Abstract
Aims
The Hamilton Depression Rating Scale (HAMD) and the Beck Depression Inventory (BDI) are the most frequently used observer-rated and self-report scales of depression, respectively. It is important to know what a given total score or a change score from baseline on one scale means in relation to the other scale.
Methods
We obtained individual participant data from the randomised controlled trials of psychological and pharmacological treatments for major depressive disorders. We then identified corresponding scores of the HAMD and the BDI (369 patients from seven trials) or the BDI-II (683 patients from another seven trials) using the equipercentile linking method.
Results
The HAMD total scores of 10, 20 and 30 corresponded approximately with the BDI scores of 10, 27 and 42 or with the BDI-II scores of 13, 32 and 50. The HAMD change scores of −20 and −10 with the BDI of −29 and −15 and with the BDI-II of −35 and −16.
Conclusions
The results can help clinicians interpret the HAMD or BDI scores of their patients in a more versatile manner and also help clinicians and researchers evaluate such scores reported in the literature or the database, when scores on only one of these scales are provided. We present a conversion table for future research.
Key words: Assessment, Beck Depression Inventory, depressive disorder, Hamilton Rating Scale for Depression, rating instrument
Introduction
It is important to evaluate the course of major depressive disorder (MDD) using quantitative rating scales of symptoms. Various rating scales have been developed to evaluate the severity of MDD in research and clinical settings. These measures can be categorised as clinician-rated scales such as the Hamilton Rating Scale for Depression (HAMD) (Hamilton, 1960; Williams et al., 2008), Montgomery Åsberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) or Quick Inventory of Depression Symptomatology Clinician Rating (Rush et al., 2003), and self-report scales such as the Beck Depression Inventory (BDI) (Beck et al., 1961) and its revised version (BDI-II) (Beck et al., 1996), Patient Health Questionnaire-9 (Kroenke et al., 2001) or Quick Inventory of Depression Symptomatology self-report version (Rush et al., 2003). Although numerous scales for rating depression severity have been developed to date, the HAMD is the most commonly used clinician-rated scale in research and clinical settings. The HAMD has been used as a main outcome measure in randomised controlled trials of pharmacotherapy and psychotherapy for depression. In the latest network meta-analysis of antidepressant medications for MDD, 464 of 522 eligible studies reported baseline severity scores using the HAMD (Cipriani et al., 2018). Similarly, the network meta-analysis of psychotherapy for MDD showed that 75 of 198 studies reported outcomes using the HAMD (Barth et al., 2013). On the other hand, the BDI is one of the most widely used self-rating scales. The BDI/BDI-II have been used particularly often as the outcome measure in psychotherapy trials. According to the above-mentioned network meta-analysis studies of psychotherapies for depression, 116 of 198 studies used the BDI and 25 of 198 studies used the BDI-II as an outcome measure of the trial (Barth et al., 2013).
Although both the HAMD and the BDI/BDI-II are standard measures to assess depression severity, no study has yet examined how scores on the HAMD can be converted to the BDI/BDI-II scores or vice versa. It is important to link these two most commonly used scales for comparison of the baseline severity or treatment outcome. Several studies identified the corresponding scores of simultaneous HAMD and other scales such as MADRS (Leucht et al., 2018) and the Clinical Global Impression (Leucht et al., 2013a) using the equipercentile linking method (Linn, 1993). The equipercentile linking method has been used extensively for various other scales in previous publications (Leucht et al., 2005, 2006, 2013b, 2016; Furukawa et al., 2009; Levine and Leucht, 2013; Samara et al., 2014). In the current study, we attempted to link the HAMD and the BDI/BDI-II applying the same procedure.
Method
Database
We used an existing database of psychological treatments for depression which is updated annually through comprehensive literature searches in the bibliographic databases of PubMed, PsycINFO, EMBASE and the Cochrane Library (Cuijpers et al., 2008). Appendix A provides the full search strings used. This database has been used in a series of previously published meta-analyses (Bower et al., 2013; Furukawa et al., 2017; Karyotaki et al., 2017). For this linking study, we focused on the individual participant data (IPD) that we had assembled to conduct IPD meta-analytic studies comparing cognitive-behavioural therapy (CBT), antidepressant pharmacotherapy and their combination (Weitz et al., 2017).
Rating scales
The HAMD is based on clinical interviews. We used the HAMD 17-item version in this analysis. The 17 items consists of nine symptoms (depressed mood, self-depreciation and guilt feelings, suicidal impulses, work and interests, psychomotor retardation, agitation, anxiety psychic, anxiety somatic, hypochondriasis) rated between 0 (absent) to 4 (very severe), and eight symptoms (initial insomnia, middle insomnia, delayed insomnia, gastrointestinal, general somatic, sexual interests, loss of insight, weight loss) rated between 0 (absent) to 2 (clearly present) (Hamilton, 1960). The maximum score of the HAMD is therefore 52. A meta-analysis showed that the HAMD has sufficient internal consistency (Cronbach's α = 0.79), inter-rater reliability (intra-class correlation coefficient (ICC) = 0.94) and test–retest reliability (ICC = 0.93) (Trajkovic et al., 2011).
The BDI is a 21-item patient's self-report questionnaire that measures the depression severity (Beck et al., 1961). All items of the BDI are rated on a four-point Likert scale ranging from 0 to 3, and the total score therefore ranges from 0 to 63. Beck et al. developed the revised version of the BDI to harmonise its item contents with the modern diagnostic criteria for MDD in Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV, while maintaining the same number of items and range of scale as the BDI (Beck et al., 1996). The BDI has sufficient internal consistency in psychiatric patients (Cronbach's α ranging from 0.76 to 0.95) and non-psychiatric populations (Cronbach's α ranging from 0 .73 to 0.92) (Beck et al., 1988). The BDI-II also has sufficient internal consistency (α = 0.93 among college students, α = 0.92 among outpatients) (Beck et al., 1996). According to a survey of 1022 undergraduate students, the mean score of the BDI-II was 1.54 points higher than that of the BDI (Dozois et al., 1998). However, the two scales showed high correlation (r = 0.93), suggesting convergence of the two scales.
Statistical analysis
We first drew scatterplots and calculated Spearman correlation coefficients between HAMD and BDI or BDI-II, at baseline and at end of treatment. We then applied the equipercentile linking procedure (Linn, 1993), which is a technique that identifies those scores on the HAMD and the BDI or the BDI-II that have the same percentile ranks, thus allowing for a nominal translation from HAMD scores to BDI or BDI-II scores or vice versa by using their percentile values. We used Microsoft Excel® to realise the analytical procedures described in Chapter 2 of Kolen and Brennan (1995) and to draw the diagrams. We merged the baseline and endpoint measurements to produce the final linking curves and the table of conversion.
Because many trials take the change scores from baseline to end of treatment, instead of raw scores at end of treatment, as the primary outcome, we also examined the linking relationships between change scores of the HAMD and the BDI/BDI-II.
Subgroup and sensitivity analyses
In order to examine possible subgroup differences, we conducted the same analyses for men and women separately, and also for dropouts.
