Abstract
Background
There are limited data regarding the relationship between interstitial lung disease (ILD) and the natural course of COVID-19. In this study, we investigate whether patients with ILD are more susceptible to COVID-19 than those without ILD and evaluate the impact of ILD on disease severity in patients with COVID-19.
Methods
A nationwide cohort of patients with COVID-19 (n=8070) and a 1:15 age-, sex- and residential area-matched cohort (n=121 050) were constructed between 1 January 2020 and 30 May 2020 in Korea. We performed a nested case–control study to compare the proportions of patients with ILD between the COVID-19 cohort and the matched cohort. Using the COVID-19 cohort, we also evaluated the risk of severe COVID-19 in patients with ILD versus those without ILD.
Results
The proportion of patients with ILD was significantly higher in the COVID-19 cohort than in the matched cohort (0.8% versus 0.4%; p<0.001). The odds of having ILD was significantly higher in the COVID-19 cohort than in the matched cohort (adjusted OR 2.02, 95% CI 1.54–2.61). Among patients in the COVID-19 cohort, patients with ILD were more likely to have severe COVID-19 than patients without ILD (47.8% versus 12.6%), including mortality (13.4% versus 2.8%) (all p<0.001). The risk of severe COVID-19 was significantly higher in patients with ILD than in those without ILD (adjusted OR 2.23, 95% CI 1.24–4.01).
Conclusion
The risks of COVID-19 and severe presentation were significantly higher in patients with ILD than in those without ILD.
Short abstract
The risks of COVID-19 and severe presentation were significantly higher in patients with ILD than in those without ILD. Clinicians should be aware of the increased risk of COVID-19 in their ILD patients and manage them appropriately amid this pandemic. https://bit.ly/3guOE6d
Introduction
The COVID-19 pandemic currently imposes a serious health-related burden worldwide [1]. The disease severity of COVID-19 varies widely according to the underlying conditions of infected patients [2]. Of the underlying diseases, chronic underlying respiratory comorbidities have significant impacts on the course of COVID-19 [3–6]. Chronic obstructive pulmonary disease (COPD) [7, 8] and thoracic malignancy [9–11] have been shown to be associated with more severe presentations and poor prognoses of COVID-19. Asthma is suggested to impose no major additional risk of severity of COVID-19 [12, 13]. However, there are very limited data on the natural course of COVID-19 in patients with interstitial lung disease (ILD).
Although it seems logical to assume that patients with ILD would be more susceptible to COVID-19 than patients without ILD, no studies have clearly revealed such a relationship [14, 15]. Regarding the disease course [16], patients with ILD seem more likely to experience respiratory failure and death compared with those without ILD [17, 18]. However, previous studies were performed in single centres. Accordingly, the question of whether patients with ILD are more susceptible to COVID-19 and experience more severe clinical courses than those without ILD remains unanswered. In addition, the susceptibility to and severity of COVID-19 in patients with ILD compared with other viral infections, such as seasonal influenza virus infection, have also not been well elucidated to date.
In this study, we investigated whether patients with ILD are more susceptible to COVID-19 than those without ILD and evaluate the impact of ILD on the severity of COVID-19. Furthermore, we investigated whether patients with ILD are more susceptible to influenza virus infection than those without ILD and evaluate the impact of ILD on the severity of influenza virus infection.
Methods
Database and study population
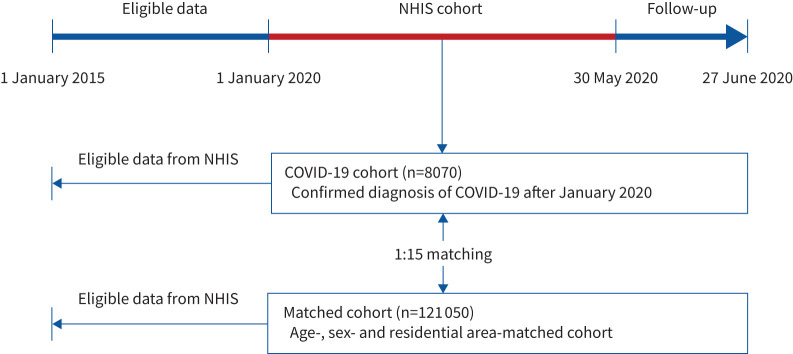
The Korean government provided the researchers with anonymised national patient data for the evaluation of COVID-19. This large cohort comprises Korean National Health Insurance Service (NHIS) claims made between 1 January 2015 and 30 May 2020, and consists of three sub-cohorts: 1) the COVID-19 cohort comprised of patients who had confirmed diagnoses of COVID-19 (positive real-time reverse transcriptase PCR results for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)) after 1 January 2020 (n=8070); 2) an inspection control group comprised of patients who were finally confirmed not to have COVID-19 (negative real-time reverse transcriptase PCR results for SARS-CoV-2; claims for COVID-19 tests are made using a special “public crisis” code (MT043) and can thus identify all individuals tested for COVID-19 in Korea) (n=222 257); and 3) a matched cohort that included a 15-fold population matched with the COVID-19 cohort by age, sex and residential area (excluding confirmed patients and test controls) (n=121 050) [11, 19]. Of these data, we used data for the COVID-19 cohort and the matched cohort in the present study (figure 1).
