Abstract
Background
Generic antidepressants are approved on the market based on evidence of bioequivalence to their brand-name versions. We aimed to assess whether generic antidepressants exert equal effectiveness as their brand-name counterparts for treating patients with depressive disorders.
Methods
In a nationwide, population-based cohort in Taiwan from 1997 through 2013, patients with a diagnosis of a depressive disorder aged between 18 and 65 years who were new users of antidepressant drugs were classified into either the brand-name group or the generic group. All patients were followed up until medication discontinuation or the end of the study period. We assessed the risk for hospitalization as a primary outcome and augmentation therapy, daily dose, medication discontinuation, or switching to another antidepressant as secondary outcomes.
Results
A total of 277 651 brand-name users (35.8% male; mean age: 41.2 years) and 270 583 generic users (35.8% male; mean age: 41.0 years) were divided into 10 different antidepressant groups (fluoxetine, sertraline, paroxetine, escitalopram, citalopram, venlafaxine, mirtazapine, moclobemide, imipramine, and bupropion). We found that patients treated with the generic form of sertraline, paroxetine, escitalopram, venlafaxine, mirtazapine, and bupropion demonstrated significantly higher risks of psychiatric hospitalization (adjusted hazard ratios ranged from 1.20–2.34), compared to their brand-name counterparts. The differences between brand-name antidepressants and their generic counterparts in secondary outcomes varied across different drugs.
Conclusions
Compared to most generic antidepressants, brand-name drugs exhibited more protective effects on psychiatric hospitalization for depressive patients. These findings could serve as an important reference for clinicians when encountering patients with depressive disorder.
Keywords: antidepressant, effectiveness, formulation, pharmacoepidemiology, psychiatry
Significance Statement.
Generic antidepressants are approved on the market based on evidence of bioequivalence to their brand-name versions. However, whether generic antidepressants provide long-term effectiveness equivalent with brand-name drugs in the real world remains unclear. In this study, Taiwan’s nationwide, population-based data were used to examine whether generic antidepressants exert equal effectiveness as their brand-name counterparts for treating patients with depressive disorders. A total of 548 234 patients with depressive disorders were categorized into 10 antidepressants groups. The results revealed that patients treated with generic products of sertraline, paroxetine, escitalopram, venlafaxine, mirtazapine, and bupropion were at a higher risk of psychiatric hospitalization than those treated with their brand-name counterpart. Compared to most generic antidepressants, brand-name drugs exhibited more protective effects on psychiatric hospitalization for depressive patients. These findings could serve as an important reference for clinicians choosing an antidepressant for depressive patients.
Introduction
Depressive disorders (DD) are common psychiatric disorders and are a growing public health issue. The lifetime risk is about 15%; DD were estimated to be the third leading cause of worldwide disability in 2015 and are projected to rank first by 2030 (Vos et al., 2016; Malhi and Mann, 2018). Pharmacotherapy has been a pillar of depression treatments (Park and Zarate, 2019), and a recent network meta-analysis of 522 trials involving 21 antidepressants indicated that all the assessed drugs were more effective than placebo (Cipriani et al., 2018). Since the late 1930s, various brand-name antidepressants have been introduced to treat depression (Pereira and Hiroaki-Sato, 2018); however, as the patents of the original drugs expired, corresponding generic counterparts entered the market as competing options (Kesselheim et al., 2017). Current studies still debate whether brand-name and generic medications are clinically equivalent (Borgheini, 2003; Desmarais et al., 2011; Cessak et al., 2016). Therefore, understanding the treatment effectiveness of brand-name antidepressants and their generic products for patients with DD is crucial from the clinical aspect.
In 1984, the United States Food and Drug Administration was authorized to approve generic medications based on evidence of average bioequivalence, which is defined as the absence of a significant difference in the bioavailability of the active ingredient of the brand-name versions (Chow, 2014). Several studies from different countries and of different populations that have investigated branded and generic antidepressants have shown that their blood concentrations or hemodynamics and the participants’ tolerability or safety were almost identical (Chenu et al., 2009; Niyomnaitham et al., 2009; Shi et al., 2010; Zheng et al., 2012; Główka et al., 2019). However, the populations of these studies were restricted to healthy subjects and thus many not extend to patients with DD. Although 1 study proved both the bioequivalence (plasma concentration) and therapeutic equivalence (depression symptoms) of brand and generic bupropion among patients with DD, its sample size was fewer than 100 people (Kharasch et al., 2019).
Furthermore, generic versions may still differ from brand-name products in peripheral features, such as excipients (inert binders or fillers) and appearance (pill shape or color; Strom, 1987). Many patients and physicians continue to have negative perceptions of generic drugs and subjectively consider them less effective and safe than brand-name medications in clinical experience (Shrank et al., 2011; Kesselheim et al., 2016). A longitudinal case series in Canada revealed that patients had symptom re-emergence and developed new adverse events after switching to generic citalopram (Van Ameringen et al., 2007). A study of 2 databases in the United States found that generic users of escitalopram and sertraline had higher rates of psychiatric hospitalization (Desai et al., 2019). However, the antidepressants of these studies were limited to selective serotonin reuptake inhibitors (SSRIs; Bolton et al., 2012); therefore, whether therapeutic inequivalence exists in other generic antidepressants remains unclear.
The objective of our study was to comprehensively determine the long-term therapeutic outcomes of brand-name and generic antidepressants in patients with DD. We used a claims database consisting of the nationwide population in Taiwan, which should overcome the limitations mentioned above (only healthy participants, small sample sizes, and only SSRIs). We compared the risks of hospitalization, augmentation therapy, medication discontinuation, and switching to another antidepressant, as well as the average daily doses, between patients treated with brand-name antidepressants and their generic counterparts.
Methods
Ethical Statement
The protocol for this study conformed to the Helsinki Declaration, and was approved by the Institutional Review Board of Chang Gung Memorial Hospital. Patient records/information were anonymized and de-identified prior to analysis, and the need for written informed consent was waived by the Institutional Review Board.
