Key Points
Question
What are the clinical features of mogamulizumab-associated rash (MAR) during treatment of mycosis fungoides (MF) and Sézary syndrome (SS)?
Findings
In this retrospective case series, MAR exhibited heterogeneous features that often clinically mimicked cutaneous MF or SS; in addition, MAR had a predilection for the head and neck and demonstrated 4 predominant patterns: folliculotropic MF–like scalp plaques with alopecia, papules and/or plaques, photoaccentuated dermatitis, and morbilliform dermatitis, and histopathologic patterns were variable. Ancillary testing (immunohistochemistry and T-cell clonality studies) can help differentiate MAR from disease.
Meaning
Distinguishing MAR from cutaneous MF or SS is essential but difficult because clinical and histopathologic features can be variable in MAR.
Abstract
Importance
Mogamulizumab is a monoclonal antibody against CCR4 approved for treatment for mycosis fungoides (MF) and Sézary syndrome (SS). Mogamulizumab-associated rash (MAR) is difficult to differentiate from cutaneous MF or SS, which can lead to unnecessary discontinuation of drug use because of concern for severe drug reaction or incorrect presumption of disease relapse or progression in the skin.
Objective
To examine the most common clinical presentations of MAR in patients with MF or SS and the diagnostic and management challenges.
Design, Setting, and Participants
This retrospective case series assessed patients from a multidisciplinary cutaneous lymphoma clinic and supportive oncodermatology clinic at a major academic referral center who had a diagnosis of MF or SS and received mogamulizumab from January 1, 2013, to January 1, 2020. Treatment was followed by new or worsening rash with skin biopsy results compatible with drug eruption determined by clinicopathologic correlation and molecular testing to exclude active malignant disease.
Exposures
At least 1 dose of mogamulizumab.
Main Outcomes and Measures
Mogamulizumab-associated rash was characterized by clinical features, including time to onset, clinical presentation, histopathologic features, and management approach.
Results
The study included 19 patients with MF or SS who developed MAR (median age, 65 years; age range, 38-82 years; 10 [52.6%] male). Median time to MAR onset was 119 days (range, 56 days to 3.8 years). Patients with MAR exhibited 4 predominant clinical presentations: (1) folliculotropic MF–like scalp plaques with alopecia, (2) papules and/or plaques, (3) photoaccentuated dermatitis, and (4) morbilliform or erythrodermic dermatitis. The most common anatomical region involved was the head and neck, including the scalp. Histopathologic findings were variable and did not correspond to primary clinical morphologic findings. Immunohistochemistry and T-cell clonality ancillary testing were helpful to distinguish MAR from disease. Most patients with MAR (14 of 19) discontinued mogamulizumab treatment; however, no life-threatening severe cutaneous adverse drug reactions occurred, and the decision for drug therapy cessation was usually multifactorial. Four patients were treated again with mogamulizumab with no life-threatening drug-related events. Approaches to management of MAR include topical corticosteroids, systemic corticosteroids, and/or methotrexate.
Conclusions and Relevance
This case series found that mogamulizumab-associated rash had a heterogeneous clinical presentation with variable and delayed onset in patients with MF or SS. Mogamulizumab-associated rash exhibited a predilection for the head and neck and was difficult to clinically distinguish from relapse or progression of disease. Recognition of the most common clinical presentations can help prevent unnecessary discontinuation of mogamulizumab treatment. The presence of MAR does not necessitate permanent discontinuation of or avoidance of retreatment with mogamulizumab.
This case series examines the most common clinical presentations of mogamulizumab-associated rash in patients with mycosis fungoides and Sézary syndrome and the diagnostic and management challenges.
Introduction
Mogamulizumab is a defucosylated humanized monoclonal antibody against CCR4 approved by the US Food and Drug Administration in 2018 for previously treated adult patients with mycosis fungoides (MF) or Sézary syndrome (SS) after the international, open-label, randomized, controlled, phase 3 Mogamulizumab Versus Vorinostat in Previously Treated Cutaneous T-Cell Lymphoma (MAVORIC) trial, which compared mogamulizumab and vorinostat.1 Expression of CCR4 is found on skin-homing malignant T cells in MF and SS and a subset of helper T cells type 2 (TH2) and T-regulatory cells.2,3 Mogamulizumab selectively binds to CCR4 and has direct antibody-dependent cellular cytotoxicity against malignant MF and SS cells and may also exert anticancer activity through immune modulation via collateral destruction of immunosuppressive TH2 and T-regulatory cells.3,4
In the initial reports from Japan,5,6 rash was commonly reported with mogamulizumab treatment, and 24% to 63% of patients with adult T-cell leukemia-lymphoma (ATLL), relapsed peripheral T-cell lymphoma, or MF or SS developed rash with mogamulizumab treatment. Most rashes were low grade, although rare reports of life-threatening severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been described in the ATLL and peripheral T-cell lymphoma populations.7,8,9 The kinetics of rash during mogamulizumab treatment follow an unpredictable wide and delayed range, with rash onset ranging from immediately after the first infusion to up to 2 years after treatment initiation, and even many months after drug therapy discontinuation. In the ATLL population, rash develops most commonly between cycles 4 and 8 of mogamulizumab treatment.10,11,12
Diagnosis of mogamulizumab-associated rash (MAR) in cutaneous T-cell lymphoma (CTCL) can be challenging because of a heterogeneous clinical presentation in the skin, including variable clinical morphologic and anatomical distribution.13 In the pivotal MAVORIC clinical trial, drug eruption was reported in 24% of patients receiving mogamulizumab for MF and SS, with a median time to onset of 107 days. Despite being mostly mild to moderate in severity (20% grade 1-2 and 4% grade 3) without any report of SJS or TEN, drug rash was the most common adverse event that led to mogamulizumab therapy discontinuation.1,13 Systemic corticosteroid use for treatment of adverse reactions was prohibited in the MAVORIC trial.
