Abstract
Background
The key-ancestor approach has been frequently applied to prioritize individuals for whole-genome sequencing based on their marginal genetic contribution to current populations. Using this approach, we selected 70 key ancestors from two lines of the Swiss Large White breed that have been selected divergently for fertility and fattening traits and sequenced their genomes with short paired-end reads.
Results
Using pedigree records, we estimated the effective population size of the dam and sire line to 72 and 44, respectively. In order to assess sequence variation in both lines, we sequenced the genomes of 70 boars at an average coverage of 16.69-fold. The boars explained 87.95 and 95.35% of the genetic diversity of the breeding populations of the dam and sire line, respectively. Reference-guided variant discovery using the GATK revealed 26,862,369 polymorphic sites. Principal component, admixture and fixation index (FST) analyses indicated considerable genetic differentiation between the lines. Genomic inbreeding quantified using runs of homozygosity was higher in the sire than dam line (0.28 vs 0.26). Using two complementary approaches, we detected 51 signatures of selection. However, only six signatures of selection overlapped between both lines. We used the sequenced haplotypes of the 70 key ancestors as a reference panel to call 22,618,811 genotypes in 175 pigs that had been sequenced at very low coverage (1.11-fold) using the GLIMPSE software. The genotype concordance, non-reference sensitivity and non-reference discrepancy between thus inferred and Illumina PorcineSNP60 BeadChip-called genotypes was 97.60, 98.73 and 3.24%, respectively. The low-pass sequencing-derived genomic relationship coefficients were highly correlated (r > 0.99) with those obtained from microarray genotyping.
Conclusions
We assessed genetic diversity within and between two lines of the Swiss Large White pig breed. Our analyses revealed considerable differentiation, even though the split into two populations occurred only few generations ago. The sequenced haplotypes of the key ancestor animals enabled us to implement genotyping by low-pass sequencing which offers an intriguing cost-effective approach to increase the variant density over current array-based genotyping by more than 350-fold.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-021-07610-5.
Keywords: Swiss large white, Genetic diversity, Low-pass sequencing, Genotyping by sequencing, Key ancestor animals
Background
Swine production follows the classical breeding pyramid. Genetic gain is generated in nucleus herds and transmitted via the multiplier to the production unit. Swiss pig production relies on maternal and paternal Swiss Large White (SLW) lines at the top level of the breeding pyramid. For decades, the SLW breed has been maintained as a universal breed, selected for production and fertility traits. In 2002, the population was divided into sire and dam lines that have been divergently selected for fattening and reproduction since then. Approximately 32.5 and 30% of the genes of 2.5 million fattening pigs slaughtered in 2020 in Switzerland originate from the dam and sire line, respectively [1]. Both lines are maintained in purebred nucleus herds. However, little is known about the genetic diversity within the lines.
The SLW breeding boars are selected based on genome-based breeding values that are predicted using genotypes obtained with a customized version of the Illumina PorcineSNP60 BeadChip. Apart from a small number of putatively causal variants that are included in the custom part, the content of the currently used microarray was designed in a way that it is useful for mainstream breeds [2]. However, the genetic constitution of the SLW breed beyond the microarray-derived SNP remains largely unknown. The sequencing of key ancestor animals has been proposed as a cost-efficient way to assess sequence variation within a population. The genomes of key ancestor individuals maximally represent the genetic diversity of the target population [3, 4]. Due to the use of individual boars in artificial insemination and intense selection in nucleus herds, the effective population size of most pig breeding populations is low. Thus, most common polymorphic sites segregating in the population can be traced back to the genomes of important contributors to the current population [5, 6]. The key ancestor approach was frequently applied to identify the most important contributors to current cattle breeding populations [6]. Recently it was also used to prioritize animals for sequencing in commercial pig breeding lines [7].
The availability of sequence variant genotypes from key ancestor animals enables imputing sequence-level genotypes for animals that had been genotyped at lower density [8–10]. In livestock populations that are routinely genotyped using 60 K genotyping arrays, sequence variant genotypes are typically imputed using stepwise imputation [11]. In a first step, 60 K genotypes are imputed to higher density (e.g., 700 K) using animals that have been genotyped with high-density genotyping arrays. In a second step, the partially imputed high-density genotypes are imputed to the sequence level based on a sequenced reference panel. The accuracy of imputing 60 K genotypes directly to the sequence level is low, particularly for rare variants, rendering most of them uninformative for downstream analyses such as genomic prediction and association testing [12, 13]. Reference-guided variant phasing and imputation from low-pass sequencing data offers an intriguing alternative approach to the two-step imputation approach in pedigreed populations [14]. This approach utilises a sequenced haplotype reference panel that represents the diversity of the target population. Sequence variant genotypes of animals sequenced at very shallow coverage are then inferred conditional on the observed haplotypes of the reference panel. This method is particularly useful in species for which dense microarray-derived genotypes are not available. Recent investigations [8, 15, 16] suggest that a sequencing coverage less than 1-fold is sufficient to accurately infer genotypes at known loci - provided an informative haplotype reference panel is available.
Here we obtain whole-genome sequencing data from key ancestor animals to characterize genetic diversity, population structure, and signatures of selection in two divergently selected commercial pig breeds. Using the haplotypes of the key ancestor animals as a reference panel, we accurately genotype more than 22 million variants in animals that have been sequenced at low coverage.
Results
Using pedigree records, the average inbreeding coefficients of the active breeding animals of the sire and dam line were 0.06 ± 0.02 and 0.05 ± 0.01, respectively. Based on these values and the inbreeding coefficients of the parents, we estimated the effective population size of the sire and dam line of the Swiss Large White (SLW) breed to 44 and 72, respectively. In order to assess sequence variation within the two lines, we prioritized 70 boars for whole-genome sequencing based on their marginal genetic contributions to the active breeding populations with a key ancestor approach. Of the 70 boars, 38 and 32 represent the sire and dam line, respectively, explaining 95.35 and 87.95% of the genetic diversity of the active breeding populations.
Following quality control (removal of adapter sequences, reads and bases of low sequencing quality), between 81.15 and 377.01 million read pairs (2 × 150 bp) per sample (mean: 165.55 ± 60.32 million read pairs) were aligned to the SSC11.1 assembly of the porcine genome. Using reads with high mapping quality (reads with mapping quality < 10 and SAM bitwise flag 1796 were not considered), the average sequencing coverage of the 70 boars was 16.69 ± 5.93-fold across all autosomes. Raw sequence read data of 70 pigs have been deposited at the European Nucleotide Archive (ENA) of the EMBL at BioProject PRJEB38156 and PRJEB39374.
A reference-guided multi-sample variant discovery and genotyping approach yielded genotypes at 28,407,060 sites (22,191,375 biallelic SNP, 4,379,470 biallelic INDEL, and 1,836,215 others, Table 1). We applied GATK’s VariantFiltration module for site-level hard filtration using parameters recommended in the best practice guidelines [17]. Subsequently, we applied Beagle (version 4.1 [18];) phasing and imputation to improve the genotype calls from GATK and to impute sporadically missing genotypes. Following the imputation, we retained 26,862,369 variants including 21,592,583 SNP and 5,269,786 INDEL. The number of polymorphic sites that were seen in the heterozygous (singletons) and homozygous (doubletons) state only once was 2,026,088 (7.54%) and 72,100 (0.27%), respectively. To prevent bias resulting from flawed genotypes in repetitive regions, we excluded 1,710,337 variants for which an excess of sequencing coverage was evident for downstream analyses. The transition/transversion (Ti/Tv)-ratio estimated from filtered and imputed variants was 2.28.
Table 1.
Variants detected in 70 sequenced key ancestor animals
| Raw | Filtered & imputed | Dam line | Sire line | |
|---|---|---|---|---|
| Number of animals | 70 | 70 | 32 | 38 |
| Sequence coverage1 | 16.69 (8.72–36.85) | 16.69 (8.72–36.85) | 18.02 (9.31–36.85) | 15.57 (8.72–27.73) |
| Number of variants | ||||
| All | 28,407,060 | 26,862,369 | 24,358,047 | 24,093,052 |
| Biallelic SNP | 22,191,375 | 21,209,725 | 19,456,000 | 19,232,692 |
| Biallelic INDEL | 4,379,470 | 4,339,947 | 3,960,976 | 3,928,684 |
| Others2 | 1,836,215 | 1,312,697 | 941,071 | 931,676 |
| Autosomal variants | ||||
| All | 27,582,843 | 26,198,587 | 23,774,053 | 23,531,919 |
| Biallelic SNP | 21,553,323 | 20,715,354 | 19,015,058 | 18,808,294 |
| Biallelic INDEL | 4,248,742 | 4,211,012 | 3,846,008 | 3,817,622 |
| Others2 | 1,780,778 | 1,272,221 | 912,987 | 906,003 |
1 estimated from the autosomes
2 this category contains multi-allelic SNP, multi-allelic INDEL, as well as sites that may contain both SNP and INDEL
The resulting data were separated into two datasets containing 23,774,053 and 23,531,919 autosomal variants detected in 32 and 38 boars from the dam and sire line, respectively. Of the variants, 1,049,689 and 1,594,775 were fixed for the alternate allele in the dam and sire line, respectively. On average, we detected 11,119,760 ± 176,113 biallelic variants per animal (Fig. 1a), of which 6,258,456 ± 280,127 and 4,861,304 ± 135,524 were heterozygous and homozygous for the reference allele, respectively. The average nucleotide diversity (π) across 452,444 overlapping windows (10 kb in size with 5 kb steps), spanning 22,840,217 and 22,529,446 biallelic variants, respectively, was 2.81 × 10− 3 in the dam and 2.72 × 10− 3 in the sire line.
