Abstract
Background:
Age of migration has been shown to have a robust association with Latino immigrant health outcomes; however, the relationship between timing of migration and cognition is less understood.
Objective:
To examine associations between race/ethnicity, nativity, age of migration, and cognitive aging among US-born (USB) non-Latino Whites (NLW) and USB and foreign-born Latinos 50 years and older.
Methods:
We used longitudinal biennial data from the Health and Retirement Study (HRS; 2006–2014) to fit generalized linear and linear latent growth curve models for: 1) global cognition (Modified Telephone Interview for Cognitive Status; TICS-M); 2) memory and attention subdomains of TICS-M; and 3) cognitive dysfunction. We also tested for sex modifications.
Results:
In age and sex adjusted models, all Latino subgroups, independent of nativity and age of migration, had lower global and domain-specific cognitive scores and higher propensity of cognitive impairment classification compared to USB-NLWs. Differences between USB Latinos, but not other Latino subgroups, and USB-NLWs remained after full covariate adjustment. Latinas, independent of nativity or age of migration, had poorer cognitive scores relative to NLW females. Differences between all Latinos and USB-NLWs were principally expressed at baseline. Racial/ethnic, nativity, and age of migration grouping was not associated with slope (nor explained variance) of cognitive decline.
Conclusion:
Older US-born Latinos, regardless of sex exhibit poorer cognitive function than older USB-NLWs and foreign-born Latinos. Social determinants that differentially affect cognitive function, particularly those that compensate for education and sex differences among US-born Latinos and foreign-born Latinos, require further exploration.
Keywords: Alzheimer’s disease and related dementias, cognitive function, immigration, Latino, nativity, sex differences
INTRODUCTION
As many as 5.8 million people in the United States (US) may be living with Alzheimer’s disease (AD) [1]. This number is projected to increase to 13.8 million by 2050, due in part, to rapid population aging [1] that coincides with growing racial and ethnic diversity [2]. For instance, the percentage of US adults 65 years and older who identify as non-Latino White is expected to decrease from 77.4% in 2016 to 55.1% in 2060; whereas the percentage who identify as Latino is expected to increase from 8.1% to 21% [3]. Importantly, Latinos residing in the US have been shown to have a higher prevalence rate of AD [4] and longer post-diagnosis survival [5] than non-Latino Whites. Recent projections put the number of Latinos with AD at 3.5 million by 2060 [6]. Thus, identifying protective and risk factors for cognitive functioning and cognitive impairment in this rapidly growing and aging population is critical for promoting healthy aging and developing effective policies aimed at reducing the burden of AD among US Latinos [7, 8].
Evidence on racial and ethnic disparities in cognitive function among US adults has grown extensively in the last decade [7, 9–12]. However, we know considerably less about how individual characteristics associated with nativity and immigration experiences influence cognitive aging within the US Latino population [13]. Longer life expectancy, higher prevalence of metabolic and cardiovascular disease, and lower educational attainment among Latinos relative to non-Latino Whites are contributing risk factors to a higher prevalence of AD; however, these risks have been shown to vary by nativity status, age of migration, and sex among Latino subgroups [6, 14–18].
Prior research examining risks for cognitive function and cognitive impairment among US Latinos has been limited in two important ways. First, most nationally representative work examines Latinos as a monolithic group when making comparisons to non-Latino Whites, obscuring complex nativity and immigration patterns that influence cognitive aging across the life course [9, 19, 20]. This is particularly problematic as approximately 58% of US Latinos 50 years and older in 2017 were foreign-born [21]. Second, evidence from studies restricted to specific geographic locations that focus on particular Latino populations have been inconclusive regarding associations between nativity and cognition and have limited generalizability [18, 22–24]. Moreover, neither of these approaches have comprehensively examined the association of age of migration, and sex (significant social determinants of Latino health) as risk factors for cognitive function and impairment.
Nativity and timing of migration influence individuals’ health across the life course by impacting processes of acculturation and incorporation which, shape early life experiences and influence cognitive health outcomes in later life [25, 26]. Among the foreign-born, age of migration captures, factors that include: (a) type of migration (labor versus family); (b) degree of health selectivity (healthy immigrant effect); and (c) duration and magnitude of exposure to the biopsychosocial risks and protective factors in both the host and origin societies [23, 27–29].
The effects of nativity and age of migration on health vary by sex. For example, existing evidence suggests that positive health selection (i.e., cognitive resiliency, and favorable physical health and mortality profiles) is strongest among foreign-born Latino men who migrate in early- and mid-adulthood in search of employment [23, 28, 30, 31]. Conversely, positive health selection has been shown to be weakest among the foreign-born who migrate as children as they are more likely to accompany a parent or other adult family member; thus any health selection reflects parental rather than individual characteristics [32, 33]. Individual characteristics that contribute to patterns of selective migration among foreign-born Latinos may also contribute to higher cognitive ability and a reduced risk of cognitive decline and cognitive impairment [17, 23, 28]. Though Early-life migration has been found to be associated with more opportunities to incorporate educationally and occupationally [30, 34] which may be beneficial to cognitive function in later life, leading to similar cognitive health experiences as their US-born co-ethnics [23, 27, 28].