Despite the frequency of MAR, the specific clinical features of MAR during treatment for MF and SS have not been fully defined, and data are limited to guide clinical management of MAR outside the clinical trial setting. A cutaneous granulomatous drug eruption, which clinically mimics CTCL, has been described in a subset of patients from the MAVORIC trial,14 whereas other case reports8,9,10,12,14,15,16,17 describe a photosensitive dermatitis. Given the importance of distinguishing drug rash from malignant disease in the skin, histopathologic features of MAR may help facilitate this differentiation. Although histopathologic analysis may provide some useful clues, initial clinical suspicion is essential to make an accurate diagnosis.
To enable timely recognition of MAR and prevent unnecessary drug therapy discontinuation because of incorrect presumption of disease relapse or progression, we reviewed our clinical experience with MAR. This report characterizes the most common clinical presentations of MAR in patients with MF and SS and describes the unique challenges in diagnosis and potential management strategies for MAR in these patients.
Methods
Patients treated with mogamulizumab at Stanford University from January 1, 2013, to January 1, 2020, were identified from the Stanford University Multidisciplinary Cutaneous T-Cell Lymphoma Program database. Inclusion criteria included diagnosis of MF or SS with new or worsening rash (documented in the clinical record and confirmed by 2 of the investigators [M.S.K. and Y.H.K.]) and with skin biopsy compatible with drug eruption determined by clinicopathologic correlation, immunohistochemistry, and molecular testing to exclude active malignant disease, as suggested by the presence of a dominant T-cell clone previously identified in the patient’s MF or SS. Medical record review was completed, including review of all medication exposures from 4 weeks before mogamulizumab therapy initiation until the date of rash onset. Patients were excluded if an alternative drug culprit was identified, if histopathologic and molecular testing from skin biopsy showed CTCL disease progression, or if clinicopathologic correlation revealed a dermatitis unrelated to drug eruption. Skin biopsy specimens from the resultant patient cohort were analyzed and have been reported as described by Wang et al.17 Briefly, immunohistochemistry for CD3, CD4, CD7, and CD8 was performed as indicated for clinical care, and specimens were retrospectively evaluated for the CD4/CD8 ratio within the epidermis, dermis, and hair follicles and for the loss or retention of CD7 expression, where available. Molecular testing was performed on tissue biopsy samples using T-cell receptor (TCR) high-throughput DNA sequencing (HTS) of β and γ genes (Adaptive Biotechnologies Inc), which detects minimal residual disease with greater sensitivity and specificity compared with other comparable tests.18 Approval from the Stanford University Institutional Review Board was obtained for this retrospective case series. All patients provide written informed consent for inclusion in the Stanford University Multidisciplinary Cutaneous T-Cell Lymphoma Program database. The institutional review board did not require additional patient consent given the retrospective nature of the study. Data were not deidentified to the principal author (K.E.H.) because notes and images had to be reviewed to obtain data, but data were deidentified to the remainder of the authors. This study followed the reporting guideline for case series.
Results
Clinical Features of MAR
Of 58 patients who received mogamulizumab for MF or SS, 19 (1 of 13 with MF and 18 of 45 with SS; median age, 65 years; age range, 38-82 years; 10 [52.6%] male) (Table 1) had worsening or new skin eruption with skin biopsy findings compatible with MAR. Most patients had near-complete clearing of their MF or SS skin disease and complete blood response to mogamulizumab at the time of new rash onset. Time from mogamulizumab therapy initiation to rash onset had a wide, variable range (median, 119 days; range 56 days to 3.8 years), including delayed presentation of rash 2 months after mogamulizumab therapy discontinuation in 1 patient. The median number of mogamulizumab cycles before rash onset date was 5 (range, 2-51). No patients in this single-institution study developed life-threatening severe cutaneous adverse drug reactions, such as acute generalized exanthematous pustulosis, SJS, TEN, drug-induced hypersensitivity syndrome, or drug reaction with eosinophilia and systemic symptoms. Moderate to severe pruritus was commonly associated with rash (63.2% of patients).