Results
Included studies
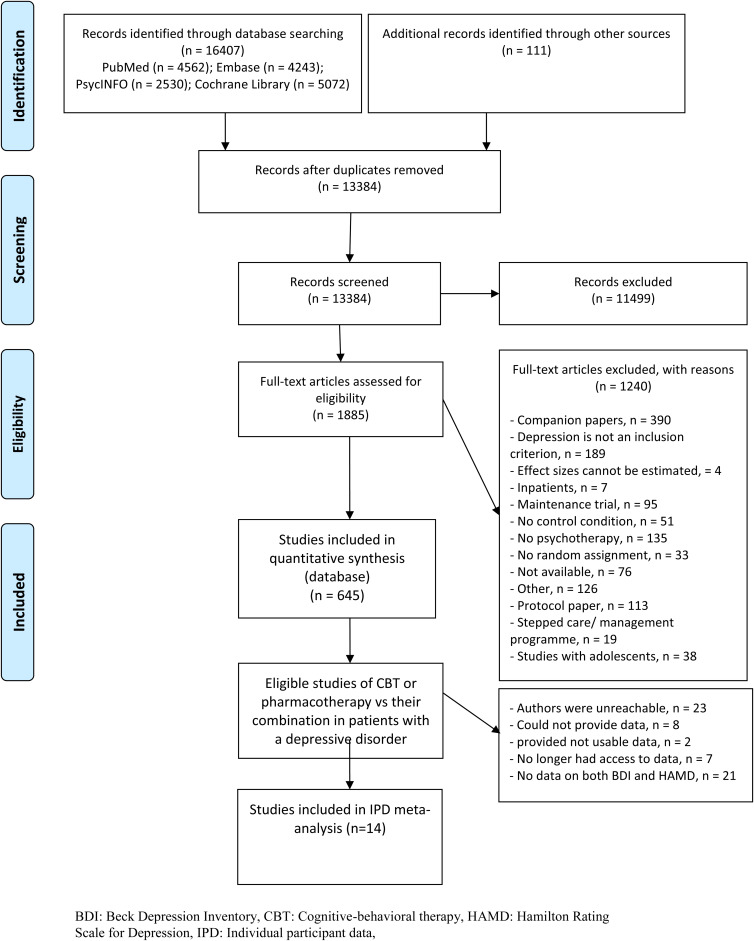
Figure 1 presents the flow of the literature search. For this study we used the search that was conducted in January 2016. After removing the duplicates from different data sources, two independent reviewers examined 13 384 titles and abstracts, retrieved 1885 full-text articles and finally identified 75 studies that compared CBT, antidepressant pharmacotherapy or their combination in the acute phase treatment of depression.
Fig. 1.
Flowchart of study identification.
Of these, authors of 14 studies provided IPD including both HAMD and BDI (Rush et al., 1977; Murphy et al., 1984; Elkin et al., 1989; Hollon et al., 1992; Jarrett et al., 1999; Reynolds et al., 1999; Mohr et al., 2001) or BDI-II (DeRubeis et al., 2005; Dimidjian et al., 2006; Lesperance et al., 2007; McBride et al., 2007; Dozois et al., 2009; Hegerl et al., 2010; Quilty et al., 2014) (Table 1). Studies using the BDI were published mainly before 2000, while those using the BDI-II were all published after 2000. The 14 studies included 1536 participants: their mean age was around 40 years, and 61% were women. The treatment lasted between 10 and 24 weeks, typically for 16 weeks. At baseline, participants presented with average HAMD scores around 20, which dropped to scores around 9 at end of treatment. Seven studies used the BDI, which dropped from around 26 to around 10; another seven studies used the BDI-II, which dropped from around 30 to 12, on average.
Table 1.
Included studies and their characteristics
| Studies | N | Age | Sex (male/female) | Treatments | Treatment duration (weeks) | Scale used for inclusion | Baseline | Endpoint | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Using BDI | HAMD | BDI | HAMD | BDI | ||||||
| Elkin et al. (1989) | 116 | 34.5 | 35/81 | CBT v. ADM | 16 | HAMD ⩾ 14 | 19.5 (4.2) | 26.7 (8.5) | 7.8 (6.8) | 9.0 (9.7) |
| Hollon et al. (1992) | 107 | 32.6 | 21/86 | CBT v. ADM v. CBT + ADM | 12 | HAMD ⩾ 14 and BDI ⩾ 20 | 23.9 (4.9) | 30.7 (7.0) | 7.7 (8.2) | 9.4 (9.9) |
| Jarrett et al. (1999) | 72 | 39.3 | 21/51 | CBT v. ADM | 10 | HAMD ⩾ 14 | 16.6 (3.1) | 25.3 (7.9) | 8.1 (6.6) | 8.6 (7.8) |
| Mohr et al. (2001) | 45 | 43.9 | 9/35 | CBT v. ADM | 16 | HAMD ⩾ 16 and BDI ⩾ 16 | 19.5 (3.8) | 20.7 (5.3) | 13.4 (6.5) | 13.5 (9.5) |
| Murphy et al. (1984) | 33 | – | 6/27 | CBT v. ADM | 12 | HAMD ⩾ 14 and BDI ⩾ 20 | 18.9 (2.9) | 28.9 (6.2) | 6.0 (5.1) | 8.1 (8.5) |
| Reynolds et al. (1999) | 58 | 67.3 | 12/46 | IPT v. ADM v. IPT + ADM | 16 | SADS-L and RDC and SCID | 19.7 (4.1) | 17.7 (8.0) | 12.4 (4.1) | 11.6 (8.1) |
| Rush et al. (1977) | 41 | – | 15/26 | CBT v. ADM | 12 | HAMD ⩾ 14 and BDI ⩾ 20 | 21.3 (4.0) | 30.2 (6.1) | 7.3 (5.4) | 9.2 (9.8) |
| All patients with BDI | 472 | 40.5 | 117/350 | 20.2 (4.7) | 26.2 (8.5) | 8.9 (6.9) | 9.8 (9.2) | |||
| Using BDI-II | HAMD | BDI-II | HAMD | BDI-II | ||||||
| DeRubeis et al. (2005) | 180 | 39.9 | 75/105 | CBT v. ADM | 16 | HAMD ⩾ 20 | 21.5 (4.0) | 32.6 (9.4) | 8.3 (6.3) | 10.3 (10.4) |
| Dimidjian et al. (2006) | 145 | 38.9 | 44/101 | CBT v. ADM | 16 | HAMD ⩾ 14 and BDI-II ⩾ 20 | 18.5 (4.1) | 31.9 (7.4) | 7.1 (5.6) | 9.9 (10.5) |
| Dozois et al. (2009) | 48 | 45.3 | 12/36 | ADM v. CBT + ADM | 15 | SCID | 18.0 (4.0) | 28.6 (9.9) | 7.0 (6.6) | 12.6 (11.3) |
| Hegerl et al. (2010) | 144 | 46.7 | 47/97 | CBT v. ADM | 10 | HAMD ⩾ 8 ⩽ 22 | 16.5 (4.3) | 21.1 (8.3) | 9.0 (6.7) | 11.2 (8.3) |
| Lesperance et al. (2007) | 142 | 57.9 | 109/33 | ADM v. IPT + ADM | 12 | HAMD ⩾ 20 | 22.3 (4.9) | 30.3 (9.1) | 11.7 (7.9) | 15.4 (11.1) |
| McBride et al. (2007) | 301 | 37.4 | 133/168 | CBT v. ADM | 24 | SCID | 19.3 (3.7) | 31.9 (9.2) | 6.6 (4.9) | 11.4 (10.7) |
| Quilty et al. (2014) | 104 | 33.5 | 50/54 | CBT v. ADM | 16 | SCID | 16.6 (5.1) | 29.8 (8.6) | 7.9 (6.2) | 12.0 (10.8) |
| All patients with BDI-II | 1064 | 42.0 | 447/527 | 19.3 (4.7) | 30.4 (9.4) | 8.5 (6.5) | 12.0 (10.6) |
ADM, antidepressant medication; BDI, Beck Depression Inventory; CBT, cognitive-behavioural therapy; HAMD, Hamilton Rating Scale for Depression; IPT, interpersonal psychotherapy; RDC, Research Diagnostic Criteria; SADS-L, Schedule for Affective Disorders and Schizophrenia-Lifetime Version; SCID, Structured Interview for DSM.