FIGURE 1.
Flowchart of the study population. NHIS: National Health Insurance Service.
The data in this cohort were combined with claims-based data from the NHIS for the period between 1 January 2015 and 30 May 2020. South Korea has a single-payer universal health system; the NHIS maintains claims data on all reimbursed inpatient and outpatient visits, procedures and prescriptions. We extracted information on age, sex and region of residence from insurance eligibility data. Therefore, the datasets analysed in this study include personal data, healthcare records of inpatients and outpatients from the past 5 years (including healthcare visits, prescriptions, diagnoses and procedures), pharmaceutical visits, COVID-19-related outcomes, and death records.
The Institutional Review Board of Hanyang University Hospital (Seoul, Korea) approved this study (HYUH 2020-06-029). Since the NHIS database was constructed after anonymisation, the requirement for informed consent from participants was waived.
Definitions
Laboratory confirmation of SARS-CoV-2 infection was defined as a positive result on a real-time reverse transcriptase PCR assay of nasal or pharyngeal swabs, under the guidelines for laboratory diagnosis of COVID-19 in Korea [17]. ILD was defined as one or more claims under the International Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) diagnosis code J84. Using ICD-10 codes, we further specified types of ILD as connective tissue disease (CTD)-related ILD and CTD-unrelated ILD. CTD-related ILD was diagnosed when patients had ICD-10 diagnosis codes for ILD (J84) plus CTD (M05, M06, M31.5, M32, M33, M34, M35.1, M35.3 or M36.0). CTD-unrelated ILD (J84 without codes for CTD) was further classified into idiopathic pulmonary fibrosis (IPF) (J84.1 and J84.18), hypersensitivity pneumonitis (D67) and other ILD (CTD-unrelated ILD without IPF and hypersensitivity pneumonitis).
Comorbidities were defined as two or more claims under ICD-10 diagnosis codes as major diagnoses during the study period (1 January 2015 to 30 May 2020), as follows: angina pectoris (I20), myocardial infarction (I21, I22 or I25.2), cerebrovascular disease (G45–G46, I60–I69 or H34.0), diabetes mellitus (E10–E14), hypertension (I10–I15), heart failure (I43, I50, I09.9, I11.0, I25.5, I13.0, I13.2, I42.0, I42.5–I42.9 or P29.0), lung cancer (C34) and malignancy other than lung cancer (C00–C97, except for C34) [20]. Severe COVID-19 disease was defined as cases requiring oxygen therapy, intensive care unit (ICU) admission, mechanical ventilation treatment or extracorporeal membrane oxygenation (ECMO) in addition to individuals who died after a confirmed COVID-19 diagnosis [5, 10, 21].
Patients with CTD who used biological agents were defined as those who used infliximab, etanercept, adalimumab, abatacept, anakinra, tocilizumab, golimumab, certolizumab, rituximab, tofacitinib, baricitinib, upadacitinib, ustekinumab, secukinumab, ixekizumab or guselkumab.
Main outcomes and measures
The main objectives of this study were to evaluate whether patients with ILD are more susceptible to COVID-19 than those without ILD and whether COVID-19 patients with ILD suffer from more severe COVID-19 disease courses than those without ILD.
Statistical analysis
Categorical variables are presented as number (percentage) and were compared using the Chi-squared test or Fisher's exact test, as appropriate. To evaluate the effect of ILD on susceptibility to COVID-19, the odds ratio for the presence of ILD in the COVID-19 cohort relative to that in the age-, sex- and residential area-matched cohort was evaluated using univariable and multivariable logistic regression analyses. In multivariable analysis, covariates which not only may affect the relationship between ILD and the susceptibility of COVID-19, but also were significant in the univariable analysis (p<0.05), were adjusted: type of insurance, reflecting socioeconomic status [22], and comorbidities (lung cancer, CTD, hypertension, diabetes mellitus, cardiovascular disease (angina pectoris, myocardial infarction and heart failure) and cerebrovascular disease). Additionally, to evaluate the effect of ILD on the occurrence of severe COVID-19, we evaluated the odds ratio for severe COVID-19 in patients with ILD relative to those without ILD using the COVID-19 cohort. In multivariable analysis, we adjusted for potential confounders which may affect the relationship between ILD and severe COVID-19, and were significant in univariable analysis (p<0.05): age, sex and comorbidities (lung cancer, CTD, hypertension, diabetes mellitus, cardiovascular disease, cerebrovascular disease and malignancy other than lung cancer). All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Graphs were compiled using Prism version 9.0.2 (GraphPad, San Diego, CA, USA).
Analysis of influenza virus infection
The data source for analysing the impact of ILD on influenza virus infection was the Korean 2017 Health Insurance Review and Assessment Service, National Patient Sample (HIRA-NPS), which is nationally representative and open to the public for research purposes [23]. The database includes approximately 1 400 000 individuals each year drawn by 3% stratified random sampling by age and sex from the entire population who had claims records during the year [20, 24]. We used the HIRA-NPS, which was different from the dataset used for analysing the impact of COVID-19 on patients with ILD, because there was dramatically decreased seasonal influenza activity during the COVID-19 pandemic [25]. In addition, as the current dataset was composed of the COVID-19 and matched cohorts, we could not establish the influenza cohort and matched cohorts using this database.