Table 1. Characteristics of Patients With MAR.
| Characteristic | Finding (N = 19)a |
|---|---|
| Age, median (range), y | 65 (38-82) |
| Male sex | 10 (52.6) |
| Clinical stage | |
| MF IIIA | 1 (5.3) |
| SS IVA1 | 16 (84.2) |
| SS IVA2 | 2 (10.5) |
| Clinical presentation at mogamulizumab cycle 1 day 1 | |
| Patch-type disease | 3 (15.8) |
| Plaque-type disease | 3 (15.8) |
| Mixed patch and plaque disease | 3 (15.8) |
| Erythrodermab | 10 (52.6) |
| Enrolled in clinical trial | 5 (26.3) |
| MAR onset, median (range) | 119 (56 d to 3.8 y) |
| No. of mogamulizumab cycles completed before MAR onset | |
| 2-3 | 6 (31.6) |
| 4-5 | 6 (31.6) |
| ≥6 | 7 (36.8) |
| Skin response before MAR onset | |
| Stable disease | 2 (10.5) |
| Partial response | 15 (78.9) |
| Complete response | 2 (10.5) |
| Blood response before MAR onset | |
| Partial response | 2 (10.5) |
| Complete response | 16 (84.2) |
Abbreviations: MAR, mogamulizumab-associated rash; MF, mycosis fungoides; SS, Sézary syndrome.
Data are presented as number (percentage) of patients unless otherwise indicated.
Erythroderma is defined as greater than 80% body surface area (patch-type and/or plaque-type disease).
Clinical appearance of MAR was heterogeneous and variable. Four predominant clinical presentations were observed (Table 2 and Figure 1): (1) folliculotropic–MF-like scalp plaques with alopecia (8 of 19 patients [42.1%]); (2) papules and/or plaques (6 of 19 patients [31.6%]) presenting as a scaly psoriasiform dermatitis, lichenoid dermatitis, or smooth indurated papules and plaques; (3) photoaccentuated dermatitis (5 of 19 patients [26.3%]); and (4) morbilliform or erythrodermic dermatitis (5 of 19 patients [26.3%]). Of the 19 patients, 4 (21.0%) had coexistence of more than 1 clinical presentation class, and evolution from one presentation to another over time also occurred.
Table 2. Clinical Characteristics and Management of Mogamulizumab-Associated Rash.
| Patient No. | Clinical presentation | Anatomical region | Symptoms (moderate-severe pruritus) | MAR management | Skin biopsy (histopathologic patterns identified) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F-MF–like scalp plaques with alopecia | Papules and/or plaquesa | Photoaccentuated | Morbilliform or erythrodermic | Head and neck | Trunk | Arms | Legs | Topical corticosteroids | Systemic corticosteroids | Methotrexate | Continued | Discontinued | |||
| 1b | X | Xc | X | X | X | Psoriasiform, spongiotic, interface | |||||||||
| 2b | X | X | X | X | X | X | X | X | Psoriasiform, spongiotic, granulomatous, lichenoid | ||||||
| 3 | X | X | Xc | X | X | X | X | X | X | X | Psoriasiform, spongiotic, interface | ||||
| 4 | X | X | X | X | X | X | X | X | X | Granulomatous | |||||
| 5 | X | X | X | Xc | X | X | X | X | X | X | X | Psoriasiform, spongiotic, granulomatous, interface | |||
| 6 | X | X | X | X | X | X | X | X | X | Spongiotic, lichenoid, interface | |||||
| 7 | X | Xc | X | X | X | Spongiotic | |||||||||
| 8 | X | X | X | X | X | X | X | X | X | Spongiotic, granulomatous | |||||
| 9b | X | X | X | X | Psoriasiform, spongiotic, granulomatous, lichenoid | ||||||||||
| 10 | X | X | X | X | X | X | X | X | X | Xd | Interface, lichenoid | ||||
| 11 | X | Xc | X | X | X | X | X | Spongiotic, lichenoid, interface | |||||||
| 12 | X | Xc | X | X | X | X | X | X | Lichenoid, spongiotic | ||||||
| 13 | X | Xc | X | X | X | Psoriasiform, spongiotic | |||||||||
| 14 | X | Xc | X | X | X | X | X | Spongiotic | |||||||
| 15 | X | Xc | X | X | Spongiotic, interface | ||||||||||
| 16 | X | Xc | X | X | X | Spongiotic | |||||||||
| 17 | X | Xc | X | X | X | X | X | X | X | X | Psoriasiform, spongiotic | ||||
| 18b | X | X | X | X | X | X | X | X | X | X | Psoriasiform, spongiotic, interface | ||||
| 19b | X | X | Xc | X | X | X | X | X | X | Spongiotic | |||||
| Total No. (N = 19) | 8 | 6 | 5 | 5 | 17 | 15 | 12 | 8 | 12 | 19 | 12 | 7 | 5 | 14 | NA |
Abbreviations: F-MF, folliculotropic mycosis fungoides; MAR, mogamulizumab-associated rash; NA, not applicable.
Variable morphologic findings, including psoriasiform, lichenoid, or smooth indurated papules and/or plaques.
Patient was enrolled in the Mogamulizumab Versus Vorinostat in Previously Treated Cutaneous T-Cell Lymphoma (MAVORIC) clinical trial.
Prominent scalp involvement.
Mogamulizumab-associated rash had a delayed onset of 2 months after last dose of mogamulizumab.
Figure 1. Clinical Characteristics of Patients With Mogamulizumab-Associated Rash (MAR).