Standard deviations in parentheses.
Correlations between HAMD and BDI, BDI-II
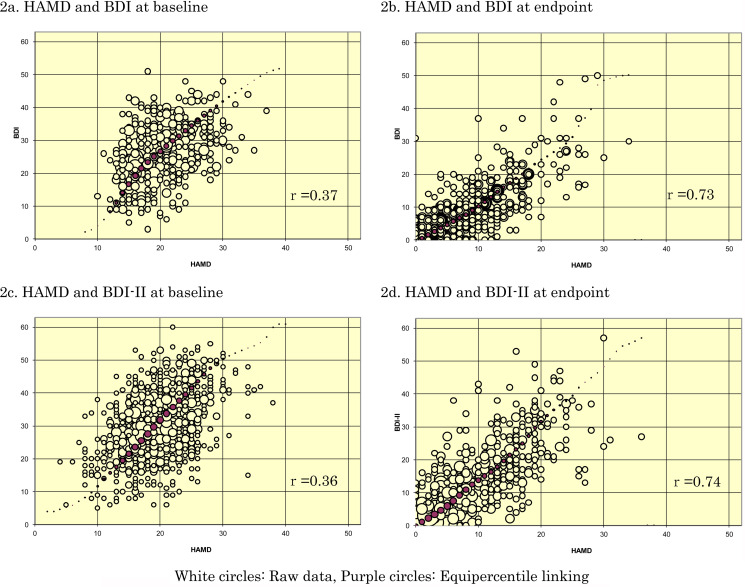
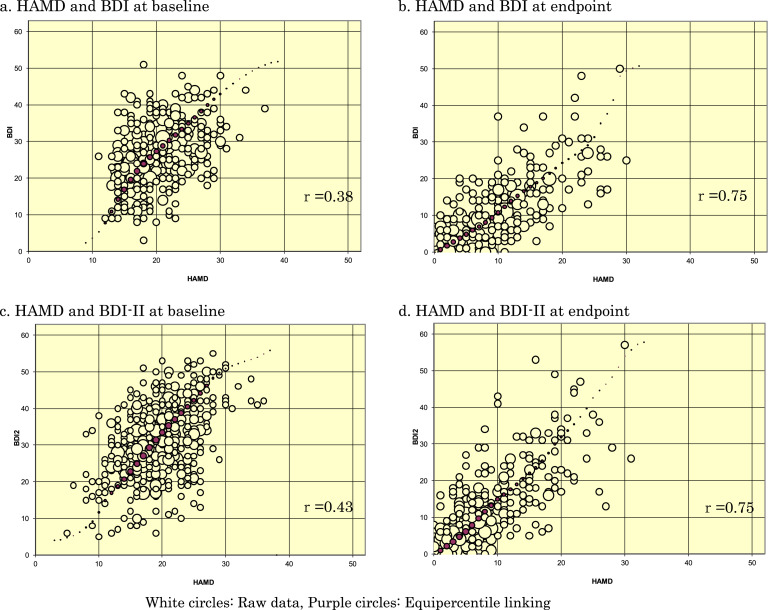
Figure 2 presents the scatterplots between HAMD and BDI or BDI-II at baseline and at end of treatment. The correlations between the HAMD and BDI or BDI-II were relatively weak, with Spearman correlation coefficients of 0.37 and 0.36, respectively: the raw data were scattered relatively widely, and there were few data points with a HAMD score of 10 or below, or 30 or higher. At the end of treatment, the correlations between the HAMD and BDI or BDI-II were stronger (r = 0.73 and 0.74, respectively), with raw data distributed in a more elliptic manner predominantly below a HAMD score of 20. When the baseline observations and end-of-treatment observations were combined, the correlations between the scales rose to 0.77 and 0.76, respectively.
Fig. 2.
Scatterplots of HAMD and BDI, BDI-II, superimposed with equipercentile linking.
There were moderately strong correlations between change scores: the Spearman correlation coefficients were 0.69 and 0.61 for the HAMD and the BDI or BDI-II change scores, respectively.
