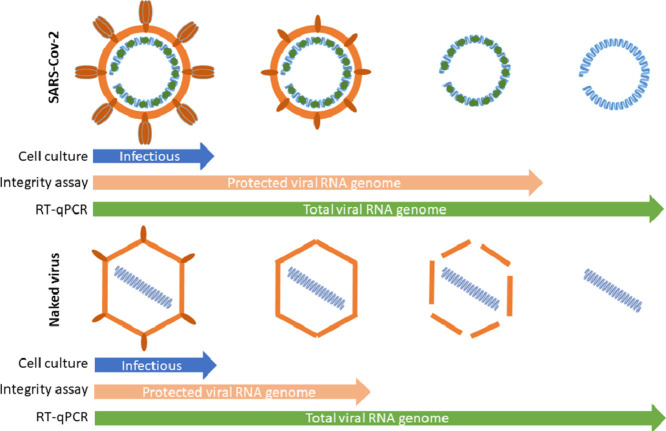
Abstract
The ongoing global pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been a public health emergency of international concern. Although SARS-CoV-2 is considered to be mainly transmitted by inhalation of contaminated droplets and aerosols, SARS-CoV-2 is also detected in human feces and to a less extent in urine, and in raw wastewaters (to date viral RNA only) suggesting that other routes of infection may exist. Monitoring SARS-CoV-2 genomes in wastewaters has been proposed as a complementary approach for tracing the dynamics of virus transmission within human population connected to wastewater network. The understanding on SARS-CoV-2 transmission through wastewater surveillance, the development of epidemic modeling and the evaluation of SARS-CoV-2 transmission from contaminated wastewater are largely limited by our knowledge on viral RNA genome persistence and virus infectivity preservation in such an environment. Using an integrity based RT-qPCR assay this study led to the discovery that SARS-CoV-2 RNA can persist under several forms in wastewaters, which provides important information on the presence of SARS-CoV-2 in raw wastewaters and associated risk assessment.
Keywords: SARS-CoV-2, Particle integrity, Quantification, Wastewater, Infectious risk
Graphical abstract
1. Introduction
Coronaviruses (CoVs) belong to Coronaviridae, a large family of enveloped single-stranded positive RNA viruses. CoVs are usually considered as moderate pathogens for humans. Four of them (229E, NL63, OC43, HKU1) are responsible for seasonal common cold or mild respiratory infections. However, three novel and highly pathogenic CoVs recently emerged in human population causing severe zoonotic diseases i.e. Severe Acute Respiratory Syndrome (SARS) (Peiris et al., 2003), Middle East Respiratory Syndrome (MERS) (Zaki et al., 2012) and more recently COronaVIrus Disease-19 (COVID-19). SARS-CoV-2, the etiological agent of COVID-19 (Huang et al., 2020; Zhou et al., 2020; Zhu et al., 2020), is responsible for a pandemic that caused more than 400 million cases and nearly 3 million deaths so far (John Hopkins university data by April 13, 2021). Although SARS-CoV-2 transmission mainly occurs by direct transmission through inhalation of contaminated respiratory droplets, aerosols or surfaces (World Health Organization, 2020a), the potential for alternative transmission pathway should be investigated. Indeed, large amounts of viral RNA have been identified in human stools from infected patients presenting with severe COVID-19 symptoms which occasionally led to the isolation of infectious virus from feces (Chen et al., 2020; Holshue et al., 2020; Huang et al., 2020; Lescure et al., 2020; Pan et al., 2020; Tang et al., 2020; Wang et al., 2020; Wölfel et al., 2020; Wu et al., 2020; Xiao et al., 2020; Zhang et al., 2020). SARS-CoV-2 can also be detected in stools from asymptomatic, pre-and mild symptomatic carriers with a largely unknown prevalence (Du et al., 2020; Park et al., 2020). This may reflect SARS-CoV-2 replication in the gut (Luz et al., 2020). Accordingly high levels of viral RNA have been detected in wastewaters in different countries and potential cases of transmission via wastewater have been reported (Yeo et al., 2020a; Yuan et al., 2020). In addition to the risk of exposure for sewage workers, wastewaters containing potentially infectious SARS-CoV-2 may enter the aquatic environment via wastewater discharge thus potentially resulting in pollution of surface waters (Kumar et al., 2021, 2020; Naddeo and Liu, 2020; Rimoldi et al., 2020; Wurtzer et al., 2020a) and to a lesser extent groundwaters, even if infectious SARS-CoV-2 were not able to be isolated from wastewater on cell culture (Rimoldi et al., 2020). Such a pollution by infectious viruses could locally affect the quality of water resources used for the production of water intended to human consumption. Moreover, the detection of SARS-CoV-2 genome in treated effluents of wastewater treatment plant could cause problems for agricultural activities through the reuse of treated wastewater or the spreading of sludge if the virus could remained partially infectious or if its infectivity could not be evaluated (Balboa et al., 2021). Consequently, the contamination of wastewater by SARS-CoV-2 could raise the same concerns as human seasonal enteric viruses (Okoh et al., 2010).
The monitoring of SARS-CoV-2 genomes in raw wastewater was successfully used for estimating the dynamics of viral pandemic in population linked to a wastewater network (Medema et al., 2020; Nemudryi et al., 2020; Randazzo et al., 2020; Sebastien Wurtzer et al., 2020). However many questions remain to be answered to better assess the risk of transmission of SARS-CoV-2 through wastewaters (Elsamadony et al., 2021; Lodder and de Roda Husman, 2020). Indeed RT-qPCR protocoles that are currently used can not distinguish between partial or full-length, virion associated or free viral genomes (Prevost et al., 2016). It is commonly admitted that enveloped viruses are less persistent in hydric matrices and less resistant to inactivation treatments than naked viruses (World Health Organization, 2020b). Gundy and collaborators showed that human seasonal coronavirus survival in tap water and wastewater was strongly reduced compared to poliovirus. The survival ranged from days to weeks depending on the virus, type of water and temperature (Casanova et al., 2009; Casanova and Weaver, 2015; Gundy et al., 2009). An experimental study showed that SARS-CoV-1 stability under an infectious form was only 2 days at 20°C, but 14 days at 4°C (Wang et al., 2005). So far only a few studies investigated SARS-CoV-2 stability on solid surfaces (Chin et al., 2020; van Doremalen et al., 2020) or in water matrix (Bivins et al., 2020). While the decay of SARS-CoV-2 infectivity appears to be different according to the nature of matrix, these studies reported an important impact of the heat on the virus infectivity. Moreover they suggested that SARS-CoV-2 could be more persistent than other coronaviruses (seasonal and epidemic CoV) and more resistant in environmental conditions, such as temperature, humidity, sunlight exposure or on various surfaces (Aboubakr et al., 2020; van Doremalen et al., 2020). Conversely risk assessment for SARS-CoV-2 was mainly based on results obtained for other coronaviruses or for SARS-CoV-2 surrogates (enteric viruses or bacteriophage indicators) (Rosa et al., 2020; Silverman and Boehm, 2020; Ye et al., 2016). So far, despite the presence of SARS-CoV-2 RNA in raw wastewaters, no infectious virus was isolated from the same samples, suggesting that the detection of viral RNA overestimated the risk of infection or resulting from methodological limitations for cultivating SARS-CoV-2 on cell culture from such samples (Rimoldi et al., 2020).