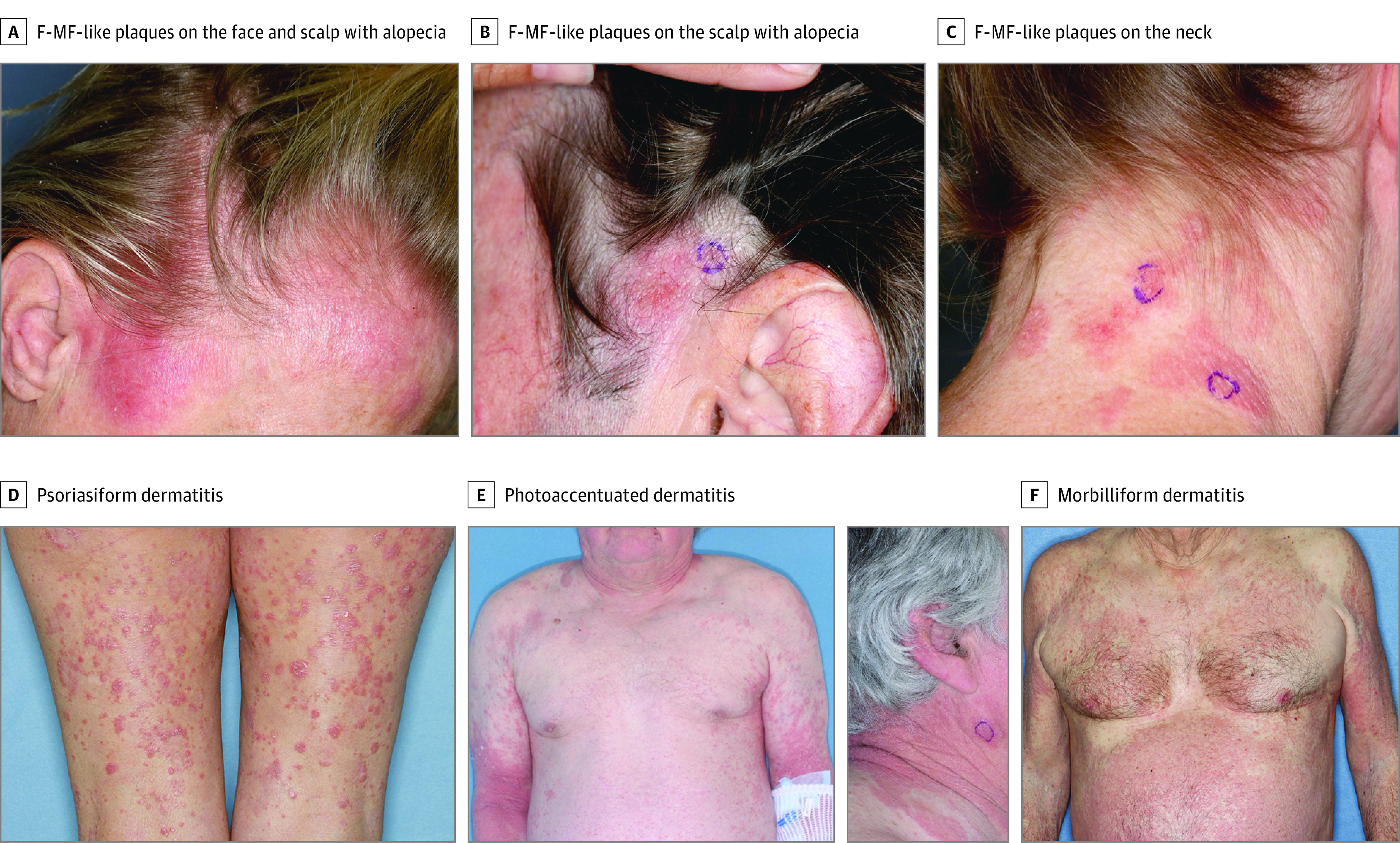
Patients with MAR exhibit 4 predominant clinical presentations, including (1) folliculotropic mycosis fungoides (F-MF)–like plaques with alopecia on the head and neck, including the scalp (A-C); (2) papules and/or plaques, often with lichenoid or psoriasiform features (D); (3) photoaccentuated dermatitis (E); and (4) morbilliform or erythrodermic dermatitis (F).
The anatomical distribution of MAR was also variable, with 8 patients (42.1%) exhibiting localized dermatitis (1 or 2 anatomical regions) and 11 (57.9%) having multifocal or widespread involvement (3-4 anatomical regions involved). The most common anatomical region involved by MAR was the head and neck (17 of 19 patients [89.5%]), with a striking and unique involvement of the scalp in most patients, followed by involvement of the trunk (15 of 19 patients [78.9%]), arms (12 of 19 patients [63.2%]), and legs (8 of 19 patients [42.1%]).