Subgroup and sensitivity analyses
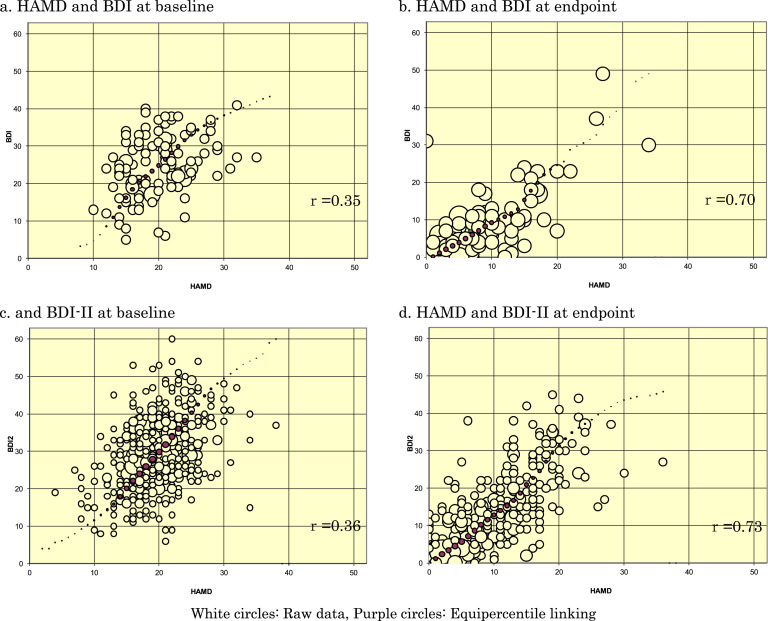
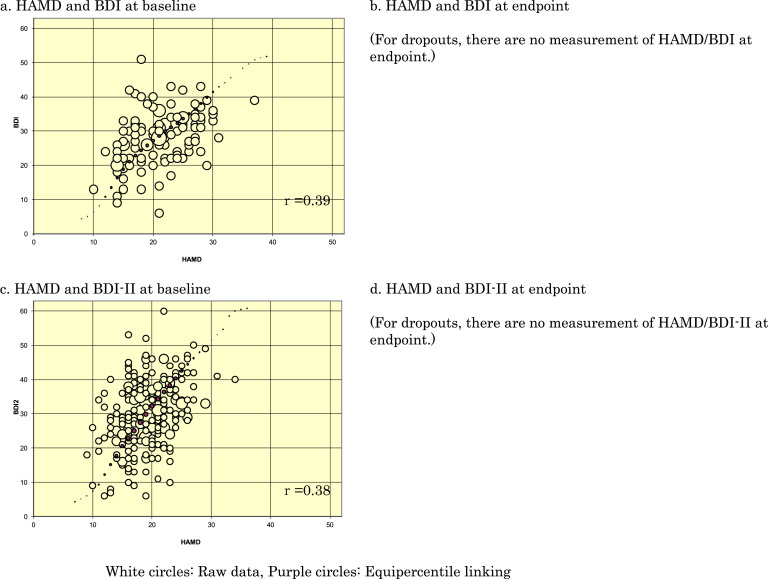
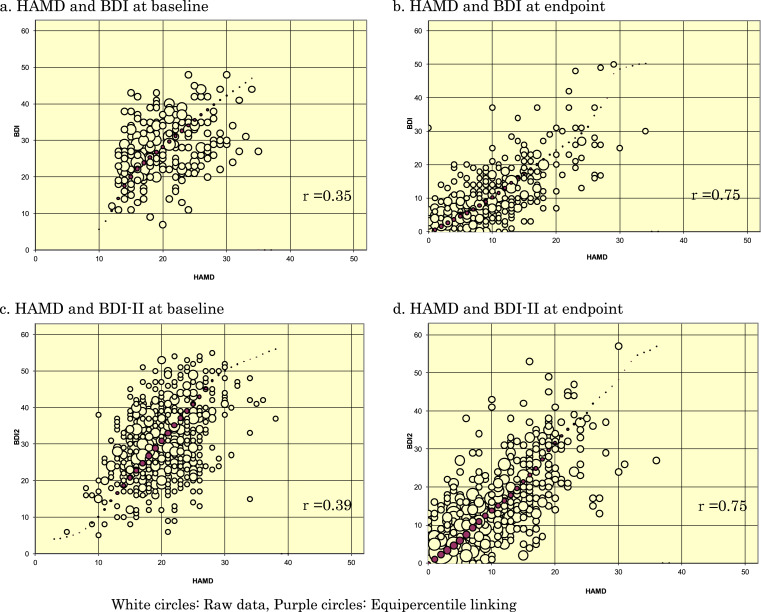
Appendix B shows the scatterplots for men and women separately at baseline and at endpoint. Appendix C provides the scatterplots for those who would later drop out and those who would complete the studies separately. The equipercentile linkings were essentially similar across these subgroups, and hence with the overall findings.
Linking HAMD and BDI, BDI-II
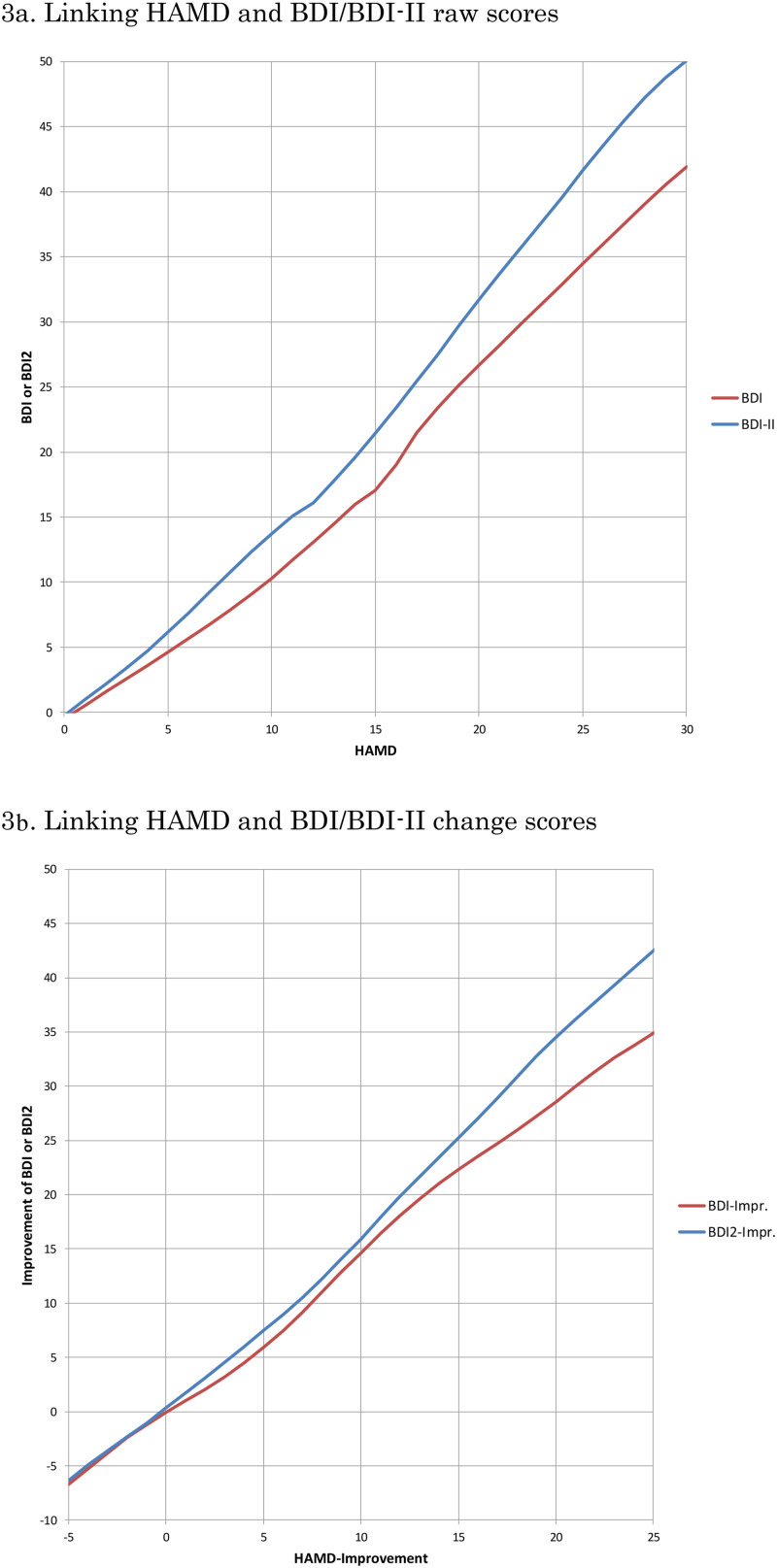
Figure 3 depicts the linking curves between HAMD and BDI or BDI-II: 3a in terms of raw scores and 3b in terms of change scores. Table 2 summarises the correspondences on each of these scales. Outside of the ranges displayed and tabled; there were too few data for linking.
