Abstract
Feline infectious peritonitis (FIP) is a fatal systemic disease of felids caused by a Coronavirus (CoV) (FIPV). In spite of its clinical relevance and impact on feline health, currently the therapeutic possibilities for treatment of FIP in cats are limited. The emergence of the pandemic Severe Respiratory Syndrome (SARS) coronavirus (CoV) type 2 (SARS-CoV-2), etiological agent of the 2019 Coronavirus Disease (COVID-19), able to infect a broad spectrum of animal species including cats, triggered the interest for the development of novel molecules with antiviral activity for treatment of CoV infections in humans and animals.
Essential oils (EOs) have raised significant attention for their antiviral properties integrating and, in some cases, replacing conventional drugs. Thymus vulgaris EO (TEO) has been previously shown to be effective against several RNA viruses including CoVs. In the present study the antiviral efficacy of TEO against FIPV was evaluated in vitro.
TEO at 27 μg/ml was able to inhibit virus replication with a significant reduction of 2 log10 TCID50/50 μl. Moreover, virucidal activity was tested using TEO at 27 and 270 μg/ml, over the cytotoxic threshold, determining a reduction of viral titre as high as 3.25 log10 TCID50/50 μl up to 1 h of time contact. These results open several perspectives in terms of future applications and therapeutic possibilities for coronaviruses considering that FIPV infection in cats could be a potential model for the study of antivirals against CoVs.
Keywords: Thymus Essential Oil, Feline Coronavirus, Thymus vulgaris, FIP
The pandemic 2019 Coronavirus Disease (COVID-19) caused by Severe Respiratory Syndrome (SARS) coronavirus (CoV) type 2 (SARS-CoV-2) (WHO, 2020) prompted the research on therapy and immune-prophylaxis, taking advantage of previous knowledge accumulated on SARS-CoV-1 and animal CoVs (Decaro et al., 2020). To date, no specific drug has been approved for the treatment of patients with COVID-19. However, remdesivir, an inhibitor of RNA-dependent RNA polymerase (RdRp), demonstrated promising results (Kabir et al., 2020). Moreover, studies to evaluate the efficacy of teicoplanin and monoclonal and polyclonal antibodies against SARS-CoV-2 are currently ongoing (Kabir et al., 2020).
Drugs investigated in cats for the therapy of Feline Infectious Peritonitis (FIP) have been also tested against COVID-19 in human patients (Pedersen et al., 2018, Pedersen et al., 2019).
CoVs have been long known for the FIP, a fatal systemic disease of felids. FIP virus (FIPV) is a virulent pathotype of feline enteric coronavirus (FCoV) (Kummrow et al., 2005). In spite of its impact on feline health, the therapeutic possibilities for treatment of FIP in cats are limited and effective vaccines are not available. Moreover, vaccine adverse effects have been reported (Tizard, 2020). The development of novel molecules with antiviral activity for treatment of CoV infections is now perceived as a priority in both human and animal medicine. FIPV infection in cats is considered a potential model for the study of antivirals against CoVs (Amirian and Levy, 2020).
Herbal medicinal products have sparked the interest of consumers and researchers (Hosseinzadeh et al., 2015) and essential oils (EOs) extracted from aromatic and medicinal plants have raised particular attention for their beneficial properties (de Sousa Barros et al., 2015). EOs have been reported to exhibit significant antiseptic, antibacterial, antiviral, antioxidant, anti-parasitic, antifungal and insecticidal activities (Chouhan et al., 2017; Ma and Yao, 2020). Recently, in catfish experimentally intoxicated with Thiamethoxam (TMX), Thymus vulgaris EO (TEO) administration partially decreased the toxic impacts of TMX (El Euony et al., 2020). EOs are also a potential reservoir of innovative therapeutic solutions that integrate and, in some cases, replace conventional drugs (Reichling et al., 2009). For instance, Laurus nobilis EO inhibited SARS CoV type 1 (SARS-CoV-1) (Loizzo et al., 2008) and a mixture of EOs was effective against the avian CoV infectious bronchitis virus (IBV) (Jackwood et al., 2010). Recently, EOs have been also shown to possess antiviral activity against SARS-CoV-2 (Asif et al., 2020). TEO has been shown to be effective against several RNA viruses including CoVs (Lelešius et al., 2019; Nadi et al., 2020).
