Abstract
Reduction of health care-associated infections is trending in the right direction after decades of work by those involved in infection prevention and control and antibiotic stewardship. With institutional priorities currently pivoting to meet the needs of COVID-19 patients, this may be an advantageous time to promote integration of facility-level antibiotic stewardship and infection prevention and control programs. We propose a team science framework as a tool to leverage the complementary expertise of stewardship and infection prevention and control professionals. This framework considers stages of team development and fluidity needed when working with shifting priorities and can be used by leaders and team members throughout all phases of team building—from developing and launching the team, through evaluating and modifying team activities to best suit local needs.
Key Words: Antimicrobial stewardship, Infection control, Team building, Team science, Leadership
We cannot afford to take our “foot off the gas” when it comes to reducing health care-associated infections (HAIs), particularly those caused by antibiotic-resistant bacteria and Clostridioides (Clostridium) difficile . We have gained ground regarding the reduction of C. difficile infection (CDI), according to a recent Centers for Disease Control and Prevention report published in the New England Journal of Medicine—indicating a 24% decrease in CDI and associated hospitalizations during 2011-2017.1 According to the HAI progress report for 2018 published by the Centers for Disease Control and Prevention, we have also made strides with other HAIs such as central line-associated bloodstream infections and catheter-associated urinary tract infections and antibiotic-resistant HAIs such as methicillin-resistant Staphylococcus aureus bacteremia.2
To build upon this progress, we portend that facility-level integration of stakeholders (with complementary subject matter expertise) is needed more than ever to accelerate progress amid rapidly shifting priorities. Evidence suggests that while antibiotic stewardship programs (ASPs) and infection prevention and control programs (IPCs) work well independently,3 there is much to gain when these programs are integrated; stewardship strategies and infection prevention measures appear to be more effective. In fact, similar approaches (CDI prevention bundles) that include both IPC and stewardship strategies have been consistently associated with reductions in CDI.4 , 5 There are other examples where IPC and ASP logically intersect such as monitoring antibiotic use to prevent infections caused by antibiotic-resistant organisms, refining testing practices for infections (urinary tract infections) to prevent unnecessary antibiotic use, and reporting process and outcome measures (surveillance for antibiotic use and resistant organisms). Recognizing the potential for quality improvement outcomes by integrating these 2 disciplines, national infection control organizations have been calling for increased interaction between ASPs and IPC programs.6
What does it mean to integrate these two programs? Using a framework based on team science to explore this question may aid in building, launching and sustaining a successful combined effort.7 Team science is defined as a strategy to leverage experience and expertise of research professionals from varying disciplines for the purpose of scientific discovery.8 Although the science of team science (SciTS) focuses on how integrated research teams operate, much of what has been learned can be applied to integrating the frontline clinical work of AS and IPC programs.9 The SciTS explores how integrated teams work together to produce discoveries that may not be possible when only one type of expertise or one discipline is involved in a project.10 , 11 Evidence suggests that teams are influenced by contextual factors such as funding trends, organizational communication, organizational policies and dynamics related to collaboration.8 , 9, 12, 13, 14
Team science underscores elements that make up an effective team such as effective leadership, trust between leaders and team members, open communication, shared expectations, well-defined roles, understanding others’ professional language, and promoting disagreement while minimizing conflict.15 Teams are fluid, with new priorities, new members, and new leadership coming and going. Therefore, teams operate on a continuum with stages of interaction and integration. This was described decades ago as regularly occurring stages of team development—forming (members are excited to be part of team), storming (team members learn conflict management), norming (members feel acceptance as part of team) and performing (members develop confidence in team perform at a higher level).16 These stages can be directly applied to the integration of AS and IPC professional stakeholders to reduce HAIs.