The present work intended to evaluate SARS-CoV-2 stability both under an infectious form or by quantifying viral RNA in wastewaters. We first demonstrated that SARS-CoV-2 RNA can be quantified without significant loss in wastewaters samples for up to 7 days at 4°C or 20°C, suggesting that viral RNA is largely protected from environmental degradation. This led us to combine cell culture isolation and integrity based RT-qPCR assay to investigate the status of viral RNA in wastewater samples (Prevost et al., 2016). We propose that SARS-CoV-2 genomes can exist under three different states at least: genomic RNA protected within an infectious particle, genomic RNA protected in a non-infectious structure, free total or partial genomic RNA. SARS-CoV-2 persistence and integrity were compared to an enteric virus – Coxsackievirus B5 – that is commonly found in feces and wastewater. The analysis of 87 raw wastewater samples collected from April to July 2020 in Paris area confirmed that total viral RNA can be detected under both a protected and an unprotected form.
2. Material and methods
2.1. Virus stock preparation
Coxsackievirus B5 (CV-B5) (ATCC VR-185) was cultivated on confluent monolayer cultures of Buffalo Green Monkey kidney (BGMK) cells (#90092601, ECACC) at 37°C with 5% CO2. Cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) high glucose (Dutscher) supplemented with 2% fetal bovine serum (PanBiotech), non-essential amino acids (Dutscher), penicillin (100 U/ml) and streptomycin (100µg/ml) (PanBiotech). The supernatant was clarified by centrifugation at 2,000 x g for 15min, then ultracentrifuged at 150,000 x g at 4°C for 2 hours through a 40 % sucrose cushion. The pellet was resuspended in 1x phosphate-buffered saline (PBS) pH 7.4. Further purification was performed by ultracentrifugation on cesium chloride gradient at 100,000 x g for 18 hours. The fraction containing the viruses was desalted with Vivaspin 20 (Sartorius) concentrators according the manufacturer’s recommandations. Viruses were stored at - 80°C before using.
SARS-CoV-2, strain SARS Cov-2 20/0001 (BetaCoV/France/IDF0372/2020/SARS-CoV-2 isolated by Pasteur Institute, France), was cultivated on confluent monolayer cultures of VERO E6 cells (ATCC® CRL-1586), kindly provided by Dr. Le Gouil and Pr. Vabret (Virology laboratory of universitary hospital of Caen, France) at 37°C with 5% CO2. Cells were grown in Dulbecco’s Modified Eagle’s Medium GlutaMAX (Gibco) supplemented with penicillin (50U/mL) and streptomycin (50µg/mL), TPCK trypsin (1µg/mL) without fetal bovin serum. The supernatant, collected after cytopathic effect observation, was clarified by centrifugation at 2,000 x g for 15min and stored at - 80°C before using.
2.2. Detection of SARS-CoV-2 in raw wastewater
Raw wastewater samples were homogenized by agitation, then 11ml were centrifugated at 200 000 x g for 1 hour at 4°C using a XPN80 Coulter Beckman ultracentrifuge equipped with a swing rotor (SW41Ti). Viral pellets were resuspended in 200μL of PBS 1X buffer as previsouly described (Wurtzer et al., 2020).
2.3. Spiking assays
Five raw wastewater samples were collected in July 2020 in different WWTP and scored negative for SARS-CoV-2 and enterovirus detection by RT-qPCR. These <24h old samples were centrifugated at 4,000 xg for 15min for removing the largest particles and supernatants were filtered through membrane with 0,45µm porosity (Low protein binding Durapore membrane, #SLHVM25NS, Millex, Germany). The filtrates were stored at 4°C and used within the following 24h.
CV-B5 or SARS-CoV-2 were spiked in the filtrated samples. Virus titration was immediately done after spiking or after incubation at 4°C or 20°C for the indicated period of time. As a control, spiking experiments were done in DMEM. Virus infectivity, virus integrity and viral RNA detection were assessed after incubation by endpoint dilution assay, PMAxx treatment coupled to RT-qPCR and RT-qPCR respectively.
2.4. Virus quantification by endpoint dilution assay
Infectious viruses (CV-B5 and SARS-CoV-2) were titrated by standard 10-fold dilutions in 96-well plates on VERO E6 cells (ATCC® CRL-1586™) (105 cells per well), with twelve replicates per dilution. After a 6-day incubation, cytopathic effects were observed and positive wells were counted. Viral titer was estimated using the Spearman-Kärber method. The results are expressed as 50% tissue culture infective dose (TCID50) per ml.
2.5. Virus integrity assay
Each sample was mixed with propidium monoazide (PMAxx, Biotium), an intercalating dyes that binds only to free accessible sites within nucleic acids and after photoactivation, making them unable to be amplified by RT-qPCR. Briefly PMAxx was added at 100µM final concentration. The samples were incubated on ice in the dark for 30min and then photoactivated at using PhastBlue system (GenIUL, Spain) for 15min. Samples were extracted as follow.
2.6. Viral RNA detection
2.6.1. Spiking assays
The spiked samples were lysed by adding two volumes of TRIZOL (Lifetechnologies) and extracted using QIAsymphony DSP/ Pathogen kit on a QIAsymphony automated extractor (QIAGEN) according to a modified manufacturer’s protocol for handling larger volumes.
2.6.2. Environmental samples
The whole viral concentrate (~200µL) was lysed and extracted using Qiasymphony PowerFecal Pro kit on a QIAsymphony automated extractor (QIAGEN) according to a modified manufacturer’s protocol. Extracted nucleic acids were filtered through OneStep PCR inhibitor removal kit (Zymoresearch) according the manufacturer’s instructions for handling larger volumes.
2.6.3. Viral RNA titration
The oligonucleotides and amplification conditions used herein have been previously described by Corman & al. for the detection of SARS-CoV-2 genome (Corman et al., 2020), and by Wurtzer & al. for the detection of enterovirus genome (Wurtzer et al., 2014). The amplifications were done using Fast virus 1-step Master mix 4x (Lifetechnologies). Detection and quantification were carried on the gene E for SARS-CoV-2 and 5’-UTR for enterovirus. Positive results for SARS-CoV-2 were confirmed by amplification of viral RNA-dependent RNA polymerase (RdRp, IP4 set) (World Health Organization, 2020c) and nucleoprotein (N1 set) genes (Center for Disease Control and Prevention, 2020). An internal positive control (IPC) was added to evaluate the presence of residual inhibitors. The IPC consists in a plasmid containing beta-acting gene flanked by enterovirus-specific primers (Wurtzer et al., 2014). Briefly, a sample was considered as inhibited when the Cq of IPC was increased by at least 2 cycles. This value corresponded to 2xSD of the Cq-value in control condition. The detection limit was estimated to be around 10 genome units per amplification reaction.