Fig. 3.
Linking curves between HAMD and BDI, BDI-II.
Table 2.
Conversion from HAMD to BDI or BDI-II scores
| Total scores | Change scores | ||||
|---|---|---|---|---|---|
| HAMD | BDI | BDI-II | HAMD | BDI | BDI-II |
| 0 | 0 | 0 | −5 | −7 | −6 |
| 1 | 1 | 1 | −4 | −5 | −5 |
| 2 | 2 | 2 | −3 | −4 | −4 |
| 3 | 3 | 3 | −2 | −2 | −2 |
| 4 | 4 | 5 | −1 | −1 | −1 |
| 5 | 5 | 6 | 0 | 0 | 0 |
| 6 | 6 | 8 | 1 | 1 | 2 |
| 7 | 7 | 9 | 2 | 2 | 3 |
| 8 | 8 | 11 | 3 | 3 | 5 |
| 9 | 9 | 12 | 4 | 4 | 6 |
| 10 | 10 | 13 | 5 | 6 | 7 |
| 11 | 12 | 15 | 6 | 7 | 9 |
| 12 | 13 | 16 | 7 | 9 | 11 |
| 13 | 15 | 18 | 8 | 11 | 12 |
| 14 | 16 | 20 | 9 | 13 | 14 |
| 15 | 17 | 21 | 10 | 15 | 16 |
| 16 | 19 | 23 | 11 | 16 | 18 |
| 17 | 21 | 25 | 12 | 18 | 20 |
| 18 | 23 | 27 | 13 | 20 | 22 |
| 19 | 25 | 30 | 14 | 21 | 23 |
| 20 | 27 | 32 | 15 | 22 | 25 |
| 21 | 28 | 34 | 16 | 24 | 27 |
| 22 | 30 | 36 | 17 | 25 | 29 |
| 23 | 31 | 38 | 18 | 26 | 31 |
| 24 | 33 | 40 | 19 | 27 | 33 |
| 25 | 34 | 42 | 20 | 29 | 35 |
| 26 | 36 | 44 | 21 | 30 | 36 |
| 27 | 38 | 45 | 22 | 31 | 38 |
| 28 | 39 | 47 | 23 | 33 | 39 |
| 29 | 41 | 49 | 24 | 34 | 41 |
| 30 | 42 | 50 | 25 | 35 | 43 |
BDI, Beck Depression Inventory; BDI-II, Beck Depression Inventory, 2nd Edition; HAMD, Hamilton Rating Scale for Depression.
Discussion
We have obtained IPD from 14 randomised controlled trials of psychotherapies for the acute phase treatment of depression (total n = 1536 participants), in which the HAMD and the BDI/BDI-II were administered concurrently both at baseline and at end of treatment. The equipercentile linking between the HAMD and the BDI/BDI-II raw scores or change scores established that the HAMD scores of 10, 20 and 30 corresponded approximately with the BDI of 10, 27 and 42 or with the BDI-II of 13, 32 and 50; the HAMD change scores of −20 and −10 with the BDI of −29 and −15 and with the BDI-II of −35 and −16.
It is worthwhile to note that the BDI-II tended to produce higher scores than the original BDI. This was noted originally when the BDI-II was first developed (Beck et al., 1996) and replicated subsequently (Dozois et al., 1998), as the BDI-II dropped or reworded items that poorly reflected depression severity in the original BDI. Our linking analyses correctly reflected this difference between the BDI and the BDI-II.
Possible weaknesses of this study include the following. First, our IPD dataset included only trials that compared psychotherapies against pharmacotherapies or their combinations. Some might suspect that the relationship between the HAMD and the BDI/BDI-II could be different if the data were derived from pharmacotherapy trials. Likewise, the datasets were limited to individuals with major depression who sought treatment. It is possible that linking results could be different among people in the community suffering from major depression but not seeking treatment. However, as this linking study is not about treatments but about measurements, we do not foresee any strong reason that there would be major differences. Second, the correlations between the HAMD and the BDI/BDI-II were only moderate at baseline. This is reflected by rounder, rather than elliptic, scatterplots between the HAMD and the BDI/BDI-II at baseline (Fig. 2). We originally suspected that there may have arisen some ‘baseline inflation’ through which people tended to overestimate the depression severity at baseline when a certain threshold on that scale was used as a cutoff criterion for eligibility. Focusing on the four studies that did not use a cutoff (cf Table 1), however, did not improve the correlation coefficients at baseline. A possibility remains that the observed low correlation at baseline is due to range restriction of the available scores on HAMD and BDI/BDI-II as indicated by smaller standard deviations of these scores at baseline than at endpoint. Another possibility is that participants may have been engaging in impression management, either by overreporting or underreporting their symptoms in the self-reports, especially at the start of the trial: as the trial progresses, they may feel less need for such impression management. Third, it must be pointed out that observer- and self-ratings of depression severity do not in general show perfect correlations and that their contrasts can sometimes provide clinically useful information (Petkova et al., 2000; Targum et al., 2013). The conversion algorithm as presented in this study must therefore serve as a rough guide when only one of HAMD/BDI/BDI-II is available and one wishes to know the approximately equivalent scores. Last, the linking above the HAMD scores of 30, where there were few endpoint measurements, may require appropriate caution. Alternatively it may be safer to convert the change scores rather than raw scores when researchers would like to use one common scale across different studies.
On the other hand, the current study also has several major strengths. This is the first study to empirically link the most representative observer-rated instrument and the most frequently used self-rating instrument for depression, based on data from over 1500 participants. The conversion table will help clinicians interpret the HAMD or BDI/BDI-II scores of their patients in a more versatile manner as they can now convert each scale into another. Clinicians will also find it easier to compare their patients’ scores with those reported in the literature when the latter only reports one of these scales while they have only scores from the other scales for their patients. The conversion table will also be informative for researchers when they compare trials using one but not the other of these scales; in particular, the table will allow researchers to convert these scales onto the common scale so that they would need less assumption when they conduct IPD meta-analysis (Furukawa et al., 2018); without the conversion the only way to pool individual data was via standardisation assuming a consistent and common standard deviation (Bower et al., 2013). For the latter purpose one might prefer to use the conversion of the change scores as they showed higher correlations among the scales.
In conclusion, this study provided the first empirically-derived conversion table between the HAMD and the BDI/BDI-II. The table is expected to be of help to both clinicians and researchers.
Acknowledgement
None.