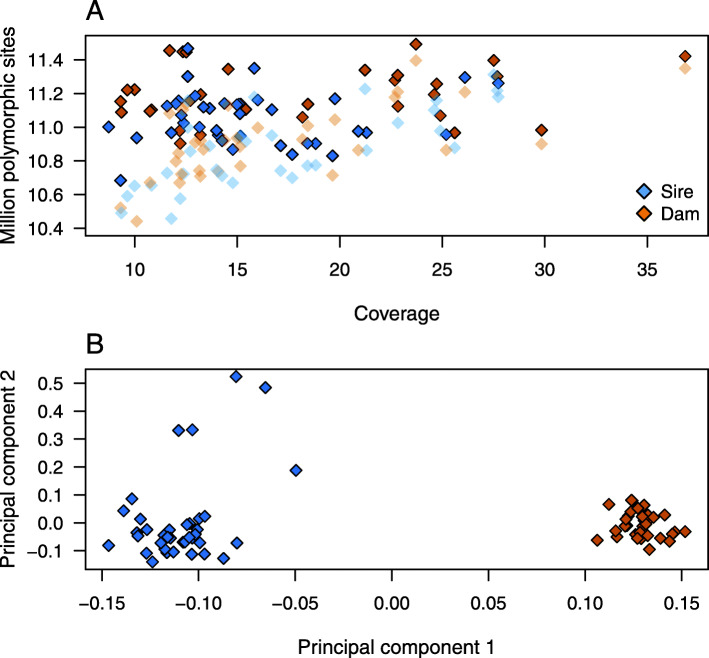
Fig. 1.
Sequencing of key ancestor animals from two pig lines. a Number of polymorphic sites detected in the 70 boars as a function of depth of coverage based on imputed and filtered non-imputed data (transparency). b Plot of the first two principal components showing the separation of animals by breed and the relationship between both lines. Blue and orange symbols indicate 38 and 32 boars from the sire and dam line, respectively
Comparison between array-called and sequence-called genotypes
Sixty-eight boars (32 and 36 from the dam and sire line, respectively) that had average sequencing coverage between 8.72 and 36.85-fold (average: 16.79-fold) also had Illumina PorcineSNP60 BeadChip-called genotypes. Using the array-called genotypes at 54,600 autosomal SNP for which we were able to determine reference and alternate alleles as a truth set, we calculated genotype concordance, non-reference sensitivity and non-reference discrepancy between array-called and sequence-called genotypes as proposed by DePristo et al. [19]. Of the 54,600 SNP, 6376 and 1029 were fixed for the reference and alternate allele, respectively, and 47,195 were polymorphic in the array-called genotypes of the 68 pigs.
Of the 48,224 SNP that were either polymorphic or fixed for the alternate allele in the array-called genotypes, 46,009 (95.41%) and 45,951 (95.29%) were also present in the raw and filtered sequence variants, respectively. 1232 SNP of the Illumina PorcineSNP60 BeadChip complement were missing in the sequenced set because they were either genotyped as INDEL or multiallelic sites using GATK and thus excluded from the comparison due to incompatible alleles. 983 and 1041 SNP were not among the raw and filtered sequence variants, respectively, although the frequency of the minor allele was > 5% in the array-called genotypes for most (> 80%) of them. It is likely that these variants could not be matched with the sequence set due to either incompatible or ambiguous map coordinates.
Non-reference sensitivity was greater than 99% and non-reference discrepancy around 1% for the raw genotypes called by the GATK, suggesting that the high sequencing coverage facilitated accurate variant discovery (Table 2). The concordance between sequence- and array-called genotypes improved slightly after applying site-level hard filtration. Beagle phasing and imputation further increased the concordance and non-reference sensitivity as well as decreased the non-reference discrepancy of the filtered sequence variant genotypes.
Table 2.
Comparison between sequence- and array-called genotypes at corresponding positions
| Dataset | Genotype concordance (%) | Non-reference sensitivity (%) | Non-reference discrepancy (%) |
|---|---|---|---|
| Raw | 99.18 | 99.75 | 1.11 |
| Filtered | 99.19 | 99.77 | 1.09 |
| Filtered & imputed | 99.82 | 99.95 | 0.24 |
Population structure and genetic diversity
To investigate the population structure, ancestry and genetic diversity among the 70 sequenced pigs, we performed principal component, admixture and fixation index (FST) analyses. The principal components were extracted from a genomic relationship matrix constructed from 23,691,198 autosomal sequence variants that had minor allele frequency greater than 0.01.
The first principal component of the genomic relationship matrix explained 8.61% of the variation and separated the animals by lines (Fig. 1b). The second principal component explaining 2.68% of the variation revealed variability within the sire line. Five outlier animals along the second axis of variation descended from imported Large White boars.
We performed an admixture analysis using 1,207,189 independent biallelic SNP to assess gene flow between both lines. As expected, K = 2 was the most plausible number of genetically distinct clusters (Supplementary Fig. S1). The cross-validation error for K = 1, K = 2 and K = 3 was 0.561, 0.546 and 0.564, respectively.
In order to investigate if pronounced allele frequency differences exist between both lines, we performed a SNP-based genetic differentiation analysis. We observed multiple 10 kb sliding windows scattered throughout the genome with FST values greater than 0.25, indicating genetic divergence of both lines (Supplementary Fig. S2). The average weighted FST value across all windows was 0.07.
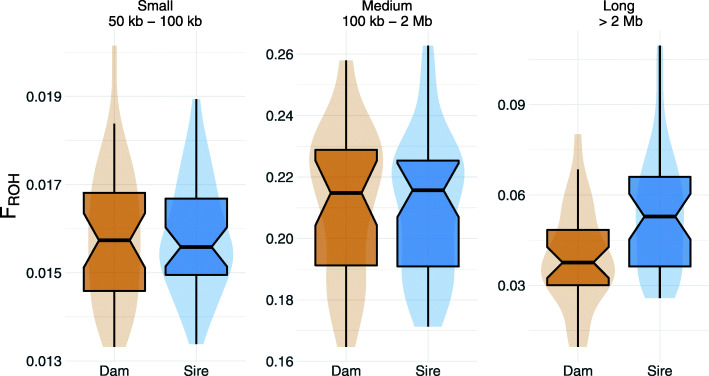
We estimated runs of homozygosity (ROH) for 19,146,365 biallelic SNP to investigate genomic inbreeding in both lines. In total 111,201 ROH with an average length of 391.28 kb (ranging from 50 kb to 11.1 Mb) were detected (Phred-scaled likelihood > 70). The ROH contained an average number of 3176 SNP (ranging from 29 to 87,699). The boars from the dam and sire line had 1604 ± 133 and 1575 ± 91 ROH with an average size of 377,928 and 402,731 bp, respectively. The genomic inbreeding (FROH, i.e., the fraction of the autosomal genome covered by ROH), was 0.26 ± 0.03 and 0.28 ± 0.03 for the dam and sire line, respectively. We classified the ROH into short (50–100 kb), medium (100 kb - 2 Mb) and long ROH (above 2 Mb) (Fig. 2). Most ROH belonged to the medium length class. The average FROH was similar in both lines for small and medium ROH. However, FROH was higher for long ROH in the sire line.
Fig. 2.
Genomic inbreeding in the two lines. FROH in dam and sire line, estimated for three groups of ROH classified based on their length: small (50 kb – 100 kb), medium (100 kb – 2 Mb) and long (> 2 Mb)
Variant annotation
In 32 boars from the dam line, we annotated 23,774,053 (19,087,807 SNP; 4,038,170 INDEL) variants, including 2,567,754 variants that were not detected in the sire line. In 38 boars of the sire line, we annotated 23,531,919 (18,881,067 SNP; 4,009,043 INDEL) variants, including 2,325,620 that were not detected in the dam line. When compared to 63,832,658 germline variants listed for Sus scrofa in the Ensembl database (release 101), 5,745,790 (24.17%, dam line) and 5,693,068 (24.19%, sire line) variants were novel, of which the majority were INDEL and 14.66 and 14.64% were biallelic SNP.
We used the Ensembl Variant Effect Predictor software (VEP, release 98 [20];) to predict functional consequences for the sequence variants (Table 3). In total, 2.96% (dam line) and 2.94% (sire line) of the variants were in exons. Putative impacts of missense variants on protein function were predicted using the SIFT (sorting intolerant from tolerant) scoring algorithm [21] as implemented in the VEP software. The scoring algorithm classified 12,024 and 11,958 amino acid substitutions in the dam and sire line, respectively, as “deleterious” (SIFT score < 0.05).
Table 3.