Histopathologic Features of MAR
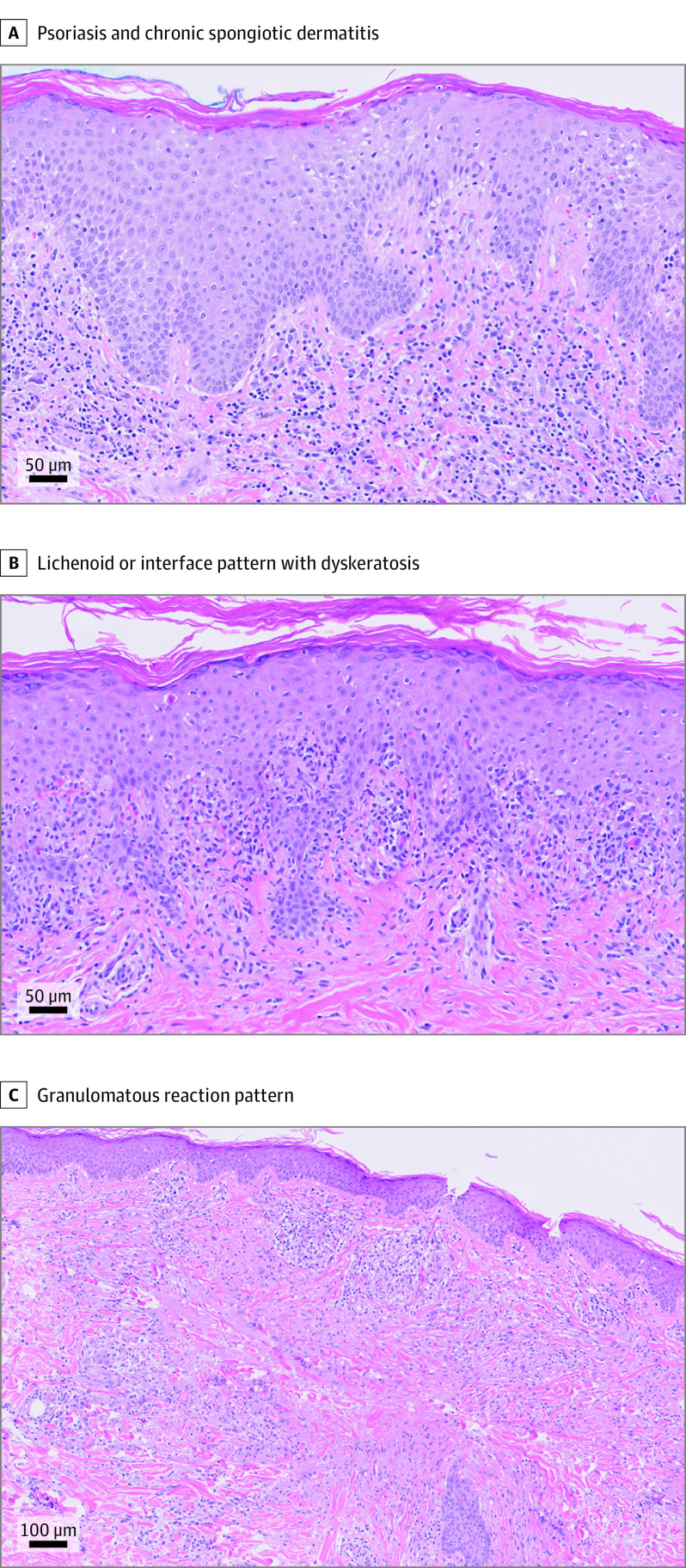
Skin biopsy specimens from patients with MAR demonstrated 3 primary histopathologic reaction patterns as recently described: spongiotic or psoriasiform dermatitis, interface dermatitis, and granulomatous dermatitis (Figure 2).17 Two or more patterns were frequently present concurrently, and MF-like features, including lymphocyte exocytosis into the epidermis and follicular epithelium, lymphocyte tagging of the basal layer, and lamellar fibroplasia, were focally identified in all cases. Of interest, no significant association between clinical and histopathologic features could be identified, given the numerous combinations of clinicopathologic presentations observed (Table 2). In contrast to the CD4-predominant T-cell populations observed in biopsy specimens from the patients’ MF or SS, most MAR biopsy specimens demonstrated exocytosis of CD8-positive T cells and a normal ratio of CD4- to CD8-positive T cells in the dermis, compatible with a reactive process.
Figure 2. Histopathologic Features of Mogamulizumab-Associated Rash (MAR).
Three major histopathologic reaction patterns of MAR were identified. The most common pattern showed overlapping features of psoriasis and a chronic spongiotic dermatitis (hematoxylin-eosin, original magnification ×20) (A). Other cases showed a lichenoid or interface pattern with dyskeratosis (hematoxylin-eosin, original magnification ×10) (B). A smaller subset of cases had a granulomatous reaction pattern with moderately to well-formed granulomas. Some granulomas were palisading in appearance, reminiscent of granuloma annulare (hematoxylin-eosin, original magnification ×20) (C).
In this cohort of patients, MAR diagnosis required that biopsy specimens demonstrate polyclonality of TCR β and γ genes by traditional polymerase chain reaction or HTS. In all patients, HTS had been performed before mogamulizumab therapy initiation for baseline characterization of their MF or SS. When HTS was performed in MAR biopsy specimens, the previously identified disease-associated TCR clone was not detected or was present at exceedingly low, nondominant levels, arguing against involvement by active disease.
Management of MAR
Mogamulizumab therapy was discontinued in 14 of the 19 patients (73.7%) with MF and SS who developed MAR. Time from mogamulizumab therapy discontinuation to MAR resolution typically occurred over months; however, accurate determination was difficult in many cases because of overlapping features of MAR and malignant disease in the skin, especially with relapse or progression of MF or SS with time.