TEO (Specchiasol Bussolengo, Verona - Italy) composition was determined in three independent experiments using the gas chromatography hyphenated with mass spectrometry (GC-MS) technique, as recently reported (Rosato et al., 2020). Details of sample preparation, instruments and GC-MS analysis methods have been previously reported (Salvagno et al., 2020; Rosato et al., 2018). Data from GC/MS analyses were expressed as area % ± Structural Equation Modeling (SEM). In all cases SEM was below 10%. Statistical analysis for SEM was performed using Microsoft Excel Office 2010 (Windows 7 Home Premium, Microsoft Corporation, USA). A total of 26 components were identified in TEO sample corresponding to 98.7% of the whole mixture. The detailed chemical composition of TEO was reported in Supplementary Table 1.
TEO at stock concentration of 928 mg/ml was initially diluted in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, Missouri, USA) and subsequently in Dulbecco-MEM (D-MEM).
Crandell Reese Feline Kidney (CRFK) cells were cultured in DMEM and FCoV-II strain 25/92 (Buonavoglia et al., 1995), with a titre of 105.25 Tissue Culture Infectious Doses (TCID50)/50 μl, was used for the experiments.
TEO cytotoxicity was assessed by XTT assay (Denizot and Lang, 1986) using the In Vitro Toxicology Assay Kit (Sigma–Aldrich Srl, Milan, Italy) after exposing the cells to various compound concentrations (7.25, 14.5, 29, 58, 116, 232, 464, 928, 1856 μg/ml) for 72 h. Cytotoxicity was assessed by measuring the absorbance signal (optical density, OD), spectrophotometrically. In all experiments untreated cells were used as negative control and considered at 0% cytotoxicity. Cells treated with equivalent dilutions of DMSO were used as vehicle control. After logarithmic conversion of TEO concentrations, data obtained in the cytotoxicity assays were analyzed by a non-linear curve fitting procedure. Goodness of fit was tested by non-linear regression analysis of the dose-response curve. The maximum non-cytotoxic concentration was considered as the compound concentration at which viability of treated CRFK cells decreased by no more than 20% (CC20) with respect to the negative control.
The CC20 value of TEO was assessed at 27 μg/ml and calculated on the basis of mean ± standard deviation (SD) of three experiments. In all the experiments, DMSO did not show any effect on cells.
On the basis of the cytotoxicity assay results, the antiviral activity against the FCoV-II strain 25/92 was evaluated using TEO at 27 μg/ml and also below the cytotoxic threshold (13.5 μg/ml). The use of the substance below the cytotoxic threshold allows us to reduce toxicity and to obtain effective results at a lower cost. Confluent monolayers of CRFK cells of 24 h in 24-well plates were infected with 100 μl of FCoV-II containing 10 TCID50, with a Multiplicity of Infection (MOI) of 0.14. After virus adsorption for 1 h at 37 °C, the inoculum was removed, cell monolayers were washed once and TEO was added. In untreated infected cells, D-MEM was used to replace the inoculum and used as virus control. After 72 h, aliquots of supernatants were collected for viral titration (Lanave et al., 2019) and RNA quantification (Gut et al., 1999).
Virucidal activity of TEO against FCoV-II was evaluated by pre-treatment of the virus (10,000 TCID50) with TEO at 27 μg/ml and over the cytotoxic threshold (270 μg/ml) since, if used as virucide, the molecule is not posed into direct contact with the cells. In detail, 100 μl of FCoV-II were treated with TEO (1 ml) at room temperature. Virus control was used for the experiments. After 10 min, 30 min, and 1 h, aliquots of each mixture of virus-TEO and virus control were subjected to viral titration (Lanave et al., 2019).
Data from antiviral and virucidal activity assays were expressed as mean ± SD and analyzed by Analysis of Variance (ANOVA) using Tukey test as post hoc test (statistical significance set at 0.05).
Statistical analyses were performed with the software GraphPad Prism v.8.0.0 (GraphPad Software, San Diego, CA, USA).
