Abstract
Aims
Various drugs increase the risk of out-of-hospital cardiac arrest (OHCA) in the general population by impacting cardiac ion channels, thereby causing ventricular tachycardia/fibrillation (VT/VF). Dihydropyridines block L-type calcium channels, but their association with OHCA risk is unknown. We aimed to study whether nifedipine and/or amlodipine, often-used dihydropyridines, are associated with increased OHCA risk, and how these drugs impact on cardiac electrophysiology.
Methods and results
We conducted a case–control study with VT/VF-documented OHCA cases with presumed cardiac cause from ongoing population-based OHCA registries in the Netherlands and Denmark, and age/sex/index date-matched non-OHCA controls (Netherlands: PHARMO Database Network, Denmark: Danish Civil Registration System). We included 2503 OHCA cases, 10 543 non-OHCA controls in Netherlands, and 8101 OHCA cases, 40 505 non-OHCA controls in Denmark. To examine drug effects on cardiac electrophysiology, we performed single-cell patch-clamp studies in human-induced pluripotent stem cell-derived cardiomyocytes. Use of high-dose nifedipine (≥60 mg/day), but not low-dose nifedipine (<60 mg/day) or amlodipine (any-dose), was associated with higher OHCA risk than non-use of dihydropyridines [Netherlands: adjusted odds ratios (ORadj) 1.45 (95% confidence interval 1.02–2.07), Denmark: 1.96 (1.18–3.25)] or use of amlodipine [Netherlands: 2.31 (1.54–3.47), Denmark: 2.20 (1.32–3.67)]. Out-of-hospital cardiac arrest risk of (high-dose) nifedipine use was not further increased in patients using nitrates, or with a history of ischaemic heart disease. Nifedipine and amlodipine blocked L-type calcium channels at similar concentrations, but, at clinically used concentrations, nifedipine caused more L-type calcium current block, resulting in more action potential shortening.
Conclusion
High-dose nifedipine, but not low-dose nifedipine or any-dose amlodipine, is associated with increased OHCA risk in the general population. Careful titration of nifedipine dose should be considered.
Keywords: Sudden cardiac arrest, Nifedipine, Amlodipine, Epidemiology
Introduction
Sudden cardiac arrest causes up to 50% of all cardiovascular deaths in industrialized countries1 and most often occurs in the general population (out-of-hospital cardiac arrest, OHCA). OHCA predominantly results from lethal cardiac arrhythmias [ventricular tachycardia/ventricular fibrillation (VT/VF)] following disruptions in cardiac electrophysiology.2 Numerous factors may cause such disruptions by impacting cardiac ion channels. Many commonly prescribed drugs, even those prescribed for non-cardiac disease, impact cardiac ion channels and are associated with increased OHCA risk.3 The best-known risk drugs are drugs that block cardiac potassium channels, thereby impairing cardiac repolarization (QT-prolonging drugs).4–9
Emerging evidence also demonstrates the risk of non-cardiac drugs that impair cardiac depolarization10 by blocking cardiac sodium-channels.3,11,12 The possible OHCA risk of another type of depolarization-blocking drugs is less known: dihydropyridine calcium-channel blocking drugs. Dihydropyridines block L-type calcium-channels, primarily but not exclusively in vascular smooth muscle,13 and are generally prescribed to treat ischaemic heart disease (IHD) or hypertension. Use of nifedipine has been associated with increased risk of all-cause mortality.14 One proposed explanation was increased sympathetic stimulation and catecholamine release.15 Although these changes may trigger VT/VF, it is unknown whether this excess mortality resulted from OHCA.
In this study, we aimed to establish whether use of nifedipine or amlodipine (the two most widely used dihydropyridines in the Netherlands) is associated with increased OHCA risk. We performed a case–control study in the Netherlands and a replication case–control study in Denmark, using population-based emergency medical services (EMS)-attended OHCA registries in both settings to study whether these drugs are associated with increased OHCA risk. We performed subgroup analyses to address confounding by indication of IHD and/or use of beta-blocking drugs. In addition, we assessed cellular electrophysiologic properties of nifedipine and amlodipine, by performing single-cell patch-clamp studies in human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs).
Methods
Design and setting
We used a case–control design with OHCA cases from ongoing population-based EMS-attended OHCA registries in the Netherlands (Amsterdam REsuscitation STudies, ARREST) and Denmark (replication cohort: Danish Cardiac Arrest Registry, DANCAR), and non-OHCA controls (Netherlands: PHARMO Database Network, Denmark: general population through the Danish Civil Registration System). Both OHCA registries are part of the ESCAPE-NET consortium that studies OHCA across Europe.16 Cases were OHCA victims aged ≥18 years with documented VT/VF from presumed cardiac causes (excluding obvious non-cardiac causes). For both registries, each case was matched using exact matching on age at the date of OHCA (index-date), sex, and index-date with up to five controls who were alive on the index-date.
The study was approved by the Institutional Review Boards of the Academic Medical Center Amsterdam and the Danish Data Protection Agency (Ref.no. 2007-58-0015, local ref.no. GEH-2014-017, I-Suite.nr. 02735). In Denmark, ethical approval is not required for retrospective register-based studies in which individual patients cannot be identified.
The Netherlands
ARREST registry is an ongoing population-based observational registry that prospectively includes all OHCAs in one contiguous region of the Netherlands (2.4 million inhabitants, urban, and rural). Details of this registry are described elsewhere.17 In short, the ARREST study centre is notified by the dispatch centre of every suspected OHCA in which EMS are involved. ECGs are collected from automated external defibrillators or EMS manual defibrillators, whichever defibrillated first. Complete drug-dispensing records 1 year before index-date are obtained from the community pharmacist. All OHCA cases from 1 June 2005 to 31 December 2011 were included. Non-OHCA controls were sampled from PHARMO Database Network, which contains drug-dispensing records from community pharmacies.18 In the Netherlands, nearly all patients are registered at a single community pharmacy; therefore, medication records were considered complete.