Using the 2017 HIRA-NPS database, we assessed patients with influenza virus infection (influenza cohort) (n=27 745) and a matched cohort that included a 10-fold sample of age-, sex- and residential area-matched patients (n=277 450). The aforementioned definitions and statistical analysis were applied to the analysis of influenza virus infection like those of COVID-19.
Results
Characteristics of COVID-19 cohort and matched cohort
Table 1 presents the characteristics of the study population. The COVID-19 and matched cohorts were well balanced in terms of age and sex. The proportion of patients who received medical aid was higher in the COVID-19 cohort than in the matched cohort (7.3% versus 3.7%; p<0.001). Regarding pulmonary comorbidities, the rates of ILD (0.8% versus 0.4%; p<0.001) and lung cancer (0.5% versus 0.3%; p=0.002) were higher in the COVID-19 cohort than in the matched cohort. The proportions of all extrapulmonary comorbidities, except for malignancy other than lung cancer (p=0.716), were also significantly higher in the COVID-19 cohort than in the matched cohort.
TABLE 1.
Baseline characteristics of patients
| Total | COVID-19 cohort | Matched cohort | p-value | |
| Patients | 129 120 | 8070 | 121 050 | |
| Age years | 0.999 | |||
| <10 | 1296 (1.0) | 81 (1.0) | 1215 (1.0) | |
| 10–19 | 4416 (3.4) | 276 (3.4) | 4140 (3.4) | |
| 20–29 | 32 912 (25.5) | 2057 (25.5) | 30 855 (25.5) | |
| 30–39 | 13 312 (10.3) | 832 (10.3) | 12 480 (10.3) | |
| 40–49 | 16 576 (12.8) | 1036 (12.8) | 15 540 (12.8) | |
| 50–59 | 25 072 (19.4) | 1567 (19.4) | 23 505 (19.4) | |
| 60–69 | 19 184 (14.9) | 1199 (14.9) | 17 985 (14.9) | |
| 70–79 | 9872 (7.7) | 617 (7.7) | 9255 (7.7) | |
| ≥80 | 6480 (5.0) | 405 (5.0) | 6075 (5.0) | |
| Sex | 0.999 | |||
| Male | 51 776 (40.1) | 3236 (40.1) | 48 540 (40.1) | |
| Female | 77 344 (59.9) | 4834 (59.9) | 72 510 (59.9) | |
| Type of insurance | <0.001 | |||
| Self-employed health insurance | 36 978 (28.6) | 2271 (28.1) | 34 707 (28.7) | |
| Employee health insurance | 87 095 (67.5) | 5212 (64.6) | 81 883 (67.6) | |
| Medical aid | 5047 (3.9) | 587 (7.3) | 4460 (3.7) | |
| Comorbidities | ||||
| ILD | 488 (0.4) | 67 (0.8) | 421 (0.4) | <0.001 |
| CTD-related ILD | 87 (0.1) | 13 (0.2) | 74 (0.1) | 0.001 |
| Biologics used | 3 (0.00) | 0 | 3 (0.00) | 0.999 |
| Biologics not used | 84 (0.1) | 13 (0.2) | 71 (0.1) | 0.001 |
| CTD-unrelated ILD | 401 (0.3) | 54 (0.7) | 347 (0.3) | <0.001 |
| IPF | 101 (0.1) | 14 (0.2) | 87 (0.1) | 0.002 |
| Sarcoidosis | 2 (0.00) | 0 | 2 (0.00) | 0.999 |
| Hypersensitivity pneumonitis | 11 (0.01) | 2 (0.02) | 9 (0.01) | <0.001 |
| Others | 287 (0.2) | 38 (0.5) | 249 (0.2) | <0.001 |
| Lung cancer | 362 (0.3) | 37 (0.5) | 325 (0.3) | 0.002 |
| Hypertension | 28 895 (22.4) | 1934 (24.0) | 26 961 (22.3) | <0.001 |
| Diabetes mellitus | 21 895 (17.0) | 1632 (20.2) | 20 263 (16.7) | <0.001 |
| Cerebrovascular diseases | 9482 (7.3) | 687 (8.5) | 8795 (7.3) | <0.001 |
| Angina pectoris | 6046 (4.7) | 424 (5.3) | 5622 (4.6) | 0.012 |
| Myocardial infarction | 954 (0.7) | 101 (1.3) | 853 (0.7) | <0.001 |
| Heart failure | 3985 (3.1) | 357 (4.4) | 3628 (3.0) | <0.001 |
| CTD | 5849 (4.5) | 461 (5.7) | 5388 (4.5) | <0.001 |
| Malignancy other than lung cancer | 6930 (5.4) | 426 (5.3) | 6504 (5.4) | 0.716 |
Data are presented as n or n (%), unless otherwise stated. ILD: interstitial lung disease; CTD: connective tissue disease; IPF: idiopathic pulmonary fibrosis.