Appendix
Translating the BDI and BDI-II into the HAMD and vice versa with equipercentile linking
Appendix A. Search string for PubMed
(Psychotherapy [MH] OR psychotherap*[All Fields] OR cbt[All Fields] OR “behavior therapies”[All Fields] OR “behavior therapy”[All Fields] OR “behavior therapeutic”[All Fields] OR “behavior therapeutical”[All Fields] OR “behavior therapeutics”[All Fields] OR “behavior therapeutist”[all Fields] OR “behavior therapeutists”[All Fields] OR “behavior treatment”[All Fields] OR “behavior treatments”[All Fields] OR “behaviors therapies”[All Fields] OR “behaviors therapy”[All Fields] OR “behaviors therapeutics”[All Fields] OR “behaviors therapeutic”[All Fields] OR “behaviors therapeutical”[All Fields] OR “behaviors therapeutist”[All Fields] OR “behaviors therapeutists”[All Fields] OR “behaviors treatment”[All Fields] OR “behaviors treatments”[All Fields] OR “behavioral therapies”[All Fields] OR “behavioral therapy”[All Fields] OR “behavioral therapeutics”[All Fields] OR “behavioral therapeutic”[All Fields] OR “behavioral therapeutical”[All Fields] OR “behavioral therapeutist”[All Fields] OR “behavioral therapeutists”[All Fields] OR “behavioral treatment”[All Fields] OR “behavioral treatments”[All Fields] OR “behaviour therapies”[All Fields] OR “behaviour therapy”[All Fields] OR “behaviour therapeutic”[All Fields] OR “behaviour therapeutical”[All Fields] OR “behaviour therapeutics”[All Fields] OR “behaviour therapeutist”[all Fields] OR “behaviour therapeutists”[All Fields] OR “behaviour treatment”[All Fields] OR “behaviour treatments”[All Fields] OR “behaviours therapies”[All Fields] OR “behaviours therapy”[All Fields] OR “behaviours therapeutics”[All Fields] OR “behaviours therapeutic”[All Fields] OR “behaviours therapeutical”[All Fields] OR “behaviours therapeutist”[All Fields] OR “behaviours therapeutists”[All Fields] OR “behaviours treatment”[All Fields] OR “behaviours treatments”[All Fields] OR “behavioural therapies” [All Fields] OR “behavioural therapy”[All Fields] OR “behavioural therapeutics”[All Fields] OR “behavioural therapeutic”[All Fields] OR “behavioural therapeutical”[All Fields] OR “behavioural therapeutist”[All Fields] OR “behavioural therapeutists”[All Fields] OR “behavioural treatment”[All Fields] OR “behavioural treatments”[All Fields] OR “cognition therapies”[All Fields] OR “cognition therapie”[All Fields] OR “cognition therapy”[All Fields] OR “cognition therapeutical”[All Fields] OR “cognition therapeutic”[All Fields] OR “cognition therapeutics”[All Fields] OR “cognition therapeutist”[All Fields] OR “cognition therapeutists”[All Fields] OR “cognition treatment”[All Fields] OR “cognition treatments”[All Fields] OR psychodynamic[All Fields] OR Psychoanalysis[MH] OR psychoanalysis[All Fields] OR psychoanalytic*[All Fields] OR counselling[All Fields] OR counseling[All Fields] OR Counseling[MH] OR “problem-solving”[All Fields] OR mindfulness[All Fields] OR (acceptance[All Fields] AND commitment[All Fields]) OR “assertiveness training”[All Fields] OR “behavior activation”[All Fields] OR “behaviors activation”[All Fields] OR “behavioral activation”[All Fields] OR “cognitive therapies”[All Fields] OR “cognitive therapy”[All Fields] OR “cognitive therapeutic”[All Fields] OR “cognitive therapeutics”[All Fields] OR “cognitive therapeutical”[All Fields] OR “cognitive therapeutist”[All Fields] OR “cognitive therapeutists”[All Fields] OR “cognitive treatment”[All Fields] OR “cognitive treatments”[All Fields] OR “cognitive restructuring”[All Fields] OR ((“compassion-focused”[All Fields] OR “compassion-focussed”[All Fields]) AND (therapy[SH] OR therapies[All Fields] OR therapy[All Fields] OR therape*[All Fields] OR therapis*[All Fields]OR Therapeutics [OR treatment*[All Fields])) OR ((therapy[SH] OR therapies[All Fields]
OR therapy [All Fields] OR therape*[All Fields] OR therapis*[All Fields] OR Therapeutics[MH] OR treatment*[All Fields]) AND constructivist*[All Fields]) OR “metacognitive therapies”[All Fields] OR “metacognitive therapy”[All Fields] OR “metacognitive therapeutic”[All Fields] OR “metacognitive therapeutics”[All Fields] OR “metacognitive therapeutical”[All Fields] OR “metacognitive therapeutist”[All Fields] OR “metacognitive therapeutists”[All Fields] OR “metacognitive treatment”[All Fields] OR “metacognitive treatments”[All Fields] OR “meta-cognitive therapies”[All Fields] OR “meta-cognitive therapy”[All Fields] OR “meta-cognitive therapeutic”[All Fields] OR “meta-cognitive therapeutics”[All Fields] OR “meta-cognitive therapeutical”[All Fields] OR “meta-cognitive therapeutist”[All Fields] OR “meta-cognitive therapeutists”[All Fields] OR “meta-cognitive treatment”[All Fields] OR “meta-cognitive treatments”[All Fields] OR “solution-focused therapies”[All Fields] OR “solution-focused therapy”[All Fields] OR “solution-focused therapeutic”[All Fields] OR “solution-focused therapeutics” [All Fields] OR “solution-focused therapeutical”[All Fields] OR “solution focused therapies”[All Fields] OR “solution focused therapy”[All Fields] OR “solution focused therapeutic”[All Fields] OR “solution focused therapeutics”[All Fields] OR “solution focused therapeutical”[All Fields]OR “solution-focussed therapies”[All Fields] OR “solution-focussed therapy”[All Fields] OR “solution-focussed therapeutic”[All Fields] OR “solution-focussed therapeutics”[All Fields] OR “solution-focussed therapeutical”[All Fields]OR “solution focussed therapies”[All Fields] OR “solution focussed therapy”[All Fields] OR “solution focussed therapeutic”[All Fields] OR “solution focussed therapeutics”[All Fields] OR “solution focussed therapeutical”[All Fields] OR “self-control therapies”[All Fields] OR “self-control therapy”[All Fields] OR “self-control therapeutics”[All Fields] OR “self-control therapeutical”[All Fields] OR “self-control therapeutic”[All Fields] OR “self-control training”[All Fields] OR “self-control trainings”[All Fields] OR “self control therapies”[All Fields] OR “self control therapy”[All Fields] OR “self control therapeutics”[All Fields] OR “self control therapeutical”[All Fields] OR “self control therapeutic”[All Fields] OR “self control training”[All Fields] OR “self control trainings”[All Fields] AND (Depressive Disorder[MH] OR Depression[MH]OR dysthymi*[All Fields] OR “affective disorder”[All Fields]OR “affective disorders”[All Fields] OR “mood disorder”[All Fields] OR “mood disorders”[All Fields] OR depression*[All Fields] OR depressive*[All Fields] OR “dysthymic disorder”[MeSH Terms]) AND ((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR randomly [tiab] NOT (animals[mh] NOT (animals[mh] AND humans [mh]))
Appendix B. Subgroup analyses by sex
B1. Men
B2. Women
Appendix C. Subgroup analyses by completers and dropouts
C1. Dropouts
C2. Completers
Data and materials
Data used in this study are not available for sharing due to ethical and data management requirements. The researchers are open to collaboration.