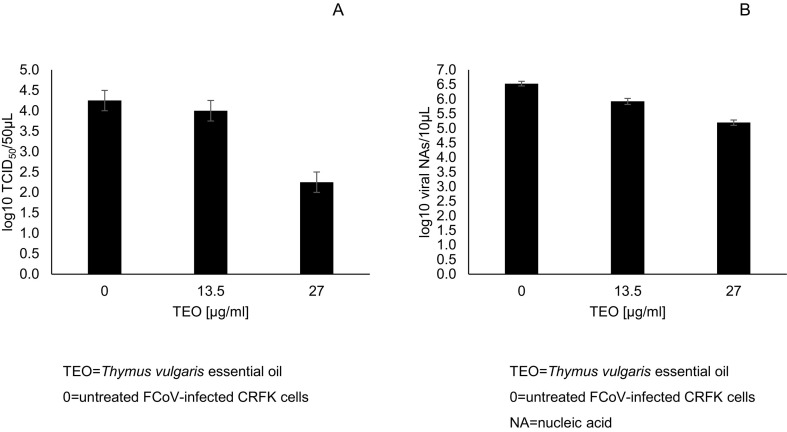
Viral titres of infected CRFK cells treated with TEO and of untreated infected cells (virus control) were expressed as the log10 TCID50/50 μl and plotted against the drug concentrations. By comparing the viral titre of untreated infected cells (4.25 log10 TCID50/50 μl) with infected cells treated with TEO at 13.5 and 27 μg/ml, a decrease of 0.25 (p > 0.05) and 2.25 log10 TCID50/50 μl (p < 0.0001), respectively was induced (Fig. 1A). This suggests that TEO at 27 μg/ml is able to significantly inhibit virus replication. The antiviral activity of TEO against FIPV parallels results obtained with Thymus vulgaris hydrosols in vitro against Porcine Reproductive and Respiratory Syndrome virus (PRRSV) (Kaewprom et al., 2017).
Fig. 1.
Viral titres of the supernatants collected at 72 h post infection from FCoV (10 TCID50)-infected CRFK cells untreated and treated with Thymus vulgaris essential oil (TEO) at different concentrations (13.5 and 27 μg/ml).
Viral titres were evaluated by endpoint dilution method, expressed as log10 TCID50/50 μl and plotted against TEO at different concentrations (A). Viral nucleic acids were quantified by qPCR, expressed as log10 viral NAs /10 μl and plotted against TEO at different concentrations (B). Bars in the figures indicate the means. Error bars indicate the standard deviation.
Viral nucleic acids (NAs) were expressed as log10 viral NAs/10 μl of infected cells treated with TEO and of virus control and plotted against the non-cytotoxic drug concentrations. By comparing viral load of untreated infected cells (6.53 log10 viral NAs /10 μl) with infected cells treated with TEO at 13.5 and 27 μg/ml a decrease of 0.61 (p = 0.0005) and 1.34 (p < 0.0001) log10 NAs/10 μl, respectively was observed (Fig. 1B).
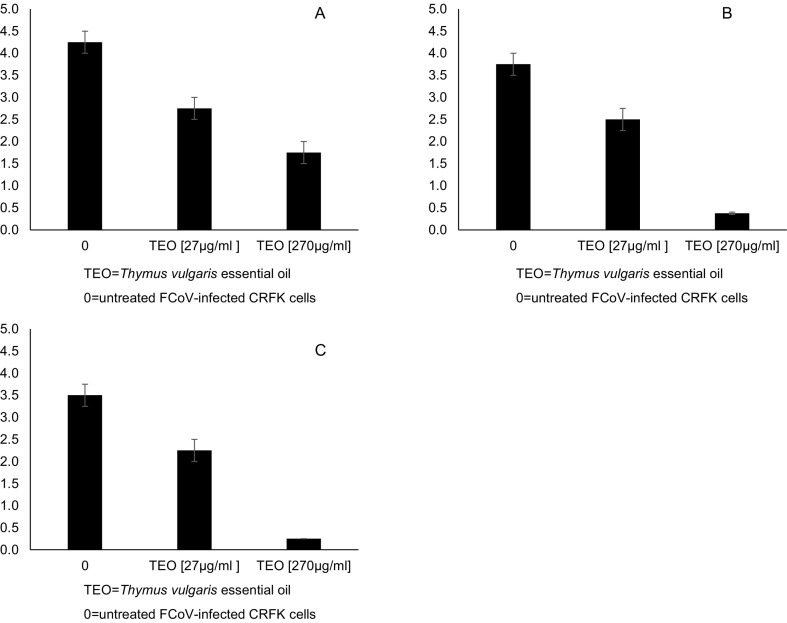
The virucidal activity of TEO at different concentrations and for different contact times with FCoV-II was assessed (Fig. 2 ). After 10 min, TEO at 27 and 270 μg/ml determined a reduction of 1.5 (p = 0.0008) and 2.5 (p < 0.0001) log10 TCID50/50 μl, respectively, compared to the virus control (4.25 log10 TCID50/50 μl) (Fig. 2A). After 30 min, TEO at 27 and 270 μg/ml induced a decrease of 1.25 (p = 0.0007) and 3.375 (p < 0.0001) log10 TCID50/50 μl, respectively, compared to the virus control (3.75 log10 TCID50/50 μl) (Fig. 2B). After 1 h, TEO at 27 and 270 μg/ml determined a decrease of 1.25 (p = 0.0007) and 3.25 (p < 0.0001) log10 TCID50/50 μl compared to the virus control (3.50 log10 TCID50/50 μl). Viral inactivation occurred in a dose- and time-dependent fashion, starting from 33.33% and reaching 92.86% when TEO was used at the highest concentration (270 μg/ml), after 1 h (Fig. 2C). The virucidal activity of TEO could be explained by the ability of damaging viral envelope, thus preventing adsorption and penetration into host cells (Reichling et al., 2009) as observed by electron microscopy in herpesvirus envelope after pre-treatment with EOs (Shogan et al., 2006). Accordingly, TEO could be a valuable tool for disinfection of surfaces and it could be proposed as additive in some food preparations.