Predicted consequences of variants segregating in two lines. The table shows only the most sever consequence for a variant
| Consequence type (most severe) | Dam line | Sire line |
|---|---|---|
| Splice donor variant | 1396 | 1421 |
| Splice acceptor variant | 1126 | 1096 |
| Stop gained | 1615 | 1604 |
| Frameshift variant | 10,912 | 11,043 |
| Stop lost | 595 | 587 |
| Start lost | 423 | 421 |
| Inframe insertion | 990 | 987 |
| Inframe deletion | 1164 | 1186 |
| Protein altering variant | 62 | 62 |
| Missense variant | 70,758 | 69,983 |
| Splice region variant | 22,493 | 22,148 |
| Incomplete terminal codon variant | 12 | 11 |
| Synonymous variant | 76,977 | 75,279 |
| Stop retained variant | 149 | 135 |
| Start retained variant | 4 | 4 |
| Coding sequence variant | 98 | 96 |
| Mature miRNA variant | 12 | 16 |
| 5′ - UTR variant | 168,000 | 164,866 |
| 3′ - UTR variant | 348,135 | 344,514 |
| Non-coding transcript exon variant | 277,002 | 275,909 |
| Intron variant | 12,213,614 | 12,092,056 |
| Non-coding transcript variant | 11 | 10 |
| Upstream gene variant | 878,779 | 869,207 |
| Downstream gene variant | 757,364 | 750,548 |
| Intergenic variant | 8,942,362 | 8,848,730 |
Known trait-associated variants
The catalogue of Mendelian traits in Sus scrofa curated in the OMIA database (https://omia.org/home/, [22]) contained records of 47 likely causal variants (as of September 2020). However, the genomic coordinates were available for only 33 likely causal variants. Using functional annotations and sequence coverage analyses, we detected OMIA-listed variants affecting the KIT, MC1R and FUT1 genes in the sequenced key ancestor animals that occurred at alternate allele frequencies between 0.013 and 1 (Supplementary Table S3).
A duplication of the KIT gene and a splice site variant in intron 17 of the KIT gene are associated with the dominant white phenotype [23, 24]. Because the genotyping of larger structural and copy number variants from short-read sequencing data is notoriously difficult, we visually inspected the depth of sequencing coverage at the SSC8 region encompassing KIT. An increase in coverage between 41.22 and 41.78 Mb confirmed the presence of a previously reported 560 kb duplication (DUP1; Supplementary Fig. S3, [25, 26]. The duplication also encompasses a copy of KIT that carries a splice donor site variant (SSC8: 41486012G > A, rs345599765) which manifests in a dominant white phenotype [23, 24]. The splice variant segregated at a frequency of 0.49 and 0.42 in the sire and dam line, respectively. Seven animals that carried either one or two copies of DUP1 did not carry the splice site variant and all others were heterozygous carriers. Because this variant is located within the 560 kb duplication, we observed allelic imbalance in heterozygous animals.
We detected three OMIA-listed pigmentation-associated variants in the MC1R gene in the sequenced pigs. All boars were homozygous carriers of a 2-bp insertion (SSC6: 182,120–182,121 bp), that causes a frameshift and premature translation termination, which is associated with recessive white color [27]. All animals were also homozygous carriers of two missense variants in the MC1R gene (SSC6: 181461 T > C, ENSSSCP00000027395.1: p.Thr243Ala and SSC6: 181697A > G, ENSSSCP00000027395.1: p.Val164Ala), for which the reference alleles had been associated with red color in the Duroc breed [28].
A missense variant (SSC6: 54079560 T > C; ENSSSCP00000062180.1: p.Thr102Ala; rs335979375) in the FUT1 gene enables adhesion of enterotoxigenic Escherichia coli F18 fimbriae (ETEC F18) to receptors at the brush border membranes of the intestinal mucosa [29]. The allele that facilitates ETEC F18 adhesion causes diarrhea in neonatal and recently weaned piglets. Since a strong selection against the ETEC F18 susceptible allele takes place in both SLW lines, we observed the disease-associated allele only in one boar from the sire line in the heterozygous state.
Signatures of selection
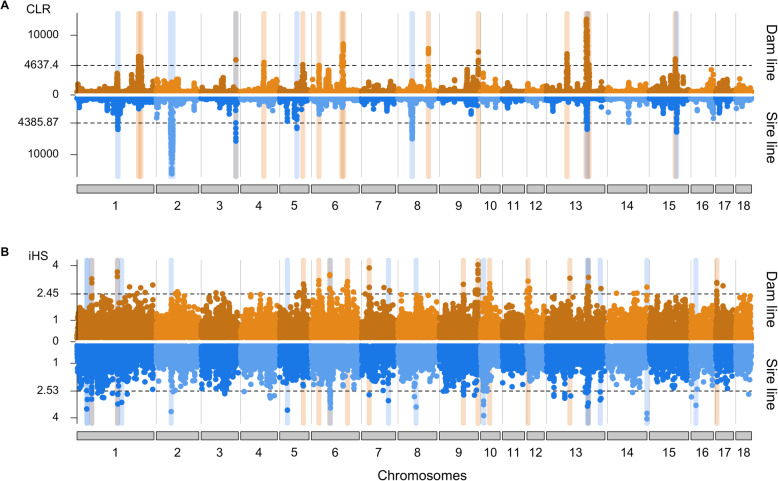
We detected signatures of past selection using the composite likelihood ratio (CLR) test. Signatures of ongoing selection were identified by the integrated haplotype score (iHS) test. For both analyses, we used biallelic autosomal SNP (Ndam = 19,015,058, Nsire = 18,808,294) that were grouped into non-overlapping 100 kb windows. For the CLR tests, we considered an empirical 0.5% significance threshold to identify putative signatures of selection (Fig. 3a). The number and length of candidate selection regions was higher in the dam than the sire line (14 vs. 7; 38.1 Mb vs. 26.1 Mb). Two regions on SSC3 (from 122.6 to 124.9 Mb) and SSC13 (from 140.0 to 146.1 Mb) showed evidence of selection in both lines. For the iHS analyses, we used an empirical 0.1% significance threshold to detect putative signatures of selection (Fig. 3b). We detected 14 and 16 candidate regions of selection in the dam and sire line, respectively, encompassing 28.5 Mb and 32.5 Mb. Four regions on SSC1 (from 51.1 to 53.7 Mb, from 142.7 to 146.2 Mb), SSC6 (from 64.9 to 69.3 Mb) and SSC13 (from 148.0 to 150.6 Mb) were shared between both lines.
Fig. 3.
Signatures of selection detected in the sire and dam line of the SLW breed. Signatures of selection detected in the sire and dam line of the SLW breed using CLR (a) and iHS (b) Dotted lines indicate the empirical 0.5 (CLR) and 0.1% (iHS) thresholds. Blue, orange and grey vertical bars highlight signatures of selection detected in the sire, dam and both lines, respectively
Considering both statistics, we detected more signatures of selection in the dam than sire line (28 vs. 23). Only 6 regions, detected by either CLR or iHS, overlapped between both lines. A strong signature of selection was detected in both lines with both methods on SSC13 between 140 and 152.4 Mb. The candidate region encompassed 125 genes (Supplementary Table S2), as well as 63,480 and 55,835 polymorphic sites in the dam and sire line, respectively, precluding to readily prioritize candidate genes and variants responsible for the sweep.
Reference-based genotyping from low-coverage sequencing data
In order to investigate if the 70 sequenced key ancestor animals may serve as a reference panel for genotyping by low-coverage sequencing, we sequenced the genomes of 175 pigs (84 from the sire line and 91 from the dam line) at low coverage using Gencove’s low-pass sequencing solution. The pigs also had Illumina PorcineSNP60 BeadChip-called genotypes. A principal component (Supplementary Fig. S4) analysis of a genomic relationship matrix constructed from microarray-derived genotypes showed that the 175 pigs cluster with the 70 key ancestor animals.
Following quality control, we aligned a median number of 16,153,314 (between 5,950,534 and 21,168,683) read pairs (2 × 150 bp) to the porcine reference genome, achieving an average depth of coverage of 1.11-fold (from 0.38 to 1.51). On average, 54% of the reference nucleotides were covered with at least one read. Following the reference-guided low-pass sequence variant genotyping approach (GLIMPSE) proposed by Rubinacci et al. [8], we utilized the haplotypes of the 70 sequenced key ancestor animals as a reference panel to call genotypes at 22,618,811 polymorphic sites in the 175 low-pass sequenced samples.
We assessed the accuracy of genotyping by low-pass sequencing based on Illumina PorcineSNP60 BeadChip-called genotypes at 54,600 SNP, for which we were able to determine reference and alternate alleles. Of the 54,600 SNP, 6176 and 965 were fixed for the reference and alternate allele, respectively, in the 175 pigs according to the array-called genotypes. Of 48,424 SNP that were either polymorphic or fixed for the alternate allele, 46,001 (94.99%) were also among the GLIMPSE-imputed genotypes. 2423 SNP had microarray-derived genotypes but were missing in the GLIMPSE-imputed genotypes because these SNP were missing in the haplotype reference panel constructed from the key ancestor animals.