Data Source
The Taiwan National Health Insurance (NHI) program was established in 1995 as the sole payer for health-care services. As of 2010, approximately 23 million individuals were enrolled, covering 99% of Taiwan’s population. In this study, we used the NHI Research Database (NHIRD), which is derived from the reimbursement medical claims records of the NHI program. The NHIRD provides comprehensive information about the insured subjects, such as demographic characteristics (gender, date of birth, and income status) and claims data (clinical diagnostic codes, visiting medical institutions, outpatient and inpatient care, and such prescription records as prescription date, use of brand-name or generic medications, form and dosage, and duration of drug supply). The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), was used for disease codes in this study. To protect individual privacy, all information from NHIRD that may have been used to identify individual patients or medical care institutions was anonymized to ensure confidentiality.
Study Subjects
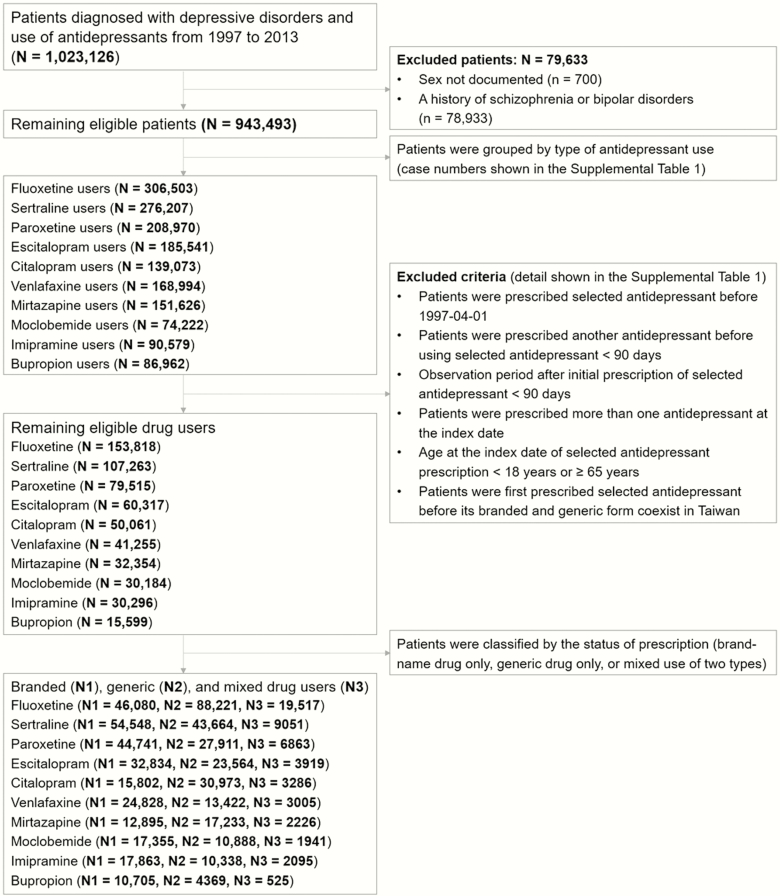
This cohort included all antidepressant users (with at least 1 antidepressant prescription) with a diagnosis of DD (ICD-9-CM: 296.2, 296.3, 300.4 or 311; diagnosed at least twice by psychiatrists based on their diagnostic interview and clinical judgment) that were registered in the NHIRD between 1 January 1997 and 31 December 2013; a similar definition was adopted in a previous study (Hsu et al., 2018). In this study, we used the code N06A from the Anatomical Therapeutic Chemical Classification System of the World Health Organization Collaborating Centre for Drug Statistic Methodology in 2019 (https://www.whocc.no/atc_ddd_index/) to identify antidepressants, finding a total of 21 antidepressants in Taiwan. Patients with unknown gender status or diagnoses of either schizophrenia spectrum disorders (ICD-9-CM: 295) or bipolar disorders (ICD-9-CM: 296 except 296.2, 296.3) were excluded. In total, 943 493 antidepressant users with DD were identified.
The index date of this cohort study was defined as the date of the antidepressant prescription with a concurrent diagnosis of DD. We established the following exclusion criteria to eliminate the confounding effect of drug interactions: (1) patients who had been prescribed the selected antidepressant before 1 April 1997 (at least a 90-day washout period); (2) patients who had been prescribed another antidepressant within 90 days before using the selected antidepressant (at least a 90-day washout period); (3) patients who had been prescribed the selected antidepressant after 2 October 2013 (at least a 90-day observation period); (4) patients who had been prescribed multiple antidepressant drugs at the index date (polypharmacy); (5) patients aged <18 years or ≥65 years at the index date of the selected antidepressant prescription; and (6) patients who received a brand-name antidepressant prior to the date when its generic counterpart has been marketed. We then categorized the remaining patients according to the selected antidepressant.
Of the remaining patients that received the 21 selected antidepressants, some users received both brand-name and generic antidepressant treatment during the course of their diseases (mixed group). To eliminate any cross-over effect, we excluded patients with mixed use for further data analysis. The detailed case numbers of patients receiving the 21 antidepressants are listed in Supplementary Table S1. Afterward, those that met the following criteria were further excluded: (1) only the brand-name antidepressant and no generic form was available in Taiwan; (2) the sum of the selected antidepressant users who had been prescribed only a brand-name or generic drug was less than 10 000; and (3) the ratio of patients between the brand-name drug and generic counterpart was greater than 4. We ultimately narrowed down our data to a total of 10 antidepressants, and Figure 1 shows the flowchart of the participant selection procedure.
Figure 1.
Flowchart showing the selection procedure of study subjects.