Fig. 2.
Virucidal effect of TEO at different concentrations (27 and 270 μg/ml) against FCoV (10,000 TCID50). The virus was incubated with TEO for 10 min (A), 30 min (B) and 60 min (C) at room temperature and subsequently titrated in CRFK cells. Viral titres of FCoV were expressed as log10 TCID50/50 μl and plotted against TEO at different concentrations. Bars in the figures indicate the means. Error bars indicate the standard deviation.
Thymus vulgaris is a Mediterranean aromatic plant, that contains EOs and lipophilic substances (Nabavi et al., 2015) and its extracts are rich in thymol, carvacrol, p-cymene, and γ-terpinene (Kowalczyk et al., 2020).
Thymus vulgaris has demonstrated antiviral activity against herpes simplex virus (HSV) (Nolkemper et al., 2006), influenzavirus (Vimalanathan and Hudson, 2014), Newcastle Disease virus (Rezatofighi et al., 2014), PRRSV (Kaewprom et al., 2017) and IBV (Lelešius et al., 2019), even if the antiviral mechanism has yet to be clarified. Conversely, the inhibition of replication of Human Immunodeficiency Virus in vitro by TEO was elucidated (Feriotto et al., 2018).
The chemical composition of TEO revealed the presence of 26 distinct molecules, the main fractions of which were thymol, p-cymene, γ-terpinene, β-linalool, caryophyllene. In order to reduce the cytotoxicity of TEO, it would be interesting to identify the active molecules and to test them individually. As expected, the major component of the TEO used in this study was represented by thymol. Thymol fraction has proved to have efficacy against HSV (Sharifi-Rad et al., 2017) and influenzavirus (Alburn et al., 1972). Other less represented fractions of TEO should be tested to assess their antiviral activity.
In conclusion, we demonstrated the in vitro antiviral and virucidal effect of TEO against FCoV in CrFK cells. These studies open several perspectives in terms of future applications and therapeutic possibilities for human and animal coronaviruses.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rvsc.2021.04.024.
Appendix A. Supplementary data
Table 1: chemical composition of Thymus vulgaris essential oil
References
- Alburn H.E., Chester W., Greenspan G. 1972. Thymol as an Anti-Influenza Agent. U.S. Patent 3,632,782. [Google Scholar]
- Amirian E.S., Levy J.K. Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses. One Health. 2020;9:100128. doi: 10.1016/j.onehlt.2020.100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asif M., Saleem M., Saadullah M., Yaseen H.S., Al Zarzour R. COVID-19 and therapy with essential oils having antiviral, anti-inflammatory, and immunomodulatory properties. Inflammopharmacology. 2020;28:1153–1161. doi: 10.1007/s10787-020-00744-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonavoglia C., Sagazio P., Cirone F., Tempesta M., Marsilio F. Isolamento e caratterizzazione di uno stipite di virus della peritonite infettiva felina [Isolation and characterization of a feline infectious peritonitis strain] Veterinaria. 1995;9:91–93. [Google Scholar]
- Chouhan S., Sharma K., Guleria S. Antimicrobial activity of some essential oils-present status and future perspectives. Medicines (Basel). 2017;4:58. doi: 10.3390/medicines4030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Martella V., Saif L.J., Buonavoglia C. COVID-19 from veterinary medicine and one health perspectives: what animal coronaviruses have taught us. Res. Vet. Sci. 2020;131:21–23. doi: 10.1016/j.rvsc.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- El Euony O.I., Elblehi S.S., Abdel-Latif H.M., Abdel-Daim M.M., El-Sayed Y.S. Modulatory role of dietary Thymus vulgaris essential oil and Bacillus subtilis against thiamethoxam-induced hepatorenal damage, oxidative stress, and immunotoxicity in African catfish (Clarias garipenus) Environ. Sci. Pollut. Res. Int. 2020;27:23108–23128. doi: 10.1007/s11356-020-08588-5. [DOI] [PubMed] [Google Scholar]
