression, bipolar disorder, and schizophrenia, available for the entire Swedish population, performed well in separating major affective and psychotic disorders on larger and more representative patient samples than has hitherto been possible. In so doing, these FGRSs provide possible validation for these major, albeit imperfect, diagnostic categories. The application of FGRS was also able to replicate and extend prior observations based on smaller samples of (1) higher genetic loading for early-onset and highly recurrent cases of major depression, bipolar disorder, and schizophrenia; (2) differences in genetic risk profiles for psychotic and nonpsychotic major depression and bipolar disorder; and (3) the association of bipolar disorder and schizophrenia FGRSs with diagnostic conversions from major depression to bipolar disorder and from ONAPs to schizophrenia, respectively. Our findings suggest that FGRSs, which are available in countries with high-quality population and medical registries, could have many additional potentially valuable research applications.
eMethods 1. Description of Registers
eTable 1. Definition of Phenotypes
eFigure 1. Diagnostic Hierarchy
eTable 2. First Decision Table
eTable 3. Second Decision Table
eMethods 2. Sensitivity Analyses for Diagnostic Hierarchy
eTable 4. Sensitivity Analysis for Separating Individuals With BD and ONAP
eTable 5. Sensitivity Analysis for Separating Individuals With SZ and ONAP
eFigure 2. FGRS Results Depicted in Figure 1 With and Without Diagnostic Hierarchies
eFigure 3. Flowchart for the Calculation of the Family Genetic Risk Score (FGRS)
eTable 6. Results from the Logistic Regression Models for the Cohabitation Effects
eMethods 3. Sensitivity Analysis for the Genetic Risk Score
eTable 7. Additional Sensitivity Analyses for the Calculation of the Family Genetic Risk Score
eTable 8. Stability of FGRSs for MD, BD and SZ by Median Splits for Cohort and Geographical Region Within Sweden
eFigure 4. Rates of MD in 50 Equaled Sized Groups of the MD FGRS
eFigure 5. Rates of BD in 50 Equaled Sized Groups of the BD FGRS
eFigure 6. Rates of SZ in 50 Equaled Sized Groups of the SZ FGRS
eFigure 7. Similarity of FGRSs for MD, BD, and SZ Across Sexes
eFigure 8. Stability of FGRSs for MD, BD and SZ by Median Splits for Cohort and Geographical Region Within Sweden
References
- 1.Kendler KS. The pre-history of psychiatric genetics: 1780-1910. Published online October 15, 2020. Am J Psychiatry. [Google Scholar]
- 2.Kendler KS, Klee A. The turn to controls and the refinement of the concept of hereditary burden: the 1895 study of Jenny Koller. Am J Med Genet B Neuropsychiatr Genet. 2020;183(7):433-442. doi: 10.1002/ajmg.b.32819 [DOI] [PubMed] [Google Scholar]
- 3.Robins E, Guze SB. Establishment of diagnostic validity in psychiatric illness: its application to schizophrenia. Am J Psychiatry. 1970;126(7):983-987. doi: 10.1176/ajp.126.7.983 [DOI] [PubMed] [Google Scholar]
- 4.Tsuang MT, Faraone SV. The Genetics of Mood Disorders. Johns Hopkins University Press; 1990. [Google Scholar]
- 5.Angst J, Frey R, Lohmeyer B, Zerbin-Rüdin E. Bipolar manic-depressive psychoses: results of a genetic investigation. Hum Genet. 1980;55(2):237-254. doi: 10.1007/BF00291773 [DOI] [PubMed] [Google Scholar]
- 6.Kety SS. The types and prevalence of mental illness in the biological and adoptive families of adopted schizophrenics. J Psychiatr Res. 1968;6:345-362. doi: 10.1016/0022-3956(68)90026-5 [DOI] [Google Scholar]
- 7.Kendler KS, Gruenberg AM, Strauss JS. An independent analysis of the Copenhagen sample of the Danish adoption study of schizophrenia, II: the relationship between schizotypal personality disorder and schizophrenia. Arch Gen Psychiatry. 1981;38(9):982-984. doi: 10.1001/archpsyc.1981.01780340034003 [DOI] [PubMed] [Google Scholar]
