Abstract
The presence of SARS-CoV-2 RNA in wastewater was first reported in March 2020. Over the subsequent months, the potential for wastewater surveillance to contribute to COVID-19 mitigation programmes has been the focus of intense national and international research activities, gaining the attention of policy makers and the public. As a new application of an established methodology, focused collaboration between public health practitioners and wastewater researchers is essential to developing a common understanding on how, when and where the outputs of this non-invasive community-level approach can deliver actionable outcomes for public health authorities. Within this context, the NORMAN SCORE “SARS-CoV-2 in sewage” database provides a platform for rapid, open access data sharing, validated by the uploading of 276 data sets from nine countries to-date. Through offering direct access to underpinning meta-data sets (and describing its use in data interpretation), the NORMAN SCORE database is a resource for the development of recommendations on minimum data requirements for wastewater pathogen surveillance. It is also a tool to engage public health practitioners in discussions on use of the approach, providing an opportunity to build mutual understanding of the demand and supply for data and facilitate the translation of this promising research application into public health practice.
1. Introduction
Research continues apace into many aspects of the use of wastewater surveillance for the detection of SARS-CoV-2 and how data generated can be utilised within local public health decision-making. Also known as sewage or environmental surveillance, the approach has an established literature in terms of monitoring the occurrence and concentration of chemicals arriving at a wastewater treatment plant (WWTP) (Choi et al., 2018). Determined chemical concentrations, loads and population normalised loads of illicit (González-Mariño et al., 2020a, González-Mariño et al., 2020b; Ort et al., 2014) and licit drugs including tobacco, caffeine and alcohol (Castiglioni et al., 2015; Gracia-Lor et al., 2017; Ryu et al., 2016, Thomaidis et al., 2016) are used to provide quantitative longitudinal data sets on the use at a catchment level. It is also possible to evaluate the rates of exposure to environmental or food contaminants using the same approach (Rousis et al., 2017; Lopardo et al., 2019). Furthermore, wastewater surveillance can be used to evidence changes overtime in relation to the implementation of new policy initiatives. The practical utility of chemical wastewater surveillance data sets is demonstrated by its use within local and national monitoring and public health programmes (EMCDDA, 2020; Riva et al. 2020; Lai et al., 2018). Prior to 2020, the use of wastewater surveillance for monitoring pathogens was gaining ground only slowly. Most notably, enterovirus wastewater surveillance systems have been established in several locations (Sedmak et al., 2003; Majumdar et al., 2018), with wastewater surveillance identified as playing a key role in polio eradication schemes in Israel, India and Egypt (WHO, 2020; Ashgar et al., 2014; Holm-Hansson et al., 2017). The first SARS-CoV-2 wastewater surveillance studies were undertaken in the Netherlands, with viral RNA material detected in wastewater treatment influent samples in seven Dutch cities and the international airport (Medema et al., 2020a). This landmark study included data on the detection of viral fragments in wastewater in one city prior to the detection of any clinical cases. This potential to provide an early warning on the presence of the virus within a community is a proof-of-concept and an evidence base that could be used by public health teams as a trigger to intensify clinical testing, facilitating the identification and isolation of positive cases (Thompson et al., 2020; POST, 2020). Hence, the use of wastewater surveillance for SARS-CoV-2 as a tool to address the COVID19 pandemic is a new application of an established method in a rapidly moving field.
SARS-CoV-2 wastewater surveillance studies to date have demonstrated the occurrence of its RNA genome in a range of compartments, primarily WWTP influents but it has also been reported in sludge and effluents as well as within receiving waters (Jones et al., 2020; Randazzo et al., 2020). In terms of infectivity potential of wastewater containing SARS-CoV-2 RNA, initial studies (Westhaus et al., 2021; Bivins et al., 2020a) and expert opinion (WHO, 2020; Jones et al., 2020) indicate that detected RNA materials do not occur in the form of an infectious viral particle. Further studies also looked to establish a quantitative relationship between viral load and number of clinical cases reported within a catchment (Vallejo et al., 2020; Ahmed et al., 2020). However, variations in the load and duration of viral material shed in faeces by asymptomatic, pre-symptomatic and symptomatic cases, together with limited understanding of the fate of viral particles within sewer systems (which vary significantly in design and flow dynamics), and variations in analytical protocols and their associated extraction efficiencies, generates considerable uncertainty in terms of directly relating viral loads to numbers of cases. Hence, many open challenges exist within this research area and use of data by public health teams. Within the field, key research questions encompass the potential for viral materials to adsorb to biofilm and particles, degrade in the sewage system and optimising sample collection processes, including collection location and frequency (WHO, 2020). Moreover, the need to standardise and optimise analytical protocols has been clearly identified (Michael-Kordatou et al., 2020). In terms of interpreting data, key issues include data comparability between studies (e.g. use of a common marker for normalisation and how contextual data e.g. flow and other parameters are included in data interpretation), the identification of a SARS-CoV-2 RNA threshold value and the actions that exceeding a threshold value should trigger (Medema et al., 2020b). Variations in the amount of viral RNA excreted per person are a further unknown, and inherent levels of variability in shedding may make accurate predictions of prevalence impossible. However, the absence of an absolute understanding of shedding rate behaviour does not preclude the use of this approach in public health contexts, where relative changes in signal (as opposed to its absolute value) can provide public health teams with valuable data. Further open questions remain over ethical aspects related to the use of wastewater surveillance, and the need to develop a social license to operate if the approach is to be successfully adopted. Whilst ethical aspects have been largely overlooked during the current health emergency, developments in near source tracking e.g. analysis of wastewater from aeroplanes, hospitals and schools (Ahmed et al., 2020; Gonçalvesa et al., 2021; Hassard et al., 2020, Hong et al., 2021) are rapidly pushing this issue up the research and practice agenda. In this article a bottom-up, collaborative approach to enabling researchers to systematically and rapidly share raw data on traditional wastewater parameters, the occurrence of SARS-CoV-2 and clinical case numbers is presented, as both a resource for researchers and a tool to facilitate discussion with public health teams.