- Feriotto G., Marchetti N., Costa V., Beninati S., Tagliati F., Mischiati C. Chemical composition of essential oils from Thymus vulgaris, Cymbopogon citratus, and Rosmarinus officinalis, and their effects on the HIV-1 Tat protein function. Chem. Biodivers. 2018;15:2. doi: 10.1002/cbdv.201700436. [DOI] [PubMed] [Google Scholar]
- Gut M., Leutenegger C.M., Huder J.B., Pedersen N.C., Lutz H. One-tube fluorogenic reverse transcription-polymerase chain reaction for the quantitation of feline coronaviruses. J. Virol. Methods. 1999;77:37–46. doi: 10.1016/S0166-0934(98)00129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinzadeh S., Jafarikukhdan A., Hosseini A., Armand R. The application of medicinal plants in traditional and modern medicine: A review of Thymus vulgaris. Int. J. Clin. Med. 2015;6:635–642. [Google Scholar]
- Jackwood M.W., Rosenbloom R., Petteruti M., Hilt D.A., McCall A.W., Williams S.M. Avian coronavirus infectious bronchitis virus susceptibility to botanical oleoresins and essential oils in vitro and in vivo. Virus Res. 2010;149:86–94. doi: 10.1016/j.virusres.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir M.T., Uddin M.S., Hossain M.F., Abdulhakim J.A., Alam M.A., Ashraf G.M., Bungau S.G., Bin-Jumah M.N., Abdel-Daim M.M., Aleya L. nCOVID-19 pandemic: from molecular pathogenesis to potential investigational therapeutics. Front. Cell Dev. Biol. 2020;10:616. doi: 10.3389/fcell.2020.00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaewprom K., Chen Y.H., Lin C.F., Chiou M.T., Lin C.N. Antiviral activity of Thymus vulgaris and Nepeta cataria hydrosols against porcine reproductive and respiratory syndrome virus. Thai. J. Vet. Med. 2017;47:25–33. [Google Scholar]
- Kowalczyk A., Przychodna M., Sopata S., Bodalska A., Fecka I. Thymol and thyme essential oil-new insights into selected therapeutic applications. Molecules. 2020;9:4125. doi: 10.3390/molecules25184125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummrow M., Meli M.L., Haessig M., Goenczi E., Poland A., Pedersen N.C., Hofmann-Lehmann R., Lutz H. Feline coronavirus serotypes 1 and 2: seroprevalence and association with disease in Switzerland. Clin. Diagn. Lab. Immunol. 2005;12:1209–1215. doi: 10.1128/CDLI.12.10.1209-1215.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanave G., Lucente M.S., Siciliano P., Zizzadoro C., Trerotoli P., Martella V., Buonavoglia C., Tempesta M., Camero M. Antiviral activity of PHA767491 on caprine alphaherpesvirus 1 in vitro. Res. Vet. Sci. 2019;126:113–117. doi: 10.1016/j.rvsc.2019.08.019. [DOI] [PubMed] [Google Scholar]
- Lelešius R., Karpovaitė A., Mickienė R., Drevinskas T., Tiso N., Ragažinskienė O., Kubilienė L., Maruška A., Šalomskas A. In vitro antiviral activity of fifteen plant extracts against avian infectious bronchitis virus. BMC Vet. Res. 2019;29:178. doi: 10.1186/s12917-019-1925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizzo M.R., Saab A.M., Tundis R., Statti G.A., Menichini F., Lampronti I., Gambari R., Cinatl J., Doerr H.W. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem. Biodivers. 2008;5:461–470. doi: 10.1002/cbdv.200890045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Yao L. Antiviral effects of plant-derived essential oils and their components: an updated review. Molecules. 2020;5:2627. doi: 10.3390/molecules25112627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S.M., Marchese A., Izadi M., Curti V., Daglia M., Nabavi S.F. Plants belonging to the genus Thymus as antibacterial agents: from farm to pharmacy. Food Chem. 2015;173:339–347. doi: 10.1016/j.foodchem.2014.10.042. [DOI] [PubMed] [Google Scholar]
