Patients with chronic lymphocytic leukemia (CLL) have been previously shown to have poor responses to antibacterial and antiviral vaccines. In a Plenary Paper that is also this month’s CME article, Herishanu and colleagues discuss the outcome of vaccination against COVID-19 in 167 patients with CLL, again demonstrating a significantly impaired vaccine response. The lowest response rates were seen in patients undergoing active treatment, while the highest responses were seen in patients who were in complete remission after therapy; however, even treatment-naïve patients had lower responses than healthy controls. In this series, no responses were achieved in patients treated with anti-CD20 antibodies within 12 months of vaccination.
Key Points
Antibody response to BNT162b2 mRNA COVID-19 vaccine in patients with CLL is markedly impaired and affected by disease activity and treatment.
In patients treated with either Bruton’s tyrosine kinase inhibitors or venetoclax ± anti-CD20 antibody, responses are relatively low.
Visual Abstract
Abstract
Patients with chronic lymphocytic leukemia (CLL) have an increased risk for severe COVID-19 disease and mortality. The goal of this study was to determine the efficacy of COVID-19 vaccine in patients with CLL. We evaluated humoral immune responses to the BNT162b2 messenger RNA (mRNA) COVID-19 vaccine in patients with CLL and compared responses with those obtained in age-matched healthy control subjects. Patients received 2 vaccine doses, 21 days apart, and antibody titers were measured by using the Elecsys Anti-SARS-CoV-2 S assay after administration of the second dose. In a total of 167 patients with CLL, the antibody response rate was 39.5%. A comparison between 52 patients with CLL and 52 sex- and aged-matched healthy control subjects revealed a significantly reduced response rate among patients (52% vs 100%, respectively; adjusted odds ratio, 0.010; 95% confidence interval, 0.001-0.162; P < .001). The response rate was highest in patients who obtained clinical remission after treatment (79.2%), followed by 55.2% in treatment-naive patients and 16.0% in patients under treatment at the time of vaccination. In patients treated with either Bruton’s tyrosine kinase inhibitors or venetoclax ± anti-CD20 antibody, response rates were considerably low (16.0% and 13.6%). None of the patients exposed to anti-CD20 antibodies <12 months before vaccination responded. In a multivariate analysis, the independent predictors of response were younger age, female sex, lack of currently active treatment, immunoglobulin G levels ≥550 mg/dL, and immunoglobulin M levels ≥40 mg/dL. In conclusion, antibody-mediated response to the BNT162b2 mRNA COVID-19 vaccine in patients with CLL is markedly impaired and affected by disease activity and treatment. This trial was registered at www.clinicaltrials.gov as #NCT04746092.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 3311.
Disclosures
Associate Editor Michael Hallek and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests. Author Yair Herishanu reports honoraria from AbbVie Inc, Janssen Israel, AstraZeneca, and Roche outside of submitted work. Author Irit Avivi reports speakers bureau participation for Gilead, Novartis, AbbVie, and Janssen and served as a consultant for Janssen, Merck Sharp & Dohme, AbbVie, Novartis, and Roche outside the submitted work. Author Erel Joffe reports advisory board participation for AstraZeneca and Epizyme. Author Paolo Ghia reports grants and personal fees from AbbVie, Gilead, Janssen, Pharmacyclics, and Sunesis and personal fees from AbbVie, Gilead, Janssen, Pharmacyclics, and Sunesis and personal fees from AstraZeneca, Adaptive Biotechnologies, ArQule/Merck Sharp & Dohme, BeiGene, Celgene/Juno/Bristol Myers Squibb, and Lilly/Loxo Oncology outside the submitted work. The remaining authors declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
-
1.
Describe humoral immune responses to the tBNT162b2 mRNA COVID-19 vaccine and adverse events in patients with chronic lymphocytic leukemia (CLL), alone and in comparison with sex- and age-matched healthy control participants, according to a clinical and serologic study
-
2.
Determine the effects of treatment and other clinical factors on humoral immune responses to the BNT162b2 mRNA COVID-19 vaccine in patients with CLL, according to a clinical and serologic study
-
3.