2. The use of wastewater surveillance data within public health decision-making
Wastewater surveillance can be used to non-invasively screen ‘hard to test’ communities (i.e. where uptake of testing is low or challenging for resource reasons) at a sewer catchment level as a new public health tool to understand COVID-19 spread (CDC, 2020; POST, 2020). Detection of SARS-CoV-2 RNA fragments in wastewater is independent of clinical testing strategy bias (Thompson et al., 2020), can be used as an early warning of the need for further testing (e.g. reallocating/increasing local testing resources such as drive-through test facilities) or the implementation of wastewater surveillance upstream of the WWTP i.e. near-source tracking to identify location of cases (Hassard et al., 2020). For example, the detection of SARS-CoV-2 RNA concentrations can indicate the (re-) emergence of the virus in a catchment following a period of no clinical cases and an increase in viral RNA load can indicate the occurrence of new outbreaks, requiring the urgent tracing of infected individuals and their subsequent support to isolate (DEFRA, 2020). Likewise decreasing prevalence can indicate that infected individuals are ‘known’ and isolation/public health interventions are effective. Further, an increase in viral load over time against a trend of ‘no-change’ in daily positive case numbers could indicate that the clinical testing regime should be intensified (i.e. new cases are not being detected) (Thompson et al., 2020). Wastewater surveillance data sets can also be used to evidence the effect of alternative policy actions e.g. curfew vs local lockdown vs national lockdown at a community level, as well as track progress of vaccination campaigns.
To deliver these types of actionable outcomes i.e. to enable public health authorities to use wastewater surveillance data within their community level decision-making processes requires activities on several fronts. As well as addressing the wastewater surveillance methodological and analytical challenges identified earlier, data from wastewater needs to be collected frequently and available rapidly in a format that is useful and useable by public health practitioners. Further collaboration between wastewater and public health practitioners is required to ensure that public health teams can access the type of data they require in a timeframe and format that integrates with current pandemic mitigation measures i.e. addressing public health data requirements needs to be front and centre of operationalising this new development in wastewater surveillance. The format and sampling strategies underpinning wastewater data sets may need to morph in terms of the locations and frequency of sample collection, quality assurance/quality control processes, scale at which data is generated and made available and the aspects of primary value from a public health perspective i.e. absolute values or trends analysis. Delivering this type of integrated data share ‘dashboard’ is already challenging under usual working conditions; working across disciplines during a pandemic when public health teams are at (or beyond) full capacity is extremely challenging. However, collaboration between public health and wastewater researchers – where public health practitioners take a lead role in determining dashboard development - is happening. For example, in Australia, the development of a SARS-CoV-2 wastewater surveillance dashboard was led by a collaboration between the Victorian state public health team and Water Research Australia. This has already matured from a research and development phase to an operational tool for day-to-day use with functional dashboards for both internal and external communications (Victoria State Government, 2020). Other countries with established monitoring programs include Canada (https://cwn-rce.ca/covid-19-wastewater-coalition/), Finland (https://www.thl.fi/episeuranta/jatevesi/jatevesiseuranta_viikkoraportti.html), Luxembourg (https://www.list.lu/en/covid-19/), Greece (http://trams.chem.uoa.gr/covid-19/), the Netherlands (https://www.rivm.nl/en/covid-19/sewage), and Spain (https://www.miteco.gob.es/es/agua/temas/concesiones-y-autorizaciones/vertidos-de-aguas-residuales/alerta-temprana-covid19/default.aspx). In the UK, sharing of data between a government-led wastewater surveillance project and the national COVID-19 ‘track and trace’ programme led to the identification of an increase in SARS-CoV-2 RNA in wastewater despite relatively low numbers of people taking clinical tests (DEFRA, 2020). This data was used to alert local health professionals to contact people in the area to warn of the increase in cases and encourage local populations to engage with clinical testing programmes.
The need for and benefits of collaboration among wastewater researchers has been recognised and several international and national collaborations rapidly established (e.g. Bivins et al., 2020b; WRF, 2020; WHO, 2020; JRC, 2020; Réseau Obépine, 2020; WRA, 2020; UCMERCED, 2020). These have focused primarily on technical and analytical issues, facilitating opportunities for rapid discussion on a range of topics from recent publications to method development, predictive modelling and risk assessment. However, collaboration activities to-date have yet to address two key issues: firstly, the development of an open-access data platform to enable and facilitate the rapid sharing and critical evaluation of multiple wastewater meta-data sets to address technical issues (Bivins et al., 2020a). Secondly, engagement with public health authorities i.e. development of a critical mass of public health and wastewater researchers to collaboratively identify and deliver an operational SARS-CoV-2 wastewater surveillance public health system.