Demographics and Potential Confounders
In this cohort study, we evaluated the patients’ characteristics, which included gender, age, cohort entry date, medical comorbidities, psychiatric comorbidities, benzodiazepines use, socioeconomic status, and properties medical institution at the time of prescription. We employed the Charlson Comorbidity Index as a proxy for medical comorbidities to determine general health status (Deyo et al., 1992), which was calculated using diagnostic codes from outpatient and inpatient records and has been widely applied for confounders in epidemiological research (Schneeweiss et al., 2001). Psychiatric comorbidities included substance use disorders (ICD-9-CM: 291, 292, 303, 304, 305 [except 305.1], 357.5, 425.5, 535.3, or 571.0–571.3), anxiety disorders (ICD-9-CM: 300 [except 300.4]), and sleep disorders (ICD-9-CM: 307.4 and 780.5). We reported monthly income in New Taiwan dollars to represent socioeconomic status, which was calculated according to the premium paid. In 2008, the approximate exchange rate of the New Taiwan dollar to the United States dollar was 31.5. Medical institution properties were grouped into 2 categories, based on the accreditation level in Taiwan: hospital and clinic.
Outcome Variables
All antidepressant users were observed from the index date of the selected antidepressant to its discontinuation date or 31 December 2013. The primary outcome of treatment effectiveness was hospitalization (overall and psychiatric) before discontinuation of the antidepressant. The secondary outcomes included the occurrence of augmentation therapy with another drug (another antidepressant, antipsychotic, or mood stabilizer) before discontinuation of the antidepressant, the average daily dose, discontinuation of the selected antidepressant, and switching to another antidepressant after discontinuing the initial antidepressant. We defined discontinuation of an antidepressant as the cessation of the selected brand-name or generic antidepressant for 90 days or longer. The average daily dose was defined as the dose of the last prescription before antidepressant discontinuation or the end of follow-up, which was also converted into a ratio of the average daily dose to the defined daily dose (DDD) for standardization. We adopted the DDD, determined by the World Health Organization Collaborating Centre for Drug Statistics Methodology, to assume the average maintenance dose per day for an antidepressant when used for its main indication in adults (Sinnott et al., 2016). Switching was defined as changing to another antidepressant within 90 days after discontinuing the initial antidepressant.
Statistical Methods and Sensitivity Analyses
We used descriptive statistics to compare patients’ characteristics and the average daily doses of different antidepressants. We used Chi-square and independent t-tests to compare categorical and continuous variables, respectively, between the users of brand-name and generic antidepressants. For the treatment outcomes (hospitalization, medication discontinuation, antidepressant switching, augmentation therapy), we constructed Cox proportional hazards regression models to calculate hazard ratios (HRs) and 95% confidence intervals for the occurrences of such outcomes, which were further adjusted for potential confounders with gender, age, cohort entry date of the selected antipsychotic, Charlson Comorbidity Index scores, psychiatric comorbidities, benzodiazepines use, income status, and prescription medical institution.
To minimize any potential indication bias from the severity of DD, we performed a subgroup analysis to evaluate the robustness of our results. We narrowed down this cohort study to antidepressant users who were diagnosed with major depressive disorders (MDD; ICD-9-CM: 296.2 or 296.3) and repeated the primary analysis. All analyses were conducted with SAS 9.4 software (SAS Institute) and MedCalc Statistical Software version 18.11.3 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2019). A 2-tailed P < .05 was considered statistically significant.
Results
This study included a total of 548 234 patients with DD who received 1 of the following antidepressants in either its brand-name or generic form: fluoxetine, sertraline, paroxetine, escitalopram, citalopram, venlafaxine, mirtazapine, moclobemide, imipramine, and bupropion. Table 1 summarizes the demographic data of the patients with DD treated with the brand-name versus generic form of an antidepressant, pooled across all 10 drugs. We found that the hospitals prescribed significantly more brand-name and fewer generic antidepressants, compared to clinics (brand-name: hospital 91.66% vs clinic 8.34%; generic: hospital 41.79% vs clinic 58.21%; P < .0001). The individual drug comparisons and the year-by-year data on the characteristics of patients with DD and MDD are shown in Supplementary Tables S2 and S3.
Table 1.
Characteristics of Patients with Depressive Disorders Treated with Brand-Name and Generic Formulas of 10 Selected Antidepressants in Taiwan, from 1997 to 2013
| All Antidepressants | |||
|---|---|---|---|
| Brand-Name | Generic | P Value | |
| n = 277 651 | n = 270 583 | ||
| Sex, male/female | 99 430/178 221 (35.81/64.19) | 96 897/173 686 (35.81/64.19) | NS |
| Age, years | 41.21 ± 13.04 | 41.03 ± 12.69 | * b |
| Charlson Comorbidity Index | 1.41 ± 1.91 | 1.37 ± 1.81 | NS |
| Substance use disorders | 16 278 (5.86) | 17 395 (6.43) | * a |
| Anxiety disorders | 123 134 (44.35) | 126 749 (46.84) | * a |
| Sleep disorders | 125 952 (62.68) | 139 821 (65.08) | * a |
| Benzodiazepines use | 174 024 (49.70) | 176 092 (50.30) | * a |
| Monthly income, NTD | 16 887 ± 17 869 | 16 180 ± 16,635 | NS |
| Medical institution, hospital/clinic | 254 499/23 152 (91.66/8.34) | 113 083/157 500 (41.79/58.21) | * b |
Data were expressed as n (%) or mean ± standard deviation. *P < .0001.
Abbreviations: NS, not significant; NTD, New Taiwan Dollar.
aDrug-G data are significantly higher than drug-B data.
bDrug-B data are significantly higher than drug-G data.