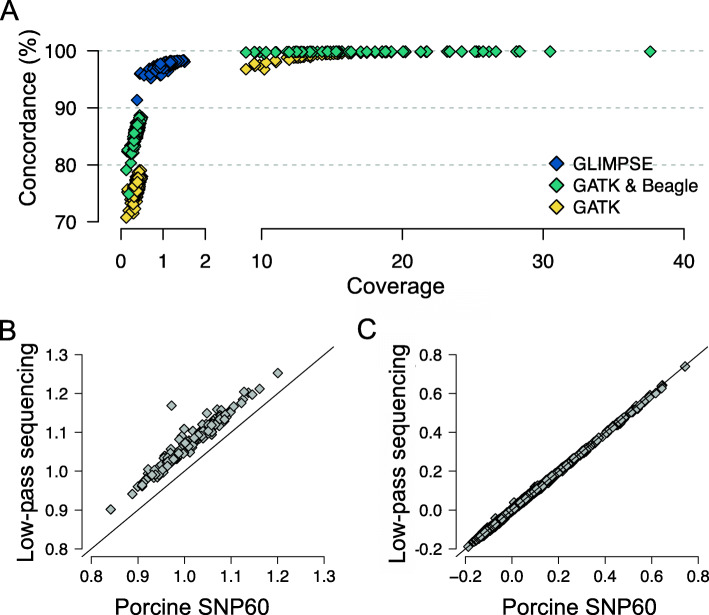
The genotype concordance, non-reference sensitivity and non-reference discrepancy between GLIMPSE-imputed and array-called genotypes at 46,001 autosomal SNP was 97.60, 98.73 and 3.24% in 175 low-pass sequenced pigs (Table 4, Fig. 4a). When the sequence variant calling of the 175 samples was performed together with the 70 key ancestor animals using the multi-sample approach implemented in the GATK, all concordance metrics were considerably worse. Although, Beagle imputation improved the genotype calls of GATK for the low-pass sequenced samples, the genotype concordance and non-reference sensitivity was lower and non-reference discrepancy higher using GATK than GLIMPSE. Using the GLIMPSE approach improved the genotype concordance over GATK filtered & Beagle imputed variants by 13.83% and this improvement is mostly due to a lower non-reference discrepancy (Table 4).
Table 4.
Accuracy of sequence variant genotyping in low-coverage (1.11-fold) sequencing data
| Variant genotyping approach | Genotype concordance | Non-reference sensitivity | Non-reference discrepancy |
|---|---|---|---|
| GLIMPSE | 97.60 | 98.73 | 3.24 |
| GATK raw | 75.90 | 52.35 | 30.20 |
| GATK filtered | 75.89 | 52.36 | 30.22 |
| GATK filtered & imputed | 85.74 | 96.56 | 19.34 |
Fig. 4.
Accuracy of genotyping by low-coverage sequencing. a Concordance between array-called and sequence-called genotypes at 46,001 biallelic autosomal SNP in 243 pigs that had been sequenced at either low (N = 175; < 1.5-fold) or medium to high (N = 68; 8.88–37.60-fold) coverage. Correlations of (b) diagonal (r = 0.96) and (c) off-diagonal (r = 0.99) elements of genomic relationship matrices constructed from array- and GLIMPSE-called genotypes at 44,268 SNP that had minor allele frequency > 0.01
We constructed genomic relationship matrices (GRM) from the microarray-derived and GLIMPSE-imputed genotypes of the 175 sequenced pigs based on a subset of 44,268 SNP that were detected at minor allele frequency greater than 0.01 in both datasets. Both the off-diagonal and the diagonal elements of the GRM constructed from array-derived genotypes had greater variance (σ2diag = 3.37 × 10− 3, σ2off = 9.34 × 10− 3) than corresponding elements of the GRM constructed from low-pass sequencing data (σ2diag = 3.30 × 10−3, σ2off = 9.15 × 10− 3). While the correlation of the off-diagonal (r = 0.99) and diagonal (r = 0.96) elements was high between both GRMs, the values of the diagonal elements were higher for all samples using the GLIMPSE-imputed than microarray-derived genotypes (Fig. 4b and c). The average value of the diagonal elements of the GRM was 1.01 ± 0.06 and 1.05 ± 0.06 for the microarray- and low-pass sequencing-derived genotypes, respectively. On average, the 175 boars were homozygous for 65.58 ± 1.39% and 67.27 ± 1.49% of the 44,268 SNP when the genotypes were called from the microarray and low-pass sequencing data, respectively.
Discussion
We applied a key ancestor animal approach to prioritize 38 and 32 boars that accounted for 95.35 and 87.95% of the genetic diversity of the SLW sire and dam line, respectively. The contributions of the SLW key ancestor animals to the current populations are considerably higher than reported for other populations. For instance, 43 key ancestor animals explained 69% of the genetic diversity of the Fleckvieh cattle population [5]. Neuditschko et al. [30] selected 41 and 55 key contributors, respectively, that explained 78 and 75% of the genetic relationship structure of the Swiss Franches-Montagnes horse and Australian Holstein-Friesian cattle population. The effective population size of the SLW sire and dam line is 44 and 72, respectively, which is less than half the effective population size of the Fleckvieh cattle and Swiss Franches-Montagnes horse population [31, 32]. Thus, a few animals that are selected based on their marginal genetic contribution to the active breeding population, account for a large fraction of the population’s haplotype diversity. It is worth mentioning that approaches other than the key ancestor animal approach may increase the haplotype diversity among the sequenced animals [33]. Nevertheless, the catalogue of 26.86 million polymorphic sites detected from the 70 sequenced boars of our study contains most alleles that segregate in the SLW populations, particularly those that occur at not too low frequency. A Ti/Tv-ratio of 2.28 indicates that the variants were of high quality [34]. In spite of the low effective population size, the nucleotide diversity (π) was high in both lines (πdam = 2.24 × 10− 3; πsire = 2.23 × 10− 3), which agrees well with estimates obtained in other European pig populations [35–37]. The nucleotide diversity in the SLW populations is higher than in cattle (1.77 × 10− 3 – 1.90 × 10− 3) and human (0.98 × 10− 3 – 1.41 × 10− 3) populations that have considerably larger current effective population sizes [38].
Although our sequencing cohort contained more animals from the sire line, we detected somewhat more autosomal variants in the dam line (Nsire = 23,531,919; Ndam = 23,774,053). While the average number of heterozygous variants detected per animal was higher in the dam line (Nsire = 6,180,048; Ndam = 6,351,565), the number of variants homozygous for the alternate allele was higher in the sire line (Nsire = 4,873,994; Ndam = 4,646,236). Because the average depth of sequencing was similar in the sire and dam line, these differences are unlikely to be due to uneven coverage between the lines. These differences are likely attributable to a smaller effective population size and higher genomic inbreeding in the sire line. The presence of many long ROH (> 2 Mb) suggests that recent inbreeding is higher in the sire than the dam line. Small effective population size and increasing inbreeding make both lines susceptible to the phenotypic manifestation of recessive alleles. For instance, a recessive sperm defect has recently been discovered in the sire line [39]. The management of an ever-increasing number of recessive traits is a challenge to domestic animal breeding populations [40–42]. Efficient and sustainable strategies are required to prevent the frequent manifestation of recessive diseases in populations with low effective population size.
Surprisingly, Cai et al. [43] detected fewer variants (between 20.68 and 22.11 million variants) in a considerably larger cohort of pigs (between 61 and 89) from three commercial Danish lines. Considering that Cai et al. also sequenced key ancestor animals, this difference to our study suggests higher genetic diversity in SLW. However, the depth of coverage, sequencing strategy, sequence variant genotyping and filtration approaches have major impacts on detecting polymorphic sites [44, 45]. While the effective depth of coverage realized by Cai et al. [43] is unknown to us, our samples were sequenced at an average depth of coverage greater than 16-fold. This depth of coverage enabled us to accurately detect both homozygous and heterozygous sites as evidenced by high non-reference sensitivity and genotype concordance at low non-reference discrepancy.
The principal components of a genomic relationship matrix constructed from whole-genome sequence variants revealed a separation of the animals by line. While the differentiation between the two populations might be less evident if diverse samples or an outgroup were considered in the analysis [7, 37, 46, 47], an average FST value of 0.07 corroborated that both lines diverged considerably. In fact, the average FST value observed between two SLW lines is similar to values reported between distinct European pig breeds [37, 43, 48]. The differentiation between the sire and dam line might result from distinct breeding objectives with negative genetic correlations [49]. While the sire line is mainly selected for meat and fattening traits, the dam line is mainly selected for reproduction traits. Using CLR and iHS, we detected 51 candidate signatures of selection, of which only six overlapped between both lines, suggesting that different loci are under selection in the sire and dam line. However, previous research indicates that selection for complex traits, such as production and reproduction, acts on many loci, thus barely leaves strong footprints in the genome [50, 51]. Moreover, both lines diverged only few generations ago, rendering limited time for shifts in allele frequency due to selection. We suspect that the strong differentiation between the SLW sire and dam line is also a result of genetic drift [52–54] due to very small effective population size and pronounced founder effects resulting from the unbalanced use of individual boars in artificial insemination.