Author ORCIDs
Mirjam Reijnders, 0000-0002-4272-2576
Financial support
This study was supported in part by a grant-in-aid from Japan Agency for Medical Research and Development (AMED) to TAF (grant number 18dk0307072). The funder had no role in study design, data collection or analysis, decision to publish or preparation of the manuscript.
Ethical standards
All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees about studies on human participants and with the Declaration of Helsinki and its amendments. The investigators of the primary trials have obtained ethical approval for the data used in the current study and for sharing the data, if this was necessary, according to local requirements and was not covered from the initial ethic assessment.
Conflict of interest
TAF has received lecture fees from Meiji, Mitsubishi-Tanabe, MSD and Pfizer. He has received research support from Mitsubishi-Tanabe. Bristol Meyers Squib and Pfizer have provided pharmaceutical supplies for CFRs NIH sponsored research. UH was an advisory board member for Lundbeck, Janssen and Servier; a consultant for Bayer Pharma; and a speaker for Servier. RBJs medical centre collects the payments from the cognitive therapy she provides to patients. RBJ is a paid consultant to the National Institutes of Health and is a paid reviewer for UpToDate. DCM received consulting fees from Apple Inc., Optum Behavioral Health and the One Mind Foundation. He also has an ownership interest in Actualize Therapy. SL has received honoraria for consulting from LB Pharma, Lundbeck, Otsuka, Roche, and TEVA, for lectures from AOP Orphan, ICON, Janssen, Lilly, Lundbeck, Otsuka, Sanofi, Roche, and Servier, and for a publication from Roche. All the other authors declare that they have no conflict of interest.
References
- Barth J, Munder T, Gerger H, Nuesch E, Trelle S, Znoj H, Juni P and Cuijpers P (2013) Comparative efficacy of seven psychotherapeutic interventions for patients with depression: a network meta-analysis. PLoS Medicine 10, e1001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J and Erbaugh J (1961) An inventory for measuring depression. Archives of General Psychiatry 4, 561–571. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA and Garbin MG (1988) Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review 8, 77–100. [Google Scholar]
- Beck AT, Steer RA and Brown GK (1996) BDI-II: Beck Depression Inventory, Second Edition, Manual. San Antonia: The Psychological Corporation [Google Scholar]
- Bower P, Kontopantelis E, Sutton A, Kendrick T, Richards DA, Gilbody S, Knowles S, Cuijpers P, Andersson G, Christensen H, Meyer B, Huibers M, Smit F, van Straten A, Warmerdam L, Barkham M, Bilich L, Lovell K and Liu ET (2013) Influence of initial severity of depression on effectiveness of low intensity interventions: meta-analysis of individual patient data. BMJ (Clinical Research Ed.) 346, f540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JPT, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JPA and Geddes JR (2018) Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 391, 1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, van Straten A, Warmerdam L and Andersson G (2008) Psychological treatment of depression: a meta-analytic database of randomized studies. BMC Psychiatry 8, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, O'Reardon JP, Lovett ML, Gladis MM, Brown LL and Gallop R (2005) Cognitive therapy vs medications in the treatment of moderate to severe depression. Archives of General Psychiatry 62, 409–416. [DOI] [PubMed] [Google Scholar]
- Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, Gallop R, McGlinchey JB, Markley DK, Gollan JK, Atkins DC, Dunner DL and Jacobson NS (2006) Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. Journal of Consulting and Clinical Psychology 74, 658–670. [DOI] [PubMed] [Google Scholar]
- Dozois DJ, Dobson KS and Ahnberg JL (1998) A psychometric evaluation of the Beck Depression Inventory-II. Psychological Assessment 10, 83–89. [Google Scholar]
- Dozois DJ, Bieling PJ, Patelis-Siotis I, Hoar L, Chudzik S, McCabe K and Westra HA (2009) Changes in self-schema structure in cognitive therapy for major depressive disorder: a randomized clinical trial. Journal of Consulting and Clinical Psychology 77, 1078–1088. [DOI] [PubMed] [Google Scholar]
- Elkin I, Shea MT, Watkins JT, Imber SD, Sotsky SM, Collins JF, Glass DR, Pilkonis PA, Leber WR, Docherty JP, Fiester SJ and Parloff MB. (1989) National Institute of Mental Health treatment of depression collaborative research program. General effectiveness of treatments. Archives of General Psychiatry 46, 971–982, discussion 983. [DOI] [PubMed] [Google Scholar]
- Furukawa TA, Shear MK, Barlow DH, Gorman JM, Woods SW, Money R, Etschel E, Engel RR and Leucht S (2009) Evidence-based guidelines for interpretation of the Panic Disorder Severity Scale. Depression and Anxiety 26, 922–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa TA, Weitz ES, Tanaka S, Hollon SD, Hofmann SG, Andersson G, Twisk J, DeRubeis RJ, Dimidjian S, Hegerl U, Mergl R, Jarrett RB, Vittengl JR, Watanabe N and Cuijpers P (2017) Initial severity of depression and efficacy of cognitive-behavioural therapy: individual-participant data meta-analysis of pill-placebo-controlled trials. British Journal of Psychiatry 210, 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa TA, Efthimiou O, Weitz ES, Cipriani A, Keller MB, Kocsis JH, Klein DN, Michalak J, Salanti G, Cuijpers P and Schramm E (2018) Cognitive-Behavioral Analysis System of Psychotherapy, drug, or their combination for persistent depressive disorder: personalizing the treatment choice using individual participant data network metaregression. Psychotherapy and Psychosomatics 87, 140–153. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960) A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegerl U, Hautzinger M, Mergl R, Kohnen R, Schutze M, Scheunemann W, Allgaier AK, Coyne J and Henkel V (2010) Effects of pharmacotherapy and psychotherapy in depressed primary-care patients: a randomized, controlled trial including a patients’ choice arm. The International Journal of Neuropsychopharmacology 13, 31–44. [DOI] [PubMed] [Google Scholar]
- Hollon SD, DeRubeis RJ, Evans MD, Wiemer MJ, Garvey MJ, Grove WM and Tuason VB (1992) Cognitive therapy and pharmacotherapy for depression. Singly and in combination. Archives of General Psychiatry 49, 774–781. [DOI] [PubMed] [Google Scholar]