As the primary outcome, DD patients receiving the generic form of sertraline, paroxetine, escitalopram, venlafaxine, mirtazapine, and bupropion demonstrated significantly higher adjusted HRs of psychiatric admission, compared to those treated with their branded counterparts (HRs ranged from 1.20 to 2.34), as shown in Table 2. The subgroup analysis of only patients with MDD also indicated that those treated with the generic forms of sertraline, paroxetine, escitalopram, venlafaxine, mirtazapine, moclobemide, and bupropion revealed significantly higher adjusted HRs of psychiatric hospitalization than those treated with their brand-name counterparts (HRs ranged from 1.27 to 2.66). For admission due to all causes, those treated with the generic forms of paroxetine, escitalopram, venlafaxine, and bupropion demonstrated significantly higher risks of overall hospitalization than those treated with brand-name products. Furthermore, in the subgroup analysis, brand-name paroxetine, escitalopram, venlafaxine, moclobemide, and bupropion outperformed their generic counterparts in preventing all-cause hospitalization in MDD patients.
Table 2.
Comparison of Overall and Psychiatric Hospitalization in Depressive Patients Treated with Brand-Name and Generic Formulas of 10 Antidepressants
| Antidepressant | Group | Subgroup | Overall n (PY) | Overall aHR (95% CI)c | Psychiatric n (PY) | Psychiatric aHR (95% CI)a |
|---|---|---|---|---|---|---|
| Fluoxetine | DD | Brand-name | 1192 (11 408) | 1.00 [Reference] | 828 (11 489) | 1.00 [Reference] |
| Generic | 1631 (24 231) | .94 (.87–1.01) | 1045 (24 407) | .90 (.82–.99)* b | ||
| MDD | Brand-name | 587 (5178) | 1.00 [Reference] | 500 (5203) | 1.00 [Reference] | |
| Generic | 978 (14 256) | 1.04 (.94–1.16) | 814 (143 03) | 1.06 (.94–1.19) | ||
| Sertraline | DD | Brand-name | 1404 (13 945) | 1.00 [Reference] | 759 (14 117) | 1.00 [Reference] |
| Generic | 652 (11 086) | 1.03 (.93–1.14) | 392 (111 63) | 1.20 (1.05–1.37)* c | ||
| MDD | Brand-name | 826 (6899) | 1.00 [Reference] | 589 (6987) | 1.00 [Reference] | |
| Generic | 335 (4488) | 1.10 (.95–1.26) | 267 (4508) | 1.27 (1.08–1.49)* c | ||
| Paroxetine | DD | Brand-name | 1081 (11 731) | 1.00 [Reference] | 666 (11 857) | 1.00 [Reference] |
| Generic | 623 (7054) | 1.55 (1.38–1.73)*** c | 481 (7101) | 1.82 (1.59–2.08)*** c | ||
| MDD | Brand-name | 628 (5259) | 1.00 [Reference] | 494 (5302) | 1.00 [Reference] | |
| Generic | 387 (2583) | 1.70 (1.46–1.97)*** c | 360 (2593) | 1.89 (1.61–2.22)*** c | ||
| Escitalopram | DD | Brand-name | 828 (7999) | 1.00 [Reference] | 508 (8066) | 1.00 [Reference] |
| Generic | 305 (5332) | 1.22 (1.05–1.42)* c | 224 (5346) | 1.39 (1.17–1.66)** c | ||
| MDD | Brand-name | 495 (3261) | 1.00 [Reference] | 383 (3288) | 1.00 [Reference] | |
| Generic | 189 (1712) | 1.34 (1.11–1.61)* c | 167 (1716) | 1.42 (1.16–1.73)** c | ||
| Citalopram | DD | Brand-name | 427 (3744) | 1.00 [Reference] | 239 (3789) | 1.00 [Reference] |
| Generic | 396 (8588) | .81 (.69–.96)* b | 265 (8636) | 1.01 (.82–1.24) | ||
| MDD | Brand-name | 242 (1887) | 1.00 [Reference] | 179 (1902) | 1.00 [Reference] | |
| Generic | 185 (3081) | .98 (.79–1.23) | 157 (3087) | 1.14 (.89–1.45) | ||
| Venlafaxine | DD | Brand-name | 635 (6392) | 1.00 [Reference] | 360 (6482) | 1.00 [Reference] |
| Generic | 223 (3058) | 1.38 (1.15–1.64)** c | 158 (3074) | 1.74 (1.39–2.16)*** c | ||
| MDD | Brand-name | 450 (3971) | 1.00 [Reference] | 325 (4013) | 1.00 [Reference] | |
| Generic | 164 (1900) | 1.30 (1.06–1.61)* c | 142 (1903) | 1.55 (1.23–1.96)** c | ||
| Mirtazapine | DD | Brand-name | 540 (2739) | 1.00 [Reference] | 248 (2795) | 1.00 [Reference] |
| Generic | 348 (3928) | .96 (.82–1.12) | 224 (3958) | 1.36 (1.11–1.68)* c | ||
| MDD | Brand-name | 311 (1573) | 1.00 [Reference] | 200 (1596) | 1.00 [Reference] | |
| Generic | 220 (1888) | 1.18 (.97–1.44) | 172 (1903) | 1.49 (1.18–1.88)** c | ||
| Moclobemide | DD | Brand-name | 277 (3778) | 1.00 [Reference] | 110 (3830) | 1.00 [Reference] |
| Generic | 128 (2576) | 1.29 (.97–1.70) | 57 (2595) | 1.48 (.97–2.27) | ||
| MDD | Brand-name | 90 (1415) | 1.00 [Reference] | 70 (1417) | 1.00 [Reference] | |
| Generic | 53 (982) | 1.93 (1.24–3.02)* c | 38 (986) | 1.92 (1.14–3.21)* c | ||
| Imipramine | DD | Brand-name | 251 (3808) | 1.00 [Reference] | 111 (3857) | 1.00 [Reference] |
| Generic | 112 (2513) | .99 (.78–1.24) | 41 (2564) | .88 (.61–1.27) | ||
| MDD | Brand-name | 75 (922) | 1.00 [Reference] | 56 (926) | 1.00 [Reference] | |
| Generic | 26 (493) | .85 (.54–1.33) | 20 (494) | .91 (.54–1.54) | ||
| Bupropion | DD | Brand-name | 197 (2352) | 1.00 [Reference] | 108 (2378) | 1.00 [Reference] |
| Generic | 43 (833) | 1.67 (1.16–2.41)* c | 31 (835) | 2.34 (1.51–3.62)** c | ||
| MDD | Brand-name | 107 (978) | 1.00 [Reference] | 76 (987) | 1.00 [Reference] | |
| Generic | 30 (269) | 2.30 (1.45–3.64)** c | 27 (269) | 2.66 (1.61–4.39)** c |
*P < .05; **P < .001; ***P < .0001.