A reference panel of less than 70 sequenced key ancestor animals facilitated imputing sequence variant genotypes at high accuracy and detecting trait-associated nucleotides using genome-wide association testing in cattle populations [6, 55]. Sequence variant genotypes are typically inferred using two-step imputation approaches. This requires the presence of a representative number of animals that had been genotyped at high density [11]. However, routine genotyping in the SLW populations is performed using a customized PorcineSNP60 BeadChip. Genotypes from high-density microarrays (e.g., 600 K) are not available. Thus, precluding the accurate imputation of sequence variant genotypes from the key ancestor animals using the well-established stepwise imputation approach [13]. This limitation prompted us to investigate an alternative approach to reference-guided sequence variant imputation. We considered the 70 key ancestor animals as a reference to call genotypes from low-pass sequencing data (1.11-fold) of genetically similar pigs. In agreement with previous studies in human and cattle populations, the genotyping accuracy from the low-pass sequencing data was very high [8, 15, 56]. Moreover, the low-pass sequencing-derived genomic relationship coefficients were highly correlated with those obtained using microarray genotyping. This suggests that the low-pass sequencing-derived imputed genotypes may readily be used for genomic prediction [56, 57]. However, the diagonal elements of the genomic relationship matrix were higher and had less variance using the genotypes from low-pass sequencing than microarray genotyping, likely because the sequenced key ancestor animals do not represent the full haplotype diversity of the SLW populations which precludes the imputation of rarer sites that predominantly occur in the heterozygous state. High-coverage sequencing of few additional animals that carry rare haplotypes may mitigate this ascertainment bias [58] and increase the accuracy of genotyping by low-pass sequencing, particularly for rare alleles. While a subset of the 22.62 million variants obtained is sufficient to accurately predict genomic breeding values, the full variant catalogue, once available for a large mapping cohort, will facilitate powerful genome-wide association studies at nucleotide resolution.
Conclusions
The high-coverage sequencing of 70 key ancestor animals from two SLW lines and subsequent reference-guided variant discovery revealed 26,862,369 polymorphic sites. Population-genetic analyses suggest considerable genetic differentiation between both lines. Our results indicate that the key ancestor genomes may serve as a haplotype reference panel for genotyping by low-pass sequencing at high accuracy in the Swiss pig breeds. Using genotyping by low-pass sequencing increases the variant density over the currently used microarray by > 350-fold, thus providing a valuable resource for powerful genome-wide association testing.
Methods
Animals and whole-genome sequencing
Whole genome sequence data were generated for 70 boars. Sixty-five boars (32 from the dam line and 33 from the sire line) were selected based on their marginal genetic contribution to the current breeding populations using a key ancestor approach [3, 4]. The marginal genetic contribution was estimated based on a numerator relationship matrix that was constructed using the PyPedal python package [59]. The effective population size of the sire and dam line was estimated based on the difference in pedigree-derived inbreeding coefficients between active breeding animals and their parents following eq. (3) presented in Leroy et al. [60]. The inbreeding coefficients were extracted from the numerator relationship matrix. Animals born after 01.01.2018 were considered as active breeding animals. In addition, we considered whole-genome sequence data from five boars from the sire line that were generated previously [39]. DNA was prepared from preserved blood samples that were provided by SUISAG (the Swiss competence center for pig breeding). No animals were specially sampled for the present study. Illumina TruSeq PCR-free libraries with insert sizes of 350 bp were prepared and sequenced with an Illumina NovaSeq6000 instrument using 2 × 150 bp paired-end reads.
Alignment quality, read mapping and depth of coverage
We used the fastp software [61] to remove adapter sequences and reads that had Phred-scaled quality less than 15 for more than 15% of the bases. Subsequently, the filtered reads were aligned to the SSC11.1 assembly of the porcine genome [62] using the mem-algorithm of the BWA software [63]. The Picard tools software suite [64] and Sambamba [65] were applied to mark duplicate reads and sort the alignments by coordinates, respectively. To calculate depth of coverage, we extracted the number of reads covering a genomic position using the mosdepth software [66]. For the coverage calculation, we discarded reads with mapping quality < 10 and SAM bitwise flag value of 1796.
Variant calling
We used the BaseRecalibrator module of the Genome Analysis Toolkit (GATK - version 4.1.0 [19];) to adjust the base quality scores while supplying 63,881,592 unique positions from the porcine dbSNP version 150 as known variants. We applied the HaplotypeCaller, GenomicsDBImport and GenotypeGVCFs modules from the GATK to discover and genotype SNP and INDEL in the 70 SLW pigs together with 28 samples from various breeds that were sequenced earlier. Subsequently, we applied the VariantFiltration module of the GATK according to best practice recommendations for site-level hard filtration to retain high-quality variants. Beagle (version 4.1 [18];) haplotype phasing and imputation was applied to impute sporadically missing sites and improve the primary genotypes obtained using the GATK.
The concordance between sequence- and array called genotypes was calculated for 68 pigs that also had Illumina PorcineSNP60 BeadChip microarray-derived genotypes. We considered only autosomal SNP. We converted the TOP/BOT alleles of the microarray-derived genotypes to REF/ALT allele coding to make them compatible with the sequence-derived genotypes. This was possible for 54,600 SNP. Sequence variant genotyping accuracy was quantified using genotypic concordance, non-reference sensitivity and non-reference discrepancy [19, 44].
Invariant sites and variants within regions with an excessive depth of coverage (> mean coverage + 2 * SD) were removed using VCFtools (v. 0.1.16 [67];). The resulting data were split into two datasets containing 23,774,053 and 23,531,919 variants segregating in 32 boars from the dam line and 38 boars from the sire line, respectively.
Functional annotation
Functional consequences of the variants (including SIFT scores [21] for missense variants) were predicted with the Ensembl Variant Effect Predictor (VEP, version 91.3 [20];) using local cache files from Ensembl release 98. The transition to transversion ratio (Ti/Tv) was calculated using BCFtools command stats (version 1.8 [68];).
Detection of mendelian trait-associated variants and coverage analysis
We downloaded genomic coordinates of 47 likely causal variants from the Online Mendelian Inheritance in Animals (OMIA) database [22]. Genes harboring likely causal variants for which the genomic coordinates were not annotated according to SSC11.1 were manually inspected. Read alignments and sequence coverage in regions harboring known larger structural variants were manually inspected.
Population structure and genetic diversity analysis
The structure of the two lines was investigated using ADMIXTURE (v1.3.0 [69];). To avoid confounding due to extensive linkage disequilibrium (LD), we removed correlated loci based on high levels (r2 > 0.6) of pairwise LD using PLINK (version 1.9 [70];) with the “--indep-pairwise 100 25 0.6” option before running the ADMIXTURE analysis. The number of ancestral clusters (K) was set from 1 to 3, and five-fold cross-validation was performed to determine the K value with the lowest cross-validation error.
A genomic relationship matrix was built using 23,691,198 autosomal sequence variants that had a minor allele frequency higher than 0.01 using PLINK. The principal components of the genomic relationship matrix were calculated using the GCTA (version 1.92.1 [71];) software. We applied the GCTA flag “--grm-singleton” to identify four pairs of animals with relationship coefficients ranging from 0.32 to 0.37. One animal from each pair was removed for the FST and signature of selection analyses (1 from the dam line and 3 from the sire line).
We calculated the weighted genome wide fixation index (FST, [72]) based on pairwise differences in the variances of allele frequencies using 24,926,366 biallelic variants. FST values were calculated in 10 kb sliding windows with an overlap of 5 kb using the “--weir-fst-pop” flag of VCFtools (v.1.2.11 [67];). The manhattan plot was constructed using the R package qqman [73].
Nucleotide diversity (π) was calculated over all biallelic autosomal variants in 10 kb sliding windows with an overlap of 5 kb using VCFtools.
Runs of homozygosity (ROH) were estimated with BCFtools/ROH [74] using the GATK-derived genotypes (containing the Phred-scaled likelihoods). We considered biallelic SNP that had non-missing genotypes in all animals (maximal missing count per site was set to 0). According to Tortereau et al. [75], we assumed a constant recombination rate of 0.7 cM/Mb along the chromosomes. Average genomic inbreeding (FROH) was calculated assuming an autosomal genome length of 2,265,774,640 bases. Following a recent study by Bhati et al. [76], we classified the ROH based on their length (short: 50–100 kb, medium: 100 kb - 2 Mb, long: > 2 Mb).
Signatures of selection (CLR and iHS) and candidate regions
Putative signatures of selection were detected using integrated Haplotype Scores (iHS) and composite likelihood ratios (CLR). The iHS [77] reveals ‘soft sweeps’, i.e., signatures of selection where selection for beneficial alleles is still ongoing. The CLR [78] reveals ‘hard sweeps’, i.e., signatures of selection where beneficial alleles recently reached fixation. We considered 24,926,366 autosomal biallelic SNP from 31 and 35 boars from the dam and sire line, respectively. The genotypes were phased using Beagle (version 5.1 [79];) with disabled imputation and effective population size set to 50. The CLR statistic was calculated chromosome-wise with the SweepFinder2 software [80] using a pre-computed empirical allele frequency spectrum and 100 kb spacing between test sites (−lg 100,000). Using the R package rehh 2.0 [81], we applied the function scan_hh to estimate the integrated extended haplotype homozygosity (EHH) on variants with MAF > 0.05 for each chromosome separately. Subsequently, we applied the function ihh2ihs to obtain standardized iHS values in 100 kb non-overlapping windows.