Rash was not the sole contributor to the decision to discontinue mogamulizumab therapy in at least 7 of 14 patients (50.0%) who stopped mogamulizumab therapy when MAR was present. Concomitant factors that contributed to mogamulizumab therapy discontinuation in patients with MAR included (1) clinical trial parameters that required discontinuation of mogamulizumab therapy if systemic corticosteroids were used (3 of 14 patients [21.4%]); (2) mogamulizumab treatment no longer needed because of global clinical remission (2 of 14 patients [14.3%]); (3) disease progression (1 of 14 patients [7.1%]); and (4) change of systemic management to methotrexate to treat MAR and MF or SS (1 of 14 patients [7.1%]).
Mogamulizumab treatment was continued in 5 of the 19 patients (26.3%). In 1 of these patients, onset of MAR occurred 2 months after mogamulizumab therapy was discontinued (delayed-onset MAR). After completing 5 cycles of mogamulizumab, this patient presented with a photoaccentuated dermatitis triggered by significant and prolonged sun exposure, and after thorough medication review, mogamulizumab was the most likely contributing factor in his drug eruption.
All 19 patients with MAR received skin-directed management with topical corticosteroids. Six patients (31.6%) were managed with topical corticosteroid monotherapy, and 12 patients (63.2%) required systemic corticosteroids (starting at 60 mg of prednisone daily, followed by a taper as tolerated), with a range of systemic corticosteroid treatment of 1 to 8 months. Persistence of MAR often led to the need for prolonged systemic corticosteroid treatment, and 6 patients (31.6%) were also treated with oral methotrexate (target dosage of 25-30 mg weekly) over a range of 2 to at least 4 months. One patient directly initiated methotrexate monotherapy, without systemic corticosteroid use, to treat MF or SS and MAR. Methotrexate was an attractive choice as a corticosteroid-sparing agent because of its additional known antitumor activity against MF and SS.
Retreatment With Mogamulizumab After MAR
Because of persistence or progression of disease, 4 patients with a history of MAR were treated with mogamulizumab again at a later date, 3 months to 5 years after the last cycle of their first course of mogamulizumab. During retreatment, patients who experienced rash recrudescence were observed to have multifocal and widespread anatomical distribution, but no patients developed life-threatening severe cutaneous adverse drug reactions. When patients with a history of MAR were treated again with mogamulizumab, clinical presentation was similar to the first episode of MAR, with heterogeneous and variable morphologic and anatomical distribution and delayed rash onset from the start of mogamulizumab treatment. Rash was managed with topical corticosteroids, systemic corticosteroids starting at 60 mg of prednisone daily followed by a taper (duration of systemic steroids of 2-5 months), and oral methotrexate (target dosage of 25-30 mg weekly for 2-4 months) to aid in tapering of systemic corticosteroids. In all 4 patients treated again, mogamulizumab therapy was discontinued because of complete response of MF or SS or disease progression not attributable to MAR.
Discussion
In this single-institution case series of 19 patients with MF or SS treated with mogamulizumab, MAR was observed in 33% of patients, mostly in the patients with SS, and demonstrated heterogeneous features, including diverse clinical presentation with delayed onset and persistent course. Time to onset of MAR can be weeks to even months after drug therapy discontinuation. Unique to MAR is that nearly all patients exhibit involvement of hair-bearing areas of the scalp. In some of these cases, the head and neck–predominant presentation was distinct from the patient’s original disease morphologic and anatomical distribution, enabling astute clinical recognition of MAR as confirmed by histopathologic analysis and TCR clonality studies.
A cutaneous granulomatous drug eruption presentation of patches and plaques clinically indistinguishable from MF, as well as a photosensitizing eruption, was previously reported during mogamulizumab treatment.14,19 Our cohort expands the clinical presentation of MAR with 4 predominant categories: (1) folliculotropic MF–like scalp plaques with alopecia, (2) papules and/or plaques, (3) photoaccentuated dermatitis, and (4) morbilliform or erythrodermic dermatitis. Pruritus was common in patients with MAR. Skin biopsy specimens revealed a range of histopathologic reaction patterns, which did not appear to correlate with clinical morphologic findings, although clinicopathologic associations may be difficult to discern in our small cohort.
If MAR is clinically suspected, careful consideration of risks and benefits of holding or discontinuing mogamulizumab therapy is important for each patient. Mogamulizumab therapy was discontinued in most patients with MAR in this study. In at least half of patients who discontinued mogamulizumab therapy, the decision to stop use of the drug involved additional contributing factors, such as disease progression, global improved clinical response, or clinical trial parameters that required drug therapy discontinuation with initiation of systemic corticosteroid therapy. No life-threatening drug reactions, including SJS or TEN, which was previously reported with mogamulizumab in the ATLL population, were observed during mogamulizumab treatment in our cohort, even after retreatment with mogamulizumab after MAR.