Impact of ILD on the susceptibility of COVID-19 and influenza
As shown in table 2, the adjusted OR for the presence of ILD in the COVID-19 cohort relative to the matched cohort was 2.02 (95% CI 1.54–2.61) and the adjusted OR for the presence of ILD was 5.65 (95% CI 4.27–7.45) in the influenza cohort relative to the matched cohort. Subgroup analyses stratified by age, sex and CTD are summarised in table 2.
TABLE 2.
Odds ratios for the presence of interstitial lung disease (ILD) in the influenza and COVID-19 cohorts relative to the matched cohort
| OR for the presence of ILD | OR for the presence of ILD | |||||
| Matched cohort | Influenza cohort | Matched cohort | COVID-19 cohort | |||
| Crude model | Adjusted model | Crude model | Adjusted model | |||
| Patients | 277 450 | 27 745 | 121 050 | 8070 | ||
| ILD overall | Reference | 7.50 (5.74–9.78) | 5.65 (4.27–7.45) | Reference | 2.40 (1.84–3.08) | 2.02 (1.54–2.61) |
| Age group | ||||||
| <60 years | Reference | 8.47 (4.76–14.9) | 7.42 (4.12–13.20) | Reference | 3.25 (1.99–5.04) | 2.82 (1.73–4.42) |
| ≥60 years | Reference | 7.30 (5.38–9.86) | 5.57 (4.03–7.64) | Reference | 2.14 (1.54–2.90) | 1.85 (1.33–2.52) |
| Sex | ||||||
| Male | Reference | 7.21 (5.09–10.14) | 4.99 (3.46–7.15) | Reference | 2.74 (1.90–3.82) | 2.23 (1.54–3.15) |
| Female | Reference | 7.98 (5.21–12.13) | 6.75 (4.36–10.37) | Reference | 2.07 (1.37–3.00) | 1.78 (1.17–2.59) |
| CTD# | ||||||
| No | Reference | 7.52 (5.70–9.88) | 5.74 (4.30–7.64) | Reference | 2.38 (1.76–3.14) | 2.04 (1.51–2.70) |
| Yes | Reference | 5.37 (1.75–15.60) | 4.60 (1.44–13.85) | Reference | 2.09 (1.10–3.66) | 1.92 (1.00–3.41) |
Data are presented as n or OR (95% CI), unless otherwise stated. CTD: connective tissue disease. The adjusted model was adjusted for type of insurance, lung cancer, CTD, hypertension, diabetes mellitus, cerebrovascular disease and cardiovascular disease. #: CTD was excluded from the adjusted model.
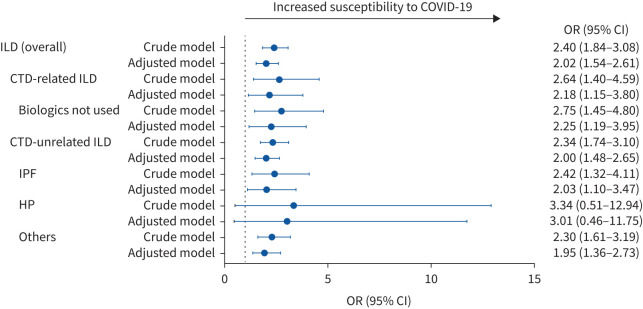
Figure 2 shows the odds ratio for each type of ILD in the COVID-19 cohort relative to the matched cohort. Regardless of the type of ILD, except for hypersensitivity pneumonitis, the odds ratios for the presence of each type of ILD in the COVID-19 cohort relative to the matched cohort were significantly increased (adjusted OR 2.25 (95% CI 1.19−3.95) for CTD-related ILD in which biological agents were not used, adjusted OR 2.03 (95% CI 1.10−3.47) for IPF and adjusted OR 1.95 (95% CI 1.36−2.73) for others).
FIGURE 2.
The impact of each type of interstitial lung disease (ILD) on the susceptibility to COVID-19. CTD: connective tissue disease; IPF: idiopathic pulmonary fibrosis; HP: hypersensitivity pneumonitis. The adjusted model was adjusted for type of insurance, lung cancer, CTD, hypertension, diabetes mellitus, cardiovascular disease (angina pectoris, myocardial infarction and heart failure) and cerebrovascular disease.
Characteristics of COVID-19 patients according to the presence or absence of ILD
Of the 8070 COVID-19 patients, 67 (0.8%) had ILD. COVID-19 patients with ILD were characterised by significantly older age (especially 60–69 years; p<0.001) and male sex (56.7% versus 40.0%; p=0.005) compared with those without ILD. Regarding comorbidities, COVID-19 patients with ILD were more likely to have lung cancer (3.0% versus 0.4%; p=0.002), hypertension (50.8% versus 23.7%; p<0.001), diabetes mellitus (47.8% versus 20.0%; p<0.001), cerebrovascular diseases (23.9% versus 8.4%; p<0.001), angina pectoris (19.4% versus 5.1%; p<0.001), heart failure (19.4% versus 4.3%; p<0.001), CTD (19.4% versus 5.6%; p<0.001) and malignancy other than lung cancer (11.9% versus 5.2%; p=0.024) than those without ILD (table 3).