The function calc_candidate_regions from the rehh 2.0 package [81] was applied to select candidate signatures of selection in 100 kb windows using the parameters “window_size = 1E6”, “overlap = 1E5”, “pval = F” and “min_n_extr_mrk = 1”. Empirical significance thresholds were chosen after visual inspection of the distribution of the test statistics (0.1% in iHS and 0.5% in CLR). Genes overlapping with candidate signatures of selections were determined based on the Ensembl (release 98) annotation of the porcine genome.
Analysis of low-pass sequence data
A median number of 16,131,419 paired-end (2x150bp) reads were generated for 96 pigs from the dam line and 96 pigs from the sire line. Adapter sequences and bases and reads with low sequencing quality were removed with fastp [61]. Subsequently, the reads were aligned to the porcine reference genome (SSC11.1) using the mem-algorithm of BWA [63] and duplicate reads were marked using Samblaster [82]. Following the read alignment, six samples were excluded because the mapping rate and the proportion of properly paired reads was less than 70 and 75%, respectively. Additionally, we excluded 10 samples for which the average coverage was less than 0.2-fold and one sample for which ancestry could not be verified.
To compile the reference haplotypes, we retained 22,618,811 biallelic autosomal SNP that were polymorphic (minor allele count ≥1) among the 70 key ancestor pigs. Following the approach proposed by Rubinacci et al. [8], we used the mpileup and call commands of BCFtools [68] to calculate genotype likelihoods at the 22,618,811 polymorphic sites in the 175 low-pass sequenced and reference-aligned samples. Subsequently, we applied the phasing and imputation algorithm implemented in GLIMPSE_phase [8] to refine the BCFtools-derived genotype calls using the previously established haplotype reference panel. This approach produced genotypes at 22,618,811 sites for the 175 low-pass sequenced samples. A genomic relationship matrix among the low-pass sequenced animals was constructed from the low-pass sequencing data-derived genotypes using GCTA [71].
Supplementary Information
Additional file 1 Fig. S1. Admixture analysis. Ancestry of 70 pigs with K = 2 ancestral populations estimated using 1,207,189 biallelic SNPs after LD pruning. Ancestry proportions were estimated using the ADMIXTURE software. Each bar represents an individual and the colors indicate the proportion of genes originating from K ancestral populations. The animals are ordered by population.
Additional file 2 Fig. S2. Manhattan plot of FST values. Weir and Cockerham FST estimates were calculated in 10 kb sliding windows between 31 dam and 35 sire boars. Black dotted line indicates a value of 0.25.
Additional file 3 Table S1. List of variants listed in the OMIA database and their corresponding frequency in the two pig lines.
Additional file 4 Fig. S3. Depth of coverage at a region on SSC8 encompassing the KIT gene. Representative plots of different depth of coverage detected in the sequenced pigs at a large duplication (DUP1 - SSC8: 41,223,212 - 41,783,660 bp) encompassing the KIT gene. Grey vertical bars represent the absolute coverage observed in four animals. The green dotted lines represent the median and 2*median coverage along SSC8. In order to determine the number of extra copies, we divided for each sequenced animal the average coverage observed at SSC8 by the average coverage observed at DUP1 (chr8: 41,223,212 - 41,783,660 bp). The number of additional copies ranged from 1 to 4. 16, 27, 4, 20, 2 and 1 animal had 1.5–1.9x, 2x, 2.1–2.4x, 2.5–2.9x and 3x the average coverage of SSC8 at DUP1, respectively. The average copy number was 2.07 and 2.19 in the dam and sire line, respectively. The 560 kb duplication (DUP1) encompasses two smaller duplications DUP2 and DUP3/4. DUP2 is 4.3 kb long and upstream, while DUP3 and DUP4 are 23 kb and 4.3 kb duplications downstream of the KIT gene.
Additional file 5 Table S2. Candidate signatures of selection based on CLR and iHS analyses. Genes annotated to the region are given for each signature of selection.
Additional file 6 Fig. S4. Principal components analysis of key ancestor and low-pass sequenced animals. Plot of the first two principal components showing the relationship of 96 dam and 96 sire animals sequenced at low (< 1.5-fold) coverage and 32 dam and 38 sire animals sequenced at high (~ 16.5-fold) coverage.
Acknowledgements
We acknowledge Gencove (https://gencove.com/) for providing support to obtain low-pass sequencing data.
Abbreviations
- CLR
Composite likelihood ratio
- FST
Fixation index
- iHS
Integrated haplotype score
- INDEL
Insertions and deletions
- LD
Linkage disequilibrium
- MAF
Minor allele frequency
- OMIA
Online Mendelian Inheritance in Animals
- PCA
Principal component analysis
- QTL
Quantitative trait loci
- ROH
Run of homozygosity
- SLW
Swiss Large White
- SNP
Single nucleotide polymorphism
- Ti/Tv
Transition/transversion ratio
- WGS
Whole-genome sequencing
Authors’ contributions
Analysis of whole-genome sequencing data: AN MB NKK DC HP; Conceived and designed the experiments: AN HP; Conceptualisation: HP AH; Secured funding: HP SN; Wrote the paper: AN HP; Critically revised the manuscript: all authors; Read and approved the final version of the manuscript: all authors.
Funding
This study was financially supported by SUISAG, Micarna SA and the ETH Zürich Foundation. The funding body was not involved in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
Raw sequencing read data of all key ancestor animals are available at the European Nucleotide Archive (ENA) (http://www.ebi.ac.uk/ena) of the EMBL at BioProject PRJEB38156 and PRJEB39374.
Declarations
Ethics approval and consent to participate
No animals were sampled for the present study. Thus, no ethical approval was required.
Consent for publication
Not applicable.
Competing interests
AH is employee of SUISAG (the Swiss pig breeding and competence centre). HP is a member of the editorial board of BMC Genomics. All other authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.SUISAG. https://www.suisag.ch/. Accessed 5 Apr 2021.
- 2.Ramos AM, Crooijmans RPMA, Affara NA, Amaral AJ, Archibald AL, Beever JE, et al. Design of a high density SNP genotyping assay in the pig using SNPs identified and characterized by next generation sequencing technology. PLoS One. 2009;4:e6524. doi: 10.1371/journal.pone.0006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonen S, Ros-Freixedes R, Battagin M, Gorjanc G, Hickey JM. A method for the allocation of sequencing resources in genotyped livestock populations. Genet Sel Evol. 2017;49:1–16. doi: 10.1186/s12711-017-0322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goddard ME, Hayes BJ. Genomic selection based on dense genotypes inferred from sparse genotypes. Proc Assoc Advmt Anim Breed Genet. 2009;18.
- 5.Jansen S, Aigner B, Pausch H, Wysocki M, Eck S, Benet-Pagès A, et al. Assessment of the genomic variation in a cattle population by re-sequencing of key animals at low to medium coverage. BMC Genomics. 2013;14:446. doi: 10.1186/1471-2164-14-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daetwyler HD, Capitan A, Pausch H, Stothard P, van Binsbergen R, Brøndum RF, et al. Whole-genome sequencing of 234 bulls facilitates mapping of monogenic and complex traits in cattle. Nat Genet. 2014;46:858–865. doi: 10.1038/ng.3034. [DOI] [PubMed] [Google Scholar]
- 7.Bovo S, Ribani A, Muñoz M, Alves E, Araujo JP, Bozzi R, et al. Whole-genome sequencing of European autochthonous and commercial pig breeds allows the detection of signatures of selection for adaptation of genetic resources to different breeding and production systems. Genet Sel Evol. 2020;52:1–19. doi: 10.1186/s12711-020-00553-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubinacci S, Ribeiro DM, Hofmeister RJ, Delaneau O. Efficient phasing and imputation of low-coverage sequencing data using large reference panels. Nat Genet. 2021;53:120–126. doi: 10.1038/s41588-020-00756-0. [DOI] [PubMed] [Google Scholar]
- 9.Gusev A, Shah MJ, Kenny EE, Ramachandran A, Lowe JK, Salit J, et al. Low-pass genome-wide sequencing and variant inference using identity-by-descent in an isolated human population. Genetics. 2012;190:679–689. doi: 10.1534/genetics.111.134874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zan Y, Payen T, Lillie M, Honaker CF, Siegel PB, Carlborg Ö. Genotyping by low-coverage whole-genome sequencing in intercross pedigrees from outbred founders: a cost-efficient approach. Genet Sel Evol. 2019;51:1–11. doi: 10.1186/s12711-019-0487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pausch H, MacLeod IM, Fries R, Emmerling R, Bowman PJ, Daetwyler HD, et al. Evaluation of the accuracy of imputed sequence variant genotypes and their utility for causal variant detection in cattle. Genet Sel Evol. 2017;49:24. doi: 10.1186/s12711-017-0301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Berg S, Vandenplas J, van Eeuwijk FA, Bouwman AC, Lopes MS, Veerkamp RF. Imputation to whole-genome sequence using multiple pig populations and its use in genome-wide association studies. Genet Sel Evol. 2019;51:1–13. doi: 10.1186/s12711-019-0445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Binsbergen R, Bink MCAM, Calus MPL, van Eeuwijk FA, Hayes BJ, Hulsegge I, et al. Accuracy of imputation to whole-genome sequence data in Holstein Friesian cattle. Genet Sel Evol. 2014;46:41. doi: 10.1186/1297-9686-46-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ros-Freixedes R, Whalen A, Gorjanc G, Mileham AJ, Hickey JM. Evaluation of sequencing strategies for whole-genome imputation with hybrid peeling. Genet Sel Evol. 2020;52. [DOI] [PMC free article] [PubMed]