Identify clinical implications of humoral immune responses to the BNT162b2 mRNA COVID-19 vaccine in patients with CLL, according to a clinical and serologic study
Release date: June 10, 2021; Expiration date: June 10, 2022
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a single-stranded RNA virus that causes COVID-19, a disease with variable presentations ranging from a mild common cold to severe respiratory failure.1,2 An increased risk for severe disease and death has been noted among elderly patients and persons with preexisting medical conditions. The coronavirus genome encodes 4 main structural proteins, designated spike (S), envelope, membrane, and nucleocapsid. The virus penetrates the host through binding of the viral S protein to angiotensin-converting enzyme 2 presented on oral mucosa epithelial cells and the lung alveolar type II cells.3,4
In general, patients with chronic lymphocytic leukemia (CLL) are predisposed to develop infections, both bacterial and viral, due to inherent immune defects related to their primary disease and as a result of therapy.5 The mechanisms underlying the immunodeficiency in CLL includes abnormal humoral and cellular immune responses due to quantitative and qualitative defects in immune effector cells, which may also reduce response to vaccines.6 During the previous months of the COVID-19 pandemic, 2 large multicenter retrospective studies reported high rates of severe COVID-19 disease and mortality in both untreated (“watch and wait”) as well as treated patients with CLL.7,8
Recently, 2 vaccines (BNT162b2 and mRNA-1273) were approved and recommended by the US Food and Drug Administration and the European Medicines Agency to prevent COVID-19 disease. BNT162b2 and mRNA-1273 are lipid nanoparticle–encapsulated messenger RNA (mRNA)-based vaccines that encode the full-length S protein of SARS-CoV-2.9,10 In phase 3 trials, these vaccines showed 94% to 95% efficacy in preventing symptomatic SARS-CoV-2 infection independent of age.11,12 However, until now, hemato-oncology patients were excluded from clinical trials with COVID-19 vaccines. In the current study, we evaluated antibody-mediated response to the BNT162b2 mRNA COVID-19 vaccine in patients with CLL.
Methods
This prospective study, conducted in the framework of the European Research Initiative on CLL (ERIC), investigated the efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with CLL/small lymphocytic lymphoma (SLL) followed up at the Tel Aviv Sourasky Medical Center. The study was approved by the Institutional Review Board and is registered on clinicaltrials.gov (#NCT04746092). All patients provided informed consent.
Patients were vaccinated through the national Israeli vaccination program. Eligibility criteria for the study included diagnosis of CLL/SLL per the International Workshop on Chronic Lymphocytic Leukemia guidelines,13 age ≥18 years, and no known history of SARS-CoV-2 infection. The study also included healthy volunteers, age ≥18 years, who served as a control group.
Blood serum of patients with CLL/SLL and healthy control subjects were collected 2 to 3 weeks after administration of the second vaccine. The primary end point was the proportion of subjects acquiring anti–SARS-CoV-2 S antibodies. Serum samples were evaluated by using Elecsys Anti-SARS-CoV-2 S assay on the cobas e 601 (Roche Diagnostics) analyzer for the quantitative detection of antibodies, predominantly immunoglobulin G (IgG), aimed at the SARS-CoV-2 S protein receptor–binding domain. This assay has a measurement range of 0.40 to 250 U/mL, with a concentration <0.80 U/mL considered as negative and ≥0.80 U/mL considered as positive. When sample results exceed the upper limit of the measuring range (reported as >250 U/mL), samples were on-board diluted 1:10 or 1:100 when needed (>2500 U/mL for 10-fold diluted samples). To ensure that none of the patients had been recently exposed to SARS-CoV-2, we additionally tested for the presence of antibodies to SARS-CoV-2 nucleocapsid with the Elecsys Anti-SARS-CoV-2 S assay using the cobas e 601 (Roche Diagnostics) analyzer. All subjects were queried about local or systemic adverse events that occurred within 7 days after each vaccine dose.
Relevant data were extracted from the medical records and included demographic characteristics, complete blood count, Binet stage, serum immunoglobulin levels, mutational status of the immunoglobulin heavy chain variable (IGHV) gene (using a cutoff of 98% identity to the germline sequence) and analyses of genomic aberrations by fluorescent in situ hybridization,14 categorized according to the hierarchical model reported by Döhner et al.15
Statistical analysis
SPSS version 27 (IBM SPSS Statistics, IBM Corporation) was used to perform the following: median with interquartile range (IQR), and mean for quantitative variables, and distributions. The Mann-Whitney U test and Kruskal-Wallis H test were used for comparing medians. Pearson’s χ2 test, Fisher’s exact test, and the Fisher-Freeman-Halton exact test were used for associations between 2 variables. Multivariate analysis was conducted in 2 stages: (1) multiple imputation was applied to derive estimated values for patients with randomly missing data; and (2) binary logistic regression was used for multivariate analysis regarding the response. The McNemar test was used to compare differences between paired dichotomous samples. WINPEPI version 11.65 was used to calculate the odds ratio (OR) and its 95% confidence interval (CI), and to calculate the adjusted OR when at least one cell contained a value of 0. Statistical significance was determined at α < 0.05, and all tests were 2 sided. Additional details about the statistical methods are provided in the supplemental Data (available on the Blood Web site).
GraphPad Prism version 8.0.0 for Windows was used for creating figures.
Results
Patient characteristics
From December 2020 through February 2021, a total of 167 patients with CLL/SLL and 52 age- and sex-matched control subjects were included in this study. Patient baseline demographic and disease characteristics are summarized in Table 1. The median age of the patients with CLL was 71 years (IQR, 63.0-76.0 years), and 112 (67.1%) were male. Fifty-eight patients (34.7%) were treatment naive, 75 (44.9%) were on active therapy, 24 (14.4%) were previously treated, in clinical complete remission (CR) or partial remission (PR), and 10 (6.0%) were currently experiencing disease relapse after being previously treated. The median time from CLL diagnosis to vaccination was 83.1 months (IQR, 48.5-139.5 months), and the median time from the second vaccine dose to serology testing was 15 days (IQR, 14-17 days).