The quantification was performed using a standard curve based on full-length amplicon cloned into pCR2.1 plasmid (Invitrogen, #452640). Amplification reaction and fluorescence detection were performed on Viia7 Real Time PCR system (Lifetechnologies).
2.7. Statistical analysis and plots
All statistical analysis and plots were done using GraphPad Prism 9.0 software. For comparison based on spiked samples (Fig. 2), the quantifications were compared between the different conditions using one-way ANOVA and Tukey's multiple comparisons test. Comparisons between total viral RNA (vRNA, measured by RT-qPCR) and protected RNA (pRNA, measured by PMAxx-RT-qPCR) (Fig. 4A) were performed using Wilcoxson matched-pair test and comparisons of ratio pRNA/vRNA (Fig. 4B) were tested using Kruskal-Wallis test and Dunn's multiple comparisons test.
Fig. 2.
Stability of total viral RNA, protected viral RNA and infectious SARS-CoV-2 and Coxsackievirus B5 in spiked wastewater samples. Five wastewater samples were spiked with infectious virus and incubated for 24h at 4°C or 20°C. DMEM was used as a control of matrix. Total viral RNA (vRNA) of CV-B5 (panel A) or SARS-CoV-2 (panel D) were quantified by RT-qPCR. Protected RNA (pRNA) of CV-B5 (panel B) or SARS-CoV-2 (panel E) were quantified using an integrity-based RT-PCR, as described. Infectious particles (TCID50) of CV-B5 (panel C) or SARS-CoV-2 (panel F) were titrated by cell culture.
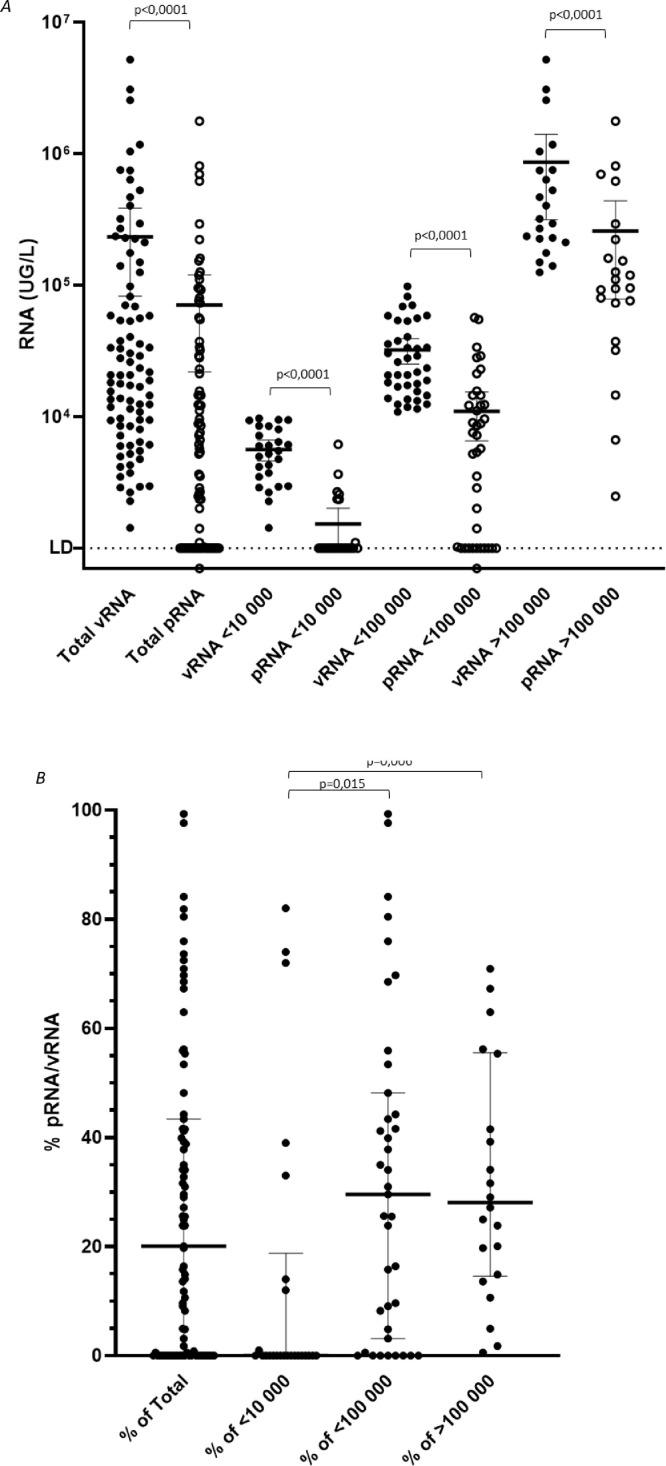
Fig. 4.
Relative proportion of protected vs unprotected SARS-CoV-2 genomes in raw wastewaters collected in Greater Paris area. Raw wastewater samples (n=87) from four WWTP were analyzed for SARS-CoV-2 genome by RT-qPCR (vRNA, filled circle) and using integrity assay (pRNA, open circle). The concentration (UG/L) was plotted on the panel A, the median values and interquartiles (25-75%) are indicated. The pRNA/vRNA ratio indicating the percentage of protected RNA over total viral RNA, is plotted for each sample on panel B. The median values and interquartiles (25-75%) are indicated.
3. Results
3.1. Stability of total viral RNA (vRNA) in wastewater samples
The quantification of SARS-CoV-2 genome highlights the importance to provide convenient tools to distinguish free viral RNA and virion-associated RNA as a first approach to evaluate the concentration of infectious virus particle in matrix from which SARS-CoV-2 is technically difficult to isolate, such as stools or wastewaters. Since viral genomes are protected by viral proteins and surrounded by a cell-derived enveloped in infectious particles, we assumed that we could distinguish between free and protected viral genomes using an integrity RT-qPCR based assay.