- 15.Li JH, Mazur CA, Berisa T, Pickrell JK. Low-pass sequencing increases the power of GWAS and decreases measurement error of polygenic risk scores compared to genotyping arrays. Genome Res. 2021:gr.266486.120. 10.1101/gr.266486.120. [DOI] [PMC free article] [PubMed]
- 16.Wasik K, Berisa T, Pickrell JK, Li JH, Fraser DJ, King K, et al. Comparing low-pass sequencing and genotyping for trait mapping in pharmacogenetics. BMC Genomics. 2021;22:197. doi: 10.1186/s12864-021-07508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Auwera GA, Carneiro MO, Hartl C, Poplin R, del Angel G, Levy-Moonshine A, et al. From fastQ data to high-confidence variant calls: The genome analysis toolkit best practices pipeline. Curr Protocols Bioinformat. 2013;43(SUPL.43). 10.1002/0471250953.bi1110s43. [DOI] [PMC free article] [PubMed]
- 18.Browning SR, Browning BL. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am J Hum Genet. 2007;81:1084–1097. doi: 10.1086/521987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Depristo MA. Banks E, poplin R, Garimella K v., Maguire JR, Hartl C, et al. a framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–501. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GRS, Thormann A, et al. The Ensembl variant effect predictor. Genome Biol. 2016;17. [DOI] [PMC free article] [PubMed]
- 21.Sim NL, Kumar P, Hu J, Henikoff S, Schneider G, Ng PC. SIFT web server: predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012;40:W452–W457. doi: 10.1093/nar/gks539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholas FW. Online Mendelian inheritance in animals (OMIA): a record of advances in animal genetics, freely available on the internet for 25 years. Anim Genet. 2021;52:3–9. doi: 10.1111/age.13010. [DOI] [PubMed] [Google Scholar]
- 23.Sun G, Liang X, Qin K, Qin Y, Shi X, Cong P, et al. Functional analysis of KIT gene structural mutations causing the porcine dominant white phenotype using genome edited mouse models. Front Genet. 2020;11:138. doi: 10.3389/fgene.2020.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marklund S, Kijas J, Rodriguez-Martinez H, Ronnstrand L, Funa K, Moller M, et al. Molecular basis for the dominant white phenotype in the domestic pig. Genome Res. 1998;8:826–833. doi: 10.1101/gr.8.8.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubin C-JJ, Megens H-JJ, Barrio AM, Maqbool K, Sayyab S, Schwochow D, et al. Strong signatures of selection in the domestic pig genome. Proc Natl Acad Sci U S A. 2012;109:19529–19536. doi: 10.1073/pnas.1217149109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Z, Deng Z, Huang M, Hou Y, Zhang H, Chen H, et al. Whole-Genome Resequencing Identifies KIT New Alleles That Affect Coat Color Phenotypes in Pigs. Frontiers Genet. 2019;10 MAR:218. doi: 10.3389/fgene.2019.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia Q, Cao C, Tang H, Zhang Y, Zheng Q, Wang X, et al. A 2-bp insertion (c.67_68insCC) in MC1R causes recessive white coat color in Bama miniature pigs. J Genet Genom. 2017;44:215–217. doi: 10.1016/j.jgg.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Kijas JMH, Wales R, Törnsten A, Chardon P, Moller M, Andersson L. Melanocortin receptor 1 (MC1R) mutations and coat color in pigs. Genetics. 1998;150:1177–1185. doi: 10.1093/genetics/150.3.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meijerink E, Neuenschwander S, Fries R, Dinter A, Bertschinger HU, Stranzinger G, et al. A DNA polymorphism influencing a(1,2) fucosyltransferase activity of the pig FUT1 enzyme determines susceptibility of small intestinal epithelium to Escherichia coli F18 adhesion. Immunogenetics. 2000;52:129–136. doi: 10.1007/s002510000263. [DOI] [PubMed] [Google Scholar]
- 30.Neuditschko M, Raadsma HW, Khatkar MS, Jonas E, Steinig EJ, Flury C, et al. Identification of key contributors in complex population structures. PLoS One. 2017;12. 10.1371/journal.pone.0177638. [DOI] [PMC free article] [PubMed]
- 31.Poncet PA, Pfister W, Muntwyler J, Glowatzki-Mullis ML, Gaillard C. Analysis of pedigree and conformation data to explain genetic variability of the horse breed Franches-Montagnes. J Anim Breed Genet. 2006;123:114–121. doi: 10.1111/j.1439-0388.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- 32.Pausch H, Aigner B, Emmerling R, Edel C, Götz KU, Fries R. Imputation of high-density genotypes in the Fleckvieh cattle population. Genet Sel Evol. 2013;45:3. doi: 10.1186/1297-9686-45-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonen S, Ros-Freixedes R, Battagin M, Gorjanc G, Hickey JM. A method for the allocation of sequencing resources in genotyped livestock populations. Genet Sel Evol. 2017;49. [DOI] [PMC free article] [PubMed]
- 34.Wang J, Raskin L, Samuels DC, Shyr Y, Guo Y. Genome measures used for quality control are dependent on gene function and ancestry. Bioinformatics. 2015;31:318–323. doi: 10.1093/bioinformatics/btu668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tong X, Hou L, He W, Mei C, Huang B, Zhang C, et al. Whole genome sequence analysis reveals genetic structure and X-chromosome haplotype structure in indigenous Chinese pigs. Sci Rep. 2020;10:1–10. doi: 10.1038/s41598-020-66061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bosse M, Megens H-JJ, Madsen O, Paudel Y, Frantz LAFF, Schook LB, et al. Regions of Homozygosity in the porcine genome: consequence of demography and the recombination landscape. PLoS Genet. 2012;8:e1003100. doi: 10.1371/journal.pgen.1003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang C, Plastow G. Genomic diversity in pig (Sus scrofa) and its comparison with human and other livestock. Curr Genom. 2011;12:138–146. doi: 10.2174/138920211795564386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crysnanto D, Pausch H. Bovine breed-specific augmented reference graphs facilitate accurate sequence read mapping and unbiased variant discovery. 2019;21:1–27. 10.1101/2019.12.20.882423. [DOI] [PMC free article] [PubMed]
- 39.Nosková A, Hiltpold M, Janett F, Echtermann T, Fang Z-H, Sidler X, et al. Infertility due to defective sperm flagella caused by an intronic deletion in DNAH17 that perturbs splicing. Genetics. 2020. 10.1093/genetics/iyaa033. [DOI] [PMC free article] [PubMed]
- 40.Cole JB. A simple strategy for managing many recessive disorders in a dairy cattle breeding program. Genet Sel Evol. 2015;47:94. doi: 10.1186/s12711-015-0174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Derks MFL, Megens HJ, Bosse M, Lopes MS, Harlizius B, Groenen MAM. A systematic survey to identify lethal recessive variation in highly managed pig populations. BMC Genomics. 2017;18:1–12. doi: 10.1186/s12864-017-4278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pausch H, Schwarzenbacher H, Burgstaller J, Flisikowski K, Wurmser C, Jansen S, et al. Homozygous haplotype deficiency reveals deleterious mutations compromising reproductive and rearing success in cattle. BMC Genomics. 2015;16:312. doi: 10.1186/s12864-015-1483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai Z, Sarup P, Ostersen T, Nielsen B, Fredholm M, Karlskov-Mortensen P, et al. Animal Genetics and Genomics Genomic diversity revealed by whole-genome sequencing in three Danish commercial pig breeds. 2020;98:1–12. [DOI] [PMC free article] [PubMed]
- 44.Crysnanto D, Wurmser C, Pausch H. Accurate sequence variant genotyping in cattle using variation-aware genome graphs. Genet Sel Evol. 2019;51:1–15. doi: 10.1186/s12711-019-0462-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor JF, Whitacre LK, Hoff JL, Tizioto PC, Kim J, Decker JE, et al. Lessons for livestock genomics from genome and transcriptome sequencing in cattle and other mammals. Genet Sel Evol. 2016;48:59. doi: 10.1186/s12711-016-0237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramos-Onsins SE, Burgos-Paz W, Manunza A, Amills M. Mining the pig genome to investigate the domestication process. Heredity. 2014;113:471–484. doi: 10.1038/hdy.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zanella R, Peixoto JO, Cardoso FF, Cardoso LL, Biegelmeyer P, Cantão ME, et al. Genetic diversity analysis of two commercial breeds of pigs using genomic and pedigree data. Genet Sel Evol. 2016;48:24. doi: 10.1186/s12711-016-0203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang J, Li W-R, Lv F-H, He S-G, Tian S-L, Peng W-F, et al. Whole-genome sequencing of native sheep provides insights into rapid adaptations to extreme environments. Mol Biol Evol. 2016;33:2576–2592. doi: 10.1093/molbev/msw129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holm B, Bakken M, Klemetsdal G, Vangen O. Genetic correlations between reproduction and production traits in swine. J Anim Sci. 2004;82:3458–3464. doi: 10.2527/2004.82123458x. [DOI] [PubMed] [Google Scholar]