The literature on Latino health largely points to a health paradox wherein US-born Latinos exhibit poorer health compared to their foreign-born Latino counterparts despite the lower socioeconomic status of the latter [17, 30, 34–38]. Mounting evidence indicates that health advantages exhibited by foreign-born Latinos appear to diminish throughout the life-course with greater acculturation and longer exposure to sociopolitical, familial and cultural changes that accompany life in the US [39, 40]. Evidence also points to sex differences in the relationship between nativity, migration, and health outcomes [17, 30, 41]. Yet, few studies have examined the Latino paradox, the potential effects of selection mechanisms, and the differential associations of nativity status, age of migration, and sex on cognitive performance [23, 28, 29].
We argue from a life course perspective that timing of migration is an important determinant of foreign-born Latino health that varies by sex. Timing of migration captures critical life stages and formative experiences that influence processes of settlement and incorporation into the host society which have important implications for late-life cognitive health outcomes [25, 26].
This study addresses a gap in the literature by using a life course perspective and longitudinal nationally representative data from the Health and Retirement Study (HRS) to comprehensively examine the associations between race/ethnicity, nativity status, age of migration, sex, and cognitive function and cognitive dysfunction. Based on previous scholarship, particularly research espousing health selection hypotheses in cognition among Latinos [17, 23, 28, 29], we hypothesize that Latinos residing in the US will exhibit poorer cognitive functioning at baseline than US-born non-Latino Whites (hereafter, NLWs) and that these associations will hold across cognitive domains that are less susceptible to education bias. We further expect foreign-born Latinos (hereafter, FBLs) who migrated to the US after their formative years (hereafter, FBLs18–34 and FBLs35+) to exhibit better cognitive functioning at baseline than US-born Latinos (hereafter, USBLs) and foreign-born Latinos who migrated to the US in their formative years (hereafter, FBLs < 18). Moreover, we expect that USBLs and FBLs < 18 will exhibit steeper declines in age- related cognitive functioning over 8 years compared to NLWs. We posit that individual characteristics related to positive health selection among FBLs who migrated after their formative years will lead to higher levels of cognitive function and a lower risk of cognitive dysfunction relative to USBLs. Given that migration and health selection processes vary by sex, we hypothesize that female USBLs and FBLs < 18 will be at higher risk for lower cognitive functioning, and higher cognitive dysfunction and cognitive decline than their male counterparts.
MATERIALS AND METHODS
Data and study population
This study uses 5 waves of biennial data (2006–2014) from the Health and Retirement Study (HRS), a nationally representative sample of adults 50 years and older in the US which initially began in 1992. The HRS sampling has a multi-stage area probability design, including geographical stratification and clustering, with an oversample of Black and Latino households. Collection of interviews occurs through a multi-modal design, inclusive of primary respondent interviews and proxy interviews when necessary. Response rates across waves of HRS have remained relatively high (~81%–89%). More detailed information regarding the HRS sample is published elsewhere [42, 44]. Our choice of study period is guided by two factors: 1) we wanted to reduce the potential for loss to follow up as a result of death for individuals who meet research criteria for dementia diagnosis during the study period. While estimates vary depending on type of dementia, severity stages, age at diagnosis, sex, and potentially race/ethnic background, a systematic review suggests a range of between 1 and 7 years [44]. As such, four biennial waves (8-years; 2006–2014) was a reasonable upper limit for longitudinal analyses. As importantly, 2) we wanted to validate our primary results using a different set of waves that stretched an equivalent and non-overlapping time period. To do so, in sensitivity analyses, we re-estimated our models using HRS data from 1998 to 2006 (4-waves). The results were consistent with the reported main findings in this study (see below, and sensitivity).
We take advantage of several HRS resources (https://hrs.isr.umich.edu/data-products) to construct our analytic sample for the current investigation; specifically, the HRS tracker file and the RAND HRS Longitudinal files [45]. The baseline (2006) sample consisted of unweighted n (u.n) = 17,106 unique respondents 50-years and older that were derived from the 2006 wave of the HRS. Given the design of the study, we focus on individuals who self-reported race/ethnic background as non-Latino Whites or Latinos and exclude foreign-born Whites and individuals that reported race/ethnic background other than non-Latino White or Latino (u.n = 13,817). Furthermore, we excluded 4% of participants (u.n = 551) due to missingness on baseline covariates. The variables with the highest prevalence of missing values were migration status (1.36%) and body mass index (BMI; 1.48%). Participants with missing values on any of the baseline covariates were more likely to be female (65.7% versus 53.5%), slightly older (66.1 versus 63.8 years), and had higher reported levels of education (16.3 years versus 13 years).