- 8.Kendler KS, Aggen SH, Knudsen GP, Røysamb E, Neale MC, Reichborn-Kjennerud T. The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM-IV Axis I and all Axis II disorders. Am J Psychiatry. 2011;168(1):29-39. doi: 10.1176/appi.ajp.2010.10030340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cross-Disorder Group of the Psychiatric Genomics Consortium . Genome-wide analysis identifies loci with shared effects on five major psychiatric disorders. Lancet. 2013;381(9875):1371-1379. doi: 10.1016/S0140-6736(12)62129-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross-Disorder Group of the Psychiatric Genomics Consortium . Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell. 2019;179(7):1469-1482.e11. doi: 10.1016/j.cell.2019.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musliner KL, Krebs MD, Albiñana C, et al. Polygenic risk and progression to bipolar or psychotic disorders among individuals diagnosed with unipolar depression in early life. Am J Psychiatry. 2020;177(10):936-943. doi: 10.1176/appi.ajp.2020.19111195 [DOI] [PubMed] [Google Scholar]
- 12.Jonas KG, Lencz T, Li K, et al. Schizophrenia polygenic risk score and 20-year course of illness in psychotic disorders. Transl Psychiatry. 2019;9(1):300. doi: 10.1038/s41398-019-0612-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bigdeli T, Peterson R, Docherty A, Kendler K, Fanous A. Genome-wide analyses of clinical features of schizophrenia in the Psychiatric Genomics Consortium. Eur Neuropsychopharmacol. 2019;29:S940. doi: 10.1016/j.euroneuro.2017.08.284 [DOI] [Google Scholar]
- 14.SAS Institute, Inc. SAS/STAT Online Documentation, Version 9.4. SAS Institute, Inc; 2012. [Google Scholar]
- 15.Lichtenstein P, Yip BH, Björk C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373(9659):234-239. doi: 10.1016/S0140-6736(09)60072-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SH, Ripke S, Neale BM, et al. ; Cross-Disorder Group of the Psychiatric Genomics Consortium; International Inflammatory Bowel Disease Genetics Consortium (IIBDGC) . Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45(9):984-994. doi: 10.1038/ng.2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kendler KS, Gruenberg AM, Tsuang MT. A DSM-III family study of the nonschizophrenic psychotic disorders. Am J Psychiatry. 1986;143(9):1098-1105. doi: 10.1176/ajp.143.9.1098 [DOI] [PubMed] [Google Scholar]
- 18.Kendler KS, McGuire M, Gruenberg AM, Walsh D. Examining the validity of DSM-III-R schizoaffective disorder and its putative subtypes in the Roscommon Family Study. Am J Psychiatry. 1995;152(5):755-764. doi: 10.1176/ajp.152.5.755 [DOI] [PubMed] [Google Scholar]
- 19.Allardyce J, Leonenko G, Hamshere M, et al. Association between schizophrenia-related polygenic liability and the occurrence and level of mood-incongruent psychotic symptoms in bipolar disorder. JAMA Psychiatry. 2018;75(1):28-35. doi: 10.1001/jamapsychiatry.2017.3485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verduijn J, Milaneschi Y, Peyrot WJ, et al. Using clinical characteristics to identify which patients with major depressive disorder have a higher genetic load for three psychiatric disorders. Biol Psychiatry. 2017;81(4):316-324. doi: 10.1016/j.biopsych.2016.05.024 [DOI] [PubMed] [Google Scholar]
- 21.Baron M, Mendlewicz J, Klotz J. Age-of-onset and genetic transmission in affective disorders. Acta Psychiatr Scand. 1981;64(5):373-380. doi: 10.1111/j.1600-0447.1981.tb00796.x [DOI] [PubMed] [Google Scholar]
- 22.Weissman MM, Wickramaratne P, Merikangas KR, et al. Onset of major depression in early adulthood: increased familial loading and specificity. Arch Gen Psychiatry. 1984;41(12):1136-1143. doi: 10.1001/archpsyc.1984.01790230022003 [DOI] [PubMed] [Google Scholar]