Denmark
Out-of-hospital cardiac arrest cases were included in the DANCAR registry if they had attempts of resuscitation by a bystander or EMS. Capture of OHCA is nearly complete as the EMS are obliged to complete a case report form for every attended OHCA. Information on the first registered heart rhythm (shockable or non-shockable) was obtained from these forms, which constitute the DANCAR registry. Details of this registry are described elsewhere.19 All OHCA cases from 1 June 2001 to 31 December 2014 were included. A unique and permanent civil registration number is assigned to all Danish citizens upon birth or immigration. This allows individual-level linkage of information between nationwide registries in Denmark. Information on age, sex, and vital status were obtained from the Danish Civil Registration System.20 Data on hospital admissions were identified using the Danish National Patient Registry (one primary diagnosis and two or more secondary diagnoses if appropriate) according to the International Classification of Diseases (ICD); since 1994 the 10th revision (ICD-10) and before 1994 the 8th revision (ICD-8).21 Causes of death according to the ICD classifications were determined using the National Causes of Death Registry.22 Information on pharmacotherapy was obtained from The Danish Registry of Medicinal Product Statistics, which includes all drug-dispensing records from Danish pharmacies since 1995.23
Exposure definition
Current use was defined as a prescription starting in (ARREST) or covering (DANCAR) a period of maximally 90 days before the index-date. In DANCAR, daily dosage was estimated by calculating mean dosages from up to five consecutive prescriptions before the prescription of interest. Treatment duration was calculated by dividing the number of tablets in the prescription of interest by daily dosage, as described previously.24 Dose-response analyses were examined using the defined daily dose (DDD, the recommended average maintenance daily dose for a medication used for its main indication).25 Drug use was defined as low-dose (DDD < 2: nifedipine <60 mg/day, amlodipine <10 mg/day) or high-dose (DDD ≥ 2: nifedipine ≥60 mg/day, amlodipine ≥10 mg/day).
Covariates for out-of-hospital cardiac arrest risk
In both registries, covariates were assessed using cardiovascular drugs, drugs used for diabetes mellitus (used within 6 months before index date, listed in Table 1), and use (index-date within prescription duration) of Vaughan-Williams Class 1 or 3 antiarrhythmic drugs and/or common (≥1000 users/year) non-cardiac QT-prolonging drugs.26 In DANCAR, covariates associated with OHCA risk were assessed using additional comorbidity information from diagnosis codes of hospital admissions up to 10 years before OHCA.
Table 1.
Baseline characteristics of cases and controls
| ARREST |
DANCAR |
|||||
|---|---|---|---|---|---|---|
| Cases | Controls | P-value | Cases | Controls | P-value | |
| Total | 2503 | 10 543 | 8101 | 40 505 | ||
| Age (years), median (interquartile range) | 67.0 (57.0–77.0) | 66.0 (57.0–76.0) | NA | 68 (58–77) | 68 (58–77) | NA |
| Male sex | 1938 (77.4) | 8167 (77.5) | NA | 6435 (79.4) | 32 175 (79.4) | NA |
| Concomitant drug use | ||||||
| Beta-blockers | 855 (34.2) | 2338 (22.2) | <0.001 | 1948 (24.1) | 5330 (13.2) | <0.001 |
| Renin-angiotensin system inhibitors | 1007 (40.2) | 2778 (26.3) | <0.001 | 3339 (41.2) | 9351 (23.1) | <0.001 |
| Diuretics | 890 (35.6) | 2356 (22.3) | <0.001 | 3695 (45.6) | 10 279 (25.4) | <0.001 |
| Nitrates | 358 (14.3) | 574 (5.4) | <0.001 | 977 (12.1) | 1343 (3.3) | <0.001 |
| Statins | 831 (33.2) | 2613 (24.8) | <0.001 | 5649 (30.8) | 7615 (18.8) | <0.001 |
| Antithrombotics | 1090 (43.5) | 3004 (28.5) | <0.001 | 3713 (45.8) | 10 136 (25.0) | <0.001 |
| Non-dihyrdopyridine calcium antagonists | 102 (4.1) | 260 (2.5) | <0.001 | 397 (4.9) | 824 (2.0) | <0.001 |
| Antidiabetics | 399 (15.9) | 1135 (10.8) | <0.001 | 1034 (12.8) | 2882 (7.1) | <0.001 |
| Antiarrhythmic drugs class 1 and 3 | 54 (2.2) | 41 (0.4) | <0.001 | 117 (1.4) | 216 (0.5) | <0.001 |
| Non-antiarrhythmic QT-prolonging drugs | 133 (5.3) | 331 (3.1) | <0.001 | 619 (7.6) | 2597 (6.4) | <0.001 |
| Comorbidities | ||||||
| Peripheral vascular disease | 808 (10.0) | 1640 (4.1) | <0.001 | |||
| Cerebral vascular disease | 957 (11.8) | 3064 (7.6) | <0.001 | |||
| Ischaemic heart disease (including previous AMI) | 2511 (31.0) | 4609 (11.4) | <0.001 | |||
| Atrial fibrillation | 1440 (17.8) | 2561 (6.3) | <0.001 | |||
| Congestive heart failure | 1718 (21.2) | 1708 (4.2) | <0.001 | |||
| Chronic kidney disease | 384 (4.7) | 799 (2.0) | <0.001 | |||
| Chronic obstructive pulmonary disease | 643 (7.9) | 1721 (4.3) | <0.001 | |||
Numbers are expressed as n (%) unless indicated otherwise.
AMI, acute myocardial infarction; NA, not applicable.
Cellular electrophysiologic studies
The current generated by L-type calcium-channels (ICa,L) and action potentials (APs) were measured in individual hiPSC-CMs, prepared as described previously.27 Electrophysiologic measurements at 36 ± 0.2°C were performed 7–9 days after plating. ICa,L was measured in response to depolarizing voltage clamp steps from −70 to 0 mV using the ruptured patch-clamp technique. Dose-response curves for nifedipine and amlodipine were fitted to the Hill equation: Idrug/Icontrol=1/[1+(dose/IC50)n], where Idrug/Icontrol is normalized ICa,L, dose is bath concentration of the drug, IC50 is dose required for 50% current block, and n is the Hill coefficient. APs were measured in spontaneously beating hiPSC-CMs using the amphotericin-B-perforated patch-clamp technique. AP parameters analysed included: maximum diastolic potential, maximum AP-amplitude (APAmax), AP-duration at 20%, 50%, and 90% repolarization (APD20, APD50, and APD90), maximal upstroke velocity (Vmax), and plateau amplitude (APAplateau). Averages were taken from 10 consecutive APs. The effects of nifedipine and amlodipine on ICa,L were tested at steady-state (5 min after bath application), with increasing concentrations within one cell. More details are provided in Supplementary material online, Methods.