TABLE 3.
Clinical characteristics of COVID-19 patients according to the presence or absence of interstitial lung disease (ILD)
| Total | With ILD | Without ILD | p-value | |
| Patients | 8070 | 67 | 8003 | |
| Age years | <0.001 | |||
| <10 | 81 (1.0) | 0 (0.0) | 81 (1.0) | |
| 10–19 | 276 (3.4) | 0 (0.0) | 276 (3.5) | |
| 20–29 | 2057 (25.5) | 2 (3.0) | 2055 (25.7) | |
| 30–39 | 832 (10.3) | 3 (4.5) | 829 (10.4) | |
| 40–49 | 1036 (12.8) | 5 (7.5) | 1031 (12.9) | |
| 50–59 | 1567 (19.4) | 12 (17.9) | 1555 (19.4) | |
| 60–69 | 1199 (14.9) | 16 (23.9) | 1183 (14.8) | |
| 70–79 | 617 (7.7) | 13 (19.4) | 604 (7.6) | |
| ≥80 | 405 (5.0) | 16 (23.9) | 389 (4.9) | |
| Sex | 0.005 | |||
| Male | 3236 (40.1) | 38 (56.7) | 3198 (40.0) | |
| Female | 4834 (59.9) | 29 (43.3) | 4805 (60.0) | |
| Type of insurance | 0.448 | |||
| Self-employed health insurance | 2271 (28.1) | 21 (31.3) | 2250 (28.1) | |
| Employee health insurance | 5212 (64.6) | 39 (58.2) | 5173 (64.6) | |
| Medical aid | 587 (7.3) | 7 (10.5) | 580 (7.3) | |
| Comorbidities | ||||
| Lung cancer | 37 (0.5) | 2 (3.0) | 35 (0.4) | 0.002 |
| Hypertension | 1934 (24.0) | 34 (50.8) | 1900 (23.7) | <0.001 |
| Diabetes mellitus | 1632 (20.2) | 32 (47.8) | 1600 (20.0) | <0.001 |
| Angina pectoris | 424 (5.3) | 13 (19.4) | 411 (5.1) | <0.001 |
| Myocardial infarction | 101 (1.3) | 2 (3.0) | 99 (1.2) | 0.205 |
| Heart failure | 357 (4.4) | 13 (19.4) | 344 (4.3) | <0.001 |
| Cerebrovascular diseases | 687 (8.5) | 16 (23.9) | 671 (8.4) | <0.001 |
| CTD | 461 (5.7) | 13 (19.4) | 448 (5.6) | <0.001 |
| Malignancy other than lung cancer | 426 (5.3) | 8 (11.9) | 418 (5.2) | 0.024 |
Data are presented as n or n (%), unless otherwise stated. CTD: connective tissue disease.
Impact of ILD on the occurrence of severe COVID-19
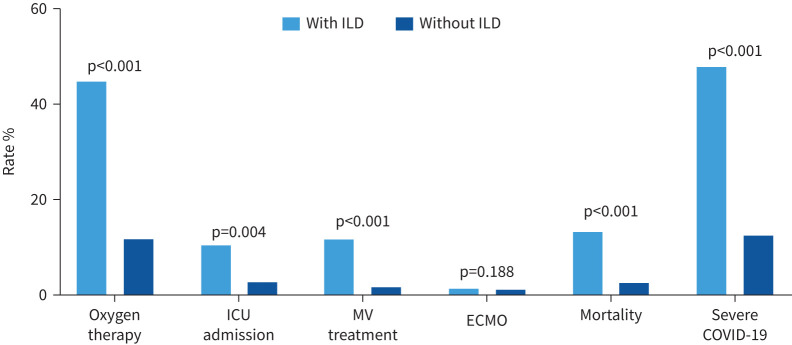
Among the COVID-19 cohort, COVID-19 patients with ILD had more severe disease courses than those without ILD (47.8% versus 12.6%; p<0.001), requiring the following treatments: oxygen therapy (cases receiving mechanical ventilation treatment or ECMO and mortality cases were not included) (44.8% versus 11.9%; p<0.001), ICU admission (10.5% versus 2.9%; p=0.004) and mechanical ventilation (cases receiving ECMO and mortality cases were not included) (11.9% versus 1.8%; p<0.001). COVID-19 patients with ILD also demonstrated significantly higher mortality than those without ILD (13.4% versus 2.8%; p<0.001) (figure 3).
FIGURE 3.
Severe COVID-19 disease according to the presence or absence of interstitial lung disease (ILD). Patients receiving oxygen therapy did not include cases receiving mechanical ventilation (MV) treatment or extracorporeal membrane oxygenation (ECMO) and mortality cases; those receiving MV treatment did not include cases receiving ECMO and mortality cases; those receiving ECMO did not include mortality cases. ICU: intensive care unit.