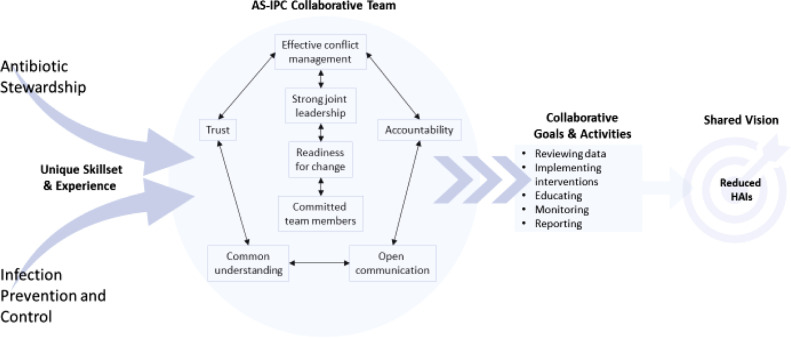
Using team science principles, we propose a framework that can be used to launch integration of AS and IPC professionals. This framework considers stages of team development and fluidity needed when working with rapidly shifting priorities (Fig 1 ). We apply our framework to an example—reduction of CDI.
Fig 1.
ASP and IPC collaborative framework.
Our framework emphasizes skills, expertise and discipline-specific goals. Although members’ priorities may vary due to facility-level priorities for the 2 disciplines, there may be many areas of overlap. The primary goals of AS and IPC programs, as defined by their respective professional societies17 , 18 are complementary and can be effectively integrated for the purpose of achieving a shared vision—reducing HAIs.
The cornerstone elements of high-functioning teams are depicted in the center of our framework, which considers contextual elements also illuminated in literature related to effective teams. Overall, readiness for change is critical to success—those participating must be prepared to engage with each other and respect the roles each will play in preventing HAIs.12 , 19 An example of this could be the incorporation of stewardship as part of the facility infection control risk assessment for preventing HAIs). Both leadership and team members must demonstrate this readiness in resources, personnel or time. As shown in our framework, leadership should be a shared role between one IPC and one stewardship professional. Joint or co-leadership is challenging but can work with the right combination of leader communication behaviors and structural characteristics of the collaborative effort. A recent case study of HAI leadership rounds led by 2 hospital executive leaders found that open communication skills (listening, asking questions, modeling fallibility, allowing time for reflection) and structural characteristics (consistency of meetings, rapid follow-up, reiterating goals, timeliness of data) are important factors related to success when co-leading an initiative.20
This case study also highlighted the necessity of building trust among team members as key to psychological safety—another key team science element.20, 21, 22 Sustaining best practices require that all members of the ASP-IPC integrated team feel safe to disclose problems and discuss solutions.23 Building trust among members as the groundwork for respectful disagreement is a cornerstone of team science principles. As such, it is essential that co-leaders pay attention to perceived power differentials between team members and facilitate conflict management without blaming.24 One approach that is gaining momentum in infection control is to perform root cause analyses on HAIs to identify system failures; these same techniques can be applied to ASP interventions.
Overall, members of the ASP-IPC team must build a common understanding of how individual skills, expertise and goals fit with the teams’ activities to reach a shared vision. This understanding should underscore all communication, strategic planning, and decision-making on the ASP-IPC integrated team. These factors will create a supportive environment for maintaining accountability and ensuring responsibilities are identified, shared and delegated, acted upon, reported, and evaluated.
Example—CDI prevention through an integrated ASP-IP team
CDI is a significant health care-associated pathogen, and the most significant cause of health care-associated diarrhea in the United States25; CDI also poses a challenging infection control problem for health care facilities: it is difficult to eliminate from the environment, pathogenesis is not fully understood, it can cause asymptomatic colonization, and it is associated with antibiotic use.26 , 27 Given the complex and multidisciplinary nature of CDI prevention, an integrated ASP-IP team may be especially effective in reducing CDI.
Stewardship professionals possess expertise in antibiotic stewardship activities such as preauthorization and postprescription audit and feedback interventions, communication related to consultation, and antibiotic use and resistance monitoring methodology. IPC professionals possess expertise in identifying and implementing evidence-based practices to prevent transmission of CDI, such as hand hygiene, early identification and isolation, transmission-based precautions, environmental and equipment cleaning and disinfection and HAI surveillance.28 Many IPC programs have already participated in national team-building collaboratives to reduce HAIs29 and believe team science is an advantageous approach to facilitate HAI reduction efforts. Both disciplines are skilled at gathering and analyzing data to provide a comprehensive picture of CDI prevention. Both disciplines are also skilled with influencing and shepherding change within organizations.