Statistical analysis
The χ2 test was used to compare baseline characteristics between cases and controls, and between the various covariates of study drug users. The Mann–Whitney test was used to compare age between cases and controls. We used conditional logistic regression to determine associations between exposure of interest and OHCA risk, applying two models. In Model 1, crude odd ratios (ORcrude) were calculated. In Model 2, the OR was adjusted (ORadj) for all confounders that were univariately significantly associated with OHCA and sufficiently powered (>5 exposed cases). Next, we performed stratified analyses regarding nitrate use (as proxy for IHD), beta-blocker use and history of IHD and/or previous acute myocardial infarction (AMI, only in DANCAR) and calculated Pinteraction using multivariable conditional logistic regression. Subgroup analysis were performed in subsets of patients classified by the presence of cardiovascular disease (in ARREST: defined as the use of any cardiovascular drugs listed in Table 1; in DANCAR: defined as patients who had a hospital contact for ≥1 cardiovascular disease up to 10 years before OHCA or the use of any cardiovascular drugs listed in Table 1). Paired and unpaired t-tests were used to test the effects of drugs and between drugs, respectively, on ICa,L and APs. We considered a two-sided P-value <0.05 statistically significant. Data are presented as OR [95% confidence interval (CI)].
Results
Patient characteristics
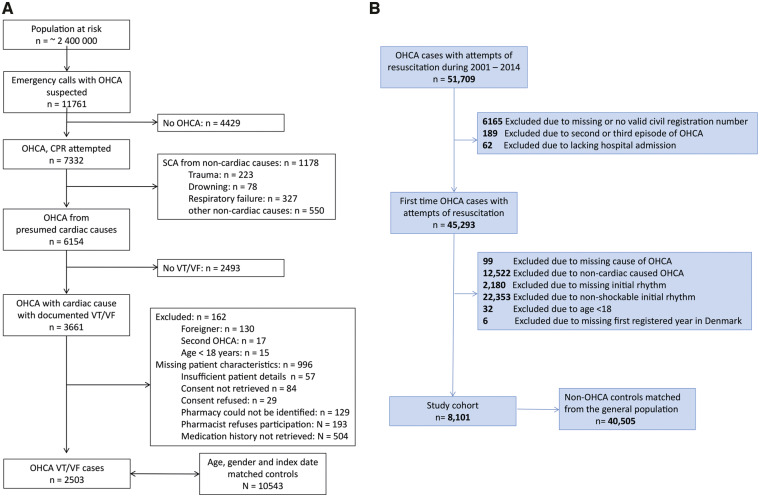
In ARREST, from a total of 3661 OHCA cases with cardiac causes and documented VT/VF, complete medication histories were obtained in 2503; these cases (median age 67.0 years, 77.4% male) were matched to 10 543 controls (Figure 1). In DANCAR, 8101 OHCA cases (median age 68.0 years, 79.4% male), were matched to 40 505 controls (Figure 1). In both registries, use of all studied drug categories and comorbidities (in DANCAR) was more prevalent among cases than controls (Table 1).
Figure 1.
Flowchart of inclusion of out-of-hospital cardiac arrest cases. (A) ARREST registry. (B) DANCAR registry. CPR, cardiopulmonary resuscitation; OHCA, out-of-hospital cardiac arrest; VT/VF, ventricular tachycardia/ventricular fibrillation.
Dihydropyridine use and out-of-hospital cardiac arrest risk
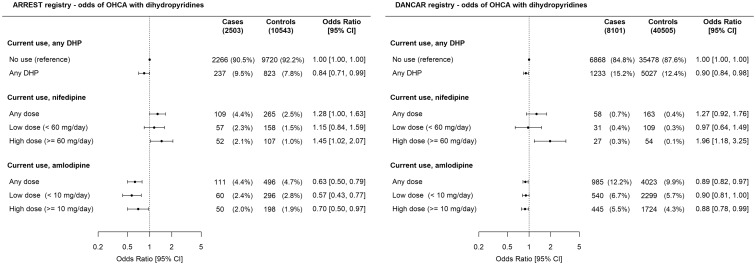
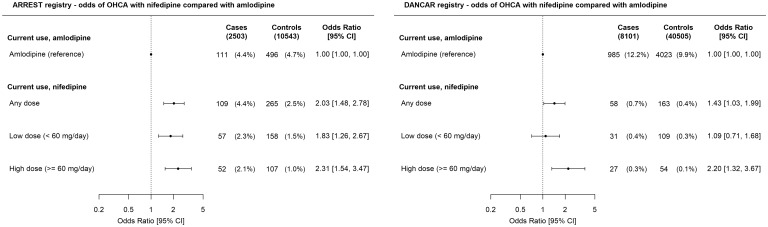
In ARREST, current use of any dihydropyridine was not associated with higher OHCA risk than no use of dihydropyridines [ORadj 0.84 (0.71–0.99), Figure 2]. Increased OHCA risk occurred among current users of nifedipine compared to no use of dihydropyridines [ORadj 1.28 (1.003–1.63), Figure 2] and was dose-dependent [low-dose ORadj 1.15 (0.84–1.59), high-dose ORadj 1.45 (1.02–2.07)]. In contrast, current use of amlodipine was associated with lower OHCA risk than no use of dihydropyridines [ORadj 0.63 (0.50–0.79)], but this lower OHCA risk was not dose-dependent [low-dose ORadj 0.57 (0.43–0.77), high-dose ORadj 0.70 (0.50–0.97)]. Accordingly, current use of nifedipine was associated with higher OHCA risk than current use of amlodipine [any-dose ORadj 2.03 (1.48–2.78), high-dose ORadj 2.31 (1.54–3.47), Figure 3]. In DANCAR, these key findings were similar: current use of high-dose nifedipine was associated with higher OHCA risk than no use of dihydropyridines [ORadj 1.96 (1.18–3.25), Figure 2] or current use of amlodipine [ORadj 2.20 (1.32–3.67), Figure 3], while current use of amlodipine was associated with lower OHCA risk than no use of dihydropyridines [ORadj 0.89 (0.82–0.97), Figure 2]. ORcrude is provided in Supplementary material online, Table S1.