Regarding MAR treatment, in patients with more widespread dermatitis, systemic rather than topical corticosteroids may be helpful. This study found that a longer corticosteroid taper of at least 1 month is often needed to avoid flaring of MAR. If systemic corticosteroids cannot be successfully tapered because of prolonged MAR, a corticosteroid-sparing agent should be considered to minimize long-term adverse effects of systemic corticosteroids and to achieve long-term improvement of MAR. Methotrexate was chosen in this cohort because of its ability to treat malignant disease and drug rash. Other considerations for management of MAR include photopheresis or phototherapy, although phototherapy should be used with caution given the photoaccentuated dermatitis and photosensitivity associated with mogamulizumab.
The clinical and histopathologic similarities with MF or SS and variable kinetics of MAR onset make it difficult but crucial to differentiate MAR from progression of MF or SS. Although the study identified certain histopathologic features suggestive of MAR, such as the presence of mixed reaction patterns, reliably distinguishing MAR from MF or SS on the basis of routine histopathologic analysis alone may be challenging. Indeed, histopathologic interpretation was frequently performed in conjunction with immunohistochemistry and TCR sequencing to enable MAR to be distinguished from disease. On the basis of this experience, a baseline immunohistochemical and/or molecular profile of the patient’s malignant T-cell population should be established to inform future evaluation of new or worsening rashes after mogamulizumab therapy initiation. Patients with persistent rash may require additional skin biopsies with molecular testing at different time points to detect emerging disease within the background of MAR. Although we used HTS to characterize the malignant clone, traditional polymerase chain reaction may perform equally well for the current purpose. Further discussion of the advantages and limitations of these techniques is presented elsewhere.17
The mechanism of MAR is unknown; however, the heterogeneous clinical presentation, including variable morphologic and anatomical distribution and delayed onset and persistence of rash even months after therapy drug discontinuation, has significant similarities to cutaneous immune-related adverse events in patients who receive immune checkpoint inhibitors.20 Mogamulizumab may play a role in depletion of immunosuppressive TH2 and T-regulatory cells, which also can express CCR4, thus leading to an immune modulating, proinflammatory effect that contributes to the development of MAR. Mogamulizumab may recruit or activate epidermotropic cytotoxic T lymphocytes, leading to a more active inflammatory response in the skin.
Whether an association exists between the presence of MAR and survival or disease progression in MF or SS is unknown. In the ATLL population, MAR may indicate a favorable prognosis and predict response to mogamulizumab,10 and granulomatous dermatitis was associated with durable clinical response in patients from the MAVORIC trial.14 Most patients in this cohort had near-complete clearing of their MF or SS skin disease and complete blood response to mogamulizumab at the time of new rash onset; however, more rigorous analysis of an expanded cohort is required to determine whether development of MAR may be a positive prognostic factor in patients with MF or SS.14,21
Although rash onset was still delayed and tended to occur in a more widespread distribution in patients who were treated again with mogamulizumab after MAR, no life-threatening drug reactions were observed in patients who received additional treatment, suggesting that development of MAR does not preclude future treatment with mogamulizumab. Recurrent MAR can be managed with a combination of topical corticosteroids, systemic corticosteroids, and methotrexate. If retreatment is beneficial for a patient’s disease, mogamulizumab can be considered in most cases.
Limitations
This study has limitations, including a single-institution cohort and retrospective medical record review protocol. This study lacked standardized grading of the severity of MAR because calculation of drug reaction–involved body surface area can often be unreliable and inaccurate because of drug reaction and skin disease being clinically indistinguishable by primary morphologic and anatomical distribution.
Conclusions
As seen in this study, MAR presents as a heterogeneous skin eruption that is crucial to recognize and distinguish from relapse or progression of disease in patients with MF or SS. No life-threatening drug-induced events were observed, and mogamulizumab therapy continuation and retreatment may be considered in the appropriate clinical context. Further studies are needed to confirm these findings, investigate the pathogenesis of MAR, and determine implications of MAR on prognosis regarding disease response to mogamulizumab.