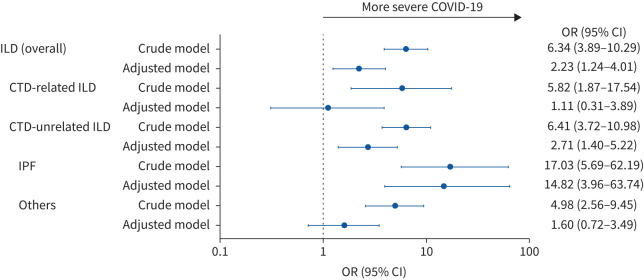
Figure 4 shows the impact of ILD on severe COVID-19 among patients in the COVID-19 cohort. Among the COVID-19 cohort, the patients with ILD were more likely to have severe COVID-19 compared with those without ILD (unadjusted OR 6.34, 95% CI 3.89–10.29; adjusted OR 2.23, 95% CI 1.24–4.01). Additionally, the patients with IPF were more likely to have severe COVID-19 compared with those without ILD (unadjusted OR 17.03, 95% CI 5.69–62.19; adjusted OR 14.82, 95% CI 3.96–63.74).
FIGURE 4.
The impact of each type of interstitial lung disease (ILD) on the occurrence of severe COVID-19. CTD: connective tissue disease; IPF: idiopathic pulmonary fibrosis. The adjusted model was adjusted for age, sex and comorbidities (lung cancer, CTD, hypertension, diabetes mellitus, cardiovascular disease (angina pectoris, myocardial infarction and heart failure), cerebrovascular disease and malignancies other than lung cancer).
Discussion
Using a nationwide COVID-19 database and an age-, sex- and residential area-matched cohort, in this study we showed that patients with ILD were 2.40-fold more likely to have COVID-19 compared with those without ILD, even after adjustments for potential confounding factors. This study revealed that ILD is associated with higher susceptibility to COVID-19 disease, and the effect was especially significant in males and younger patients. Additionally, we found that COVID-19 patients with ILD had more severe clinical courses, including higher mortality, than those without ILD. Whereas increased susceptibility to COVID-19 was observed regardless of the presence or absence of CTD, the presence of CTD was not associated with severe COVID-19 in patients with ILD. In contrast, IPF was not only associated with increased susceptibility of COVID-19 but also shows the highest risk of developing severe COVID-19.
It was previously demonstrated that pulmonary comorbidities including lung cancer and asthma are associated with increased susceptibility to COVID-19 [10, 11, 26]. Unfortunately, there was very limited information on whether patients with ILD have a higher risk of COVID-19 than those without ILD. Two previous studies evaluated this issue [14, 15]. However, since these studies were performed in single centres by indirectly comparing rates of COVID-19 with that of the general population, the results were inconclusive. Considering the limited generalisability of previous studies, our study has the advantage of using a large nationally representative dataset composed of a COVID-19 cohort and age-, sex- and residential area-matched cohort. In our study, the increased risk of COVID-19 in patients with ILD persisted even after adjusting for potential confounding factors, including the type of insurance (representing household income) and other comorbidities (hypertension, diabetes mellitus and lung cancer), a demonstration that supports the validity of our findings. Furthermore, this study has another advantage because we discovered that the increasing trend of odds for COVID-19 was more pronounced in those of younger age (<60 years) and among males.
Previous studies exploring the disease severity of COVID-19 in patients with ILD have yielded conflicting results. Whereas one study suggested that patients with ILD did not show increased occurrence of severe COVID-19 [14], the other two studies showed that patients with ILD had more severe disease and poorer prognosis than those without ILD [15, 18]. In the former study, patients who were not hospitalised were not allowed to undergo specific PCR testing for SARS-CoV-2, which was solely reserved for the most severe cases during this pandemic [14]. Thus, the number of overall ILD patients with COVID-19 could not be accurately evaluated, which limits the scope of this study. In contrast, the latter two studies compared the severity of COVID-19 according to the presence or absence of ILD and showed that COVID-19 patients with ILD had a more severe presentation of COVID-19 than those without ILD [15, 18]. Similarly, in the current study, we observed that patients with ILD suffered from more severe COVID-19 than those without ILD. Furthermore, whereas previous studies simply described poor outcomes (i.e. mortality) in ILD patients with COVID-19 [15], our study has the advantage of providing more detailed information about the clinical course of severe COVID-19 that includes oxygen therapy, mechanical ventilation treatment, ECMO and ICU admission.
Another notable aspect of the current study is our evaluation of the relationships between subgroups of ILD patients. In this study, the risk of severe COVID-19 was higher in males and younger patients compared with females and older patients, respectively. Previous studies revealed that males are more prone to acute exacerbations, of which the major aetiologies are viral infections, than females [27] and these findings are in line with our study results. However, as older patients have a higher risk of severe presentation of COVID-19 than younger patients, our findings should be confirmed in future studies [28]. In this study, whereas an increased risk of severe COVID-19 was observed in ILD patients without coexisting CTD, it was not found in ILD patients with CTD. Several previous studies have revealed that the risk of disease severity of COVID-19 is increased in patients with CTD [17, 29] and ILD [15, 30]. Accordingly, CTD patients with ILD may have a higher risk of severe COVID-19 compared with those without ILD, contrary to our findings. Because the number of ILD or CTD patients in our study was small, future studies are needed to further explore the issue.