Figure 2.
Current use of dihydropyridines, nifedipine, or amlodipine and out-of-hospital cardiac arrest risk compared with no use of any dihydropyridine. Numbers in table are n (%) unless indicated otherwise. Error bars denote 95% confidence interval. In ARREST, dose information was unavailable for one case and two controls; these individuals were not included in the Figure. CI, confidence interval; DHP, dihydropyridine.
Figure 3.
Current use of nifedipine and out-of-hospital cardiac arrest risk compared with current use of amlodipine. Numbers in table are n (%) unless indicated otherwise. Error bars denote 95% confidence interval. CI, confidence interval; OR, odds ratio.
To assess possible confounding, we studied whether concomitant medication use was different between nifedipine users and amlodipine users (Supplementary material online, Table S2). In both registries, there were no statistically significant differences. In DANCAR, there were also no statistically significant differences in comorbidities between nifedipine users and amlodipine users. In both registries, we found no statistically significant differences in concomitant medication use and comorbidities (in DANCAR) between low-dose and high-dose nifedipine users (Supplementary material online, Table S3). Moreover, in our subgroup analysis of patients with known cardiovascular disease, use of high-dose nifedipine was consistently associated with increased OHCA risk in both registries (Supplementary material online, Table S4).
We next studied whether OHCA risk was further increased in patients using nitrates (as proxy for IHD) by performing stratified analyses. We found that OHCA risk was not further increased in patients using nitrates (ARREST: any-dose nifedipine Pinteraction 0.098, high-dose nifedipine Pinteraction 0.050; DANCAR: any-dose nifedipine Pinteraction 0.546; high-dose nifedipine Pinteraction 0.857, Supplementary material online, Figure S1). Next, in DANCAR, we performed stratified analyses according to IHD or previous AMI status. We found that OHCA risk associated with any-dose nifedipine was not further increased in this group (DANCAR: any-dose nifedipine Pinteraction 0.079, high-dose nifedipine Pinteraction 0.949).
To study whether increased OHCA risk of nifedipine may be related to the effects of increased sympathetic stimulation, we conducted stratified analysis according to concomitant use of beta-blockers (which attenuate the effects of sympathetic stimulation). We found that OHCA risk associated with nifedipine was not altered in patients using beta-blockers (ARREST: any-dose nifedipine Pinteraction 0.405, high-dose nifedipine Pinteraction 0.226; DANCAR: any-dose nifedipine Pinteraction 0.766; high-dose nifedipine Pinteraction 0.796, Supplementary material online, Figure S1). Moreover, most nifedipine users among cases used its slow-release form (ARREST: N = 106 of 109, DANCAR: N = 55 of 58) which causes less sympathetic stimulation than short-acting nifedipine,28 and there were no cases who used high-dose short-acting nifedipine.
Cellular electrophysiologic studies
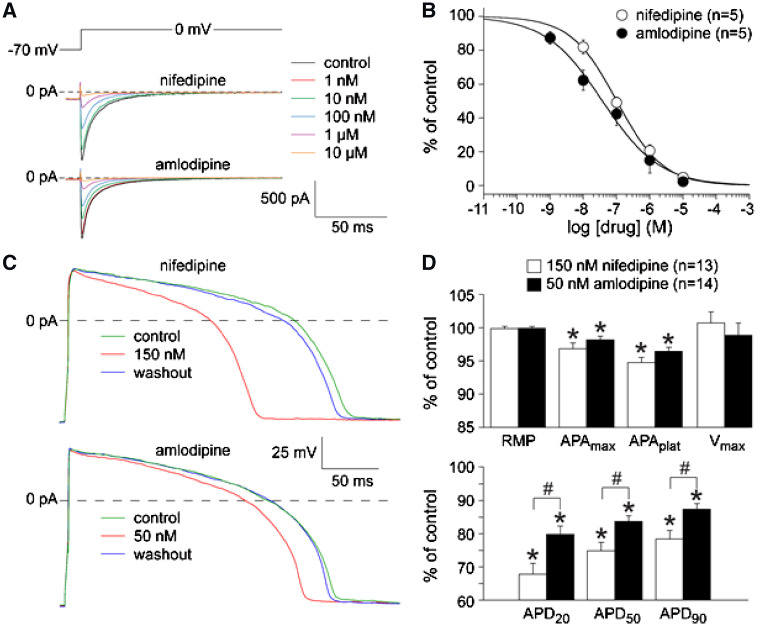
We studied whether the disparate associations between nifedipine or amlodipine use and OHCA risk could be explained by differences in cardiac electrophysiologic properties between both drugs. First, we studied the effects of nifedipine and amlodipine on ICa,L. Figure 4A shows typical total ICa,L recordings in the absence and presence of various nifedipine and amlodipine concentrations. Dose-response relationships of nifedipine and amlodipine (Figure 4B) were virtually overlapping [IC50 104 ± 13 nmol/L (nifedipine, n = 5) vs. 64 ± 30 nmol/L (amlodipine, n = 5), P = 0.22; Hill coefficients 0.66 ± 0.11 (nifedipine, n = 5) vs. 0.53 ± 0.05 (amlodipine, n = 5), P = 0.25]. Next, we tested the effects on APs of 150 nM nifedipine and 50 nM amlodipine (corresponding to maximal plasma concentrations of 60 mg/day nifedipine and 10 mg/day amlodipine, respectively29,30). Figure 4C shows typical APs, average effects are summarized in Figure 4D. Both drugs caused reversible AP-shortening and decrease of APAmax and APAplateau (Figure 4C and D), but these effects were larger for nifedipine (Figure 4D). For example, APD90 was reduced by 21.6 ± 2.5 and 12.7 ± 1.8% for nifedipine and amlodipine, respectively (P = 0.006).
Figure 4.