3. Open-access data sharing to progress collaboration across disciplines
The NORMAN/SCORE SARS-COV-2 in sewage (SC2S) database is a platform, which can contribute to meeting both these needs. This open-access database is an output of the collaboration between two international networks: the NORMAN network (www.norman-network.net/) of research organisations supporting the validation and harmonisation of measurement methods and monitoring tools and SCORE (https://score-cost.eu) a network established to harmonise methodologies for measuring human biomarkers in wastewater to evaluate lifestyle, health and exposure at the community level. The database is located within the NORMAN Database System at https://www.norman-network.com/nds/ as the latest addition to its 13 database modules within the interlinked database system series for the collection and evaluation of data / information on emerging substances in the environment (Dulio et al., 2020). The SC2S database structure follows that of the NORMAN Antibiotic Resistance Bacteria/Genes database, enabling users to freely access data at a WWTP level as well us upload new data via a customised data collection template (DCT; downloadable from the website) which facilitates its automatic uploading to the system. On accessing the database, users can search via country and/or WWTP or view the entire data set (both within the database or it can be exported into MS Excel) without any restrictions. Data displayed in the dashboard includes sampling date, gene copy (number of copies/mL and/or ng of RNA/mL), cycle threshold (Ct), WWTP and country name, population served and the number of people reported SARS-CoV-2 positive in the sewer catchment area on the day of sampling. Table 1 identifies the requested reporting parameters and provides an overview of their role in interpreting generated data sets. Finally, the full DCT containing all reported data on all parameters can be downloaded for each dataset. In terms of engaging the attention of public health authorities, as a first step it includes both wastewater and clinical case data. In addition, and perhaps more importantly, it is a starting point for further discussions with public health practitioners on what wastewater surveillance is, the types of longitudinal data sets it can produce (together with process controls), and the potential of this non-invasive approach as a tool to provide an early warning of new clusters as well as the impact of existing pandemic mitigation measures.
Table 1.
Overview of parameters recorded and their role in facilitating data analysis, interpretation and comparison.
| Type of data | Parameters | Role in data interpretation |
|---|---|---|
| Sampler information | Name, contact details | Auditability |
| Sampling site | WWTP name and country; longitude/latitude; altitude (m) | Identify sewer shed location; consider climatic influences |
| Design capacity (PE); population served (PE); catchment size (m2) | Consider drainage network size and WWTP loads/dynamics; calculate population density and population-normalised virus loads | |
| SARS-CoV-2 clinical prevalence data | No. of people SARS-CoV-2 positive on sampling date | Relationship between viral load and clinical cases on day of sampling |
| No. of people recovered from SARS-CoV-2 on sampling date | Relationship between viral load and all clinical cases to-date | |
| No. of people SARS-CoV-2 positive 2 weeks prior to sampling date | Longitudinal trends in clinical case numbers; consider shedding from active cases versus post-infection shedding | |
| No. of people recovered from SARS-CoV-2 2 weeks prior to sample date | ||
| Sample matrix | Influent wastewater | Confirmation of sample type |
| Sampling date | Start and finish: hour; day; month; year | Seasonality |
| Sampling procedure | Composite (time- or flow-weighted with intervals reported) or grab sample | Understanding of sampling errors/bias |
| Inflow characteristics | Flow (total m3; minimum/maximum m3/h); | Consider drainage network and WWTP dynamics; calculate mass loads |
| COD [mg/L]; TSS [mg/L]; Total N / NH4-N [mg N/L] | Consider effects of wastewater composition on RNA yield and occurrence of groundwater infiltration | |
| Rain (dry weather/number of days since last rain event | Occurrence of dilution due to rainfall | |
| Sample preparation | Date of analysis; storage temperature (°C) | Potential for degradation of RNA |
| Internal standard used (if so which) | Process quality control / quality assurance | |
| Method used for sample preparation | Potential differences in extraction efficiencies | |
| Volume of sample [mL] | Understanding of RNA copies per a certain wastewater volume | |
| Number of replicates | Quality control / quality assurance | |
| RNA extraction | Date of and method used for RNA extraction | Quality control / quality assurance |
| Genetic markers (N1, N2, E etc.) | Differences in sensitivity using qPCR analysis | |
| Internal standard used (if so which) | Quality control / quality assurance in understanding RNA extraction efficiency | |
| RNA [μL; ng / μL] | Quantitative identification of virus in wastewater | |
| Number of replicates | Quality control / quality assurance | |
| Analytical method | Technique e.g. Conventional PCR / Real-time PCR / Illumina Myseq / Whole genome sequencing / LAMP-PCR / non-targeted analysis. | Quality control / quality assurance |
| Limit of detection (number of copies/mL of sample) | The lowest level of virus that can be determined as present | |
| Limit of quantification (number of copies/mL of sample) | The lowest level of virus that can be quantified at a good confidence | |