- 23.Kendler KS, Gatz M, Gardner CO, Pedersen NL. Age at onset and familial risk for major depression in a Swedish national twin sample. Psychol Med. 2005;35(11):1573-1579. doi: 10.1017/S0033291705005714 [DOI] [PubMed] [Google Scholar]
- 24.Docherty AR, Edwards AC, Yang F, et al. Age of onset and family history as indicators of polygenic risk for major depression. Depress Anxiety. 2017;34(5):446-452. doi: 10.1002/da.22607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strober M. Relevance of early age-of-onset in genetic studies of bipolar affective disorder. J Am Acad Child Adolesc Psychiatry. 1992;31(4):606-610. doi: 10.1097/00004583-199207000-00005 [DOI] [PubMed] [Google Scholar]
- 26.Kalman JL, Papiol S, Forstner AJ, et al. Investigating polygenic burden in age at disease onset in bipolar disorder: findings from an international multicentric study. Bipolar Disord. 2019;21(1):68-75. doi: 10.1111/bdi.12659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pulver AE, Liang KY. Estimating effects of proband characteristics on familial risk, II: the association between age at onset and familial risk in the Maryland schizophrenia sample. Genet Epidemiol. 1991;8(5):339-350. doi: 10.1002/gepi.1370080506 [DOI] [PubMed] [Google Scholar]
- 28.Kendler KS, Tsuang MT, Hays P. Age at onset in schizophrenia: a familial perspective. Arch Gen Psychiatry. 1987;44(10):881-890. doi: 10.1001/archpsyc.1987.01800220047008 [DOI] [PubMed] [Google Scholar]
- 29.Kendler KS, Karkowski-Shuman L, Walsh D. Age at onset in schizophrenia and risk of illness in relatives: results from the Roscommon Family Study. Br J Psychiatry. 1996;169(2):213-218. doi: 10.1192/bjp.169.2.213 [DOI] [PubMed] [Google Scholar]
- 30.Meier SM, Agerbo E, Maier R, et al. ; MooDS SCZ Consortium . High loading of polygenic risk in cases with chronic schizophrenia. Mol Psychiatry. 2016;21(7):969-974. doi: 10.1038/mp.2015.130 [DOI] [PubMed] [Google Scholar]
- 31.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157(10):1552-1562. doi: 10.1176/appi.ajp.157.10.1552 [DOI] [PubMed] [Google Scholar]
- 32.Kendler KS, Gatz M, Gardner CO, Pedersen NL. Clinical indices of familial depression in the Swedish Twin Registry. Acta Psychiatr Scand. 2007;115(3):214-220. doi: 10.1111/j.1600-0447.2006.00863.x [DOI] [PubMed] [Google Scholar]
- 33.Burcusa SL, Iacono WG. Risk for recurrence in depression. Clin Psychol Rev. 2007;27(8):959-985. doi: 10.1016/j.cpr.2007.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kendler KS. Mood-incongruent psychotic affective illness: a historical and empirical review. Arch Gen Psychiatry. 1991;48(4):362-369. doi: 10.1001/archpsyc.1991.01810280078012 [DOI] [PubMed] [Google Scholar]
- 35.Coryell W, Tsuang MT, McDaniel J. Psychotic features in major depression: is mood congruence important? J Affect Disord. 1982;4(3):227-236. doi: 10.1016/0165-0327(82)90007-6 [DOI] [PubMed] [Google Scholar]
- 36.Markota M, Coombes BJ, Larrabee BR, et al. Association of schizophrenia polygenic risk score with manic and depressive psychosis in bipolar disorder. Transl Psychiatry. 2018;8(1):188. doi: 10.1038/s41398-018-0242-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bipolar Disorder and Schizophrenia Working Group of the Psychiatric Genomics Consortium. Electronic address: douglas.ruderfer@vanderbilt.edu; Bipolar Disorder and Schizophrenia Working Group of the Psychiatric Genomics Consortium . Genomic dissection of bipolar disorder and schizophrenia, including 28 subphenotypes. Cell. 2018;173(7):1705-1715.e16. doi: 10.1016/j.cell.2018.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Angst J, Sellaro R, Stassen HH, Gamma A. Diagnostic conversion from depression to bipolar disorders: results of a long-term prospective study of hospital admissions. J Affect Disord. 2005;84(2-3):149-157. doi: 10.1016/S0165-0327(03)00195-2 [DOI] [PubMed] [Google Scholar]