Abbreviations: aHR, adjusted hazard ratio; CI: confidence interval; DD, depressive disorders; MDD, major depressive disorders; n, number of hospitalizations; PY, person-year of follow-up.
aAdjusted for gender, age, entry year, Charlson Comorbidity Index, substance use disorders, anxiety disorders, sleep disorders, benzodiazepines use, monthly income, and medical institution.
bDrug-B data are significantly higher than drug-G data.
cDrug-G data are significantly higher than drug-B data.
Table 3 provides the average daily doses, the ratios of the average daily doses to the DDDs, and the adjusted HRs of augmentation therapies, antidepressant discontinuation, and antidepressant switching among the 10 antidepressants in patients with DD and MDD. We found that the users of generic sertraline were at a higher risk of receiving augmentation of a psychotropic medication, while those who received the generic forms of fluoxetine and escitalopram had lower risks of receiving any psychotropic drug when compared to their brand-name counterparts. The details of the psychotropic drugs used in augmentation therapies are provided in Supplementary Table S4.
Table 3.
Comparison of Augmentation, Average Daily Dose, Discontinuation, and Switching in Depressive Patients Treated with Brand-Name and Generic Formula of 10 Antidepressants
| Antidepressant | Group | Subgroup | Augmentation aHR (95% CI) | Dose, mg/day mean ± SD | Ratio, Dose/DDD | Discontinuation aHR (95% CI)c | Switching aHR (95% CI)a |
|---|---|---|---|---|---|---|---|
| Fluoxetine | DD | Brand-name | 1.00 [Reference] | 21.62 ± 10.82 | 1.08 | 1.00 [Reference] | 1.00 [Reference] |
| Generic | .91 (.89–.93)*** b | 22.62 ± 11.42 | 1.13*** c | 1.00 (.99–1.01) | .93 (.90–.95)*** b | ||
| MDD | Brand-name | 1.00 [Reference] | 22.97 ± 12.50 | 1.15 | 1.00 [Reference] | 1.00 [Reference] | |
| Generic | .93 (.90–.96)*** b | 23.68 ± 10.91 | 1.18*** c | .99 (.97–1.00) | .89 (.86–.93)*** b | ||
| Sertraline | DD | Brand-name | 1.00 [Reference] | 51.20 ± 27.12 | 1.02 | 1.00 [Reference] | 1.00 [Reference] |
| Generic | 1.10 (1.07–1.13)*** c | 49.00 ± 23.61 | .98*** b | 1.06 (1.04–1.07)*** c | 1.07 (1.04–1.10)*** c | ||
| MDD | Brand-name | 1.00 [Reference] | 56.04 ± 30.47 | 1.12 | 1.00 [Reference] | 1.00 [Reference] | |
| Generic | 1.11 (1.07–1.16)*** c | 54.94 ± 26.24 | 1.10*** b | 1.06 (1.03–1.09)*** c | .93 (.89–.98)* b | ||
| Paroxetine | DD | Brand-name | 1.00 [Reference] | 17.65 ± 8.88 | .88 | 1.00 [Reference] | 1.00 [Reference] |
| Generic | 1.01 (.98–1.04) | 17.98 ± 8.42 | .90*** c | 1.05 (1.03–1.07)*** c | 1.02 (.98–1.05) | ||
| MDD | Brand-name | 1.00 [Reference] | 19.54 ± 12.01 | .98 | 1.00 [Reference] | 1.00 [Reference] | |
| Generic | .97 (.92–1.01) | 20.59 ± 9.10 | 1.03** c | 1.00 (.97–1.04) | .96 (.91–1.02) | ||
| Escitalopram | DD | Brand-name | 1.00 [Reference] | 9.30 ± 4.37 | .93 | 1.00 [Reference] | 1.00 [Reference] |
| Generic | .89 (.86–.92)*** b | 9.50 ± 4.16 | .95*** c | 1.02 (1.00–1.05) | .91 (.86–.95)*** b | ||
| MDD | Brand-name | 1.00 [Reference] | 10.16 ± 4.70 | 1.02 | 1.00 [Reference] | 1.00 [Reference] | |
| Generic | .84 (.79–.89)*** b | 10.86 ± 4.58 | 1.09*** c | .98 (.94–1.02) | .90 (.84–.97)* b | ||
| Citalopram | DD | Brand-name | 1.00 [Reference] | 19.21 ± 9.19 | .96 | 1.00 [Reference] | 1.00 [Reference] |
| Generic | .92 (.89–.95)*** b | 18.42 ± 8.35 | .92*** b | .93 (.91–.95)*** b | .85 (.81–.88)*** b | ||
| MDD | Brand-name | 1.00 [Reference] | 20.74 ± 9.75 | 1.04 | 1.00 [Reference] | 1.00 [Reference] | |
| Generic | .96 (.91–1.01) | 20.58 ± 9.97 | 1.03* b | .92 (.89–.95)*** b | .87 (.81–.92)*** b | ||
| Venlafaxine | DD | Brand-name | 1.00 [Reference] | 85.00 ± 44.73 | .85 | 1.00 [Reference] | 1.00 [Reference] |
| Generic | .98 (.94–1.03) | 77.04 ± 41.69 | .77*** b | 1.01 (.98–1.04) | 1.05 (1.00–1.11)* c | ||
| MDD | Brand-name | 1.00 [Reference] | 96.27 ± 57.46 | .96 | 1.00 [Reference] | 1.00 [Reference] | |
| Generic | .83 (.78–.88)*** b | 93.39 ± 45.94 | .93*** b | .91 (.87–.95)*** b | .98 (.91–1.06) | ||
| Mirtazapine | DD | Brand-name | 1.00 [Reference] | 28.19 ± 13.42 | .94 | 1.00 [Reference] | 1.00 [Reference] |
| Generic | .96 (.92–1.00) | 24.46 ± 11.54 | .82*** b | .94 (.92–.97)** b | .91 (.86–.96)** b | ||
| MDD | Brand-name | 1.00 [Reference] | 30.45 ± 15.42 | 1.01 | 1.00 [Reference] | 1.00 [Reference] | |
| Generic | .96 (.90–1.03) | 27.64 ± 12.57 | .92*** b | .94 (.90–.98)* b | .88 (.81–.95)* b | ||
| Moclobemide | DD | Brand-name | 1.00 [Reference] | 312.08 ± 144.88 | 1.04 | 1.00 [Reference] | 1.00 [Reference] |