| Uncertainty of the quantification (%RSD) | Potential variations in qPCR measurement | |
| Extraction efficiency | Understanding of performance of selected extraction methods | |
| Concentration of RNA in which analysis performed (μL; ng/μL) | Quantitative information of virus measured in wastewater extracts | |
| Positive control used (if so which) | Process quality control / quality assurance; indication of method performance | |
| Number of replicates | Quality control / quality assurance | |
| RNA concentration / abundance | Cycle threshold (Ct) | Quality control / quality assurance |
| Gene copy [number/mL of sample or number/ng of RNA) | Trend and spatial evaluations of virus levels within and across catchments. Calculations considering concentrations, wastewater flow and population served by a WWTP. |
Key: WWTP = wastewater treatment plant
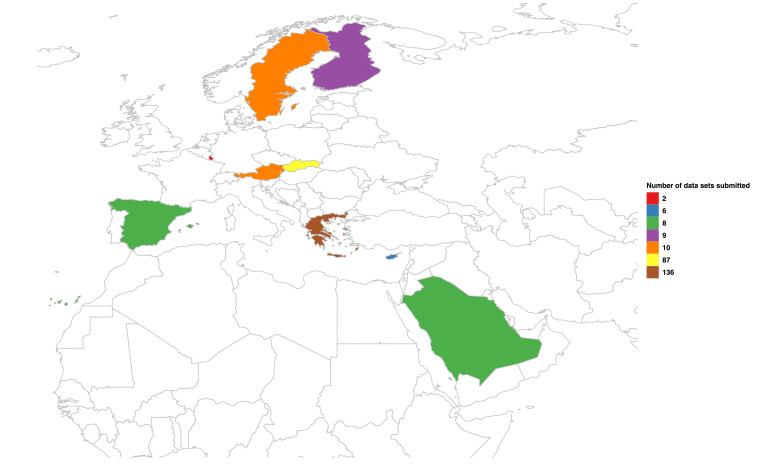
To launch the database, invitations to participate were initially shared through both the NORMAN and SCORE networks, with a request for members to disseminate further through their own networks. To harmonise activities, participants were provided with a common protocol covering sample collection, RNA extraction and analysis. The common protocol (available at https://www.norman-network.com/nds/sars_cov_2/) adopts the Medema et al., 2020a, Medema et al., 2020b methodology with an alternative simplified protocol for SARS-CoV-2 extraction from wastewater via polyethylene glycol (PEG) precipitation (recognising that many consumables/equipment currently in short supply). Given the logistical challenges and urgency to share data quickly, participating laboratories did not undertake an inter- laboratory validation procedure but were asked to report their laboratory QA/QC procedures in full. Submission of data using both methods is welcomed, with space on the DCT to identify which approach was used and the genes targeted. A further step was to establish a ‘buddy system’ for research groups who were able to collect wastewater samples but whose laboratories were under lock-down and/or were not familiar with RNA analysis. As such, the rapid sharing of a common protocol also had a capacity building effect, enabling many groups to explore opportunities to undertake wastewater surveillance for pathogens for the first time. Two scheduled sampling campaigns were held on June 1st 2020 and June 15th 2020, with data referring to further identified sampling campaigns now welcomed. To date the SC2S database contains 276 sets of data from nine different countries (see Fig. 1 ).
Fig. 1.
Overview of the number of data sets contributed to the NORMAN SCORE SC2S database per country.
The impact of pandemic mitigation measures on working conditions impacted on the ability to both collect and manage samples e.g. reduced access to WWTPs and laboratories, consumables and/or work force. Further, whilst the DCTs were developed to support systematic data reporting, not all laboratories were able to provide all requested data due to the on-going challenges experienced by many research groups in terms of access to laboratories, shortages/delays in shipping consumables and reduced work force. Nevertheless, all received data sets were uploaded to achieve the aim of rapid data share as a compliment to ongoing efforts to standardise sampling and analytical protocols. Downloading the current data set shows that 24-hour composite samples (either volume-weighted or time-weighted) were collected on several dates on or close to scheduled sampling dates (from 24th May 2020 – 16th June 2020) with grab and/or composite samples collected on further as local conditions permitted. Sample preparation date, date of analysis and storage conditions were identified, together with the method used for sample preparation, RNA extraction, analysis and the use of internal standards in the sample preparation phase (61% of samples) and the RNA extraction step (88% of samples). Reviewing the data set as a whole, a positive signal for SARS-CoV-2 was quantified in 167 of the 276 samples analysed. Of these 167 samples, the N1 gene was quantified in 18 samples, N2 gene in 8 samples, a combined measure of N1 and N2 in 133 samples and the E gene in 3 samples. Ct counts ranged from 31.9 - 41.9 (median 35), with the number of gene copies/ml ranging from 0.04 – 148 gene copies/mL (median: 10.6 gene copies/mL). In terms of quality control, reported analysis included two to six replicates per sample with the use of a positive control reported in the analyses of 268 of the 276 samples. The analytical limit of detection was reported on 173 occasions (range: 3 – 5 gene copies/ml for N1 gene; 0.5-5 gene copies/ml for N2 gene; 0.75 gene copies/ml for N1/N2 combined gene measurement; 0.5 - 100 gene copies/mL for E gene), with a study by Philo et al. (2021) suggesting that the variability in detection between target genes could be due to variations in the performance of assays or differential rates of degradation in the target genetic material. No study reported their limit of quantification. In terms of clinical data, the number of positive cases reported in the local municipality (which may/may not reflect the sewer catchment) on the day of sampling was reported for 260 of the 276 samples analysed (range: 0 – 1701; median = 239 cases). Whilst at sewer catchment level, ethical issues around participant anonymity and data protection is generally not an issue. However, as contributing areas reduce to, for example, an individual building level, the need to systematically and robustly consider the use of generated data at source and further downstream (i.e. secondary data use) becomes increasingly urgent.