Health care facilities also have regulatory requirements to conduct annual facility-level infection control risk assessments prioritizing risk based on impact and likelihood of occurring. The ASP-IPC team should define overarching goals by performing an annual risk assessment of HAIs and antibiotic use factors that can affect CDI rates. Facility IPC process measures such as compliance with hand hygiene, isolation, and environmental cleaning should also be reviewed. Once the data review has been completed, intervention planning should begin. The ASP-IPC team can tailor existing CDI prevention practices to local needs. The team can also evaluate methods for collecting data, evaluate diagnostic and testing methods and practices, and identify strategies to streamline feedback to providers and monitoring agencies.
Building integrated ASP-IPC teams
We understand that prior to integration, it will be necessary to consider local contextual factors. For example, not all institutions have separate IPC and stewardship programs and some institutions house IPC within nursing. Stewardship programs may be housed in infectious disease or pharmacy. One must also consider where the two programs overlap and where they do not overlap. Historically, the ASP role is to assist providers on how best to provide for patients by guiding antimicrobial prescribing decision-making, and the IPC role may focus more on systems-level approaches instead of individual clinical recommendations.
Our framework is a preliminary tool that can be used by ASP-IPC leaders and team members throughout all phases of team development. Future research should examine the structural and process outcomes of using a team science approach for prevention of HAIs.
Footnotes
Conflicts of interest: All authors report no conflict of interest.
Funding: This work was supported by Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development pilot (1-121-HX002692-01A1, 101 HX002332); Nasia Safdar is supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (DP2A1144244). The content is solely the responsibility of the authors and does not necessarily reflect the position or policy of the Department of Veterans Affairs, the National Institutes of Health or the United States government.
References
- 1.Guh AY, Mu Y, Winston LG, et al. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med. 2020;382:1320–1330. doi: 10.1056/NEJMoa1910215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kourtis AP, Hatfield K, Baggs J, et al. Vital signs: epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections—United States. MMWR Morb Mortal Wkly Rep. 2019;68:214–219. doi: 10.15585/mmwr.mm6809e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furuya EY, Dick AW, Herzig CT, Pogorzelska-Maziarz M, Larson EL, Stone PW. Central line-associated bloodstream infection reduction and bundle compliance in intensive care units: a national study. Infect Control Hosp Epidemiol. 2016;37:805–810. doi: 10.1017/ice.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker AK, Ngam C, Musuuza JS, Vaughn VM, Safdar N. Reducing Clostridium difficile in the inpatient setting: a systematic review of the adherence to and effectiveness of C. difficile prevention bundles. Infect Control Hosp Epidemiol. 2017;38:639–650. doi: 10.1017/ice.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louh IK, Greendyke WG, Hermann EA, et al. Clostridium difficile infection in acute care hospitals: systematic review and best practices for prevention. Infect Control Hosp Epidemiol. 2017;38:476–482. doi: 10.1017/ice.2016.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manning ML, Septimus EJ, Ashley ESD, et al. Antimicrobial stewardship and infection prevention-leveraging the synergy: a position paper update. Infect Control Hosp Epidemiol. 2018;39:467–472. doi: 10.1017/ice.2018.33. [DOI] [PubMed] [Google Scholar]
- 7.Tebes JK, Thai ND. Interdisciplinary team science and the public: steps toward a participatory team science. Am Psychol. 2018;73:549–562. doi: 10.1037/amp0000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall KL, Feng AX, Moser RP, Stokols D, Taylor BK. Moving the science of team science forward: collaboration and creativity. Am J Prev Med. 2008;35(2 suppl):S243–S249. doi: 10.1016/j.amepre.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogel AL, Hall KL, Fiore SM, et al. The team science toolkit: enhancing research collaboration through online knowledge sharing. Am J Prev Med. 2013;45:787–789. doi: 10.1016/j.amepre.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Stokols D, Hall KL, Taylor BK, Moser RP. The science of team science: overview of the field and introduction to the supplement. Am J Prev Med. 2008;35(suppl 2):S77–S89. doi: 10.1016/j.amepre.