- 39.Sharma V, Xie B, Campbell MK, et al. A prospective study of diagnostic conversion of major depressive disorder to bipolar disorder in pregnancy and postpartum. Bipolar Disord. 2014;16(1):16-21. doi: 10.1111/bdi.12140 [DOI] [PubMed] [Google Scholar]
- 40.Kessing LV, Willer I, Andersen PK, Bukh JD. Rate and predictors of conversion from unipolar to bipolar disorder: a systematic review and meta-analysis. Bipolar Disord. 2017;19(5):324-335. doi: 10.1111/bdi.12513 [DOI] [PubMed] [Google Scholar]
- 41.Rice JP, McDonald-Scott P, Endicott J, et al. The stability of diagnosis with an application to bipolar II disorder. Psychiatry Res. 1986;19(4):285-296. doi: 10.1016/0165-1781(86)90121-6 [DOI] [PubMed] [Google Scholar]
- 42.Cegla-Schvartzman FB, Ovejero S, López-Castroman J, Baca-García E. Diagnostic stability in bipolar disorder: a narrative review. Harv Rev Psychiatry. 2019;27(1):3-14. doi: 10.1097/HRP.0000000000000187 [DOI] [PubMed] [Google Scholar]
- 43.Lichtenstein P, Björk C, Hultman CM, Scolnick E, Sklar P, Sullivan PF. Recurrence risks for schizophrenia in a Swedish national cohort. Psychol Med. 2006;36(10):1417-1425. doi: 10.1017/S0033291706008385 [DOI] [PubMed] [Google Scholar]
- 44.Sellgren C, Landén M, Lichtenstein P, Hultman CM, Långström N. Validity of bipolar disorder hospital discharge diagnoses: file review and multiple register linkage in Sweden. Acta Psychiatr Scand. 2011;124(6):447-453. doi: 10.1111/j.1600-0447.2011.01747.x [DOI] [PubMed] [Google Scholar]
- 45.Ekholm B, Ekholm A, Adolfsson R, et al. Evaluation of diagnostic procedures in Swedish patients with schizophrenia and related psychoses. Nord J Psychiatry. 2005;59(6):457-464. doi: 10.1080/08039480500360906 [DOI] [PubMed] [Google Scholar]
- 46.Kendler KS, Ohlsson H, Lichtenstein P, Sundquist J, Sundquist K. The genetic epidemiology of treated major depression in Sweden. Am J Psychiatry. 2018;175(11):1137-1144. doi: 10.1176/appi.ajp.2018.17111251 [DOI] [PubMed] [Google Scholar]
- 47.Sundquist J, Ohlsson H, Sundquist K, Kendler KS. Common adult psychiatric disorders in Swedish primary care where most mental health patients are treated. BMC Psychiatry. 2017;17(1):235. doi: 10.1186/s12888-017-1381-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Description of Registers
eTable 1. Definition of Phenotypes
eFigure 1. Diagnostic Hierarchy
eTable 2. First Decision Table
eTable 3. Second Decision Table
eMethods 2. Sensitivity Analyses for Diagnostic Hierarchy
eTable 4. Sensitivity Analysis for Separating Individuals With BD and ONAP
eTable 5. Sensitivity Analysis for Separating Individuals With SZ and ONAP
eFigure 2. FGRS Results Depicted in Figure 1 With and Without Diagnostic Hierarchies
eFigure 3. Flowchart for the Calculation of the Family Genetic Risk Score (FGRS)
eTable 6. Results from the Logistic Regression Models for the Cohabitation Effects
eMethods 3. Sensitivity Analysis for the Genetic Risk Score
eTable 7. Additional Sensitivity Analyses for the Calculation of the Family Genetic Risk Score
eTable 8. Stability of FGRSs for MD, BD and SZ by Median Splits for Cohort and Geographical Region Within Sweden
eFigure 4. Rates of MD in 50 Equaled Sized Groups of the MD FGRS
eFigure 5. Rates of BD in 50 Equaled Sized Groups of the BD FGRS
eFigure 6. Rates of SZ in 50 Equaled Sized Groups of the SZ FGRS
eFigure 7. Similarity of FGRSs for MD, BD, and SZ Across Sexes
eFigure 8. Stability of FGRSs for MD, BD and SZ by Median Splits for Cohort and Geographical Region Within Sweden