References
- 1.Kim YH, Bagot M, Pinter-Brown L, et al. ; MAVORIC Investigators . Mogamulizumab Versus Vorinostat in Previously Treated Cutaneous T-Cell Lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192-1204. doi: 10.1016/S1470-2045(18)30379-6 [DOI] [PubMed] [Google Scholar]
- 2.Yoshie O, Matsushima K. CCR4 and its ligands: from bench to bedside. Int Immunol. 2015;27(1):11-20. doi: 10.1093/intimm/dxu079 [DOI] [PubMed] [Google Scholar]
- 3.Ni X, Jorgensen JL, Goswami M, et al. Reduction of regulatory T cells by mogamulizumab, a defucosylated anti-CC chemokine receptor 4 antibody, in patients with aggressive/refractory mycosis fungoides and Sézary syndrome. Clin Cancer Res. 2015;21(2):274-285. doi: 10.1158/1078-0432.CCR-14-0830 [DOI] [PubMed] [Google Scholar]
- 4.Sugiyama D, Nishikawa H, Maeda Y, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A. 2013;110(44):17945-17950. doi: 10.1073/pnas.1316796110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishida T, Joh T, Uike N, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30(8):837-842. doi: 10.1200/JCO.2011.37.3472 [DOI] [PubMed] [Google Scholar]
- 6.Ishitsuka K, Yurimoto S, Kawamura K, et al. Safety and efficacy of mogamulizumab in patients with adult T-cell leukemia-lymphoma in Japan: interim results of postmarketing all-case surveillance. Int J Hematol. 2017;106(4):522-532. doi: 10.1007/s12185-017-2270-9 [DOI] [PubMed] [Google Scholar]
- 7.Ishida T, Ito A, Sato F, et al. Stevens-Johnson syndrome associated with mogamulizumab treatment of adult T-cell leukemia / lymphoma. Cancer Sci. 2013;104(5):647-650. doi: 10.1111/cas.12116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tani N, Sugita K, Ito A, Ooi S, Yamamoto O. CD8+ T cell-mediated interface dermatitis during combination chemotherapy with mogamulizumab in a patient with adult T-cell leukaemia/lymphoma. Clin Exp Dermatol. 2018;43(6):736-737. doi: 10.1111/ced.13539 [DOI] [PubMed] [Google Scholar]
- 9.Ito A, Sugita K, Adachi K, Hosoda Y, Motokura T, Yamamoto O. CD8+ T-cell-mediated interface dermatitis after CCR4+ T-cell depletion by mogamulizumab treatment of adult T-cell leukaemia/lymphoma. Acta Derm Venereol. 2017;97(3):377-378. doi: 10.2340/00015555-2555 [DOI] [PubMed] [Google Scholar]
- 10.Yonekura K, Tokunaga M, Kawakami N, et al. Cutaneous adverse reaction to mogamulizumab may indicate favourable prognosis in adult T-cell leukaemia-lymphoma. Acta Derm Venereol. 2016;96(7):1000-1002. doi: 10.2340/00015555-2421 [DOI] [PubMed] [Google Scholar]
- 11.Shiratori S, Ohhigashi H, Ito S, et al. Late onset toxic epidermal necrolysis induced by mogamulizumab, an anti-CC chemokine receptor 4 antibody for the treatment of adult T-cell leukaemia/lymphoma. Hematol Oncol. 2017;35(1):138-140. doi: 10.1002/hon.2242 [DOI] [PubMed] [Google Scholar]
- 12.Kanno K, Honma M, Ishida-Yamamoto A. Cutaneous adverse reaction of mogamulizumab, an anti-CC chemokine receptor 4 monoclonal antibody: shared histopathological features with thymoma-associated multi-organ autoimmunity. J Dermatol. 2017;44(6):e117-e118. doi: 10.1111/1346-8138.13738 [DOI] [PubMed] [Google Scholar]
- 13.Bagot M, Dalle S, Sokol L, Kim YH. Long-term clinical benefit to anti-CCR4 mogamulizumab: results from the phase 3 MAVORIC study in previously treated cutaneous T-cell lymphoma (CTCL). Poster presented at: American Society of Hematology Conference; December 1-4, 2018; San Diego, CA. [Google Scholar]
- 14.Chen L, Carson KR, Staser KW, et al. Mogamulizumab-associated cutaneous granulomatous drug eruption mimicking mycosis fungoides but possibly indicating durable clinical response. JAMA Dermatol. 2019. doi: 10.1001/jamadermatol.2019.0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yonekura K, Kanzaki T, Gunshin K, et al. Effect of anti-CCR4 monoclonal antibody (mogamulizumab) on adult T-cell leukemia-lymphoma: cutaneous adverse reactions may predict the prognosis. J Dermatol. 2014;41(3):239-244. doi: 10.1111/1346-8138.12419 [DOI] [PubMed] [Google Scholar]
- 16.Suzuki Y, Saito M, Ishii T, et al. Mogamulizumab treatment elicits autoantibodies attacking the skin in patients with adult T-cell leukemia-lymphoma. Clin Cancer Res. 2019;25(14):4388-4399. doi: 10.1158/1078-0432.CCR-18-2575 [DOI] [PubMed] [Google Scholar]
- 17.Wang JY, Hirotsu KE, Neal TM, et al. Histopathologic characterization of mogamulizumab-associated rash. Am J Surg Pathol. 2020;44(12):1666-1676. doi: 10.1097/PAS.0000000000001587 [DOI] [PubMed] [Google Scholar]
- 18.Weng WK, Armstrong R, Arai S, Desmarais C, Hoppe R, Kim YH. Minimal residual disease monitoring with high-throughput sequencing of T cell receptors in cutaneous T cell lymphoma. Sci Transl Med. 2013;5(214):214ra171. doi: 10.1126/scitranslmed.3007420 [DOI] [PubMed] [Google Scholar]
- 19.Masuda Y, Tatsuno K, Kitano S, et al. Mogamulizumab-induced photosensitivity in patients with mycosis fungoides and other T-cell neoplasms. J Eur Acad Dermatol Venereol. 2018;32(9):1456-1460. doi: 10.1111/jdv.14797 [DOI] [PubMed] [Google Scholar]
- 20.Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25. doi: 10.1016/j.ejca.2016.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan L, Hwang SJE, Byth K, et al. Survival and prognosis of individuals receiving programmed cell death 1 inhibitor with and without immunologic cutaneous adverse events. J Am Acad Dermatol. 2020;82(2):311-316. doi: 10.1016/j.jaad.2019.06.035 [DOI] [PubMed] [Google Scholar]