4. Conclusions
The current data hosted by the SC2S provides a snapshot of the occurrence of SARS-CoV-2 in wastewater at participating WWTPs and demonstrates the ad-hoc cooperation of the scientific community on data collection. However, more importantly, the NORMAN/SCORE initiative:
-
•
demonstrates that the SC2S database is a workable multi-jurisdictional data-share platform with potential to facilitate development of an international dataset
-
•
provides a tool to engage and inform discussions with public health practitioners on the potential role of wastewater surveillance as an additional approach to integrate within community public health strategies
-
•
is open to all (contributors are warmly invited to submit data from any campaigns they are able to share, using the relevant sections on the DCT to document sample collection, storage and analytical details together with clinical case numbers)
-
•
with continued use, this collection of wastewater meta-data will support a retrospective analysis of the impact of differing sewer/catchment/population variables on the use of wastewater surveillance as a tool in public health practice
-
•
facilitated the collection of comparable data sets from an early phase of the pandemic; continued use will provides an opportunity to maximise operational insights gained during different phases of the pandemic and support development of robust best practice in wastewater surveillance.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
All authors wish to thank the WWTP operators for providing samples. LL, AH and MV would like to acknowledge the VINNOVA (Swedish Governmental Agency for Innovation Systems) DRIZZLE – Centre for Stormwater Management (Grant no. 2016-05176) and the technical expertise provided by the Stormwater&Sewers network, Nireas-International Water Research Center of the University of Cyprus would like to thank the Sewerage Board of Limassol-Amathus (SBLA), the Sewerage Board of Nicosia (SBN) and the Paralimni Sewerage Board (PSB) for the provision of influent samples, for the purpose of performing this work. MPD wishes to thank COVIDBENS Inv04020 financed by EDAR Bens S.A, A Coruña, FYL wishes to thank Z Cetecioglu Gurol (KTH) and P Haglund (Umeå University) and TM would like to acknowledge financial support from APVV-19-0250, PP-COVID-20-0019, ASS8 and VIR-SCAN. Authors from the Univ Jaume LB, FH, MB and RdL acknowledge the financial support from Dirección General del Agua, Generalitat Valenciana, to develop the project "Covid_Wastewater", as well as the help E. Santateresa and N. Zamorano from FACSA, for the invaluable support in performing this work. RdL. was funded through a Beatriz Galindo Fellowship of the Ministerio de Educación y Formación Profesional, Spanish Government (BEAGAL18/00042). TM wishes to thank the generous support of the Operational Program Integrated Infrastructure for the project "Strategic research in the field of SMART monitoring, treatment and preventive protection against coronavirus (SARS-CoV-2) ", Project no. 313011ASS8 (co-financed by the European Regional Development Fund) and the project VIR-SCAN - Wastewater monitoring data as an early warning tool to alert COVID-19 in the population (EOSCsecretariat.eu has received funding from the European Union's Horizon Program call H2020-INFRAEOSC- 05-2018-2019, grant Agreement number 831644). SK (IBISS) acknowledges the financial support from Ministry of Education, Science and Technological Development of Republic of Serbia grant No 451-03-9/2021-14/ 200007.