Interestingly, the odds (OR 7.50) for the presence of ILD in the influenza cohort relative to the matched cohort was higher than that (OR 2.40) in the COVID-19 cohort relative to the matched cohort. The results are very surprising, considering the higher contagious characteristics of SARS-CoV-2 compared with influenza. Although the reasons for this are not clear, there may be some explanations for this phenomenon. From the beginning of the COVID-19 pandemic, there have been warnings that patients with chronic lung disease may be more susceptible to COVID-19 and their prognosis would be worse than those without chronic lung disease [31]. Accordingly, it is very likely that patients with ILD had an exaggerated fear of the highly contagious characteristics of COVID-19 and concerns about poor prognosis, which might lead to very high compliance with public health interventions, including social distancing, avoiding outdoor activities and universal personal preventive measures (e.g. using face masks and handwashing) in patients with ILD [25, 32]. Such adherence to public health interventions might have substantially attenuated the impact of COVID-19 on patients with ILD, although those with ILD revealed higher susceptibility to and severity of COVID-19 than those without ILD in this study.
The major strength of this study is that we demonstrated that patients with ILD are more susceptible to COVID-19 and experience more severe COVID-19 compared with those without ILD using nationally representative data, which reflects real-world clinical outcomes. However, this study also has limitations that should be acknowledged. First, since the Korean national insurance claims dataset was used, data on the severity of ILD, including pulmonary function tests and treatment duration, were limited. Hence, this study could not elucidate whether the poor outcomes of ILD patients with COVID-19 were related to ILD alone. Future research is warranted to address this issue. Second, the small number of ILD patients using biologics did not allow us to investigate whether biologics lead to adverse outcomes in ILD patients with COVID-19. Third, since we used ICD-10 codes for diagnosis, there is a possibility of misclassification. In addition, the coding in the early phase of COVID-19 (January to May 2020), which is the study period of the current study, might be different from that in the current phase (after May 2020). However, as all Korean hospitals were obligated to report all confirmed COVID-19 cases to the Korean Centres for Disease Control and Prevention from the first case in January 2020, we carefully suggest that the coding error might be insignificant. Fourth, although we adjusted for potential confounding factors which can influence the association between ILD and COVID-19 susceptibility, there were factors not adjusted for in our model. For example, we could not adjust for smoking history and body mass index as the NHIS database did not provide these variables. Fifth, there is a possibility that patients with ILD had more concern about COVID-19 and received more tests (or repeated tests), which was followed by the increased prevalence of COVID-19 in patients with ILD compared with their counterparts. The negative test rates of COVID-19 in ILD patients were higher than in those without ILD (98.4% versus 96.5%), which might have affected our study results. Sixth, the NHIS dataset lacked detailed information, including non-respiratory conditions or specific causes of death. Thus, we could not specify non-pulmonary COVID-19 pathology (e.g. acute kidney injury) when assessing severe COVID-19.
In conclusion, patients with ILD are more susceptible to COVID-19 and experience more severe COVID-19 compared with those without ILD. Whereas the susceptibility of COVID-19 was not different by the type of ILD (except for hypersensitivity pneumonitis), ILD was associated with especially higher risk of severe COVID-19. Clinicians should be aware of the increased risk of COVID-19 in their ILD patients and manage or educate them appropriately amid this COVID-19 pandemic.
Shareable PDF
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.01956-2021
Author contributions: All authors provided substantial contributions to the conception, design, data acquisition, analysis or interpretation of this work. H. Lee, H. Choi, B. Yang, S-K. Lee and S-H. Kim drafted the manuscript. All authors participated in revising the manuscript after a critical review. S-H. Kim is the guarantor of this study.
Conflict of interest: H. Lee has nothing to disclose.
Conflict of interest: H. Choi has nothing to disclose.
Conflict of interest: B. Yang has nothing to disclose.
Conflict of interest: S-K. Lee has nothing to disclose.
Conflict of interest: T.S. Park has nothing to disclose.
Conflict of interest: D.W. Park has nothing to disclose.
Conflict of interest: J-Y. Moon has nothing to disclose.
Conflict of interest: T-H. Kim has nothing to disclose.
Conflict of interest: J.W. Sohn has nothing to disclose.
Conflict of interest: H.J. Yoon has nothing to disclose.
Conflict of interest: S-H. Kim has nothing to disclose.