Table 1.
Patient baseline demographic and disease characteristics
| Parameter | Patients with CLL (N = 167) |
|---|---|
| Age, median (IQR), y | 71.0 (63.0-76.0) |
| Age ≤65 y, N (%) | 50 (29.9) |
| Male sex, N (%) | 112 (67.1) |
| Disease/treatment status, N (%) | |
| Treatment-naive | 58 (34.7) |
| On-therapy | 75 (44.9) |
| Off-therapy in remission | 24 (14.4) |
| Off-therapy in relapse | 10 (6.0) |
| Binet stage,* N (%) | |
| A | 43 (64.1) |
| B | 14 (20.9) |
| C | 10 (14.9) |
| IGHV mutational status, N (%) | |
| Mutated | 61 (50.0) |
| Unmutated | 61 (50.0) |
| FISH, N (%) | |
| Normal | 20 (12.0) |
| del(13q) | 39 (23.4) |
| Trisomy 12 | 16 (9.6) |
| del(11q) | 31 (18.6) |
| del(17p) | 19 (11.4) |
| β 2 -microglobulin, N (%) | |
| ≤3.5 mg/L | 90 (78.3) |
| >3.5 mg/L | 25 (21.7) |
| Protocols of currently treated, N (%) | |
| BTKis | 50 (66.7) |
| Venetoclax ± anti-CD20 antibody | 22 (29.3) |
| Others | 3 (4.0) |
| Time from last anti-CD20 antibody to vaccination, N (%) | |
| <12 mo | 22 (28.6) |
| ≥12 mo | 55 (71.4) |
| Laboratory parameters, median (IQR) | |
| Absolute lymphocyte count, (109/L) | 5.0 (2.0-18.5) |
| β2-microglobulin, mg/L | 2.5 (2.0-3.5) |
| IgG, mg/dL | 723.5 (515.8-1000.3) |
| IgM, mg/dL | 32.0 (0.0-57.5) |
| IgA, mg/dL | 94.0 (49.0-146.8) |
FISH, fluorescence in situ hybridization.
Treatment-naive patients and patients in relapse.
Serologic response
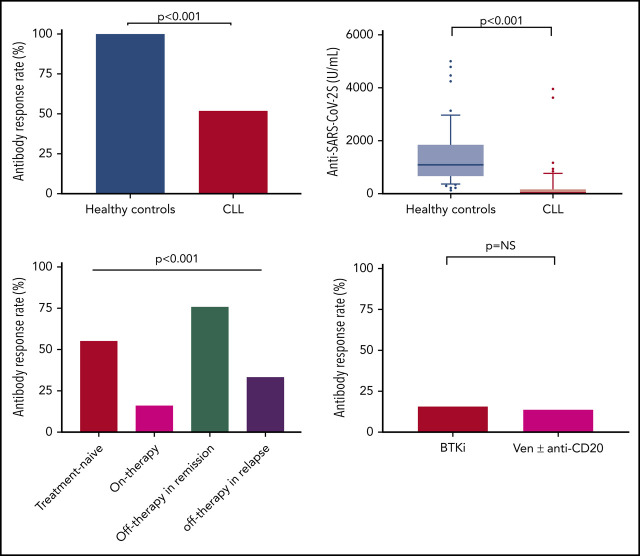
Antibody-mediated response to the vaccine was evident in only 66 (39.5%) of the 167 patients with CLL. An sex- and aged-matched analysis, comparing the response rates in 52 patients with CLL (median age, 69 years; IQR, 63.0-73.7 years) and 52 age- and sex-matched healthy control subjects (median age, 68.0 years; IQR, 64.0-74.0 years), revealed a significantly reduced response rate (52% vs 100%, respectively; adjusted OR, 0.010; 95% CI, 0.001-0.162; P < .001) (Figure 1A-C) and lower antibody titers in patients with CLL (median of 0.824 U/mL [IQR, 0.4-167.3 U/mL], including 155 U/mL [IQR, 7.6-490.3 U/mL] in responding patients with CLL vs 1084 U/mL [IQR, 128.9-1879 U/mL], respectively; P < .001) (Figure 1D). Of note, none of the patients with CLL who achieved a seropositive test result against the SARS-CoV-2 S protein receptor-binding domain had anti–SARS-CoV-2 nucleocapsid antibodies.
Figure 1.
Anti–SARS-CoV-2 antibody response in patients with CLL and healthy control subjects. (A-B) Distribution of individual responses in patients with CLL (n = 52) and sex- and age-matched control subjects (n = 52). Each column represents the level of antibodies in individual patients (red bars indicate treatment naive, green bar indicates on-therapy, blue bars indicate off-therapy in remission, and purple bars indicate off-therapy in relapse) in panel A and in individual healthy control subjects (red bars) in panel B. (C) Response rate in patients with CLL (n = 52) and sex- and age-matched control subjects (n = 52). (D) Anti–SARS-CoV-2 antibody levels in patients with CLL (n = 52) and sex- and age-matched control subjects (n = 52).