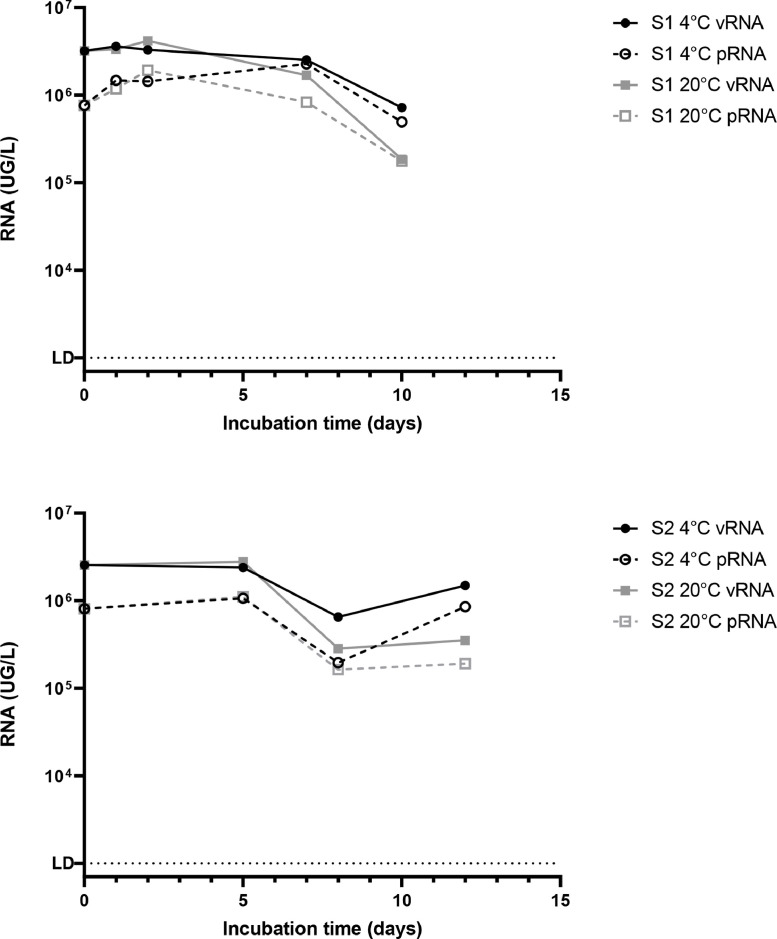
Briefly, two 100mL-raw wastewater samples were collected by the 3rd (sample S1) and the 7th (sample S2) of april 2020 in Greater Paris area, a period when SARS-CoV-2 genomes were easily detected (Wurtzer et al., 2020b). Samples were analyzed less than 24h after the time of the sampling (day 0). The rest of each sample was split into 2 parts and stored at 4°C or 20°C for 10 days and 12 days respectively. Total SARS-Cov-2 viral RNA (vRNA) and protected viral RNA (pRNA) were quantified by RT-qPCR and PMAxx-RT-qPCR, respectively. As shown on Fig. 1, less than 10% of the total viral RNA was under a protected form. SARS-CoV-2 vRNA and pRNA concentrations were relatively stable for 7 (S1) and 12 (S2) days respectively at 4°C while they were slighly less stable when stored at 20°C. As control of PMAxx pretreatment efficacy, 6 raw wastewater samples negative for SARS-CoV-2 genome by RT-qPCR were collected. Half was autoclaved at 121°C for 20 minutes. Eleven milliliters were concentrated by ultracentrifugation as previously described (Wurtzer et al., 2020) and all concentrates (200µL) were immediately seeded using the same quantity of SARS-CoV-2 RNA purified from cell culture surpernatant and stored on ice. Concentrates were incubated with PMAxx and SARS-CoV-2 RNA genomes were detected according to the described protocol. Purified RNA was strongly and quickly degraded in raw wastewater samples, whereas it was detected in autoclaved samples as expected. The PMAxx pretreatment induced a strong reduction of RNA detection (>3-log) in autoclaved samples (p<0.01 for detection of both E and RdRp genes) (supplementary data). RNA reduction was also significantly observed in raw samples when RNA input remained sufficient for being assessed.
Fig. 1.
Persistence of total or protected SARS-CoV-2 RNA of in two raw wastewater samples. Two naturally SARS-CoV-2 contaminated wastewater samples (S1 and S2) were independently incubated at 4°C or 20°C for several days. Total viral RNA (vRNA, filled forms) and protected viral RNA (pRNA, open forms) were quantified by RT-qPCR and by an integrity-based RT-PCR respectively.
3.2. Comparing Coxsackievirus B5 and SARS-CoV-2 persistence in raw wastewater
Infectious enteric viruses are commonly found in wastewaters, but the ability of enveloped virus, like SARS-CoV-2, to persist under an infectious form is still debated. To address this question, the persistence of SARS-CoV-2 in raw wastewater was compared to that of Coxsackievirus B5 (CV-B5) using three different indicators namely the quantification of vRNA, pRNA and infectious particles (TCID50). Five raw wastewater samples, that were negative for SARS-CoV-2 and enterovirus genome by RT-qPCR (data not shown) were used. The detection of infectious virus, vRNA and pRNA was performed after spiking each sample with infectious SARS-CoV-2 or CV-B5.
CV-B5 vRNA and pRNA were quantified at similar concentrations in raw wastewaters (WW) or in cell culture medium (DMEM) when analysis was done immediately after spiking (control) or after 24h-incubation at 4°C or 20°C ( Fig. 2A, B). This result was expected since CV-B5 particles were purified to homogeneity on sucrose gradient, which efficiently separates encapsidated RNA from free RNA. Infectivity of CV-B5 was not significantly altered after 24h-incubation at 4°C, while it only sightly decreased (<1-log) after a 24h-incubation at 20°C (Fig. 2C). One WW sample dramatically affected the virus infectivity (>2-log), the significance could not be statistically assessed. Strikingly, pRNA was only 10% of total vRNA for SARS-CoV-2 suggesting that unpurified SARS-CoV-2 preparation contains only a minor part of intact particles. This result was further confirmed by the relatively low level of the infectivity of the viral stock (Fig. 2F). As before pRNA was highly stable whereas SARS-CoV-2 total vRNA partly decreased over time in all conditions (Fig. 2D and E). As importantly SARS-CoV-2 infectivity was strongly (>3-log) or moderately (<1-log) reduced in 3 out of 5 WW samples and 2 over 5 samples respectively. The decrease in TCID50 was about 1-log in all samples after a 24h-incubation at 4°C. Since no similar observation was made on samples containing DMEM, this suggested that SARS-CoV-2 infectivity is strongly reduced in wastewaters likely depending of their chemical and/or microbial composition (Fig. 2F).