References
- Ahmed W, Bertsch PM, Angel N, Bibby K, Bivins A, Dierens L, Edson J, Ehret J, Gyawali P, Hamilton KA, Hosegood I, Hugenholtz P, Jiang G, Kitajima M, Sichani HT, Shi J, Shimko KM, Simpson SL, Smith WJM, Symonds EM, Thomas KV, Verhagen R, Zaug J, Mueller JF. Detection of SARS-CoV-2 RNA in commercial passenger aircraft and cruise ship wastewater: a surveillance tool for assessing the presence of COVID-19 infected travellers. Journal of Travel Medicine. 2020;27(5) doi: 10.1093/jtm/taaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar H, Diop OM, Weldegebriel G, Malik F, Shetty S, Bassioni. AO Akande LE, Lowther SA. Environmental surveillance for polioviruses in the global polio eradication initiative. Journal of Infectious Diseases. 2014;210:S294–S303. doi: 10.1093/infdis/jiu384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivins A, Greaves J, Fischer R, Yinda KC, Ahmed W, Kitajima M, Munster VJ, Bibby K. Persistence of SARS-CoV‑2 in Water and Wastewater. Environmental Science and Technology Letters. 2020 doi: 10.1021/acs.estlett.0c00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivins A, North D, Ahmad A, Ahmed W, Alm E, Been F, Bhattacharya P, Bijlsma L, Boehm AB, Brown J, Buttiglieri G, Calabro V, Carducci A, Castiglioni S, Cetecioglu Gurol Z, Chakraborty S, Costa F, Curcio S, de los Reyes FL, III, Vela JD, Farkas K, Fernandez-Casi X, Gerba G, Gerrity D, Girones R, Gonzalez R, Haramoto E, Harris A, Holden PA, Islam Md.Tahmidul, Jones DL, Kasprzyk-Hordern B, Kitajima Masaaki, Kotlarz Nadine, Kumar Manish, Kuroda Keisuke, Rosa Giuseppina La, Malpei F, Mautus M, McLellan SL, Medema G, Scott Meschke J, Mueller J, Newton RJ., Nilsson D, Noble RT, van Nuijs A, Peccia J, Perkins TA, Pickering AJ, Rose J, Sanchez G, Smith A, Stadler L, Stauber C, Thomas K, van der Voorn T, Wigginton K, Zhu K, Bibby K. Wastewater-Based Epidemiology: Global Collaborative to Maximize Contributions in the Fight Against COVID-19. Environmental Science and Technology. 2020;54:7754–7757. doi: 10.1021/acs.est.0c02388. [DOI] [PubMed] [Google Scholar]
- Castiglioni S, Senta I, Borsotti A, Davoli E, Zuccato E. A novel approach for monitoring tobacco use in local communities by wastewater analysis. Tob Control. 2015;24:38–42. doi: 10.1136/tobaccocontrol-2014-051553. [DOI] [PubMed] [Google Scholar]
- CDC (2020) National Wastewater Surveillance System (NWSS) A new public health tool to understand COVID-19 spread in a community https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/wastewater-surveillance.html
- Choi PM, Tscharke BJ, Donner E, O'Brien JW, Grant SC, Kaserzon SL, Mackie R, O'Malley E, Crosbie ND, Thomas KV, Mueller JF. Wastewater-based epidemiology biomarkers: Past, present and future. Trends in Analytical Chemistry. 2018;105:453e469. (2018) [Google Scholar]
- DEFRA . 2020. Sewage signals early warning of coronavirus outbreaks.https://www.gov.uk/government/news/sewage-signals-early-warning-of-coronavirus-outbreaks [Google Scholar]
- Dulio V, Koschorreck J, Slobodnik J. The NORMAN Association and the European Partnership for Chemicals Risk Assessment (PARC): let's cooperate! Environmental Sciences Europe. 2020;32(100) doi: 10.1186/s12302-020-00375-w. [DOI] [Google Scholar]
- EMCDDA . 2020. Wastewater-based epidemiology and drugs topic.https://www.emcdda.europa.eu/topics/wastewater_en [Google Scholar]
- Gonçalvesa J, Koritnika T, Mioča V, Trkova M, Bolješiča M, Berginca N, Prosenca K, Kotarb T, Paragi M. Detection of SARS-CoV-2 RNA in hospital wastewater from a low COVID-19 disease prevalence area. Science of the Total Environment. 2021;755 doi: 10.1016/j.scitotenv.2020.143226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Mariño I, Baz-Lomba JA, Alygizakis NA, Andrés-Costa MJ, Bade R, Barron LP, Been F, Berset JD, Bijlsma L, Bodík I, Brenner A, Brock AL, Burgard DA, Castrignanò E, Christophoridis C.E., Covaci A., de Voogt P., Devault D.A., Dias M.J., Emke E., Fatta-Kassinos D., Fedorova G., Fytianos K., Gerber C., Grabic R., Grüner S., Gunnar T., Hapeshi E., Heath E., Helm B., Hernández F., Kankaanpaa A., Karolak S., Kasprzyk-Hordern B., Krizman-Matasic I., Lai F.Y., Lechowicz W., Lopes A., López de Alda M., López-García E., Löve A.S.C., Mastroianni N., McEneff G.L., Montes R., Munro K., Nefau T., Oberacher H., O'Brien J.W., Olafsdottir K., Picó Y., Plósz B.G., Polesel F., Postigo C., Quintana J.B., Ramin P., Reid M.J., Rice J., Rodil R., Senta I., Simões S.M., Sremacki M.M., Styszko K., Terzic S., Thomaidis N.S., Thomas K.V., Tscharke B.J., van Nuijs A.L.N., Yargeau V., Zuccato E., Castiglioni S., Ort C. Spatio-temporal assessment of illicit drug use at large scale: evidence from 7 years of international wastewater monitoring. Addiction. 2020;115(1):109–120. doi: 10.1111/add.14767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Mariño I, Leticia A, Montes R, Rodil R, Cela R, López-García E, Postigo C, López de Alda M, Pocurull E, Marcé RM, Bijlsma L, Hernández F, Picó Y, Andreu V, Rico A, Valcárcel Y, Miró M, Etxebarria N, Benito Quintana J. Assessing population exposure to phthalate plasticizers in thirteen Spanish cities through the analysis of wastewater. Journal of Hazardous Materials. 2020;22(401) doi: 10.1016/j.jhazmat.2020.123272. [DOI] [PubMed] [Google Scholar]