Effects of nifedipine and amlodipine on the L-type calcium current (ICa,L) and action potentials.(A) Typical ICa,L recordings in absence and presence of nifedipine (top) and amlodipine (bottom). Inset: voltage clamp protocol. (B) Average dose-response curves of nifedipine and amlodipine on ICa,L. Note that currents are normalized to current before drug application. Solid lines: Hill equation fits of average data. (C) Typical examples of APs in absence and presence of 150 nM nifedipine (top) and 50 nM amlodipine (bottom). Both drugs caused reversible AP-shortening. (D) Average effects of nifedipine (150 nM) and amlodipine (50 nM) on AP-properties. *P < 0.05 control conditions vs. drugs; #P < 0.05 nifedipine vs. amlodipine.
Discussion
In this observational study with real-world data from two large independent population-based OHCA registries in different countries, high-dose nifedipine, but not low-dose nifedipine or any-dose amlodipine, was associated with increased OHCA risk, independent of concomitant medication use or comorbidities. OHCA risk was not further increased in the presence of IHD. Furthermore, at clinically used concentrations, nifedipine caused more L-type calcium current block, resulting in more action potential shortening.
Previous studies have raised serious concerns about the long-term safety of L-type calcium-channel blockers, mainly short-acting dihydropyridines.15,31 Increased AMI risk upon use of high-dose short-acting calcium-channel blockers was reported among hypertension patients.31 Also, increased total mortality risk was found among IHD patients using high-dose short-acting nifedipine.15 One hypothesized mechanism for these findings is increased sympathetic tone,15 most pronounced for short-acting nifedipine.28 While reflex sympathetic activation during nifedipine use may have occurred secondary to rapid blood pressure drop among nifedipine users, we found that increased VT/VF risk still occurred among nifedipine users who concomitantly used beta-blockers (Supplementary material online, Figure S1); the latter drugs would block the effects of reflex sympathetic activation. Also, the vast majority of nifedipine users used its slow-release form; this form acts slowly and gradually, thereby not provoking rapid blood pressure drop and reflex stimulation of the sympathetic system. Supporting this notion, multiple randomized controlled trials were conducted to study the association between long-acting nifedipine and mortality risk,32–35 but none found increased risk of (all-cause) mortality. Finally, the presence of coronary steal by collateral arteries has been described during nifedipine use,36 but the results seem to be inconsistent.37 For instance, one study reported pro-ischaemic effects of nifedipine in chronic stable angina patients with good collateral flow and suggested that these findings could be mediated through coronary steal.36 However, another study found opposite effects of nifedipine administration.37 Also, while increased AMI risk was reported among users of short-acting calcium blockers,31 multiple randomized controlled trials found no evidence for increased AMI risk among users of long-acting calcium channel antagonists, while the vast majority of nifedipine users among the OHCA cases in our study used its slow-release form. Our study indicates no further increased OHCA risk among patients with IHD. To our knowledge, no studies yet have investigated whether long-acting dihydropyridine use is associated with increased OHCA risk. Such studies require a dedicated study design, in particular, to ascertain that OHCA resulted from cardiac arrhythmia (VT/VF) rather than from non-cardiac causes. Thus, ECG documentation of VT/VF during OHCA is required, but this is extremely challenging, because OHCA occurs suddenly and unexpectedly, and VF dissolves into asystole within minutes if left untreated. The often-used pragmatic definition of OHCA (without requirement of ECG documentation) of the European Society of Cardiology (‘event occurring within 1 h of symptom onset or, if unwitnessed, within 24 h of the victim being seen in good health’38) carries the risk of misclassification of non-cardiac causes. This is particularly relevant in our study, since hypertension patients (some of whom are treated with dihydropyridines) have increased risk of these non-cardiac causes (stroke, ruptured aneurysm). To overcome these difficulties, and to comprehensively study risk factors associated with OHCA, we set-up dedicated population-based OHCA registries (ARREST, DANCAR) and the ESCAPE-NET research network.16 These efforts now allow us to study more reliably the risk of OHCA from cardiac arrhythmia associated with dihydropyridine use.
Our findings indicate that increased OHCA risk of high-dose nifedipine may be related to specific drug effects, rather than a class effect. While we found no evidence in our epidemiologic studies that increased sympathetic stimulation is involved, our cellular electrophysiologic studies provided possible clues. Different dihydropyridines have distinct potencies to inhibit L-type calcium-channels in vascular smooth muscle cells or myocardium. For example, nifedipine has ∼10 times higher affinity for vascular cells than myocardium.39 Although nifedipine and amlodipine are prescribed because of their effects on vascular L-type calcium-channels, we found that both drugs significantly block cardiac ICa,L, thereby shortening AP-duration. AP-shortening may provoke VT/VF by facilitating re-entrant excitation, the predominant electrophysiologic mechanism of VT/VF. This is most clearly demonstrated by the rare inherited Short-QT-syndrome, which is associated with a high risk of OHCA.40 Conversely, AP-prolongation is the mechanism by which Classes IA and III antiarrhythmic drugs exert their therapeutic action. Thus, AP-shortening may contribute to the increase in OHCA risk of high-dose nifedipine. This may also explain why high-dose nifedipine, but not low-dose nifedipine or amlodipine, is associated with increased OHCA risk: high-dose nifedipine causes more AP-shortening than both other conditions. Of note, although amlodipine blocks cardiac L-type calcium-channels at similar concentrations as nifedipine, the extent of ICa,L block in clinical practice is lower for amlodipine than for nifedipine, because prescribed dosages (and plasma-concentrations) are significantly lower for amlodipine.
Our findings provide clues for the design of preventive strategies against this adverse drug effect of nifedipine. Most strategies against OHCA risk of drugs that impact on cardiac electrophysiology focus strongly on identifying vulnerable individuals, e.g. individuals with (genetic) vulnerability to excessive QT-prolongation when prescription of QT-prolonging medication is considered. Similarly, guidelines state that anti-arrhythmic sodium-channel blockers such as flecainide should be withheld from patients with acquired causes of reduced cardiac excitability, e.g. cardiac ischaemia and/or heart failure. In the case of nifedipine, the strategy may have to focus both on identifying vulnerable individuals and on limiting the height of prescribed dosages. Although dose-dependent reduction in blood pressure was shown in patients with hypertension41 and high-dose nifedipine was shown to better attenuate angina pectoris than low-dose nifedipine,42 the dose-effect relationships of nifedipine strongly differ between patients.43 Careful titration of nifedipine dose may have to be considered. Clearly, future studies are required to establish (i) whether nifedipine use confers higher OHCA risk than amlodipine use, (ii) whether vulnerable individuals are those who receive nifedipine for hypertension treatment, and (iii) whether lower dosages impact less on cardiac electrophysiology and OHCA risk, while retaining their beneficial effects on treatment for IHD and/or hypertension.