In a univariate analysis (Table 2), the variables found to be significantly associated with response included: younger age (≤65 years), female sex, early disease stage (Binet stage A), mutated IGHV, β2-microglobulin (≤3.5 mg/L), untreated/off-therapy ≥12 months from the last anti-CD20 therapy, IgG levels ≥550 mg/dL, IgM levels ≥40 mg/dL, and IgA levels ≥80 mg/dL. Treatment-naive patients had a higher response rate (55.2% [23 of 58]) and higher antibody levels (median, 1.7 U/mL; IQR, 0.4-136.0 U/mL) (Figure 2A-B; supplemental Table 1) compared with actively treated patients (16.0% [12 of 75]; OR, 0.16; 95% CI, 0.07-0.35; P < .001); median antibody levels were 0.4 U/mL (IQR, 0.4-0.4 U/mL; P < .001).
Table 2.
Univariate analysis for serologic response rate in patients with CLL
| Variable | Serologic response, N (%) | |||||
|---|---|---|---|---|---|---|
| Positive | Negative | Total | P | OR | 95% CI | |
| Age in time of vaccination | ||||||
| ≤65 y | 26 (52.0) | 24 (48.0) | 50 | .031 | 2.09 | 1.01-4.32 |
| >65 y | 40 (34.2) | 77 (65.8) | 117 | |||
| Sex | ||||||
| Female | 30 (54.5) | 25 (45.5) | 55 | .005 | 2.53 | 1.24-5.18 |
| Male | 36 (32.1) | 76 (67.9) | 112 | |||
| Treatment status (detailed) | ||||||
| Treatment-naive | 32 (55.2) | 26 (44.8) | 58 | <.001 | 1 | |
| On-therapy | 12 (16.0) | 63 (84.0) | 75 | 0.16 | 0.07-0.35 | |
| Off-therapy in remission (CR or PR) | 19 (79.2) | 5 (20.8) | 24 | 3.01 | 1.02-9.40 | |
| Off-therapy in relapse | 3 (30.0) | 7 (70.0) | 10 | 0.35 | 0.08-1.48 | |
| Binet stage | ||||||
| A | 29 (67.4) | 14 (32.6) | 43 | .001 | 6.21 | 1.80-22.96 |
| B or C | 6 (24.0) | 18 (75.0) | 24 | |||
| IGHV | ||||||
| Mutated | 29 (47.5) | 32 (52.5) | 61 | .005 | 3.04 | 1.31-7.21 |
| Unmutated | 14 (23.0) | 47 (77.0) | 61 | |||
| FISH test | ||||||
| Normal | 9 (45.0) | 11 (55.0) | 20 | .061 | 1 | |
| del(13q) | 16 (41.0) | 23 (59.0) | 39 | 0.85 | 0.28-2.52 | |
| Trisomy 12 | 6 (37.5) | 10 (62.5) | 16 | 0.73 | 0.19-2.80 | |
| del(11q) | 11 (35.5) | 20 (64.5) | 31 | 0.67 | 0.21-2.12 | |
| del(17p) | 1 (5.30) | 18 (94.7) | 19 | 0.07 | 0.01-0.61 | |
| β 2 -microglobulin | ||||||
| ≤3.5 mg/L | 43 (47.8) | 47 (52.2) | 90 | .004 | 4.80 | 1.44-20.54 |
| >3.5 mg/L | 4 (16.0) | 21 (84.0) | 25 | |||
| Current treatment status | ||||||
| Untreated | 54 (58.7) | 38 (41.3) | 92 | <.001 | 7.46 | 3.38-17.14 |
| Treated | 12 (16.0) | 63 (84.0) | 75 | |||
| Treatment protocol | ||||||
| BTKi | 8 (16.0) | 42 (84.0) | 50 | .601 | 1 | |
| Venetoclax ± anti-CD20 antibody | 3 (13.6) | 19 (86.4) | 22 | 0.82 | 0.20-3.48 | |
| Others | 1 (33.3) | 2 (66.7) | 3 | |||
| Anti-CD20 (last treatment) | ||||||
| At least 12 mo later | 25 (45.5) | 30 (54.6) | 55 | <.001 | 37.6 | 2.2-651.3 |
| Within <12 mo | 0 (0.0) | 22 (100.0) | 22 | |||
| Serum IgG level | ||||||
| ≥550 mg/dL | 53 (49.1) | 55 (50.1) | 108 | <.001 | 5.37 | 2.11-15.34 |
| <550 mg/dL | 7 (14.6) | 39 (85.4) | 46 | |||
| Serum IgM level | ||||||
| ≥40 mg/dL | 39 (59.1) | 27 (40.9) | 66 | <.001 | 4.84 | 2.27-10.37 |
| <40 mg/dL | 20 (23.0) | 67 (77.0) | 87 | |||
| Serum IgA level | ||||||
| ≥80 mg/dL | 42 (34.5) | 47 (54.5) | 89 | .012 | 2.42 | 1.15-5.19 |
| <80 mg/dL | 17 (24.5) | 46 (38.5) | 63 | |||
Figure 2.