- Jarrett RB, Schaffer M, McIntire D, Witt-Browder A, Kraft D and Risser RC (1999) Treatment of atypical depression with cognitive therapy or phenelzine: a double-blind, placebo-controlled trial. Archives of General Psychiatry 56, 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karyotaki E, Riper H, Twisk J, Hoogendoorn A, Kleiboer A, Mira A, Mackinnon A, Meyer B, Botella C, Littlewood E, Andersson G, Christensen H, Klein JP, Schroder J, Breton-Lopez J, Scheider J, Griffiths K, Farrer L, Huibers MJ, Phillips R, Gilbody S, Moritz S, Berger T, Pop V, Spek V and Cuijpers P (2017) Efficacy of self-guided internet-based cognitive behavioral therapy in the treatment of depressive symptoms: a meta-analysis of individual participant data. JAMA Psychiatry 74, 351–359. [DOI] [PubMed] [Google Scholar]
- Kolen MJ and Brennan RL (1995) Test Equating: Methods and Practices. New York: Springer. [Google Scholar]
- Kroenke K, Spitzer RL and Williams JB (2001) The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine 16, 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesperance F, Frasure-Smith N, Koszycki D, Laliberte MA, van Zyl LT, Baker B, Swenson JR, Ghatavi K, Abramson BL, Dorian P, Guertin MC and Investigators C (2007) Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian cardiac randomized evaluation of antidepressant and psychotherapy efficacy (CREATE) trial. JAMA 297, 367–379. [DOI] [PubMed] [Google Scholar]
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E and Engel RR (2005) What does the PANSS mean? Schizophrenia Research 79, 231–238. [DOI] [PubMed] [Google Scholar]
- Leucht S, Kane JM, Etschel E, Kissling W, Hamann J and Engel RR (2006) Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacology 31, 2318–2325. [DOI] [PubMed] [Google Scholar]
- Leucht S, Fennema H, Engel R, Kaspers-Janssen M, Lepping P and Szegedi A (2013a) What does the HAMD mean? Journal of Affective Disorders 148, 243–248. [DOI] [PubMed] [Google Scholar]
- Leucht S, Rothe P, Davis JM and Engel RR (2013b) Equipercentile linking of the BPRS and the PANSS. European Neuropsychopharmacology 23, 956–959. [DOI] [PubMed] [Google Scholar]
- Leucht S, Fennema H, Engel RR, Kaspers-Janssen M, Lepping P and Szegedi A (2016) What does MADRS mean? Equipercentile linking with the CGI using a company database of mirtazapine studies. Journal of Affective Disorders 210, 287–293. [DOI] [PubMed] [Google Scholar]
- Leucht S, Fennema H, Engel RR, Kaspers-Janssen M and Szegedi A (2018) Translating the HAM-D into the MADRS and vice versa with equipercentile linking. Journal of Affective Disorders 226, 326–331. [DOI] [PubMed] [Google Scholar]
- Levine SZ and Leucht S (2013) Identifying clinically meaningful symptom response cut-off values on the SANS in predominant negative symptoms. Schizophrenia Research 145, 125–127. [DOI] [PubMed] [Google Scholar]
- Linn RL (1993) Linking results of distinct assessments. Applied Measurements in Education 6, 83–102. [Google Scholar]
- McBride C, Segal Z, Kennedy S and Gemar M (2007) Changes in autobiographical memory specificity following cognitive behavior therapy and pharmacotherapy for major depression. Psychopathology 40, 147–152. [DOI] [PubMed] [Google Scholar]
- Mohr DC, Boudewyn AC, Goodkin DE, Bostrom A and Epstein L (2001) Comparative outcomes for individual cognitive-behavior therapy, supportive-expressive group psychotherapy, and sertraline for the treatment of depression in multiple sclerosis. Journal of Consulting and Clinical Psychology 69, 942–949. [PubMed] [Google Scholar]
- Montgomery SA and Asberg M (1979) A new depression scale designed to be sensitive to change. British Journal of Psychiatry 134, 382–389. [DOI] [PubMed] [Google Scholar]
- Murphy GE, Simons AD, Wetzel RD and Lustman PJ (1984) Cognitive therapy and pharmacotherapy. Singly and together in the treatment of depression. Archives of General Psychiatry 41, 33–41. [DOI] [PubMed] [Google Scholar]
- Petkova E, Quitkin FM, McGrath PJ, Stewart JW and Klein DF (2000) A method to quantify rater bias in antidepressant trials. Neuropsychopharmacology 22, 559–565. [DOI] [PubMed] [Google Scholar]
- Quilty LC, Dozois DJ, Lobo DS, Ravindran LN and Bagby RM (2014) Cognitive structure and processing during cognitive behavioral therapy vs. pharmacotherapy for depression. International Journal of Cognitive Therapy 7, 235–250. [Google Scholar]
- Reynolds CF 3rd, Miller MD, Pasternak RE, Frank E, Perel JM, Cornes C, Houck PR, Mazumdar S, Dew MA and Kupfer DJ (1999) Treatment of bereavement-related major depressive episodes in later life: a controlled study of acute and continuation treatment with nortriptyline and interpersonal psychotherapy. American Journal of Psychiatry 156, 202–208. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Beck AT, Kovacs M and Hollon S (1977) Comparative efficacy of cognitive therapy and pharmacotherapy in the treatment of depressed outpatients. Cognitive Therapy and Research 1, 17–37. [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH and Keller MB (2003) The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry 54, 573–583. [DOI] [PubMed] [Google Scholar]
- Samara MT, Engel RR, Millier A, Kandenwein J, Toumi M and Leucht S (2014) Equipercentile linking of scales measuring functioning and symptoms: examining the GAF, SOFAS, CGI-S, and PANSS. European Neuropsychopharmacology 24, 1767–1772. [DOI] [PubMed] [Google Scholar]
- Targum SD, Wedel PC, Robinson J, Daniel DG, Busner J, Bleicher LS, Rauh P and Barlow C (2013) A comparative analysis between site-based and centralized ratings and patient self-ratings in a clinical trial of major depressive disorder. Journal of Psychiatric Research 47, 944–954. [DOI] [PubMed] [Google Scholar]
- Trajkovic G, Starcevic V, Latas M, Lestarevic M, Ille T, Bukumiric Z and Marinkovic J (2011) Reliability of the Hamilton Rating Scale for Depression: a meta-analysis over a period of 49 years. Psychiatry Research 189, 1–9. [DOI] [PubMed] [Google Scholar]
- Weitz E, Kleiboer A, van Straten A, Hollon SD and Cuijpers P (2017) Individual patient data meta-analysis of combined treatments versus psychotherapy (with or without pill placebo), pharmacotherapy or pill placebo for adult depression: a protocol. BMJ Open 7, e013478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JB, Kobak KA, Bech P, Engelhardt N, Evans K, Lipsitz J, Olin J, Pearson J and Kalali A (2008) The GRID-HAMD: standardization of the Hamilton Depression Rating Scale. International Clinical Psychopharmacology 23, 120–129. [DOI] [PubMed] [Google Scholar]