Strengths and limitations
A major strength of the ARREST and DANCAR registries is that documentation of VT/VF was present; this reduces risk of misclassification of patients who suffered OHCA from non-cardiac causes. Also, the population-based real-world design minimized selection bias by prospectively including every OHCA case in large contiguous regions representative for the community at large. Finally, our cellular electrophysiologic studies supported these epidemiologic findings by revealing differential effects on the AP that may explain these findings.
The observational nature of our epidemiologic studies comes with inherent limitations, e.g. the fact that we could only detect associations without proving causality. To gain more insight into a possible mechanistic explanation, we conducted cellular electrophysiologic studies using hiPSC-CMs. Although hiPSC-CMs are relatively immature compared to cardiomyocytes from an adult heart,44 ICa,L antagonists have similar effects in hiPSC-CMs to those observed in native cardiomyocytes.45 Another limitation is the lack of completeness of data from the Dutch cohort since almost one-third of the OHCA cases was not included primarily due to lack of medication history (Figure 1). However, we expect that incomplete data were distributed proportionally between users and non-users of nifedipine. Also, while nifedipine was used by 38% of dihydropyridine users in the Netherlands, this was only the case in 4% in Denmark. As a consequence, we were able to identify only 27 users of high-dose nifedipine at the time of OHCA in the Danish registry, which is also reflected in the wide CI (Figure 2). Another limitation is that, although drug-dispensing data were complete, we had no information whether claimed medications were actually taken in both cohorts. In any case, drug-dispensing records, used in both cohorts, are already one important step closer to actual intake than drug prescription records. Furthermore, we have no reason to assume that intake behaviour between nifedipine and amlodipine users would be different. Yet, possible misclassification arising from this was probably similarly distributed between cases and controls. To mitigate the limitations associated with use of medication proxies, we also used information on comorbidities in our multivariable analyses using DANCAR-data. This approach resulted in similar findings. Finally, there may have been confounding by indication, as cases and controls are different regarding concomitant drug use (Table 1), pointing towards cases in general having more comorbidity. However, it was very hard—if not practically impossible—to obtain data to prove that possible confounding was present or absent. We addressed this problem primarily by comparing OHCA risk of nifedipine use with amlodipine use. Moreover, we found no evidence that nifedipine users differed significantly from amlodipine users in demographic variables or their comorbidities (Supplementary material online, Table S2). In addition, the robustness of our findings regarding high-dose nifedipine was confirmed by a subgroup analysis in which we examined only patients with cardiovascular disease (Supplementary material online, Table S4). However, it is still possible that (unmeasured) residual confounders might have affected our observed associations.
Conclusion
High-dose nifedipine, but not low-dose nifedipine or any-dose of amlodipine, is associated with increased OHCA risk in the general population. Differences in cellular electrophysiologic properties of clinically used concentrations between both drugs were found.
Supplementary material
Supplementary material is available at European Heart Journal – Cardiovascular Pharmacotherapy online.
Supplementary Material
Acknowledgements
The authors greatly appreciate the contributions of Paulien Homma, Remy Stieglis, and Sandra de Haas for data management of the ARREST registry, and are greatly indebted to all participating EMS dispatch centres (Amsterdam, Haarlem, and Alkmaar), regional ambulance services (Ambulance Amsterdam, GGD Kennemerland, Witte Kruis, and Veiligheidsregio Noord-Holland Noord Ambulancezorg), fire brigades, and police departments in the study region for their contribution and support. The authors would also like to thank the pharmacists, PHARMO Database Network and Stichting Farmaceutische Kerngetallen. For completion of the case reports which form the Danish Cardiac Arrest Registry, the authors thank the Danish Emergency Medical Services.
Funding
This work was supported by the European Union’s Horizon 2020 research and innovation programme under the acronym ESCAPE-NET, registered under grant agreement No 733381 (T.E.E., M.T.B., and H.L.T.) and the Netherlands CardioVascular Research Initiative (Dutch Heart Foundation, Dutch Federation of University Medical Centers, Netherlands Organization for Health Research and Development, and Royal Netherlands Academy of Sciences) grant CVON-2018-30 Predict 2 (M.T.B. and H.L.T.). The Danish Cardiac Arrest Registry is supported by Trygfonden. Work by L.v.d.B. and R.P.D. was supported by a European Research Council Starting Grant (STEMCARDIORISK; grant agreement #638030), and a VIDI fellowship from the Netherlands Organisation for Scientific Research (ILLUMINATE; grant agreement #91715303).
Conflict of interest: G.H.G. was supported by an unrestricted clinical research scholarship from the Novo Nordisk Foundation. C.T.-P. reports grants and personal fees from Bayer and grants from Biotronic. And all other authors have no conflict of interest to declare.