- 50.Johansson AM, Pettersson ME, Siegel PB, Carlborg Ö. Genome-wide effects of long-term divergent selection. PLoS Genet. 2010;6. [DOI] [PMC free article] [PubMed]
- 51.Kemper KE, Saxton SJ, Bolormaa S, Hayes BJ, Goddard ME. Selection for complex traits leaves little or no classic signatures of selection. BMC Genomics. 2014;15:246. doi: 10.1186/1471-2164-15-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feder AF, Kryazhimskiy S, Plotkin JB. Identifying signatures of selection in genetic time series. Genetics. 2014;196:509–522. doi: 10.1534/genetics.113.158220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kardos M, Luikart G, Bunch R, Dewey S, Edwards W, McWilliam S, et al. Whole-genome resequencing uncovers molecular signatures of natural and sexual selection in wild bighorn sheep. Mol Ecol. 2015;24:5616–5632. doi: 10.1111/mec.13415. [DOI] [PubMed] [Google Scholar]
- 54.Pavlidis P, Jensen JD, Stephan W, Stamatakis A. A critical assessment of storytelling: gene ontology categories and the importance of validating genomic scans. Mol Biol Evol. 2012;29:3237–3248. doi: 10.1093/molbev/mss136. [DOI] [PubMed] [Google Scholar]
- 55.Qanbari S, Pausch H, Jansen S, Somel M, Strom TM, Fries R, et al. Classic selective sweeps revealed by massive sequencing in cattle. PLoS Genet. 2014;10:e1004148. doi: 10.1371/journal.pgen.1004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snelling WM, Hoff JL, Li JH, Kuehn LA, Keel BN, Lindholm-Perry AK, et al. Assessment of imputation from low-pass sequencing to predict merit of beef steers. Genes. 2020;11:1–16. doi: 10.3390/genes11111312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Forni S, Aguilar I, Misztal I. Different genomic relationship matrices for single-step analysis using phenotypic, pedigree and genomic information. Genet Sel Evol. 2011;43:1. doi: 10.1186/1297-9686-43-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clark AG, Hubisz MJ, Bustamante CD, Williamson SH, Nielsen R. Ascertainment bias in studies of human genome-wide polymorphism. Genome Res. 2005;15:1496–1502. doi: 10.1101/gr.4107905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cole JB. PyPedal: a computer program for pedigree analysis. Comput Electron Agric. 2007;57:107–113. doi: 10.1016/j.compag.2007.02.002. [DOI] [Google Scholar]
- 60.Leroy G, Mary-Huard T, Verrier E, Danvy S, Charvolin E, Danchin-Burge C. Methods to estimate effective population size using pedigree data: examples in dog, sheep, cattle and horse. Genet Sel Evol. 2013;45:1. doi: 10.1186/1297-9686-45-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen S, Zhou Y, Chen Y, Gu J. Fastp: an ultra-fast all-in-one FASTQ preprocessor. In: Bioinformatics. Oxford University Press; 2018. p. i884–90. [DOI] [PMC free article] [PubMed]
- 62.Warr A, Affara N, Aken B, Beiki H, Bickhart DM, Billis K, et al. An improved pig reference genome sequence to enable pig genetics and genomics research. GigaScience. 2020;9:1–14. doi: 10.1093/gigascience/giaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. 2013. http://arxiv.org/abs/1303.3997. .
- 64.Picard Toolkit. Broad institute, GitHub repository. 2019.
- 65.Tarasov A, Vilella AJ, Cuppen E, Nijman IJ, Prins P. Sambamba: fast processing of NGS alignment formats. Bioinformatics. 2015;31:2032–2034. doi: 10.1093/bioinformatics/btv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pedersen BS, Quinlan AR. Mosdepth: quick coverage calculation for genomes and exomes. Bioinformatics. 2018;34:867–868. doi: 10.1093/bioinformatics/btx699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, et al. The variant call format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27:2987–2993. doi: 10.1093/bioinformatics/btr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chang CC, Chow CC, Tellier LCAM, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang J, Hong Lee S, Goddard ME, Visscher PM. Genome-Wide Complex Trait Analysis (GCTA): Methods, Data Analyses, and Interpretations. Springer. 2013;:215–36. doi:10.1007/978-1-62703-447-0_9. [DOI] [PubMed]
- 72.Weir BS, Cockerham CC. No title. Evolution. 1984;38 https://pubmed.ncbi.nlm.nih.gov/28563791/.
- 73.Turner S. qqman: an R package for visualizing GWAS results using Q-Q and manhattan plots. J Open Source Softw. 2018;3:731. doi: 10.1101/005165. [DOI] [Google Scholar]
- 74.Narasimhan V, Danecek P, Scally A, Xue Y, Tyler-Smith C, Durbin R. BCFtools/RoH: a hidden Markov model approach for detecting autozygosity from next-generation sequencing data. Bioinformatics. 2016;32:1749–1751. doi: 10.1093/bioinformatics/btw044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tortereau F, Servin B, Frantz L, Megens HJ, Milan D, Rohrer G, et al. A high density recombination map of the pig reveals a correlation between sex-specific recombination and GC content. BMC Genomics. 2012;13:586. doi: 10.1186/1471-2164-13-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bhati M, Kadri NK, Crysnanto D, Pausch H. Assessing genomic diversity and signatures of selection in original Braunvieh cattle using whole-genome sequencing data. BMC Genomics. 2020;21:1–14. doi: 10.1186/s12864-020-6446-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Voight BF, Kudaravalli S, Wen X, Pritchard JK. A map of recent positive selection in the human genome. PLoS Biol. 2006;4:0446–0458. doi: 10.1371/journal.pbio.0040446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nielsen R, Williamson S, Kim Y, Hubisz MJ, Clark AG, Bustamante C. Genomic scans for selective sweeps using SNP data. Genome Res. 2005;15:1566–1575. doi: 10.1101/gr.4252305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Browning BL, Zhou Y, Browning SR. A one-penny imputed genome from next-generation reference panels. Am J Hum Genet. 2018;103:338–348. doi: 10.1016/j.ajhg.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Degiorgio M, Huber CD, Hubisz MJ, Hellmann I, Nielsen R. SweepFinder2: increased sensitivity, robustness and flexibility. Bioinformatics. 2016;32:1895–1897. doi: 10.1093/bioinformatics/btw051. [DOI] [PubMed] [Google Scholar]
- 81.Gautier M, Klassmann A, Vitalis R. rehh 2.0: a reimplementation of the R package rehh to detect positive selection from haplotype structure. In: Molecular Ecology Resources. Blackwell Publishing Ltd; 2017. p. 78–90. [DOI] [PubMed]
- 82.Faust GG, Hall IM. SAMBLASTER: fast duplicate marking and structural variant read extraction. Bioinformatics. 2014;30:2503–2505. doi: 10.1093/bioinformatics/btu314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1 Fig. S1. Admixture analysis. Ancestry of 70 pigs with K = 2 ancestral populations estimated using 1,207,189 biallelic SNPs after LD pruning. Ancestry proportions were estimated using the ADMIXTURE software. Each bar represents an individual and the colors indicate the proportion of genes originating from K ancestral populations. The animals are ordered by population.
Additional file 2 Fig. S2. Manhattan plot of FST values. Weir and Cockerham FST estimates were calculated in 10 kb sliding windows between 31 dam and 35 sire boars. Black dotted line indicates a value of 0.25.
Additional file 3 Table S1. List of variants listed in the OMIA database and their corresponding frequency in the two pig lines.
Additional file 4 Fig. S3. Depth of coverage at a region on SSC8 encompassing the KIT gene. Representative plots of different depth of coverage detected in the sequenced pigs at a large duplication (DUP1 - SSC8: 41,223,212 - 41,783,660 bp) encompassing the KIT gene. Grey vertical bars represent the absolute coverage observed in four animals. The green dotted lines represent the median and 2*median coverage along SSC8. In order to determine the number of extra copies, we divided for each sequenced animal the average coverage observed at SSC8 by the average coverage observed at DUP1 (chr8: 41,223,212 - 41,783,660 bp). The number of additional copies ranged from 1 to 4. 16, 27, 4, 20, 2 and 1 animal had 1.5–1.9x, 2x, 2.1–2.4x, 2.5–2.9x and 3x the average coverage of SSC8 at DUP1, respectively. The average copy number was 2.07 and 2.19 in the dam and sire line, respectively. The 560 kb duplication (DUP1) encompasses two smaller duplications DUP2 and DUP3/4. DUP2 is 4.3 kb long and upstream, while DUP3 and DUP4 are 23 kb and 4.3 kb duplications downstream of the KIT gene.
Additional file 5 Table S2. Candidate signatures of selection based on CLR and iHS analyses. Genes annotated to the region are given for each signature of selection.
Additional file 6 Fig. S4. Principal components analysis of key ancestor and low-pass sequenced animals. Plot of the first two principal components showing the relationship of 96 dam and 96 sire animals sequenced at low (< 1.5-fold) coverage and 32 dam and 38 sire animals sequenced at high (~ 16.5-fold) coverage.
Data Availability Statement
Raw sequencing read data of all key ancestor animals are available at the European Nucleotide Archive (ENA) (http://www.ebi.ac.uk/ena) of the EMBL at BioProject PRJEB38156 and PRJEB39374.