| Generic | .93 (.87–.98)* b | 277.04 ± 127.13 | .92*** b | 1.01 (.98–1.05) | .87 (.82–.94)** b | ||
| MDD | Brand-name | 1.00 [Reference] | 350.00 ± 295.56 | 1.17 | 1.00 [Reference] | 1.00 [Reference] | |
| Generic | .96 (.87–1.06) | 326.79 ± 134.63 | 1.09*** b | .97 (.91–1.04) | .95 (.85–1.07) | ||
| Imipramine | DD | Brand-name | 1.00 [Reference] | 29.80 ± 24.68 | .30 | 1.00 [Reference] | 1.00 [Reference] |
| Generic | .85 (.81–.89)*** b | 39.51 ± 28.17 | .40*** c | .97 (.94–.99)* b | .90 (.86–.95)*** b | ||
| MDD | Brand-name | 1.00 [Reference] | 41.94 ± 41.41 | .42 | 1.00 [Reference] | 1.00 [Reference] | |
| Generic | 1.05 (.95–1.15) | 45.90 ± 37.58 | .46*** c | 1.12 (1.05–1.19)** c | 1.06 (.95–1.18) | ||
| Bupropion | DD | Brand-name | 1.00 [Reference] | 185.60 ± 79.71 | .62 | 1.00 [Reference] | 1.00 [Reference] |
| Generic | .96 (.88–1.04) | 168.31 ± 68.56 | .56*** b | 1.03 (.98–1.08) | 1.02 (.93–1.12) | ||
| MDD | Brand-name | 1.00 [Reference] | 200.76 ± 86.54 | .67 | 1.00 [Reference] | 1.00 [Reference] | |
| Generic | 1.02 (.90–1.15) | 185.86 ± 80.31 | .62*** b | .99 (.91–1.09) | .89 (.76–1.05) |
*P < .05; **P < .001; ***P < .0001.
Abbreviations: aHR, adjusted hazard ratio; CI: confidence interval; DD, depressive disorders; DDD, defined daily dose; MDD, major depressive disorders; SD, standard deviation.
aAdjusted for gender, age, entry year, Charlson Comorbidity Index, substance use disorders, anxiety disorders, sleep disorders, benzodiazepines use, monthly income, and medical institution.
bDrug-B data are significantly higher than drug-G data.
cDrug-G data are significantly higher than drug-B data.
In 4 of the studied antidepressants (fluoxetine, paroxetine, escitalopram, and imipramine), the daily doses of the generic drug were higher than those of the brand-name drug; in the other 6 antidepressants studied herein (sertraline, citalopram, venlafaxine, mirtazapine, moclobemide, and bupropion), the daily doses of the brand-name drug were higher than those of the generic version.
Regarding the adjusted HRs of discontinuation, the users of generic sertraline were at a higher risk than the users of the brand-name form; however, the users of generic citalopram and mirtazapine had lower risks of discontinuation than the users of the brand-name versions. As for the adjusted HRs of switching, the generic drug users of 4 antidepressants (fluoxetine, escitalopram, citalopram, and mirtazapine) had lower risks of switching than the users of the brand-name products.
Discussion
This is the first study to compare the effectiveness between brand-name and generic antidepressants for depressive patients in Taiwan using real-world evidence. Compared to brand-name antidepressants, we found that patients with DD and MDD that received generic antidepressants were at a disadvantage with regard to preventing hospitalization, for both psychiatric admission and all-cause admission, after adjusting for potential confounders. Furthermore, DD and MDD patients treated with generic or brand-name antidepressants demonstrated heterogeneous findings in the risks for combining augmentation therapy, last average daily dose, discontinuation of antidepressants, and drugs switching, which varied across different drug products.
The occurrence of hospitalization is an important outcome for evaluating treatment effectiveness in psychiatric research using a database (Desai et al., 2019; Montastruc et al., 2019). Our results demonstrate that most brand-name antidepressants have an advantage in preventing psychiatric hospitalization: a finding supported by previous studies (Wu et al., 2011; Desai et al., 2019). A nationwide study revealed that users of brand-name sertraline and escitalopram had lower psychiatric hospitalization rates, compared to those using generic products (Desai et al., 2019). Another retrospective analysis of the claims database showed that MDD patients who were switched from a brand-name SSRI to an alternative, generic version had higher hospitalization rates (Wu et al., 2011). Compared to the aforementioned studies, this study has 2 strengths. First, we used a comprehensive, nationwide database that provides a large sample size for longitudinal analysis. In the current study, we included 10 kinds of antidepressants (SSRIs and other classes of drugs) with 548 234 participants. Another strength is the direct comparison between brand-name drugs and their generic counterparts, which should yield more useful information than those studies that compare the differences between different antidepressants.