References
- 1. Myerburg RJ, Castellanos A. Cardiac arrest and sudden cardiac death. In: Libby P, Bonow RO, Mann DL, Zipes DP, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. Oxford, UK: Elsevier; 2007. pp. 933–974. [Google Scholar]
- 2. Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med 2001;345:1473–1482. [DOI] [PubMed] [Google Scholar]
- 3. Bardai A, Amin AS, Blom MT, Bezzina CR, Berdowski J, Langendijk PNJ, Beekman L, Klemens CA, Souverein PC, Koster RW, de Boer A, Tan HL. Sudden cardiac arrest associated with use of a non-cardiac drug that reduces cardiac excitability: evidence from bench, bedside, and community. Eur Heart J 2013;34:1506–1516. [DOI] [PubMed] [Google Scholar]
- 4. Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med 2004;350:1013–1022. [DOI] [PubMed] [Google Scholar]
- 5. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009;360:225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Straus SMJM, Sturkenboom MCJM, Bleumink GS, Dieleman JP, van der Lei J, de Graeff PA, Kingma JH, Stricker BHC. Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur Heart J 2005;26:2007–2012. [DOI] [PubMed] [Google Scholar]
- 7. Cheng Y-J, Nie X-Y, Chen X-M, Lin X-X, Tang K, Zeng W-T, Mei W-Y, Liu L-J, Long M, Yao F-J, Liu J, Liao X-X, Du Z-M, Dong Y-G, Ma H, Xiao H-P, Wu S-H. The role of macrolide antibiotics in increasing cardiovascular risk. J Am Coll Cardiol 2015;66:2173–2184. [DOI] [PubMed] [Google Scholar]
- 8. Weeke P, Jensen A, Folke F. Antipsychotics and associated risk of out‐of‐hospital cardiac arrest. Clin Pharmacol Ther 2014;96:490–497. [DOI] [PubMed] [Google Scholar]
- 9. Weeke P, Jensen A, Folke F, Gislason GH, Olesen JB, Andersson C, Fosbøl EL, Larsen JK, Lippert FK, Nielsen SL, Gerds T, Andersen PK, Kanters JK, Poulsen HE, Pehrson S, Køber L, Torp-Pedersen C. Antidepressant use and risk of out‐of‐hospital cardiac arrest: a nationwide case–time–control study. Clin Pharmacol Ther 2012;92:72–79. [DOI] [PubMed] [Google Scholar]
- 10. Risgaard B, Winkel BG, Jabbari R, Lynge TH, Wissenberg M, Glinge C, Haunsø S, Behr ER, Fink-Jensen A, Gislason GH, Tfelt-Hansen J. Sudden cardiac death: pharmacotherapy and proarrhythmic drugs: a Nationwide Cohort Study in Denmark. JACC: Clin Electrophysiol 2017;3:473–481. [DOI] [PubMed] [Google Scholar]
- 11. Postema PG, Wolpert C, Amin AS, Probst V, Borggrefe M, Roden DM, Priori SG, Tan HL, Hiraoka M, Brugada J, Wilde AAM. Drugs and Brugada syndrome patients: review of the literature, recommendations and an up-to-date website (www.brugadadrugs.org). Heart Rhythm 2009;6:1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bardai A, Blom MT, van Noord C, Verhamme KM, Sturkenboom M, Tan HL. Sudden cardiac death is associated both with epilepsy and with use of antiepileptic medications. Heart 2015;101:17–22. [DOI] [PubMed] [Google Scholar]
- 13. Wu J, Wang X, Chung YY, Koh CH, Liu Z, Guo H, Yuan Q, Wang C, Su S, Wei H. L-type calcium channel inhibition contributes to the proarrhythmic effects of aconitine in human cardiomyocytes. PLoS One 2017;12:e0168435.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Opie LH, Yusuf S, Kübler W. Current status of safety and efficacy of calcium channel blockers in cardiovascular diseases: a critical analysis based on 100 studies. Progr Cardiovasc Dis 2000;43:171–196. [DOI] [PubMed] [Google Scholar]
- 15. Furberg CD, Psaty BM, Meyer JV. Nifedipine: dose-related increase in mortality in patients with coronary heart disease. Circulation 1995;92:1326–1331. [DOI] [PubMed] [Google Scholar]
- 16. Tan HL, Dagres N, Böttiger BW, Schwartz PJ; ESCAPE-NET Investigators. European Sudden Cardiac Arrest network: towards Prevention, Education and New Effective Treatments (ESCAPE-NET) A major European Horizon 2020 project focused on cardiac arrest. Eur Heart J 2018;39:86–88. [Google Scholar]
- 17. Blom MT, van Hoeijen DA, Bardai A, Berdowski J, Souverein PC, De Bruin ML, Koster RW, de Boer A, Tan HL. Genetic, clinical and pharmacological determinants of out-of-hospital cardiac arrest: rationale and outline of the AmsteRdam Resuscitation Studies (ARREST) registry. Open Heart 2014;1:e000112.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herings R, Pedersen L. Pharmacy-based medical record linkage systems. In: Strom B, Kimmel S, eds. Pharmacoepidemiology, 5 ed. Chichester: John Wiley & Sons, Ltd; 2012. pp. 270–286. [Google Scholar]
- 19. Wissenberg M, Lippert FK, Folke F, Weeke P, Hansen CM, Christensen EF, Jans H, Hansen PA, Lang-Jensen T, Olesen JB, Lindhardsen J, Fosbol EL, Nielsen SL, Gislason GH, Kober L, Torp-Pedersen C. Association of national initiatives to improve cardiac arrest management with rates of bystander intervention and patient survival after out-of-hospital cardiac arrest. JAMA 2013;310:1377–1384. [DOI] [PubMed] [Google Scholar]
- 20. Pedersen CB. The Danish civil registration system. Scand J Public Health 2011;39(7_Suppl):22–25. [DOI] [PubMed] [Google Scholar]
- 21. Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health 2011;39(7_suppl):30–33. [DOI] [PubMed] [Google Scholar]
- 22. Helweg-Larsen K. The Danish register of causes of death. Scand J Public Health 2011;39(7_suppl):26–29. [DOI] [PubMed] [Google Scholar]
- 23. Wallach Kildemoes H, Toft Sørensen H, Hallas J. The Danish national prescription registry. Scand J Public Health 2011;39(7_suppl):38–41. [DOI] [PubMed] [Google Scholar]
- 24. Gislason GH, Rasmussen JN, Abildstrøm SZ, Gadsbøll N, Buch P, Friberg J, Rasmussen S, Køber L, Stender S, Madsen M, Torp-Pedersen C. Long-term compliance with beta-blockers, angiotensin-converting enzyme inhibitors, and statins after acute myocardial infarction. Eur Heart J 2006;27:1153–1158. [DOI] [PubMed] [Google Scholar]
- 25.ATC index with DDDs. Oslo, Norway: WHO collaborating Centre for Drug Statistics Methodology; 2018. https://www.whocc.no/atc_ddd_index (1 February 2018). [Google Scholar]
- 26. Woosley R, Heise CW, Romero KA. QTdrugs List. Oro Valley, AZ: AZCERT, Inc. http://www.Crediblemeds.org (11 February 2017).