A detailed scheme showing the exclusion criteria and the analytic sample inclusion is provided in Supplementary Figure 1. Since most attrition in the HRS is a result of death, we used the HRS tracker file to construct an indicator measure capturing whether respondents were 1) alive and responding by end of each wave respectively; 2) attrited from the sample as a result of death; and/or 3) were lost to follow-up but not due to death. A table detailing the rates and causes of attrition in the HRS in the study period is provided as a supplement (Supplementary Table 1). We include death as an outcome category in analyses of longitudinal cognitive classification to minimize bias arising from attrition. All analyses accounted for the complex design of the HRS, including probability weighting, stratification, and clustering to allow appropriate generalization and correct inferences relative to the target population [42]. Given the scope of this study we used the baseline weights reflective of the target population of individuals who were not institutionalized or incarcerated at baseline. Data included in analyses are publicly available data, which received institutional review board (IRB) approval from the University of Michigan [HUM00061128]; therefore, the corresponding authors’ IRB determined our research was exempt from IRB consideration. Below we describe our analytic sample in more detail.
Outcome(s)
Our primary baseline and longitudinal analyses focus on cognitive functioning based on an abbreviated version of the Telephone Interview of Cognitive Status (TICS-M) [46–49]. The HRS TICS-M includes 10 word immediate and delayed recall tests of memory, a serial 7s subtraction test for working memory, and counting backwards to assess attention and processing speed. We exclude the object naming items used to assess language, and the probes for date and president/vice-president used to assess orientation as these were only administered to respondents 65 years and older. The composite score values were normally distributed (see Supplementary Figure 2) and ranged between 0 and 27. In secondary analyses we independently model the 1) memory (sum of immediate and delayed recall; range = 0–20) and 2) processing speed, working memory, and attention subcomponents (total serial 7s and backward counting from 20; range = 0–7) to assess potential education induced bias on cognitive performance. Finally, we consider baseline and longitudinal (i.e., 8-years later) cognitive status classification based on the Langa-Weir (LW) algorithm. The cut points for the LW classification are derived through extrapolations from more comprehensive adjudication processes using data from the Aging, Demographics, and Memory Study (a subsample of the HRS) and allow distinction between a) normal cognition; b) cognitive impairment no dementia (CIND); and c) dementia. LW classifications have been used extensively [9, 15, 16, 19]; details and rationale for the underlying algorithm are published elsewhere [9, 50–53]. For the longitudinal classification outcome, we include an additional category to group individuals that died between the baseline wave and 2014 (1 = normal cognition; 2 = CIND; 3 = dementia; and 4 = deceased). The outcome is set to missing if respondent status indicates loss to follow-up [35, 54].
Exposure
The primary exposure is a measure constructed to distinguish US-born NLWs, USBLs, and FBLs and capture life course migration patterns among FBLs. This was coded as follows: 0 = NLWs; 1 = USBLs; 2 = FBLs who migrated to the US prior to age 18 (FBLs < 18); 3 = FBLs who migrated to the US between 18 and 34 years (FBLs18–34); and 4 = FBLs who migrated to the US after age 35 (FBLs35+).
Baseline covariates
Our conceptual framework is guided by two theoretical underpinnings. First, social determinants of health, and education in particular, are critical for explaining differences between groups. Second, we are also guided by a lifecourse perspective which links early life factors to late-life health of immigrants and underserved groups in general. The variables that we chose to control for, given availability in the HRS, are reflective of these conceptual frame-works. In line with previous work [12, 18, 23], we account for a series of measures that capture potential differences in sociodemographic and economic characteristics as well as comorbid health conditions, and health behaviors known to affect cognitive functioning. Sociodemographic and economic measures included: (a) participant sex (0 = male; 1 = female); (b) age in years at baseline interview; (c) educational attainment, based on the numbers of years of schooling completed; (d) marital status using a binary measure for whether the respondent was married or partnered versus not (married but spouse absent, separated, divorced, widowed, and never married); (e) and health insurance status using a 4-category indicator that differentiates participants with 1) government supplied insurance only; 2) government-supplied and private insurance; 3) private insurance only; and 4) no health insurance. We also account for geographical residence using a 4-category census region indicator: a) Northeast; b) Midwest; c) South; and d) West. For chronic comorbid health conditions, we used the count of eight self-reported conditions based on history of diagnoses including high blood pressure, diabetes, cancer, lung disease, arthritis, stroke, heart, and psychiatric problems. These conditions were included since they account for the largest share of health, social, and economic burden in the US [55]. Additionally, we included BMI (kg/cm) as another health condition as it has been shown to influence cognitive aging processes [56, 57]. Health behavior measures known to influence cognitive aging processes were also included; namely, smoking status, and alcohol consumption [58]. BMI was mean centered and entered into the models as a continuous linear measure. In supplementary models (available upon request) we accounted for BMI as a categorical variable using four groups (underweight, typical, overweight, and obese), as well as using quadratic and cubic forms. The results were unchanged. Smoking status was a binary measure indicating whether the respondent had a history of smoking. Alcohol consumption was ascertained using a binary self-reported measure of ever drinking alcoholic beverages. Finally, we also controlled for a count of the number of waves participated by an individual. We did not adjust for language of interview (Spanish versus English) as this measure is completely confounded with the NLW referent group.