Support statement: This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (HC19C0318), and the Bio and Medical Technology Development Program of the National Research Foundation funded by the Korean government (Ministry of Science and ICT) (2019M3E5D1A01069363). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee A, Pasea L, Harris S, et al. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Lancet 2020; 395: 1715–1725. doi: 10.1016/S0140-6736(20)30854-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513. doi: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garassino MC, Whisenant JG, Huang LC, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol 2020; 21: 914–922. doi: 10.1016/S1470-2045(20)30314-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020; 323: 1574–1581. doi: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birkmeyer JD, Barnato A, Birkmeyer N, et al. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Affairs 2020; 39: 2010–2017. doi: 10.1377/hlthaff.2020.00980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One 2020; 15: e0233147. doi: 10.1371/journal.pone.0233147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao Y, Wang H, Liu Z. Expression of ACE2 in airways: implication for COVID-19 risk and disease management in patients with chronic inflammatory respiratory diseases. Clin Exp Allergy 2020; 50: 1313–1324. doi: 10.1111/cea.13746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogado J, Pangua C, Serrano-Montero G, et al. Covid-19 and lung cancer: a greater fatality rate? Lung Cancer 2020; 146: 19–22. doi: 10.1016/j.lungcan.2020.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020; 21: 335–337. doi: 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang B, Choi H, Lee SK, et al. Risk of coronavirus disease occurrence, severe presentation, and mortality in patients with lung cancer. Cancer Res Treat 2021; 53: 678–684. doi: 10.4143/crt.2020.1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovinsky-Desir S, Deshpande DR, De A, et al. Asthma among hospitalised patients with COVID-19 and related outcomes. J Allergy Clin Immunol 2020; 146: 1027–1034. doi: 10.1016/j.jaci.2020.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izquierdo JL, Almonacid C, González Y, et al. The impact of COVID-19 on patients with asthma. Eur Respir J 2021; 57: 2003142. doi: 10.1183/13993003.03142-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guiot J, Henket M, Frix AN, et al. Single-center experience of patients with interstitial lung diseases during the early days of the COVID-19 pandemic. Respir Investig 2020; 58: 437–439. doi: 10.1016/j.resinv.2020.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang H, Zhang M, Chen C, et al. Clinical characteristics of COVID-19 in patients with preexisting ILD: a retrospective study in a single center in Wuhan, China. J Med Virol 2020; 92: 2742–2750. doi: 10.1002/jmv.26174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cottin V, Hirani NA, Hotchkin DL, et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev 2018; 27: 180076. doi: 10.1183/16000617.0076-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye C, Cai S, Shen G, et al. Clinical features of rheumatic patients infected with COVID-19 in Wuhan, China. Ann Rheum Dis 2020; 79: 1007–1013. doi: 10.1136/annrheumdis-2020-217627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esposito AJ, Menon AA, Ghosh AJ, et al. Increased odds of death for patients with interstitial lung disease and COVID-19: a case–control study. Am J Respir Crit Care Med 2020; 202: 1710–1713. doi: 10.1164/rccm.202006-2441LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huh K, Ji W, Kang M, et al. Association of prescribed medications with the risk of COVID-19 infection and severity among adults in South Korea. Int J Infect Dis 2020; 104: 7–14. doi: 10.1016/j.ijid.2020.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi H, Yang B, Nam H, et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur Respir J 2019; 54: 1900194. doi: 10.1183/13993003.00194-2019 [DOI] [PubMed] [Google Scholar]
- 21.Choi H, Lee H, Lee SK, et al. Impact of bronchiectasis on susceptibility to and severity of COVID-19: a nationwide cohort study. Ther Adv Respir Dis 2021; 15: 1753466621995043. doi: 10.1177/1753466621995043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Little C, Alsen M, Barlow J, et al. The impact of socioeconomic status on the clinical outcomes of COVID-19; a retrospective cohort study. J Community Health 2021; 46: 794–802. doi: 10.1007/s10900-020-00944-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song S, Lee SE, Oh SK, et al. Demographics, treatment trends, and survival rate in incident pulmonary artery hypertension in Korea: a nationwide study based on the health insurance review and assessment service database. PLoS One 2018; 13: e0209148. doi: 10.1371/journal.pone.0209148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim L, Kim JA, Kim S. A guide for the utilisation of health insurance review and assessment service national patient samples. Epidemiol Health 2014; 36: e2014008. doi: 10.4178/epih/e2014008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H, Lee H, Song KH, et al. Impact of public health interventions on seasonal influenza activity during the COVID-19 outbreak in Korea. Clin Infect Dis 2021; 73: e132–e140. doi: 10.1093/cid/ciaa672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang JM, Koh HY, Moon SY, et al. Allergic disorders and susceptibility to and severity of COVID-19: a nationwide cohort study. J Allergy Clin Immunol 2020; 146: 790–798. doi: 10.1016/j.jaci.2020.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leuschner G, Behr J. Acute exacerbation in interstitial lung disease. Front Med 2017; 4: 176. doi: 10.3389/fmed.2017.00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scire CA, Carrara G, Zanetti A, et al. COVID-19 in rheumatic diseases in Italy: first results from the Italian registry of the Italian Society for Rheumatology (CONTROL-19). Clin Exp Rheumatol 2020; 38: 748–753. [PubMed] [Google Scholar]
- 30.Drake TM, Docherty AB, Harrison EM, et al. Outcome of hospitalisation for COVID-19 in patients with interstitial lung disease: an international multicenter study. Am J Respir Crit Care Med 2020; 202: 1656–1665. doi: 10.1164/rccm.202007-2794OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 2020; 94: 91–95. doi: 10.1016/j.ijid.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim S, Yoon HI, Song KH, et al. Face masks and containment of COVID-19: experience from South Korea. J Hosp Infect 2020; 106: 206–207. doi: 10.1016/j.jhin.2020.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-04125-2020.Shareable (433.7KB, pdf)