In the current study, we found the mean durations between the initial prescription to discontinuation were only 91.5 days and 94.8 days for brand-name and generic drugs, respectively, in patients with DD; the difference in the risks of discontinuation between brand-name and generic was less than 12% (Table 3). In addition, we found that 72.3% of the depressive patients who discontinued drug therapy did not switch to another antidepressant (data not shown). The clinical practice guidelines recommend that the duration of antidepressant treatment for MDD should be at least 6 months (Lam et al., 2009). Our result was similar to a study using a United States database, which reported that only 27.6% of the patients continued antidepressant therapy for more than 90 days (Olfson et al., 2006). The most common reasons for premature discontinuation were non-responsiveness or intolerance of side effects (Hodgkin et al., 2007). So, we compared the risks of discontinuation between brand-name and generic antidepressants as another indication of effectiveness. It was suggested that generic medications may have lower proportions of adverse events when compared to branded medications, which also lowers the risk of discontinuation (Takami et al., 2019). However, whether a lower proportion of adverse events is related to lower efficacy still requires further study. Furthermore, high placebo response rates (ranging from 35% to 40%) in antidepressant users may also hinder the exploration of differences in efficacy between brand-name and generic versions (Furukawa et al., 2016).
If a first-line antidepressant cannot achieve satisfying treatment effects, several previous guidelines have suggested that switching to another antidepressant or augmentation therapy (combining 2 antidepressants or adding 1 antipsychotic/mood stabilizer to the original antidepressant) is a feasible strategy for physicians (Bayes and Parker, 2018). We found slight differences between brand-name and generic antidepressants with regard to the risks of switching to another antidepressant or augmentation with an antidepressant, antipsychotic, or mood stabilizer. The risks for switching and augmentation may also rely on clinical presentation and the prescribing physician’s medical resources, which were not controlled for in the current study. Regarding the differences in average daily doses of the last prescription between brand-name and generic antidepressants, our results showed varied findings (higher doses in 4 kinds of generic antidepressants compared to their brand-name counterparts), with weak significance (the ratio of an average daily dose to a DDD was less than 0.12 in the same drugs and disorders group), as shown in Table 3.
Our study also has some limitations. First, it is subject to the usual limitations of a retrospective analysis from reimbursement data. Although we attempted to control for potential confounding factors and adjust for observable baseline characteristics, unobserved confounders are not included in the current study, such as the severity of depressive symptoms, residential area, support system, or drug compliance. Second, we make multiple comparisons for effectiveness (discontinuation, dose, switching, augmentation, and hospitalization) in this study. Third, patients were prescribed either generic or brand-name drugs through clinical judgment in real-world settings, but not through random assignment, so this study may have selection bias. Fourth, the brand-name drugs were mostly prescribed by the doctors in hospitals, but not in clinics. The doctors in the hospitals may provide better care than those in the clinics, and the medical resources in hospitals may be more abundant than those in the clinics. However, patients seeking treatment in a hospital may have suffered from greater severity of depression than their counterparts who received treatment in clinics. Although we adjusted the factor of “medical institution” in the Cox proportional hazards regression models, the confounding effect may still exist. Finally, this study was only able to evaluate the effectiveness of 10 antidepressants, and our findings may not be generalizable to other types of antidepressants.
Conclusions
The real-world evidence from Taiwan revealed that depressive patients treated with most generic antidepressants were at a higher risk of psychiatric admission, compared to those treated with brand-name drugs. Furthermore, other clinical outcomes with regard to augmentation therapy, last average daily dose, medication discontinuation, and antidepressant switching were inconclusive in depressive patients treated with generic or brand-name antidepressants. These results could be an essential reference for clinical practices using antidepressants to treat depressive patients.
Supplementary Material
Acknowledgments
The authors thank Y.Y. Hsieh, MS, the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital, for the technical consultation and TransGlobe for English-language editing.
This work was supported by the Chang Gung Memorial Hospital Research Project (CFRPG8H0271). The funding source had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Statement of Interest
The authors report no financial interest or potential conflicts of interest.
References
- Bayes AJ, Parker GB (2018) Comparison of guidelines for the treatment of unipolar depression: a focus on pharmacotherapy and neurostimulation. Acta Psychiatr Scand 137:459–471. [DOI] [PubMed] [Google Scholar]
- Bolton JM, Dahl M, Sareen J, Enns MW, Leslie WD, Collins DM, Alessi-Severini S (2012) A population-based study of the use of selective serotonin reuptake inhibitors before and after introduction of generic equivalents. Can J Psychiatry 57:223–229. [DOI] [PubMed] [Google Scholar]
- Borgheini G (2003) The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther 25:1578–1592. [DOI] [PubMed] [Google Scholar]
- Cessak G, Rokita K, Dąbrowska M, Sejbuk-Rozbicka K, Zaremba A, Mirowska-Guzel D, Bałkowiec-Iskra E (2016) Therapeutic equivalence of antipsychotics and antidepressants - a systematic review. Pharmacol Rep 68:217–223. [DOI] [PubMed] [Google Scholar]
- Chenu F, Batten LA, Zernig G, Ladstaetter E, Hébert C, Blier P (2009) Comparison of pharmacokinetic profiles of brand-name and generic formulations of citalopram and venlafaxine: a crossover study. J Clin Psychiatry 70:958–966. [DOI] [PubMed] [Google Scholar]