Analytic approach
Our analyses were executed in six steps. First, we generated descriptive statistics to characterize the target population at baseline year by race/ethnicity, nativity and age of migration. We used survey adjusted chi-squared tests to assess group differences over categorical indicators (e.g., marital status) and survey-adjusted two-sided t-tests to assess differences in continuously measured indicators (e.g., age, education). Estimates can be found in Table 1.
Table 1.
2006 Baseline characteristics of target population by race/ethnicity, nativity, and age of migration
| Unweighted N= | USB |
FBL |
|||||
|---|---|---|---|---|---|---|---|
| NLW | USBL | <18L | 18–34L | 35+L | Total | p | |
| 11,984 | 676 | 355 | 200 | 51 | 13,266 | ||
| %(SE)/Mean (SD) | |||||||
| Sex* | |||||||
| Female | 53.52 (0.40) | 52.39 (2.03) | 53.34 (2.70) | 58.32 (3.90) | 61.63 (4.11) | 53.54 (0.38) | p = 0.477 |
| Age (Years)↑ | 66.19 (9.72) | 64.04 (9.58) | 63.39 (9.14) | 66.54 (10.69) | 80.01 (11.21) | 66.08 (9.78) | p < 0.001 |
| Education (Years)↑ | 13.26 (2.42) | 10.49 (4.00) | 8.01 (5.45) | 7.42 (5.38) | 6.60 (5.32) | 12.96 (2.82) | p < 0.001 |
| Cohort* | |||||||
| HRS/AHEAD Overlap | 12.03 (0.49) | 6.71 (1.51) | 4.71 (1.09) | 8.26 (1.79) | 42.58 (9.94) | 11.67 (0.47) | p = 0.016 |
| CODA | 7.71 (0.51) | 6.51 (1.36) | 4.63 (1.49) | 9.51 (2.01) | 29.19 (12.18) | 7.66 (0.47) | |
| HRS | 34.35 (0.65) | 31.87 (3.41) | 40.18 (3.78) | 42.56 (4.33) | 23.76 (5.30) | 34.43 (0.58) | |
| War Babies | 24.32 (0.61) | 27.71 (4.49) | 19.28 (2.69) | 15.91 (3.96) | 0.00 (0.00) | 24.22 (0.62) | |
| Early Boomers | 21.59 (0.90) | 27.20 (6.91) | 31.19 (4.67) | 23.77 (5.69) | 4.47 (3.74) | 22.02 (0.82) | |
| Marital Status* | |||||||
| Married | 68.01 (0.60) | 67.19 (1.98) | 66.30 (3.23) | 62.46 (2.96) | 55.61 (6.67) | 67.85 (0.57) | p = 0.282 |
| Insurance* | |||||||
| Government Only | 22.24 (0.75) | 39.95 (1.87) | 42.85 (3.89) | 52.71 (4.00) | 85.11 (6.06) | 23.88 (0.69) | p < 0.001 |
| Government & Private | 31.23 (0.88) | 12.07 (1.49) | 5.58 (0.90) | 3.54 (1.42) | 7.69 (5.26) | 29.54 (0.83) | |
| Private Only | 41.91 (0.85) | 32.51 (2.12) | 32.69 (4.01) | 13.54 (3.25) | 2.21 (2.62) | 40.93 (0.82) | |
| No Insurance | 4.63 (0.28) | 15.48 (2.30) | 18.89 (3.94) | 30.22 (3.84) | 4.99 (2.91) | 5.66 (0.37) | |
| Census Region* | |||||||
| Northeast | 17.41 (1.55) | 2.95 (0.90) | 14.96 (3.67) | 19.71 (3.79) | 14.91 (6.57) | 16.76 (1.44) | p < 0.001 |
| Midwest | 28.92 (1.76) | 5.25 (2.07) | 4.25 (1.41) | 2.46 (1.10) | 0.00 (0.00) | 27.05 (1.65) | |
| South | 35.27 (1.42) | 43.27 (10.51) | 41.67 (5.75) | 48.80 (5.51) | 58.38 (10.84) | 35.94 (1.39) | |
| West | 18.40 (1.55) | 48.53 (9.69) | 39.11 (6.08) | 29.03 (4.60) | 26.70 (9.71) | 20.25 (1.66) | |
| # of Chronic Conditions↑ | 1.88 (1.34) | 1.86 (1.41) | 1.50 (1.44) | 1.58 (1.60) | 2.15 (2.02) | 1.86 (1.35) | p < 0.001 |
| BMI* | 27.78 (5.38) | 28.84 (6.23) | 28.41 (5.25) | 28.06 (6.49) | 27.27 (6.17) | 27.84 (5.44) | p = 0.01 |
| Smoking Status* | |||||||
| Ever Smoker | 57.92 (0.76) | 58.69 (2.50) | 47.54 (3.89) | 46.33 (3.68) | 39.43 (5.10) | 57.58 (0.69) | p = 0.003 |
| Alcohol Consumption* | |||||||
| Drinker | 57.06 (0.95) | 44.32 (3.34) | 41.73 (3.10) | 34.45 (3.73) | 26.48 (6.26) | 55.89 (0.90) | p < 0.001 |
| # of Participated Waves↑ | 4.21 (1.21) | 4.25 (1.31) | 4.28 (1.27) | 4.31 (1.31) | 3.68 (2.02) | 4.21 (1.22) | p = 0.21 |
% and standard errors (SEs) are presented.