- Gracia-Lor E, Rousis NI, Zuccato E, Bade R, Baz-Lomba JA, Castrignanò E, Causanilles A, Hernández F, Kasprzyk-Hordern B, Kinyua J, McCall AK, van Nuijs ALN, Plósz BG, Ramin P, Ryu Y, Santos MM, Thomas K, de Voogt P, Yang Z, Castiglioni S. Estimation of caffeine intake from analysis of caffeine metabolites in wastewater. Sci Total Environ. 2017;609:1582–1588. doi: 10.1016/j.scitotenv.2017.07.258. [DOI] [PubMed] [Google Scholar]
- Hassard F, Lundy L, Singer AC, Grimsley J, Di Cesare M. Innovation in wastewater near-source tracking for rapid identification of COVID-19 in schools. Lancet Microbe. 2020 doi: 10.1016/S2666-5247(20)30193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm-Hansen CC, Midgley SE, Schjørring S, Fischer TK. The importance of enterovirus surveillance in a Post-polio world. Clinical Microbiology and Infection. 2017;23:352e354. doi: 10.1016/j.cmi.2017.02.010. (2017) [DOI] [PubMed] [Google Scholar]
- Hong PY, Taruna Rachmadi A, Mantilla-Calderon D, Alkahtani M, YM.Bashawri H Al Qarni, O'Reilly KM, Zhou J. Estimating the minimum number of SARS-CoV-2 infected cases needed to detect viral RNA in wastewater: To what extent of the outbreak can surveillance of wastewater tell us? Environmental Research. 2021;195 doi: 10.1016/j.envres.2021.110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Quintela Baluja M, Graham DW, Corbishley A, McDonald JE, Malham SK, Hillary LS, Connor TR, Gaze WH, Moura IB, Wilcox Mark H., Farkas K. Shedding of SARS-CoV-2 in feces and urine and its potential role in person-to-person transmission and the environment-based spread of COVID-19. Science of the Total Environment. 2020;749 doi: 10.1016/j.scitotenv.2020.141364. (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- JRC (2020) CALL NOTICE Feasibility assessment for an EU-wide Wastewater Monitoring System for SARS-CoV-2 Surveillance. https://ec.europa.eu/jrc/en/science-update/call-notice-feasibility-assessment-eu-wide-wastewater-monitoring-system-sars-cov-2-surveillance
- Lai FY, Gartner C, Hall W, Carter S, O'Brien J, Tscharke BJ, Been F, Gerber C, White J, Thai P, Bruno R, Prichard J, Kirkbride KP, Mueller JF. Measuring spatial and temporal trends of nicotine and alcohol consumption in Australia using wastewater-based epidemiology. Addiction. 2018;113(6):1127–1136. doi: 10.1111/add.14157. 2018. [DOI] [PubMed] [Google Scholar]
- Lopardo L, Petrie B, Proctor K, Youdan J, Barden R, Kasprzyk-Hordern B. Estimation of community-wide exposure to bisphenol A via water fingerprinting. Environment International. 2019;125:1–8. doi: 10.1016/j.envint.2018.12.048. [DOI] [PubMed] [Google Scholar]
- Majumdar M, Klapsa D, Wilton T, Akello J, Anscombe C, Allen D, Mee ET, Minor PD, Martin J. Isolation of Vaccine-Like Poliovirus Strains in Sewage Samples from the United Kingdom Journal of Infectious DiseasesVolume. 2018;217(8) doi: 10.1093/infdis/jix667. 28 March 20181222-123. [DOI] [PubMed] [Google Scholar]
- Medema G, Heijnen L, Elsinga Goffe, Italiaander Ronald, Brouwer Anke. Presence of SARS-Coronavirus-2 in sewage and 3 correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environmental Science and Technology Letters. 2020 doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Medema G, Been F, Heijnen L, Petterson S. Implementation of environmental surveillance for SARS-CoV-2 virus to support public health decisions: Opportunities and challenges. Current Opinion in Environmental Science & Health. 2020;17:49–71. doi: 10.1016/j.coesh.2020.09.006. http://www.sciencedirect.com/science/article/pii/S2468584420300635 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael-Kordatou I, Karaolia P, Fatta-Kassinos D. Sewage analysis as a tool for the COVID-19 pandemic response and management: the urgent need for optimised protocols for SARS-CoV-2 detection and quantification. Journal of Chemical Engineering. 2020;8 doi: 10.1016/j.jece.2020.104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort C, van Nuijs AL, Berset JD, Bijlsma L, Castiglioni S, Covaci A, de Voogt P, Emke E, Fatta-Kassinos D, Griffiths P, Hernández F, González-Mariño I, Grabic R, Kasprzyk-Hordern B, Mastroianni N, Meierjohann A, Nefau T, Ostman M, Pico Y, Racamonde I, Reid M, Slobodnik J, Terzic S, Thomaidis N, Thomas KV. Spatial differences and temporal changes in illicit drug use in Europe quantified by wastewater analysis. Addiction. 2014;109(8):1338–1352. doi: 10.1111/add.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POST . Parliamentary Office for Science and Technology; London UK: 2020. Monitoring wastewater for COVID-19.https://post.parliament.uk/ [Google Scholar]