- Chow SC (2014) Bioavailability and bioequivalence in drug development. Wiley Interdiscip Rev Comput Stat 6:304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JPT, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JPA, Geddes JR (2018) Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 391:1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RJ, Sarpatwari A, Dejene S, Khan NF, Lii J, Rogers JR, Dutcher SK, Raofi S, Bohn J, Connolly JG, Fischer MA, Kesselheim AS, Gagne JJ (2019) Comparative effectiveness of generic and brand-name medication use: a database study of US health insurance claims. PLOS Med 16:e1002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmarais JE, Beauclair L, Margolese HC (2011) Switching from brand-name to generic psychotropic medications: a literature review. CNS Neurosci Ther 17:750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613–619. [DOI] [PubMed] [Google Scholar]
- Furukawa TA, Cipriani A, Atkinson LZ, Leucht S, Ogawa Y, Takeshima N, Hayasaka Y, Chaimani A, Salanti G (2016) Placebo response rates in antidepressant trials: a systematic review of published and unpublished double-blind randomised controlled studies. Lancet Psychiatry 3:1059–1066. [DOI] [PubMed] [Google Scholar]
- Główka FK, Hermann TW, Danielak D, Zabel M, Hermann J (2019) Bioavailability of moclobemide from two formulation tablets in healthy humans. Pharmazie 74:97–100. [DOI] [PubMed] [Google Scholar]
- Hodgkin D, Volpe-Vartanian J, Alegría M (2007) Discontinuation of antidepressant medication among Latinos in the USA. J Behav Health Serv Res 34:329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CW, Lee SY, Wang LJ (2018) Comparison of the effectiveness of brand-name and generic antipsychotic drugs for treating patients with schizophrenia in Taiwan. Schizophr Res 193:107–113. [DOI] [PubMed] [Google Scholar]
- Kesselheim AS, Gagne JJ, Franklin JM, Eddings W, Fulchino LA, Avorn J, Campbell EG (2016) Variations in patients’ perceptions and use of generic drugs: results of a national survey. J Gen Intern Med 31:609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesselheim AS, Sinha MS, Avorn J (2017) Determinants of market exclusivity for prescription drugs in the United States. JAMA Intern Med 177:1658–1664. [DOI] [PubMed] [Google Scholar]
- Kharasch ED, Neiner A, Kraus K, Blood J, Stevens A, Schweiger J, Miller JP, Lenze EJ (2019) Bioequivalence and therapeutic equivalence of generic and brand bupropion in adults with major depression: a randomized clinical trial. Clin Pharmacol Ther 105:1164–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam RW, Kennedy SH, Grigoriadis S, McIntyre RS, Milev R, Ramasubbu R, Parikh SV, Patten SB, Ravindran AV; Canadian Network for Mood and Anxiety Treatments (CANMAT) (2009) Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. III. Pharmacotherapy. J Affect Disord 117Suppl 1:S26–S43. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Mann JJ (2018) Depression. Lancet 392:2299–2312. [DOI] [PubMed] [Google Scholar]
- Montastruc F, Nie R, Loo S, Rej S, Dell’Aniello S, Micallef J, Suissa S, Renoux C (2019) Association of aripiprazole with the risk for psychiatric hospitalization, self-harm, or suicide. JAMA Psychiatry 76:409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyomnaitham S, Chatsiricharoenkul S, Sathirakul K, Pongnarin P, Kongpatanakul S (2009) Bioequivalence study of 50 mg sertraline tablets in healthy Thai volunteers. J Med Assoc Thai 92:1229–1233. [PubMed] [Google Scholar]
- Olfson M, Marcus SC, Tedeschi M, Wan GJ (2006) Continuity of antidepressant treatment for adults with depression in the United States. Am J Psychiatry 163:101–108. [DOI] [PubMed] [Google Scholar]
- Park LT, Zarate CA Jr (2019) Depression in the primary care setting. N Engl J Med 380:559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira VS, Hiroaki-Sato VA (2018) A brief history of antidepressant drug development: from tricyclics to beyond ketamine. Acta Neuropsychiatr 30:307–322. [DOI] [PubMed] [Google Scholar]
- Schneeweiss S, Seeger JD, Maclure M, Wang PS, Avorn J, Glynn RJ (2001) Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol 154:854–864. [DOI] [PubMed] [Google Scholar]
- Shi S, Liu Y, Wu J, Li Z, Zhao Y, Zhong D, Zeng F (2010) Comparative bioavailability and tolerability of a single 20-mg dose of two fluoxetine hydrochloride dispersible tablet formulations in fasting, healthy Chinese male volunteers: an open-label, randomized-sequence, two-period crossover study. Clin Ther 32:1977–1986. [DOI] [PubMed] [Google Scholar]
- Shrank WH, Liberman JN, Fischer MA, Girdish C, Brennan TA, Choudhry NK (2011) Physician perceptions about generic drugs. Ann Pharmacother 45:31–38. [DOI] [PubMed] [Google Scholar]
- Sinnott SJ, Polinski JM, Byrne S, Gagne JJ (2016) Measuring drug exposure: concordance between defined daily dose and days’ supply depended on drug class. J Clin Epidemiol 69:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom BL (1987) Generic drug substitution revisited. N Engl J Med 316:1456–1462. [DOI] [PubMed] [Google Scholar]
- Takami A, Hirata K, Ishiguro C, Hanaoka H, Uyama Y (2019) Lower proportion of spontaneous adverse event reports for generic drugs by comparison with original branded drugs at the postmarket stage in Japan. Clin Pharmacol Ther. 105: 1471– 1476. [DOI] [PubMed] [Google Scholar]
- Van Ameringen M, Mancini C, Patterson B, Bennett M (2007) Symptom relapse following switch from Celexa to generic citalopram: an anxiety disorders case series. J Psychopharmacol 21:472–476. [DOI] [PubMed] [Google Scholar]
- Vos T, et al. (2016) Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1545–1602. 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu EQ, Yu AP, Lauzon V, Ramakrishnan K, Marynchenko M, Ben-Hamadi R, Blum S, Erder MH (2011) Economic impact of therapeutic substitution of a brand selective serotonin reuptake inhibitor with an alternative generic selective serotonin reuptake inhibitor in patients with major depressive disorder. Ann Pharmacother 45:441–451. [DOI] [PubMed] [Google Scholar]
- Zheng L, Yu Q, Miao J, Xiang J, Xu N (2012) Bioequivalence study of two mirtazapine oral tablet formulations in healthy Chinese male volunteers. Int J Clin Pharmacol Ther 50:368–374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.