Anti–SARS-CoV-2 antibody responses in patients with CLL according to disease status and treatment. (A-B) Response rate and anti–SARS-CoV-2 antibody levels in patients with CLL according to disease status: Treatment naive (n = 58), on-therapy (n = 75), off-therapy in remission (n = 24), and off-therapy in relapse (n = 10). (C) Response rate in patients with CLL treated with BTKi (n = 50) and venetoclax (Ven) ± anti-CD20 antibody (n = 22). NS, not significant.
Among the 75 patients on treatment at the time of vaccination, 72 (96%) were treated with novel agents, including Bruton’s tyrosine kinase inhibitor (BTKi) monotherapy (ibrutinib or acalabrutinib, n = 50) or venetoclax ± anti-CD20 antibody (n = 22; 5 patients treated with venetoclax alone, and 17 patients treated with venetoclax plus rituximab or obinutuzumab). Antibody response rate in patients receiving BTKi was 16.0% (8 of 50) compared with 13.6% (3 of 22) in patients treated with venetoclax ± anti-CD20 antibodies (P = not significant) (Figure 2C). Two (40%) of the 5 patients who received venetoclax monotherapy achieved a positive serologic response, with relatively low antibody titers (range, 2.19-4.5 U/mL). Demographic, disease-related, and treatment-associated factors (including the number of prior lines) had no statistically significant impact on response rate in actively treated patients.
A total of 77 patients with CLL had been previously exposed to anti-CD20 therapy: 22 within the last 12 months before vaccination (median, 5.3 months; IQR, 0.75-8.0 months) and 55 patients ≥12 months before vaccination (median, 53.1 months; IQR, 25.5-76.8 months). Most patients (18 of 22 [81.8%]) exposed to anti-CD20 antibodies <12 months before vaccination received it in combination with venetoclax. The majority of patients (43 of 55 [78.1%]) treated with anti-CD20 therapy ≥12 months before vaccination received chemoimmunotherapy. None of the patients treated with anti-CD20 antibodies (n = 22) within the last 12 months has responded vs 45.5% (25 of 55) of those who were exposed to anti-CD20 therapy ≥12 months before vaccination (adjusted OR, 37.6; 95% CI, 2.2-651.3; P ≤ .001). In the subgroup of patients treated with anti-CD20 antibody ≥12 months before vaccination, there was no association between the time that elapsed from the end of the anti-CD20 therapy and the patients’ serologic response.
A particularly high response rate (79.2%) and antibody levels (median, 297.6 U/mL; IQR, 1.2-1149.8 U/mL) were observed among the 24 patients who completed treatment (mostly chemoimmunotherapy or targeted therapies) and maintained their response (CR/PR) at the time of vaccination (Figure 2A-B; supplemental Tables 2-3). Serologic response rate was remarkably high in those who completed treatment beyond 12 months before vaccination compared with those who completed therapy within <12 months (94.1% vs 50.0%; P = .04) (supplemental Table 2).
In a multivariate analysis (Table 3), the independent predictors of response were age (≤65 years; OR, 3.17; 95% CI, 1.16-8.67; P = .025), sex (female; OR, 3.66; 95% CI, 1.46-9.18; P = .006), lack of active therapy (including treatment-naive and previously treated patients; OR, 6.59; 95% CI, 2.30-18.86; P < .001), IgG levels ≥550 mg/dL (OR, 3.70; 95% CI, 1.08-12.66; P = .037), and IgM levels ≥40 mg/dL (OR, 2.92; 95% CI, 1.21-7.02; P = .017).
Table 3.
Multivariate analysis for serologic response in patients with CLL
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Age ≤65 y | 3.17 | 1.16-8.67 | .025 |
| Female sex | 3.66 | 1.46-9.18 | .006 |
| Mutated IGHV | 1.39 | 0.47-4.10 | .543 |
| β2-microglobulin ≤3.5 mg/dL | 1.36 | 0.36-5.19 | .645 |
| Lack of active therapy | 6.59 | 2.30-18.86 | <.001 |
| Serum IgG level ≥550 mg/dL | 3.70 | 1.08-12.66 | .037 |
| Serum IgM level ≥40 mg/dL | 2.92 | 1.21-7.02 | .017 |
| Serum IgA level ≥80 mg/dL | 1.09 | 0.41-2.94 | .862 |
Within a median follow-up period of 75 days (IQR, 72-87 days) since the first vaccine dose, none of the patients developed COVID-19 infection.
Adverse events
Fifty-two (31.1%) and 56 (33.5%) patients reported mild local reactions after the first and second dose of the vaccine, respectively (Figure 3; supplemental Table 4). These reactions included pain at the injection site and, less commonly, local erythema or swelling. There was no statistical difference in the rates of local reactions between the first and second dose of the vaccine (P = .629) (supplemental Table 5).