3.3. Temperature-based inactivation revealed different status for viral RNA
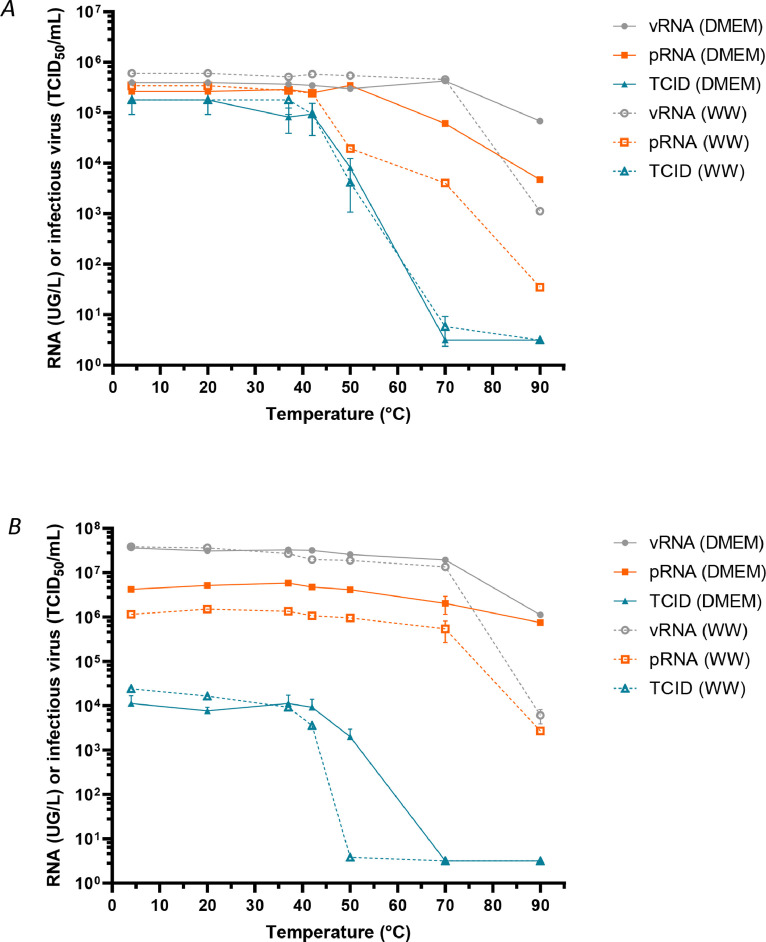
Temperature is known to affect viruses in the environment albeit to very different extent (Bertrand et al., 2012). Heat inactivation is commonly used for studying virus survival in water. In low temperature range (<50°C), the inactivation of naked viruses mainly comes from the denaturation of capsids (Waldman et al., 2020, 2017). However little is known concerning enveloped viruses. Therefore, we intended to evaluate more precisely the effect of temperature on SARS-CoV-2 using CV-B5 as a control. For this purpose, we first exposed samples spiked with infectious SARS-CoV-2 and CV-B5 to increasing temperature for 10 minutes. Then we evaluated the effect of the treatment on infectious particles or total RNA stability (vRNA). Viral genome protection was evaluated as before by an integrity RT-qPCR based assay (pRNA).
CV-B5 infectivity was preserved up to 42°C and then dramatically decreased up to 70°C, as previously described by Waldman and co-authors (Waldman et al., 2017). pRNA and vRNA were stable up to 50 and 70°C respectively in culture medium, although pRNA decreased at a lower temperature in wastewater ( Fig. 3A).
Fig. 3.
Stability to heat of total viral RNA, protected viral RNA and infectious SARS-CoV-2 and Coxsackievirus B5 in spiked wastewater. Wastewater samples were spiked with infectious CV-B5 particles (panel A) or infectious SARS-CoV-2 particles (panel B) and incubated for 10min at various temperatures. Total viral RNA (vRNA) was quantified by RT-qPCR, protected RNA (pRNA) was quantified by an integrity-based RT-PCR, as described, and infectious virus (TCID50) was titrated by cell culture.
In the same conditions, SARS-CoV-2 viability was not significantly affected until 42°C. A marked reduction in infectivity was observed both in wastewaters and culture medium that was not related with a decrease in vRNA nor pRNA (Fig. 3B). In both culture medium and wastewater samples, reduction of vRNA paralleled pRNA reduction although reduction in vRNA and pRNA was stronger in wastewater sample.
Altogether, all these experiments indicated that SARS-CoV-2 viral genomes could exist under three different forms at least: protected within infectious particles, protected within non-infectious particles or in a ribonucleoprotein complex and as free/unprotected viral RNA.
3.4. Estimating the relative proportion of protected vs unprotected SARS-CoV-2 genomes in raw wastewater
Total and protected viral RNA were quantified in 87 raw wastewater samples that were collected from April to July 2020 in Greater Paris area. vRNA and pRNA concentrations ranged from 1.4 × 103 to 5.2 × 106 GU/L and from 0.7 × 103 to 1.8 × 106 GU/L respectively ( Fig. 4A). Total viral RNA were significantly higher than protected RNA in each sample (p<0.0001). The pRNA/vRNA ratio was comprised between 0 and 100%, with a median value of 20.1%. In wastewater samples with vRNA concentrations <100,000 (n=39) and >100,000 GU/L (n=22), the median ratio was 29.6% (max=99.3%) and 28.1% (max=100%) respectively. This ratio was significantly lower in samples with low genome concentration (n=26; <10,000 GU/L; median ratio = 0%; max = 18.8%) compared to <100,000 GU/L and >100,000 GU/L samples (p=0.015 and p=0.006 respectively) (Fig. 4B).
4. Discussion
Wastewater-based epidemiology has been widely used over the world for monitoring the spreading of SARS-CoV-2 (Ahmed et al., 2020a; Medema et al., 2020; Randazzo et al., 2020; Wurtzer et al., 2020) in human populations as well as other waterborne viruses such as poliovirus (World Health Organization, 2003) and other enteric viruses (Prevost et al., 2015). A large panel of methods has been developed with various performances. SARS-CoV-2 is detected in the feces of about 50% of infected people, mainly with no or moderate symptoms (Lescure et al., 2020; Pan et al., 2020; Tang et al., 2020). It has been proposed that the presence of SARS-CoV-2 genomes in raw wastewater could reflect the virus excreted by infected people, whether they are symptomatic or not. It is of upmost importance to confirm this assumption in order to propose mathematical models that could correlate viral load in wastewaters with other individual epidemiological parameters. Modeling viral dynamics greatly depends on the quality of the analysis, but also on the half-life of total viral RNA in raw wastewater. In this study, we showed that total viral RNA (vRNA) concentration in raw wastewater was stable for at least 7 days provided that the samples were stored at 4°C until analysis, which is in agreement with previous work (Bivins et al., 2020). Ahmed and coworkers reported a comparable decrease of vRNA over 7 days (about 0.6-log and 1.3-log at 4°C and 25°C respectively) in seeded raw wastewaters (Ahmed et al., 2020b). Importantly, freezing water samples had a negative impact on the relevance of the measurement (data not shown), at least in our protocol. Such a delay is important to be taken into consideration to organize campaigns from the sampling to the analysis, including transportation to specialized laboratories. Although our study was performed on a limited number of samples, the results suggested that vRNA concentration was not dramatically affected by the composition of wastewater samples over 24h-incubation time, a period of time that is compatible with the travel of the viral genomes from emission of human feces to raw wastewater sampling at the inlet of WWTP. As importantly SARS-CoV-2 vRNA detection was unaffected in a range of temperature comprised between 4°C and at least 40°C. Altogether these results suggested that the measurement of SARS-CoV-2 vRNA concentration in wastewater is a relevant indicator of the effective level of the viral genomes excreted by infected people that is only moderately affected by temperature and travel time.