Means and Standard Deviations are presented. Note: NLW refers to US-Born (USB) non-Latino Whites; USBL refers to USB Latinos; <18L refers to Foreign Born (FB) Latinos who migrated prior to age 18; 18–34L refers to FB Latinos who migrated between the ages of 18–34 years; 35+L refers to FB Latinos who migrated at age 35 and older.
Second, we fit linear models to examine baseline global and domain-specific (e.g., memory, processing speed and working memory) performance as a function of the primary exposure and sequentially adjusting for model covariates. This modeling approach takes advantage of our outcomes’ composite summed score and includes a pooled cross-sectional orientation of the data. Specifically, we estimate (1) crude, (2) age and sex adjusted, (3) additional adjustment for education, and (4) full covariates adjusted models. Beta coefficients, standard errors, and p-values for the estimated parameters are presented in Table 2A. To facilitate interpretation of the results we estimate and plot post-hoc average marginal effects (AMEs) derived from the sequentially adjusted models and their corresponding 95% confidence intervals (see Fig. 1).
Table 2.
Association between race/ethnic, nativity, and age of migration and 2006 baseline cognitive function and status. The reference group is set to US-born non-Latino White
| M1 β [SE] | M2 β [SE] | M3 β [SE] | M4 β [SE] | |
|---|---|---|---|---|
| A) Cognitive Function | ||||
| Global Cognition | ||||
| USBL | −2.31*** [0.26] | −2.71*** [0.22] | −1.23*** [0.22] | −1.11*** [0.20] |
| <18L | −2.38*** [0.32] | −2.81*** [0.28] | −0.03 [0.28] | −0.06 [0.29] |
| 18–34L | −2.85*** [0.48] | −2.88*** [0.46] | 0.16 [0.43] | 0.13 [0.43] |
| 35+L | −5.24*** [0.63] | −3.05*** [0.59] | −0.10 [0.49] | −0.15 [0.47] |
| Memory | ||||
| USBL | −1.21*** [0.20] | −1.55*** [0.16] | −0.60*** [0.16] | −0.56*** [0.15] |
| <18L | −1.49*** [0.19] | −1.87*** [0.17] | −0.08 [0.18] | −0.13 [0.18] |
| 18–34L | −1.78*** [0.32] | −1.83*** [0.32] | 0.12 [0.28] | 0.06 [0.28] |
| 35+L | −3.34*** [0.41] | −1.50*** [0.34] | 0.40 [0.27] | 0.30 [0.27] |
| Processing speed, working memory, and attention | ||||
| USBL | −1.10*** [0.09] | −1.15*** [0.09] | −0.63*** [0.09] | −0.55*** [0.09] |
| <18L | −0.88*** [0.17] | −0.94*** [0.17] | 0.05 [0.17] | 0.08 [0.17] |
| 18–34L | −1.07*** [0.19] | −1.05*** [0.19] | 0.04 [0.20] | 0.07 [0.21] |