- Obépine Réseau. 2020. Observatoire Épidémiologique des Eaux Usées.https://www.reseau-obepine.fr/ [Google Scholar]
- Philo SE, Keim EK, Swanstrom R, Ong AQW, Burnor EA, Kossik AL, Harrison JC, Demeke BA., Zhou NA, Beck NK, Shirai JH, Meschke JS. A comparison of SARS-CoV-2 wastewater concentration methods for environmental surveillance. Science of the Total Environment. 2021;760 doi: 10.1016/j.scitotenv.2020.144215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W, Truchado P, Cuevas-Ferrando E, Simón P, Allende A, Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Research. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu Y., Barceló D., Barron LP., Bijlsma L., Castiglioni S., de Voogt P., Emke E., Hernández F., Lai FY., Lopes A., de Alda ML., Mastroianni N., Munro K., O'Brien J., Ort C., Plósz BG., Reid MJ., Yargeau V., Thomas KV. Comparative measurement and quantitative risk assessment of alcohol consumption through wastewater-based epidemiology: An international study in 20 cities. Sci Total Environ. 2016;565:977–983. doi: 10.1016/j.scitotenv.2016.04.138. [DOI] [PubMed] [Google Scholar]
- Riva F, Castiglioni S, Pacciani C, Zuccato E. Testing urban wastewater to assess compliance with prescription data through wastewater-based epidemiology: First case study in Italy. Science of the Total Environment. 2020;739 doi: 10.1016/j.scitotenv.2020.139741. 2020. [DOI] [PubMed] [Google Scholar]
- Rousis NI, Gracia-Lor E, Zuccato E, Bade R, Baz-Lomba JA, Castrignanò E, Causanilles A, Covaci A, de Voogt P, Hernàndez F, Kasprzyk-Hordern B, Kinyua J, McCall AK, Plósz BG, Ramin P, Ryu Y, Thomas KV, van Nuijs A, Yang Z, Castiglioni S. Wastewater-based epidemiology to assess pan-European pesticide exposure. Water Res. 2017;121:270–279. doi: 10.1016/j.watres.2017.05.044. [DOI] [PubMed] [Google Scholar]
- Sedmak G, Bina D, MacDonald J. Assessment of an Enterovirus Sewage Surveillance System by Comparison of Clinical Isolates with Sewage Isolates from Milwaukee, Wisconsin, Collected August 1994 to December 2002. Applied and Environmental Microbiology. 2003;69(12):7181–7187. doi: 10.1128/AEM.69.12.7181-7187.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaidis NS, Gago-Ferrero P, Ort C, Maragou NC, Alygizakis NA, Borova VL, Dasenaki ME. Reflection of Socioeconomic Changes in Wastewater: Licit and Illicit Drug Use Patterns. Environmental Science and Technology. 2016;50(18):10065–10072. doi: 10.1021/acs.est.6b02417. 2016. [DOI] [PubMed] [Google Scholar]
- Thompson JR, Nancharaiah YV, Gu X, Lee W L, Rajal VB, Haines MB, Girones R, Ching Ng L, Alm EJ, Wuertz S. Making waves: Wastewater surveillance of SARS-CoV-2 for population-based health management. Water Research. 2020;184 doi: 10.1016/j.watres.2020.116181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UCMERCED (2020) COVIDPoops19: Summary of Global SARS-CoV-2 Wastewater Monitoring Efforts. https://ucmerced.maps.arcgis.com/apps/opsdashboard/index.html#/c778145ea5bb4daeb58d31afee389082
- Vallejo JA, Rumbo-Feal S, Conde-Pérez K, López-Oriona A, Tarrío-Saavedra J, Reif R, Ladra S, Rodiño-Janeiro BK, Nasser M, Cid A, Veiga MC, Acevedo A, Lamora C, Bou G, Cao R, Poza M. 2020. Predicting the number of people infected with SARS-COV-2 in a population using statistical models based on wastewater viral load.https://www.medrxiv.org/content/10.1101/2020.07.02.20144865v3 Medrxiv available at: [Google Scholar]
- Victoria State Government . 2020. wastewater monitoring – corona virus (Covid-19)https://www.dhhs.vic.gov.au/wastewater-monitoring-covid-19 Available at. [Google Scholar]
- Westhaus S, Weber F-A, Schiwy S, Linnemann V, Brinkmann M, Widera M, Greve C, Janke A, Hollert H, Wintgens T, Ciesek S. Detection of SARS-CoV-2 in raw and treated wastewater in Germany – Suitability for COVID-19 surveillance and potential transmission risks. Science of the Total Environment. 2021;751 doi: 10.1016/j.scitotenv.2020.141750. (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . WHO Regional Office for Europe; Copenhagen, Denmark: 2020. Rapid expert consultation on environmental surveillance of Sars-Cov-2 in wastewater. [Google Scholar]
- WRA . 2020. ColoSSoS Project – Collaboration on Sewage Surveillance of SARS-CoV-2.https://www.waterra.com.au/research/communities-of-interest/covid-19/ [Google Scholar]
- WRF Wastewater Surveillance of the COVID-19 Genetic Signal in Sewersheds Recommendations from Global Experts. Water Research Foundation. 2020 https://www.waterrf.org/sites/default/files/file/2020-06/COVID-19_SummitHandout-v3b.pdf [Google Scholar]