- Nadi A., Abbas Shiravi A., Mohammadi Z., Aslani A., Zeinalian M. Thymus vulgaris, a natural pharmacy against COVID-19 and other similar infections: a molecular review. Zenodo. 2020 doi: 10.5281/zenodo.3841889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolkemper S., Reichling J., Stintzing F.C., Carle R., Schnitzler P. Antiviral effect of aqueous extracts from species of the Lamiaceae family against Herpes simplex virus type 1 and type 2 in vitro. Planta Med. 2006;72:1378–1382. doi: 10.1055/s-2006-951719. [DOI] [PubMed] [Google Scholar]
- Pedersen N.C., Kim Y., Liu H., Galasiti Kankanamalage A.C., Eckstrand C., Groutas W.C., Bannasch M., Meadows J.M., Chang K.O. Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis. J. Feline Med. Surg. 2018;20:378–392. doi: 10.1177/1098612X17729626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C., Perron M., Bannasch M., Montgomery E., Murakami E., Liepnieks M., Liu H. Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis. J. Feline Med. Surg. 2019;21:271–281. doi: 10.1177/1098612X19825701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling J., Schnitzler P., Suschke U., Saller R. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties--an overview. Forsch Komplementmed. 2009;16:79–90. doi: 10.1159/000207196. [DOI] [PubMed] [Google Scholar]
- Rezatofighi S.E., Seydabadi A., Seyyed Nejad S.M. Evaluating the efficacy of Achillea millefolium and Thymus vulgaris extracts against newcastle disease virus in ovo. Jundishapur J. Microbiol. 2014;7 doi: 10.5812/jjm.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato A., Carocci A., Catalano A., Clodoveo M.L., Franchini C., Corbo F., Carbonara G.G., Carrieri A., Fracchiolla G. Elucidation of the synergistic action of Mentha Piperita essential oil with common antimicrobials. PLoS One. 2018;13 doi: 10.1371/journal.pone.0200902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato A., Sblano S., Salvagno L., Carocci A., Clodoveo M.L., Corbo F., Fracchiolla G. Anti-biofilm inhibitory synergistic effects of combinations of essential oils and antibiotics. Antibiotics (Basel) 2020;9:637. doi: 10.3390/antibiotics9100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvagno G.L., Danese E., Lippi G. Mass spectrometry and total laboratory automation: opportunities and drawbacks. Clin. Chem. Lab. Med. 2020;58:994–1001. doi: 10.1515/cclm-2019-0723. [DOI] [PubMed] [Google Scholar]
- Sharifi-Rad J., Salehi B., Schnitzler P., Ayatollahi S.A., Kobarfard F., Fathi M., Eisazadeh M., Sharifi-Rad M. Susceptibility of herpes simplex virus type 1 to monoterpenes thymol, carvacrol, p-cymene and essential oils of Sinapis arvensis L., Lallemantia royleana Benth. and Pulicaria vulgaris Gaertn. Cell Mol. Biol. (Noisy-le-grand) 2017;30:42–47. doi: 10.14715/cmb/2017.63.8.10. [DOI] [PubMed] [Google Scholar]
- Shogan B., Kruse L., Mulamba G.B., Hu A., Coen D.M. Virucidal activity of a GT rich oligonucleotide against herpes simplex virus mediated by glycoprotein B. J. Virol. 2006;80:4740–4747. doi: 10.1128/JVI.80.10.4740-4747.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa Barros A., de Morais S.M., Ferreira P.A.T., Vieira Í.G.P., Craveiro A.A., dos Santos Fontenelle R.O., de Menezes J.E.S.A., da Silva F.W.F., de Sousa H.A. Chemical composition and functional properties of essential oils from Mentha species. Ind. Crop. Prod. 2015;76:557–564. doi: 10.1016/j.indcrop.2015.07.004. [DOI] [Google Scholar]
- Tizard I.R. Vaccination against coronaviruses in domestic animals. Vaccine. 2020;38:5123–5130. doi: 10.1016/j.vaccine.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimalanathan S., Hudson J. Anti-influenza virus activity of essential oils and vapors. Am. J. Essent Oil. 2014;2:47–53. [Google Scholar]
- World Health Organisation (WHO) Coronavirus Disease (COVID-19) Pandemic. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1: chemical composition of Thymus vulgaris essential oil