| 35+L | −1.89*** [0.31] | −1.55*** [0.31] | −0.49 [0.28] | −0.45 [0.27] |
| RRR [95% CI] | RRR [95% CI] | RRR [95% CI] | RRR [95% CI] | |
| B) Cognitive Status | ||||
| CIND vs. Normal Cognition | ||||
| USBL | 2.62*** [2.21;3.10] | 3.57*** [2.84;4.47] | 1.87*** [1.41;2.48] | 1.78*** [1.34;2.36] |
| <18L | 3.06*** [2.42;3.86] | 4.45*** [3.50;5.66] | 1.16 [0.83;1.62] | 1.24 [0.89;1.72] |
| 18–34L | 3.04*** [2.17;4.26] | 3.51*** [2.44;5.03] | 0.86 [0.56;1.32] | 0.84 [0.54;1.32] |
| 35+L | 7.50*** [3.92;14.37] | 3.52*** [1.84;6.72] | 1.12 [0.47;2.66] | 1.19 [0.51;2.79] |
| Dementia vs. Normal Cognition | ||||
| USBL | 2.57*** [1.92;3.45] | 4.23*** [3.15;5.69] | 1.49 [0.84;2.63] | 1.37 [0.80;2.34] |
| <18L | 2.40** [1.44;4.00] | 4.52*** [2.70;7.57] | 0.60 [0.32;1.09] | 0.69 [0.37;1.27] |
| 18–34L | 3.77*** [2.11;6.73] | 4.89*** [2.69;8.89] | 0.62 [0.31;1.26] | 0.62 [0.30;1.30] |
| 35+L | 13.94*** [7.68;25.29] | 4.97*** [2.73;9.03] | 0.85 [0.43;1.70] | 0.96 [0.47;1.96] |
Note: Reference category is NLW; NLW refers to US-Born (USB) non-Latino Whites; USBL refers to USB Latinos; <18L refers to Foreign Born (FB) Latinos who migrated prior to age 18; 18–34L refers to FB Latinos who migrated between the ages of 18–34 years 35+L refers to FB Latinos who migrated at age 35 and older. M1: Crude M2: Age, sex adjustment M3: M2 + Education M4: M3 + Marital status, insurance, census region, alcohol consumption, smoking, BMI, count of number of chronic conditions, and number of waves participated.
p < 0.001;
p < 0.01;
p < 0.05.
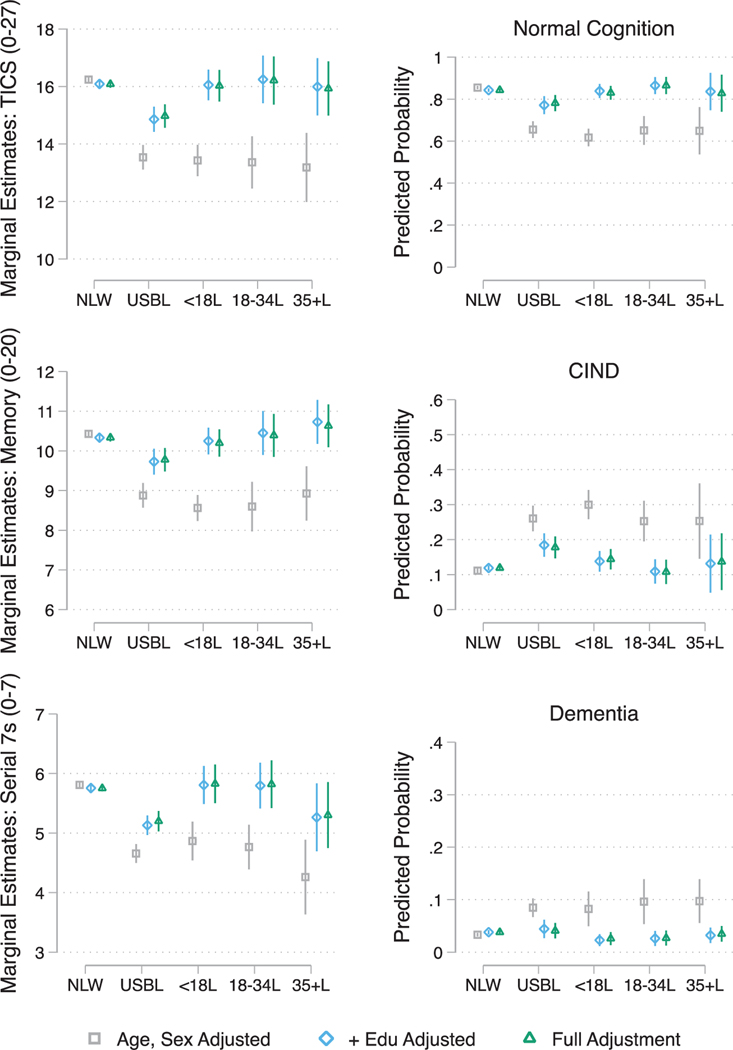
Fig. 1.
Estimated (a) average marginal means of baseline global and domain specific cognitive function and (b) marginal probabilities of baseline Langa-Weir cognitive status classification (Normal, CIND, and Dementia) by race/ethnicity, nativity, and age of migration. Note: NLW refers to US-Born (USB) non-Latino Whites; USBL refers to USB Latinos; <18L refers to Foreign Born (FB) Latinos who migrated prior to age 18; 18–34L refers to FB Latinos who migrated between the ages of 18–34 years; 35+L refers to FB Latinos who migrated at age 35 and older Full Adjustment includes age, sex, education, marital status, insurance, census region, alcohol consumption, smoking, BMI, count of number of chronic conditions, and number of participated waves.