The detection of SARS-CoV-2 genomes in stools and subsequently in wastewaters raises several other important concerns concerning the risk of transmission. RT-qPCR assays have been designed to detect specific regions of the viral genomes whatever the quantified RNA is extracted from infectious particle or not. Therefore, these approaches provide an obvious overestimate of the effective concentration of infectious viral particles within stools and wastewaters. Even though sewage is an unsanitary environment for many reasons, sewers and operators of wastewater treatment plant worried about the occupational risk of infection by SARS-CoV-2. As recently underlined by WHO (World Health Organization, 2020a), SARS-CoV-2 is a respiratory virus whose main routes of transmission are respiratory (inhalation of contaminated droplets and aerosols) and contact (with contaminated surfaces). However, if SARS-CoV-2 infection via contaminated wastewater was not unambiguously demonstrated, this possibility cannot be ruled out (Yeo et al., 2020b). To that respect, let us note that genetic evidences and case clustering led Yuan and coworkers to suggest that sewage may be a possible transmission vehicle for SARS-CoV-2 (Gormley, 2020; Kang et al., 2020; Yuan et al., 2020). Enveloped viruses were commonly thought to be less resistant than naked viruses. Due to the possible presence of detergent and other chemical agents that may degrade viral envelop, raw wastewater might be highly detrimental to the persistence of infectious SARS-CoV-2 particles. Trials have been done unsuccessfully in order to isolate and cultivate SARS-CoV-2 from fresh wastewater samples (Rimoldi et al., 2020), meaning that SARS-CoV-2 might be simply non-infectious, or that cell culture system was not adapted for such highly chemically or microbiologically contaminated samples (Cashdollar and Wymer, 2013). Efforts for concentrating and isolating infectious viruses from hydric environment are usually successful for naked virus that are less sensitive to chemicals. In this study, infectious SARS-CoV-2 was spiked in negative wastewater samples and viable viruses were quantified up to 24 hours, without pretreatment of sample before cultivation. Whereas such an exposure only mildly affected Coxsackievirus B5 viability, SARS-CoV-2 infectivity was clearly affected at 20°C depending on the nature of the sample. These results are in agreement with previous work (Bivins et al., 2020). We brought here additional evidences that sample temperature had a strong impact on virus viability since the SARS-CoV-2 infectivity was not significantly modified at 4°C for 24h whereas it is slightly affected at 20°C. Both viruses infectivity was fully preserved up to 42°C for shorter incubation times (10min). In all conditions, infectious virus persisted up to 24 hours at least in wastewater samples. Previous studies reported that infectious SARS-CoV-2 could persist for up to 28 days on various supports (glass, plastic or stainless steel for example) (Riddell et al., 2020; van Doremalen et al., 2020). An effect of temperature on viral infectivity was already reported when virus was adsorbed on solid surfaces (Riddell et al., 2020) or in transportation medium (Chin et al., 2020). More recently Bivins and collaborators brought first elements to evaluate SARS-CoV-2 viability in wastewaters and provided evidences that SARS-CoV-2 viral RNA persisted for longer period of time than infectious particles (Bivins et al., 2020).
The present study confirmed that evaluating total vRNA widely overestimated the number of infectious particles within wastewaters. Nevertheless, the relatively long persistence of SARS-CoV-2 genomes was surprising with regards to its supposed fragility compared to surrogates. Whether the regions that are amplified by RT-qPCR came from total or partial genomes cannot be assessed by such assays. A tool for assessing the integrity of naked virus particles already showed that genome of naked RNA viruses can be protected from degradation by the capsid, a structure that remains non-permeable to intercalating dye. Our comparative study on SARS-CoV-2 and CV-B5 demonstrated that viral genomes can be found in multiple states i.e. infectious protected, non-infectious protected and unprotected forms. Unpublished data and a recent study showed that such dyes (ethidium monoazide or propidium monoazide) targeted secondary structures within single-stranded RNA (hairpins or IRES for picornaviruses)(Puente et al., 2020; Wurtzer et al., 2018). In addition previous study showed that capsid integrity is lost at 42°C for CV-B5, with a maximum access of SyBR green II to viral RNA at 50°C (Waldman et al., 2017). In the case of coronaviruses, a lipid layer protects the RNA genome that is closely associated to nucleoproteins. The lipid layer is probably very labile in wastewater, which may contain detergent residues for example, and unstable at high temperature. Nonetheless integrity measurements showed that vRNA remained protected from intercalating dye up to 70°C-incubation in wastewater and up to 90°C in control condition. These results suggested other structures such as viral nucleoriboproteic complex, resulting from strong interactions between nucleoproteins and viral RNA, may limit access of the dye to SARS-CoV-2 RNA, in addition to the viral envelop. Purified RNA (without nucleoproteins) was shortly degraded in raw wastewater, confirming the role of ribonucleoproteic complex in the persistence of SARS-CoV-2 RNA. It is to note that SARS-CoV-2 and CV-B5 shared a similar profil of sensitivity to temperature, although SARS-CoV-2 genome appeared to be better protected than CV-B5 genomes. This assay was used on a large panel of samples, confirming that less than 30% of the viral genomes on the average were under a protected form in wastewater samples. Randazzo and colleagues have proposed to add a surfactant for improving the efficiency of PMAxx pretreatment in wastewater samples (Puente et al., 2020; Randazzo et al., 2018). Our estimation of viral genome protected in wastewater could be further refined. Considering that infectious particles correspond only to a subfraction of protected genomes, as illustrated by spiking experiments, it can be considered that risk assement for viral infection through wastewaters should be better evaluated using an integrity based assay if systematic cell culture isolation cannot be done. The results suggested also that the infectious risk could be mitigated by heat exposure encountered during the sludge hygienization for example. Such a technique could also be used to evaluate the relative fraction of protected genomes in other matrix such as in sputums or stools of infected patients or for other enveloped viruses, such as influenza virus (Chan et al., 2009; Hirose, 2016).
5. Conclusion
SARS-CoV-2 RNA genome quantification in raw wastewaters has been proposed as an efficient way to monitor the dynamics of COVID-19 within populations. However, the detection of SARS-CoV-2 RNA genomes by RT-qPCR in raw wastewaters raises important questions concerning SARS-CoV-2 stability and risk assessment. Using an integrity-based RT-qPCR, the present results demonstrated that SARS-CoV-2 genomes can be found in three different states in wastewater at least i.e. infectious protected, non-infectious protected and non-protected forms. Evidences show that some protected forms are associated with the long-term persistence of viral RNA in wastewaters, which may have important consequences for risk assessment since conventional RT-qPCR largely overestimated the presence of infectious viruses. The present study demonstrated that integrity-based RT-qPCR would allow to better estimate the risk of contamination through wastewaters. In addition, it may also be useful to detect viral particles in other complex matrices such as sludges and stools in which isolating infectious viruses is often technically challenging.
Authors' contribution
SW and PW made the virus measurements; SW, FRA, FVG and MB performed the infectivity assay in BSL3 laboratory; YM and JMM facilitated wastewater sampling; SW, PW, VM and LM for the redaction of the manuscript; YM, JMM and MB for critical discussion.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This research was cofunded by the French ministery of research and innovation, Eau de Paris, the French armed forces biomedical research institute (IRBA), Sorbonne university and CNRS.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.watres.2021.117183.