- 27. Sala L, van Meer BJ, Tertoolen LGJ, Bakkers J, Bellin M, Davis RP, Denning C, Dieben MAE, Eschenhagen T, Giacomelli E, Grandela C, Hansen A, Holman ER, Jongbloed MRM, Kamel SM, Koopman CD, Lachaud Q, Mannhardt I, Mol MPH, Mosqueira D, Orlova VV, Passier R, Ribeiro MC, Saleem U, Smith GL, Burton FL, Mummery CL. MUSCLEMOTION: a versatile open software tool to quantify cardiomyocyte and cardiac muscle contraction in vitro and in vivo. Circ Res 2018;122:e5–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grossman E, Messerli FH. Effect of calcium antagonists on plasma norepinephrine levels, heart rate, and blood pressure. Am J Cardiol 1997;80:1453–1458. [DOI] [PubMed] [Google Scholar]
- 29. Glasser SP, Jain A, Allenby KS, Shannon T, Pride K, Pettis PP, Schwartz LA, Mac Carthy EP. The efficacy and safety of once-daily nifedipine: the coat-core formulation compared with the gastrointestinal therapeutic system formulation in patients with mild-to-moderate diastolic hypertension. Nifedipine Study Group. Clin Ther 1995;17:12–29. [DOI] [PubMed] [Google Scholar]
- 30. Meredith PA, Elliott HL. Clinical pharmacokinetics of amlodipine. Clin Pharmacokinet 1992;22:22–31. [DOI] [PubMed] [Google Scholar]
- 31. Psaty BM, Heckbert SR, Koepsell TD, Siscovick DS, Raghunathan TE, Weiss NS, Rosendaal FR, Lemaitre RN, Smith NL, Wahl PW. The risk of myocardial infarction associated with antihypertensive drug therapies. JAMA 1995;274:620–625. [PubMed] [Google Scholar]
- 32. Yui Y, Sumiyoshi T, Kodama K, Hirayama A, Nonogi H, Kanmatsuse K, Origasa H, Iimura O, Ishii M, Saruta T, Arakawa K, Hosoda S, Kawai C, for The Japan Multicenter Investigation for Cardiovascular Diseases-B (JMIC-B) Study Group. Comparison of nifedipine retard with angiotensin converting enzyme inhibitors in Japanese hypertensive patients with coronary artery disease: the Japan Multicenter Investigation for Cardiovascular Diseases-B (JMIC-B) randomized trial. Hypertens Res 2004;27:181–191. [DOI] [PubMed] [Google Scholar]
- 33. Lubsen J, Wagener G, Kirwan B-A, de Brouwer S, Poole-Wilson PA, Investigators A. Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with symptomatic stable angina and hypertension: the ACTION trial. J Hypertens 2005;23:641–648. [DOI] [PubMed] [Google Scholar]
- 34. Poole-Wilson PA, Lubsen J, Kirwan B-A, van Dalen FJ, Wagener G, Danchin N, Just H, Fox KAA, Pocock SJ, Clayton TC, Motro M, Parker JD, Bourassa MG, Dart AM, Hildebrandt P, Hjalmarson Å, Kragten JA, Molhoek GP, Otterstad J-E, Seabra-Gomes R, Soler-Soler J, Weber S. Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): randomised controlled trial. Lancet 2004;364:849–857. [DOI] [PubMed] [Google Scholar]
- 35. Brown MJ, Palmer CR, Castaigne A, de Leeuw PW, Mancia G, Rosenthal T, Ruilope LM. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet 2000;356:366–372. [DOI] [PubMed] [Google Scholar]
- 36. Egstrup K, Andersen PE. Transient myocardial ischemia during nifedipine therapy in stable angina pectoris, and its relation to coronary collateral flow and comparison with metoprolol. Am J Cardiol 1993;71:177–183. [DOI] [PubMed] [Google Scholar]
- 37. Carboni GP, D'Ermo M, Mattioli M, Lioy E, Biffani G. The response of the coronary collateral circulation to acute administration of nifedipine: an angiographic and ergometric study. Int J Cardiol 1986;11:25–36. [DOI] [PubMed] [Google Scholar]
- 38. Members ATF, Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Endorsed by: association for European Paediatric and Congenital Cardiology (AEPC). Europace 2015;17:1601–1687. [DOI] [PubMed] [Google Scholar]
- 39. Opie LH. Calcium channel antagonists in the treatment of coronary artery disease: fundamental pharmacological properties relevant to clinical use. Prog Cardiovasc Dis 1996;38:273–290. [DOI] [PubMed] [Google Scholar]
- 40. Gaita F, Giustetto C, Bianchi F, Wolpert C, Schimpf R, Riccardi R, Grossi S, Richiardi E, Borggrefe M. Short QT syndrome: a familial cause of sudden death. Circulation 2003;108:965–970. [DOI] [PubMed] [Google Scholar]
- 41. Feig P, Gibson L, Mac EC, Pettis P, Schwartz L. The efficacy and safety of once-daily nifedipine coat-core in the treatment of mild-to-moderate hypertension. Adalat CC Cooperative Study Group. Clin Ther 1993;15:963–975. [PubMed] [Google Scholar]
- 42. Lynch P, Dargie H, Krikler S, Krikler D. Objective assessment of antianginal treatment: a double-blind comparison of propranolol, nifedipine, and their combination. Br Med J 1980;281:184–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Deanfield J, Wright C, Fox K. Treatment of angina pectoris with nifedipine: importance of dose titration. Br Med J 1983;286:1467–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Veerman CC, Kosmidis G, Mummery CL, Casini S, Verkerk AO, Bellin M. Immaturity of human stem-cell-derived cardiomyocytes in culture: fatal flaw or soluble problem? Stem Cells Dev 2015;24:1035–1052. [DOI] [PubMed] [Google Scholar]
- 45. Kang J, Chen XL, Ji J, Lei Q, Rampe D. Ca2+ channel activators reveal differential L-type Ca2+ channel pharmacology between native and stem cell-derived cardiomyocytes. J Pharmacol Exp Ther 2012;341:510–517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.