Figure 3.
Local and systemic reactions reported after injection of BNT162b2 in patients with CLL (N = 167). Reactions reported after the first vaccine dose (A) and after the second vaccine dose (B).
Overall, 21 (12.5%) and 39 (23.4%) patients reported systemic adverse events after the first and second vaccine dose, respectively (Figure 3); they were more frequent after the second dose (P = .005) (supplemental Table 5), and all were mild. The most frequently reported systemic reaction after the first dose included weakness (n = 11 [6.6%]), headache (n = 9 [5.4%]), fever (n = 4 [2.4%]), and muscle pain (n = 3 [1.8%]) (supplemental Table 4). The systemic side effects most commonly reported after the second dose were weakness (n = 14 [8.6%]), fever (n = 11 [6.6%]), chills (n = 10 [6.0%]), headache (n = 10 [5.4%]), and muscle pain (n = 8 [4.8%]). No statistically significant correlation was found between local or systemic reactions and a positive serologic response to the vaccine. In addition, there were no statistically significant differences in the rate of adverse events between patients who were actively treated at the time of vaccination vs those who were not.
Discussion
This study evaluated the serologic response to SARS-CoV-2 vaccination after a 2-dose regimen of BNT162b2 mRNA COVID-19 vaccine, given 21 days apart, in patients with CLL. The anti–SARS-CoV-2 antibody response rate in patients with CLL was considerably low. This low response rate, approaching 40%, is consistent with response rates of 20% to 40% to pneumococcal conjugated vaccine (PCV13), pneumococcal polysaccharide vaccine (PPSV23), and the HepB-CpG vaccine16-19; it is also consistent with the reduced efficacy of influenza A and B vaccination reported in patients with CLL.20
The underlying causes for poor humoral response to vaccination in patients with CLL are multifactorial and attributed to disease-related immune dysregulation and therapy-related immnuosupression.21 In our study, younger patients (aged ≤65 years), female subjects, favorable disease-related factors (mutated IGHV, β2-microglobulin ≤3.5 mg/L, and early disease stage), lack of active treatment (treatment naive or off-therapy, including ≥12 months from the last anti-CD20 therapy at the time of vaccination), and higher serum immunoglobulin levels at time of vaccination were all associated with better response rates.
Although treatment-naive patients had a better antibody response to the vaccine than actively treated patients, our findings highlight the fact that treatment-naive patients with CLL have reduced responses and lower antibody titers after vaccination compared with healthy individuals. This can be explained by qualitative and quantitative defects of the innate and adaptive immune systems, which have been well documented in almost all patients with CLL since diagnosis, along with an inefficient antibody response, often recognized in patients with CLL from the early disease stages.21
Patients actively treated with BTKis or with venetoclax ± anti-CD20 were unlikely to respond to vaccination. Because BTKis block the B-cell receptor signaling, in both malignant and normal B cells, it is not unexpected that these agents impair the humoral response to vaccination. Previous studies have described antibody-mediated response rates of 7% to 26% to influenza vaccine in patients with CLL treated with BTKis22,23; more recently, Pleyer et al showed that BTKis are associated with a decreased de novo immune response to the anti–hepatitis B vaccine HepB-CpG.19 Given the lower IgG levels obtained in patients on BTKis,24 one should also consider the possibility of postponing the start of treatment until safe for the patients and allow enough time to conclude the vaccination program. Furthermore, patients with CLL treated with anti-CD20 antibody (rituximab or obinutuzumab) within the last 12 months before vaccination failed to produce anti–SARS-CoV-2 antibodies, whereas better responses were observed in patients who completed anti-CD20 therapy at least 12 months before vaccination. These findings are consistent with previous studies showing that recent exposure to B cell–deleting agents (within the last 6-12 months) reduces response to influenza vaccine, pneumococcal polysaccharide vaccine, and other vaccines,25-28 and they have relevant bearings on the definition of the right timing for vaccination after an anti-CD20–based treatment. In addition, given that most patients today are treated with venetoclax in combination with an anti-CD20 antibody, we cannot draw firm conclusions about the effect of venetoclax monotherapy on response to the anti–COVID-19 vaccine, given the fact that only 5 patients have been treated with venetoclax alone.
Notably, patients with CLL who completed treatment and maintained their response achieved the highest immune response rate among all subgroups of patients, indicating a reconstitution of humoral immunity as the result of disease control. It is particularly interesting that all 4 of our patients who completed fixed-duration treatment with the BTKi plus venetoclax-based regimens, administered up to 1.9 to 12.6 months before vaccination, achieved very high anti–SARS-CoV-2 antibody levels (median, 2244 U/mL; range, 404-13 792 U/mL). These patients were in CR, and 3 of them also attained undetectable minimal residual disease. These findings are of practical importance as they might suggest that an additional booster dose of the vaccine could be considered for patients with CLL who have completed therapy and previously failed to respond to COVID-19 vaccine.29 The exact timing of an additional vaccine dose should be further investigated. Nevertheless, our data suggest that to achieve a humoral response, the time interval required between the last treatment and vaccination is at least 12 months after anti-CD20 therapy, including following chemoimmunotherapy, whereas after exposure to other fixed-duration targeted therapies, the required washout period might be much shorter. Moreover, in patients who are still untreated, or are planned to be treated shortly, a pretherapy vaccination policy is highly recommended, although responses might also be affected by the immunosuppression that accompanies the disease itself.