Appendix. Supplementary materials
References
- Aboubakr H.A., Sharafeldin T.A., Goyal S.M. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: a review. Transbound Emerg. Dis. tbed. 2020;13707 doi: 10.1111/tbed.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bibby K., Haramoto E., Hewitt J., Huygens F., Gyawali P., Korajkic A., Riddell S., Sherchan S.P., Simpson S.L., Sirikanchana K., Symonds E.M., Verhagen R., Vasan S.S., Kitajima M., Bivins A. Decay of SARS-CoV-2 and surrogate murine hepatitis virus RNA in untreated wastewater to inform application in wastewater-based epidemiology. Environ. Res. 2020;191 doi: 10.1016/j.envres.2020.110092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboa S., Mauricio-Iglesias M., Rodriguez S., Martínez-Lamas L., Vasallo F.J., Regueiro B., Lema J.M. The fate of SARS-COV-2 in WWTPS points out the sludge line as a suitable spot for detection of COVID-19. Sci. Total Environ. 2021;772 doi: 10.1016/j.scitotenv.2021.145268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand I., Schijven J.F., Sánchez G., Wyn-Jones P., Ottoson J., Morin T., Muscillo M., Verani M., Nasser A., de Roda Husman A.M., Myrmel M., Sellwood J., Cook N., Gantzer C. The impact of temperature on the inactivation of enteric viruses in food and water: a review. J. Appl. Microbiol. 2012;112:1059–1074. doi: 10.1111/j.1365-2672.2012.05267.x. [DOI] [PubMed] [Google Scholar]
- Bivins A., Greaves J., Fischer R., Yinda K.C., Ahmed W., Kitajima M., Munster V.J., Bibby K. Persistence of SARS-CoV-2 in water and wastewater. Environ. Sci. Technol. Lett. 2020;7:937–942. doi: 10.1021/acs.estlett.0c00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009:1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova L.M., Weaver S.R. Inactivation of an enveloped surrogate virus in human sewage. Environ. Sci. 2015:76–78. doi: 10.1021/acs.estlett.5b00029. [DOI] [Google Scholar]
- Cashdollar J.L., Wymer L. Methods for primary concentration of viruses from water samples: a review and meta-analysis of recent studies. J. Appl. Microbiol. 2013;115:1–11. doi: 10.1111/jam.12143. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention, 2020. 2019-novel coronavirus (2019-nCoV) real-time rRT-PCR panel primers and probes.
- Chan M.C.W., Lee N., Chan P.K.S., Leung T.F., Sung J.J.Y. Fecal detection of influenza A virus in patients with concurrent respiratory and gastrointestinal symptoms. J. Clin. Virol. 2009;45:208–211. doi: 10.1016/j.jcv.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Chen C., Gao G., Xu Y., Pu L., Wang Q., Wang Liming, Wang W., Song Y., Chen M., Wang Linghang, Yu F., Yang S., Tang Y., Zhao L., Wang H., Wang Y., Zeng H., Zhang F. SARS-CoV-2–positive sputum and feces after conversion of pharyngeal samples in patients with COVID-19. Ann. Intern. Med. 2020 doi: 10.7326/M20-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W., Peiris M., Poon L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1:e10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W., Bleicker T., Brunink S., Schneider J., Schmidt M.L., Mulders D.G.J.C., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.-L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P.G., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W., Yu J., Liu X., Chen H., Lin L., Li Q. Persistence of SARS-CoV-2 virus RNA in feces: a case series of children. J. Infect. Public Health. 2020;13:926–931. doi: 10.1016/j.jiph.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsamadony M., Fujii M., Miura T., Watanabe T. Possible transmission of viruses from contaminated human feces and sewage: implications for SARS-CoV-2. Sci. Total Environ. 2021;755 doi: 10.1016/j.scitotenv.2020.142575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley M. SARS-CoV-2: the growing case for potential transmission in a building via wastewater plumbing systems. Ann. Intern. Med. 2020;173:1020–1021. doi: 10.7326/M20-6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2009;1:10. doi: 10.1007/s12560-008-9001-6. [DOI] [Google Scholar]
- Hirose R. Long-term detection of seasonal influenza RNA in faeces and intestine. Clin. Microbiol. Infect. 2016;813:e1–813. doi: 10.1016/j.cmi.2016.06.015. e7. [DOI] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. First case of 2019 novel coronavirus in the United States. New Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M., Wei J., Yuan J., Guo J., Zhang Y., Hang J., Qu Y., Qian H., Zhuang Y., Chen X., Peng X., Shi T., Wang J., Wu J., Song T., He J., Li Y., Zhong N. Probable evidence of fecal aerosol transmission of SARS-CoV-2 in a high-rise building. Ann. Intern. Med. 2020;173:974–980. doi: 10.7326/M20-0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Kuroda K., Patel A.K., Patel N., Bhattacharya P., Joshi M., Joshi C.G. Decay of SARS-CoV-2 RNA along the wastewater treatment outfitted with Upflow Anaerobic Sludge Blanket (UASB) system evaluated through two sample concentration techniques. Sci. Total Environ. 2021;754 doi: 10.1016/j.scitotenv.2020.142329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Thakur A.K., Mazumder P., Kuroda K., Mohapatra S., Rinklebe J., Ramanathan Al., Cetecioglu Z., Jain S., Tyagi V.K., Gikas P., Chakraborty S., Tahmidul Islam M., Ahmad A., Shah A.V., Patel A.K., Watanabe T., Vithanage M., Bibby K., Kitajima M., Bhattacharya P. Frontier review on the propensity and repercussion of SARS-CoV-2 migration to aquatic environment. J. Hazard. Mater. Lett. 2020;1 doi: 10.1016/j.hazl.2020.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure F.-X., Bouadma L., Nguyen D., Parisey M., Wicky P.-H., Behillil S., Gaymard A., Bouscambert-Duchamp M., Donati F., Le Hingrat Q., Enouf V., Houhou-Fidouh N., Valette M., Mailles A., Lucet J.-C., Mentre F., Duval X., Descamps D., Malvy D., Timsit J.-F., Lina B., van-der-Werf S., Yazdanpanah Y. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect. Dis. 2020;20:697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol. Hepatol. 2020 doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz B.B.da, Oliveira N.M.T.de, Santos I.W.F.dos, Paza L.Z., Braga L.L.V.de M., Platner F.da S., Werner M.F.de P., Fernandes E.S., Maria-Ferreira D. An overview of the gut side of the SARS-CoV-2 infection. Intest. Res. 2020 doi: 10.5217/ir.2020.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ. Sci. 2020:511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Naddeo V., Liu H. Editorial Perspectives: 2019 novel coronavirus (SARS-CoV-2): what is its fate in urban water cycle and how can the water research community respond? Environ. Sci.: Water Res. Technol. 2020;6:1213–1216. doi: 10.1039/D0EW90015J. [DOI] [Google Scholar]