Third, we fit survey multinomial logit regression models to examine the association between baseline cognitive classification, using the normal cognitive group as the reference, and the exposure and sequentially adjusting for covariates of interest as described in step 2 above. This modeling approach takes advantage of the Langa-Weir classification cut-points indicating: a) normal cognition (reference), b) CIND, c) dementia, and d) deceased. Additionally, this modeling allowed us to assess model performance and identify any trends using the full analytic sample (2006–2014), by applying a pooled cross-sectional orientation to the data. The estimated relative risk ratios derived from these models and corresponding 95% confidence intervals are presented in Table 2B. We estimate and plot post-hoc average marginal probabilities of classification in each of the cognitive status groups derived from the sequentially adjusted models and corresponding 95% confidence intervals (see Fig. 1).
Fourth, we used latent growth curve models (LGCM) to test longitudinal associations between race/ethnicity, nativity and age of migration (NLWs, referent; USBLs, FBLs < 18; FBLs18–34; and FBLs35+) and the trajectory of global cognition (TICS-M) (Table 3A). Although the cross-sectional models described above are useful for testing associations between key measures of interests and the baseline outcome measures, these models are insufficient to clarify whether and to what extent nativity and age of migration influence cognitive change and prospective cognitive status. As with the above modeling approach, we sequentially adjusted for baseline measured covariates. LGCMs are conceptually and, in most cases, mathematically equivalent to mixed effects models of longitudinal data [59]. Detailed theoretical and applied treatments of latent growth models are provided elsewhere [60–63]. Briefly, under LGCM the repeated measures are modeled using wide data format within a structural equation framework [59, 61]. Constraints are imposed on the loadings of latent factors to model the observed variables (in this case the biennially measured cognitive outcomes) as a parametric or non-parametric function of time. In our case two, latent variables were estimated: an intercept and a slope; also, an estimate of the covariance between these two variables. Each latent variable is summarized by two parameters: a mean (the fixed effect) and a variance (the random effect). The estimated mean of the intercept is interpreted as the average cognitive score for the target population at the baseline. The estimated slope mean quantifies the average positive or negative change in cognitive scores over time. The estimated variance of the intercept is a measure of deviations of participant baseline scores from the estimated population mean. The variance of the slope measures average deviations in growth trajectories about the slope mean. A statistically significant variance estimate suggests that individual trajectories are not equivalent across participants. Since the intercept and slope are estimated as latent variables, rather than parameters, LGCM affords the flexibility of modeling these factors as a function of covariates. The interpretation of the associations between the covariates and the factors follow standard regression models [60, 61]. A linear function provided good fit to the data, and all models were estimated as such. In sensitivity analyses, we re-estimated the LCGM without imposing a parametric functional form on time (e.g., linear). Results were qualitatively unchanged. We used years since baseline wave as the time metric and estimate the models using full information maximum likelihood (FIML) models to account for all available outcome data. All LGCM models were fit using Mplus8.3 [64]. Supplementary Figure 3 provides a conceptual representation of the estimated model, and Table 3A includes the estimated fixed and random effects (variance estimates of growth parameters) and inferential statistics (SEs, and p-values). Evaluation of changes in the estimated variances/residual variances of the intercept and slope allows us to assess the contribution of the covariates to explaining individual differences in growth trajectories.
Table 3.