Protective immune responses to viral infections or vaccines usually arise from the combined actions of both humoral and cellular immune systems. It is generally considered that humoral immunity plays an important role in the protection against SARS-CoV-2.30,31 It is supported by the relatively high levels of antibody responses to the S protein in patients who have recovered from COVID-19 infection32 and the therapeutic benefit of high-titer convalescent plasma and of the anti-spike neutralizing monoclonal antibody bamlanivimab together with etesevimab.33 The serologic response can also, indirectly, represent T helper (CD4) function, given their contribution to recruitment and activation of antibody-producing B cells, beyond their role in cellular immunity. Furthermore, CD4+ T-cell responses to the viral S protein were recently shown to correlate with the degree of the anti–SARS-CoV-2 IgG and IgA titers.34 Nevertheless, the effects of SARS-CoV-2 vaccine on cellular immunity should be further studied in patients with CLL, to better understand its protective role, particularly in patients who failed to achieve an optimal antibody-mediated response. Indeed, both humoral and cellular responses seem to be important in this setting. However, there are several reports,35,36 including in patients with non-Hodgkin lymphoma who were infected with COVID-19,37 suggesting that T-cell response is generally preserved in patients who have been recently treated with B cell–depleting therapy. Moreover, a robust humoral response seems to be important for the achievement of rapid clearance of COVID-19 infection. In addition, it remains to be established if and for how long the patients with CLL who exhibited an immune response to the vaccine will maintain these levels throughout time as well as if they will be able to maintain a B-cell memory response. Only longer follow-up will tell us.
In summary, the antibody-mediated response to SARS-CoV-2 vaccine in patients with CLL is considerably impaired and affected by disease activity and treatment. Thus, vaccinated patients with CLL should continue to adhere to masking and social distancing, and vaccination of their close contacts should be strongly recommended. Serologic test results after the second injection of the COVID-19 vaccine can provide valuable information to the individual patient and perhaps may be integrated into future clinical decisions. In the future, the efficacy of booster doses should be studied in patients who failed to achieve an optimal response, especially in patients who completed treatment attaining a deep and sustained response.
Supplementary Material
Acknowledgment
The authors thank the clinical study coordinators, nurses, and laboratory staff at the Hematology Department in the Tel Aviv Sourasky Medical Center.
Footnotes
For original data, please contact the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is a Blood Commentary on this article in this issue.
Authorship
Contribution: Y.H. and I.A. initiated the trial, designed the study, analyzed data, and wrote the paper; A.A., G.S., Y.S.A., and M.M. performed the serologic testing; Y.B. collected the data; S.L., T.Z., and E.J. analyzed the data; L.S. designed the study; C.P. wrote the paper; and P.G. designed the study and wrote the paper.
Conflict-of-interest disclosure: Y.H. reports honoraria from AbbVie, Janssen, AstraZeneca, and Roche outside the submitted work. I.A. reports speakers bureau participation for Gilead, Novartis, AbbVie, and Janssen; and served as a consultant for Janssen, Merck Sharp & Dohme, AbbVie, Novartis, and Roche outside the submitted work. E.J. reports advisory board participation for AstraZeneca and Epizyme. P.G. reports grants and personal fees from AbbVie, Gilead, Janssen, Pharmacyclics, and Sunesis and personal fees from AstraZeneca, Adaptive Biotechnologies, ArQule/Merck Sharp & Dohme, BeiGene, Celgene/Juno/Bristol Myers Squibb, and Lilly/Loxo Oncology, outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Yair Herishanu, Department of Hematology Tel Aviv Sourasky Medical Center, 6 Weizmann St, Tel Aviv, Israel 64239; e-mail: yairh@tasmc.health.gov.il.