- Nemudryi A., Nemudraia A., Wiegand T., Surya K., Buyukyoruk M., Cicha C., Vanderwood K.K., Wilkinson R., Wiedenheft B. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Cell Rep. Med. 2020;1 doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoh A.I., Sibanda T., Gusha S.S. Inadequately treated wastewater as a source of human enteric viruses in the environment. Int. J. Environ. Res. Public Health. 2010:2620–2637. doi: 10.3390/ijerph7062620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Lee C.-W., Park D.-I., Woo H.-Y., Cheong H.S., Shin H.C., Ahn K., Kwon M.-J., Joo E.-J. Detection of SARS-CoV-2 in fecal samples from patients with asymptomatic and mild COVID-19 in Korea. Clin. Gastroenterol. Hepatol. 2020 doi: 10.1016/j.cgh.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S.M., Lai S.T., Poon L.L.M., Guan Y., Yam L.Y.C., Lim W., Nicholls J., Yee W.K.S., Yan W.W., Cheung M.T., Cheng V.C.C., Chan K.H., Tsang D.N.C., Yung R.W.H., Ng T.K., Yuen K.Y. Coronavirus as a possible cause of severe acute respiratory. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevost B., Goulet M., Lucas F.S., Joyeux M., Moulin L., Wurtzer S. Viral persistence in surface and drinking water: suitability of PCR pre-treatment with intercalating dyes. Water Res. 2016;91:68–76. doi: 10.1016/j.watres.2015.12.049. [DOI] [PubMed] [Google Scholar]
- Prevost B., Lucas F.S., Goncalves A., Richard F., Moulin L., Wurtzer S. Large scale survey of enteric viruses in river and waste water underlines the health status of the local population. Environ. Int. 2015;79:42–50. doi: 10.1016/j.envint.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Puente H., Randazzo W., Falcó I., Carvajal A., Sánchez G. Rapid selective detection of potentially infectious porcine epidemic diarrhea coronavirus exposed to heat treatments using viability RT-qPCR. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.01911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Cuevas-Ferrando E., Sanjuan R., Domingo-Calap P., Sanchez G. Metropolitan wastewater analysis for COVID-19 epidemiological surveillance. Int. J. Hyg. Environ. Health. 2020;230 doi: 10.1016/j.ijheh.2020.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Khezri M., Ollivier J., Le Guyader F.S., Rodríguez-Díaz J., Aznar R., Sánchez G. Optimization of PMAxx pretreatment to distinguish between human norovirus with intact and altered capsids in shellfish and sewage samples. Int. J. Food Microbiol. 2018;266:1–7. doi: 10.1016/j.ijfoodmicro.2017.11.011. [DOI] [PubMed] [Google Scholar]
- Riddell S., Goldie S., Hill A., Eagles D., Drew T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020;17:145. doi: 10.1186/s12985-020-01418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F., Pagani C., Cappelli F., Roscioli C., Moja L., Gismondo M.R., Salerno F. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744 doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa G.L., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods—a scoping review. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman A.I., Boehm A.B. Systematic review and meta-analysis of the persistence and disinfection of human coronaviruses and their viral surrogates in water and wastewater. Environ. Sci. Technol. Lett. 2020;7:544–553. doi: 10.1021/acs.estlett.0c00313. [DOI] [PubMed] [Google Scholar]
- Tang B., Wang X., Li Q., Bragazzi N.L., Tang S., Xiao Y., Wu J. Estimation of the transmission risk of the 2019-nCoV and its implication for public health interventions. J. Clin. Med. 2020;9:462. doi: 10.3390/jcm9020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman P., Lucas F.S., Varrault G., Moulin L., Wurtzer S. Hydrophobic organic matter promotes coxsackievirus B5 stabilization and protection from heat. Food Environ. Virol. 2020 doi: 10.1007/s12560-019-09418-9. [DOI] [PubMed] [Google Scholar]
- Waldman P., Meseguer A., Lucas F., Moulin L., Wurtzer S. Interaction of human enteric viruses with microbial compounds: implication for virus persistence and disinfection treatments. Environ. Sci. Technol. 2017;51:13633–13640. doi: 10.1021/acs.est.7b03875. [DOI] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.-W., Li J.-S., Jin M., Zhen B., Kong Q.-X., Song N., Xiao W.-J., Yin J., Wei W., Wang G.-J., Si B.-Y., Guo B.-Z., Liu C., Ou G.-R., Wang M.-N., Fang T.-Y., Chao F.-H., Li J.-W. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods. 2005;126:171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2020a. Transmission of SARS-CoV-2: implications for infection prevention precautions (No. WHO/2019-nCoV/Sci_Brief/Transmission_modes/2020.3).
- World Health Organization, 2020b. Water, sanitation, hygiene, and waste management for the COVID-19 virus (No. WHO/2019-nCoV/IPC_WASH/2020.3).
- World Health Organization, 2020c. Protocol: Real-time RT-PCR assays for the detection of SARS-CoV-2 Institut Pasteur, Paris.
- World Health Organization, 2003. Guidelines for environmental surveillance of poliovirus circulation. (No. WHO/V&B/03.03).
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X., Kuang L., Fang X., Mishra N., Lu J., Shan H., Jiang G., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020 doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S, Marechal V., Mouchel J., Maday Y., Teyssou R., Richard E., Almayrac J., Moulin L. Time course quantitative detection of SARS-CoV-2 in Parisian wastewaters correlates with COVID-19 confirmed cases. (preprint) Epidemiology. 2020 doi: 10.1101/2020.04.12.20062679. [DOI] [Google Scholar]
- Wurtzer S, Marechal V., Mouchel J., Maday Y., Teyssou R., Richard E., Almayrac J., Moulin L. Evaluation of lockdown impact on SARS-CoV-2 dynamics through viral genome quantification in Paris wastewaters (preprint) Epidemiology. 2020 doi: 10.1101/2020.04.12.20062679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer Sebastien, Marechal V., Mouchel J.-M., Maday Y., Teyssou R., Richard E., Almayrac J.-L., Moulin L. Evaluation of lockdown effect on SARS-CoV-2 dynamics through viral genome quantification in waste water, Greater Paris, France, 5 March to 23 April 2020. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.50.2000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Prevost B., Lucas F.S., Moulin L. Detection of enterovirus in environmental waters: a new optimized method compared to commercial real-time RT-qPCR kits. J. Virol. Methods. 2014;209:47–54. doi: 10.1016/j.jviromet.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Wurtzer, S., Waldman, P., Moulin, L., 2018. New insights for optimizing molecular detection of infectious viruses.
- Xiao F., Sun J., Xu Y., Li F., Huang X., Li H., Zhao Jingxian, Huang J., Zhao Jincun. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg. Infect. Dis. 2020;26:1920–1922. doi: 10.3201/eid2608.200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ. Sci. Technol. 2016;50:5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol. Hepatol. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol. Hepatol. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Chen Z., Gong C., Liu H., Li B., Li K., Chen X., Xu C., Jing Q., Liu G., Qin P., Liu Y., Zhong Y., Huang L., Zhu B.-P., Yang Z. Sewage as a possible transmission vehicle during a coronavirus disease 2019 outbreak in a densely populated community: Guangzhou, China, April 2020. Clin.Infect. Dis. 2020:ciaa1494. doi: 10.1093/cid/ciaa1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chen C., Zhu S., Shu C., Wang D., Song J., Song Y., Zhen W., Feng Z., Wu G. Isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19) China CDC Weekly. 2020;2:123–124. [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.