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Stokols D, Misra S, Moser RP, Hall KL, Taylor BK. The ecology of team science: understanding contextual influences on transdisciplinary collaboration. Am J Prev Med. 2008;35(suppl 2):S96–115. doi: 10.1016/j.amepre.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Hall KL, Stokols D, Moser RP, et al. The collaboration readiness of transdisciplinary research teams and centers findings from the National Cancer Institute's TREC Year-One evaluation study. Am J Prev Med. 2008;35(suppl 2):S161–S172. doi: 10.1016/j.amepre.2008.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall KL, Vogel AL, Huang GC, et al. The science of team science: a review of the empirical evidence and research gaps on collaboration in science. Am Psychol. 2018;73:532–548. doi: 10.1037/amp0000319. [DOI] [PubMed] [Google Scholar]
- 14.Weinfurt KP. Managing different intellectual personalities in scientific teams. J Clin Transl Sci. 2019;3:50–52. doi: 10.1017/cts.2019.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett LM, Gadlin H. Collaboration and team science: from theory to practice. J Investig Med. 2012;60:768–775. doi: 10.231/JIM.0b013e318250871d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuckman BW. Developmental sequence in small groups. Psychol Bull. 1965;63:384–399. doi: 10.1037/h0022100. [DOI] [PubMed] [Google Scholar]
- 17.Manning ML, Septimus EJ, Ashley ESD, et al. Antimicrobial stewardship and infection prevention- leveraging the synergy: a position paper update. Am J Infect Control. 2018;46:364. doi: 10.1016/j.ajic.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Bryant K, Harris AD, Gould C, et al. Necessary infrastructure of infection prevention and healthcare epidemiology programs: a review. Infect Control Hosp Epidemiol. 2016;37:371–380. doi: 10.1017/ice.2015.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hays TC. The science of team science: commentary on measurements of scientific readiness. Am J Prev Med. 2008;35(suppl 2):S193–S195. doi: 10.1016/j.amepre.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Knobloch MJ, Chewning B, Musuuza J, et al. Leadership rounds to reduce health care-associated infections. Am J Infect Control. 2018;46:303–310. doi: 10.1016/j.ajic.2017.08.045. [DOI] [PubMed] [Google Scholar]
- 21.Dirks KT, Ferrin DL. Trust in leadership: meta-analytic findings and implications for research and practice. J Appl Psychol. 2002;87:611–628. doi: 10.1037/0021-9010.87.4.611. [DOI] [PubMed] [Google Scholar]
- 22.Seibert SE, Wang G, Courtright SH. Antecedents and consequences of psychological and team empowerment in organizations: a meta-analytic review. J Appl Psychol. 2011;96:981–1003. doi: 10.1037/a0022676. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Liu J, Zhu Y. Humble leadership, psychological safety, knowledge sharing, and follower creativity: a cross-level investigation. Front Psychol. 2018;9:1727. doi: 10.3389/fpsyg.2018.01727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Appelbaum NP, Dow A, Mazmanian PE, Jundt DK, Appelbaum EN. The effects of power, leadership and psychological safety on resident event reporting. Med Educ. 2016;50:343–350. doi: 10.1111/medu.12947. [DOI] [PubMed] [Google Scholar]
- 25.Weiner LM, Fridkin SK, Aponte-Torres Z, et al. Vital signs: preventing antibiotic-resistant infections in hospitals—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:235–241. doi: 10.15585/mmwr.mm6509e1. [DOI] [PubMed] [Google Scholar]
- 26.Anderson DJ, Chen LF, Weber DJ, et al. Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organisms and Clostridium difficile (the Benefits of Enhanced Terminal Room Disinfection study): a cluster-randomised, multicentre, crossover study. Lancet. 2017;389:805–814. doi: 10.1016/S0140-6736(16)31588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber DJ, Anderson D, Rutala WA. The role of the surface environment in healthcare-associated infections. Curr Opin Infect Dis. 2013;26:338–344. doi: 10.1097/QCO.0b013e3283630f04. [DOI] [PubMed] [Google Scholar]
- 28.Vassallo A, Tran M-CN, Goldstein EJ. Vol. 12. Taylor & Francis; 2014. pp. 1087–1102. (Clostridium Difficile: Improving the Prevention Paradigm in Healthcare Settings). [DOI] [PubMed] [Google Scholar]
- 29.McAlearney AS, Hefner JL, Sieck CJ, et al. Searching for management approaches to reduce HAI transmission (SMART): a study protocol. Implement Sci. 2017;12:82. doi: 10.1186/s13012-017-0610-z. [DOI] [PMC free article] [PubMed] [Google Scholar]