REFERENCES
- 1.Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271-280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walls AC, Park YJ, Tortorici MA, et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein [published correction appears in Cell. 2020;183(6):1735]. Cell. 2020;181(2):281-292.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tadmor T, Welslau M, Hus I. A review of the infection pathogenesis and prophylaxis recommendations in patients with chronic lymphocytic leukemia [published correction appears in Expert Rev Hematol. 2018;11(1):ix]. Expert Rev Hematol. 2018;11(1):57-70. [DOI] [PubMed] [Google Scholar]
- 6.Ravandi F, O’Brien S. Immune defects in patients with chronic lymphocytic leukemia. Cancer Immunol Immunother. 2006;55(2):197-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mato AR, Roeker LE, Lamanna N, et al. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136(10):1134-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scarfò L, Chatzikonstantinou T, Rigolin GM, et al. COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia. 2020;34(9):2354-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh EE, Frenck RW Jr., Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson LA, Anderson EJ, Rouphael NG, et al. ; mRNA-1273 Study Group . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383(20):1920-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745-2760. [DOI] [PubMed] [Google Scholar]
- 14.Ghia P, Stamatopoulos K, Belessi C, et al. ; European Research Initiative on CLL . ERIC recommendations on IGHV gene mutational status analysis in chronic lymphocytic leukemia. Leukemia. 2007;21(1):1-3. [DOI] [PubMed] [Google Scholar]
- 15.Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910-1916. [DOI] [PubMed] [Google Scholar]
- 16.Mauro FR, Giannarelli D, Galluzzo CM, et al. Response to the conjugate pneumococcal vaccine (PCV13) in patients with chronic lymphocytic leukemia (CLL). Leukemia. 2021;35(3):737-746. [DOI] [PubMed] [Google Scholar]
- 17.Hartkamp A, Mulder AHL, Rijkers GT, van Velzen-Blad H, Biesma DH. Antibody responses to pneumococcal and haemophilus vaccinations in patients with B-cell chronic lymphocytic leukaemia. Vaccine. 2001;19(13-14):1671-1677. [DOI] [PubMed] [Google Scholar]
- 18.Svensson T, Kättström M, Hammarlund Y, et al. Pneumococcal conjugate vaccine triggers a better immune response than pneumococcal polysaccharide vaccine in patients with chronic lymphocytic leukemia A randomized study by the Swedish CLL group. Vaccine. 2018;36(25):3701-3707. [DOI] [PubMed] [Google Scholar]
- 19.Pleyer C, Ali MA, Cohen JI, et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood. 2021;137(2):185-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Velden AMT, Mulder AHL, Hartkamp A, Diepersloot RJ, van Velzen-Blad H, Biesma DH. Influenza virus vaccination and booster in B-cell chronic lymphocytic leukaemia patients. Eur J Intern Med. 2001;12(5):420-424. [DOI] [PubMed] [Google Scholar]
- 21.Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126(5):573-581. [DOI] [PubMed] [Google Scholar]
- 22.Sun C, Gao J, Couzens L, et al. Seasonal influenza vaccination in patients with chronic lymphocytic leukemia treated with ibrutinib. JAMA Oncol. 2016;2(12):1656-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Douglas AP, Trubiano JA, Barr I, Leung V, Slavin MA, Tam CS. Ibrutinib may impair serological responses to influenza vaccination. Haematologica. 2017;102(10):e397-e399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun C, Tian X, Lee YS, et al. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib [published correction appears in Blood. 2016;128(7):1020]. Blood. 2015;126(19):2213-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yri OE, Torfoss D, Hungnes O, et al. Rituximab blocks protective serologic response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood. 2011;118(26):6769-6771. [DOI] [PubMed] [Google Scholar]
- 26.Bedognetti D, Zoppoli G, Massucco C, et al. Impaired response to influenza vaccine associated with persistent memory B cell depletion in non-Hodgkin’s lymphoma patients treated with rituximab-containing regimens. J Immunol. 2011;186(10):6044-6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horwitz SM, Negrin RS, Blume KG, et al. Rituximab as adjuvant to high-dose therapy and autologous hematopoietic cell transplantation for aggressive non-Hodgkin lymphoma. Blood. 2004;103(3):777-783. [DOI] [PubMed] [Google Scholar]
- 28.Nazi I, Kelton JG, Larché M, et al. The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood. 2013;122(11):1946-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Lavallade H, Garland P, Sekine T, et al. Repeated vaccination is required to optimize seroprotection against H1N1 in the immunocompromised host. Haematologica. 2011;96(2):307-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Padron-Regalado E. Vaccines for SARS-CoV-2: lessons from other coronavirus strains [published correction appears in Infect Dis Ther. 2021;10(1):631]. Infect Dis Ther. 2020;9(2):1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du L, Tai W, Zhou Y, Jiang S. Vaccines for the prevention against the threat of MERS-CoV. Expert Rev Vaccines. 2016;15(9):1123-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juno JA, Tan HX, Lee WS, et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat Med. 2020;26(9):1428-1434. [DOI] [PubMed] [Google Scholar]
- 33.Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489-1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arad U, Tzadok S, Amir S, et al. The cellular immune response to influenza vaccination is preserved in rheumatoid arthritis patients treated with rituximab. Vaccine. 2011;29(8):1643-1648. [DOI] [PubMed] [Google Scholar]
- 36.Mouquet H, Musette P, Gougeon ML, et al. B-cell depletion immunotherapy in pemphigus: effects on cellular and humoral immune responses. J Invest Dermatol. 2008;128(12):2859-2869. [DOI] [PubMed] [Google Scholar]
- 37.Hueso T, Pouderoux C, Péré H, et al. Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19. Blood. 2020;136(20):2290-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.