Associations between race/ethnicity, nativity, and age of migration and a) cognitive function trajectories, and b) longitudinal cognitive status. Results for (a) are based on Linear latent growth curve models, and (b) on multinomial logistic regression models. The reference group is set to US-born non-Latino White
| M0 β [SE] | M1 β [SE] | M2 β [SE] | M3 β [SE] | M4 β [SE] | |
|---|---|---|---|---|---|
| A) Cognitive Function | |||||
| Fixed Effects | |||||
| Intercept | |||||
| USBL | n/a | −2.48***(0.26) | −2.85***(0.22) | −1.36***(0.21) | −1.20***(0.20) |
| <18L | n/a | −2.59***(0.31) | −3.04***(0.27) | −0.24(0.25) | −0.26(0.26) |
| 18–34L | n/a | −2.70***(0.41) | −2.71***(0.41) | 0.33(0.36) | 0.31(0.36) |
| 35+L | n/a | −5.07***(0.66) | −2.92***(0.58) | 0.09(0.40) | 0.22(0.38) |
| Slope | |||||
| USBL | n/a | 0.04(0.05) | −0.02(0.05) | 0.00(0.04) | 0.01(0.04) |
| <18L | n/a | 0.06(0.08) | 0.02(0.07) | 0.07(0.07) | 0.09(0.07) |
| 18–34L | n/a | 0.03(0.08) | 0.03(0.08) | 0.09(0.08) | 0.11(0.08) |
| 35+L | n/a | −0.18(0.17) | 0.09(0.19) | 0.13(0.20) | 0.15(0.20) |
| Random Effects | |||||
| Mean | |||||
| Intercept | 16.00***(0.08) | 16.20***(0.08) | 15.14***(0.09) | 14.68***(0.07) | 14.80***(0.18) |
| Slope | −0.34***(0.01) | −0.34***(0.01) | −0.48***(0.02) | −0.49***(0.02) | −0.56***(0.06) |
| Covariance | |||||
| Intercept with Slope | 0.33***(0.08) | 0.33***(0.08) | 0.08(0.07) | 0.04(0.06) | 0.05(0.07) |
| Variances | |||||
| Intercept | 12.29***(0.33) | 11.79***(0.31) | 8.94***(0.28) | 6.96***(0.22) | 6.28***(0.21) |
| Slope | 0.13***(0.02) | 0.13***(0.02) | 0.08***(0.02) | 0.08***(0.02) | 0.07***(0.02) |
| RRR [95% CI] | RRR [95% CI] | RRR [95% CI] | |||
| B) Longitudinal Cognitive Status | |||||
| CIND vs. Normal Cognition | |||||
| USBL | n/a | 2.58*** [1.89;3.51] | 3.30*** [2.39;4.56] | 1.85*** [1.40;2.44] | 1.60** [1.17;2.20] |
| <18L | n/a | 2.98*** [2.27;3.91] | 3.80*** [2.97;4.87] | 1.14 [0.82;1.59] | 0.97 [0.66;1.41] |
| 18–34L | n/a | 3.91*** [2.54;6.01] | 4.32*** [2.78;6.72] | 1.18 [0.74;1.87] | 0.99 [0.58;1.71] |
| 35+L | n/a | 3.78** [1.58;9.02] | 1.66 [0.61;4.47] | 0.52 [0.19;1.41] | 0.48 [0.20;1.13] |
| Dementia vs. Normal Cognition | |||||
| USBL | n/a | 3.21*** [2.32;4.46] | 5.31*** [3.55;7.94] | 2.37*** [1.62;3.46] | 2.48*** [1.64;3.76] |
| <18L | n/a | 3.66*** [2.19;6.12] | 6.22*** [3.82;10.13] | 1.29 [0.66;2.49] | 1.23 [0.67;2.29] |
| 18–34L | n/a | 4.17*** [2.33;7.46] | 5.23*** [2.93;9.34] | 0.98 [0.52;1.87] | 0.88 [0.43;1.78] |
| 35+L | n/a | 17.20*** [6.35;46.55] | 4.70* [1.38;16.05] | 1.04 [0.27;4.08] | 1.10 [0.37;3.29] |
| Death vs. Normal Cognition | |||||
| USBL | n/a | 1.07 [0.79;1.44] | 1.85*** [1.44;2.36] | 1.18 [0.95;1.45] | 0.60 [0.22;1.67] |
| <18L | n/a | 0.87 [0.56;1.37] | 1.59* [1.01;2.49] | 0.62 [0.36;1.07] | 0.31 [0.08;1.22] |
| 18–34L | n/a | 1.19 [0.57;2.49] | 1.49 [0.74;2.98] | 0.53 [0.26;1.07] | 0.33 [0.09;1.30] |
| 35+L | n/a | 5.60*** [2.85;11.02] | 1.40 [0.57;3.41] | 0.55 [0.20;1.52] | 0.88 [0.30;2.51] |
Note: Reference category is NLW; NLW refers to US-Born (USB) non-Latino Whites; USBL refers to USB Latinos; <18L refers to Foreign Born (FB) Latinos who migrated prior to age 18; 18–34L refers to FB Latinos who migrated between the ages of 18–34 years; 35+L refers to FB Latinos who migrated at age 35 and older. M0: Unconditional linear growth model; M1: Crude; M2: Age, sex adjustment; M3: M2 + Education; M4: M3 + Marital status, insurance, census region, alcohol consumption, smoking, BMI, count of number of chronic conditions, and number of participated waves.
p < 0.001;
p < 0.01;
p < 0.05.
Fifth, we fit survey multinomial logit regression models to examine the association between a combined measure of cognitive classification and death status in 2014, using the normal cognitive group as the reference and the exposure and sequentially adjusting for covariates of interest as described in step 2 above. The estimated relative risk ratios derived from these models and their 95% confidence intervals are presented in Table 3B. As with step four above, we estimate and plot post-hoc average marginal probabilities of classification in each of the cognitive status groups and death derived from the sequentially adjusted models and corresponding 95% confidence intervals in Fig. 2.
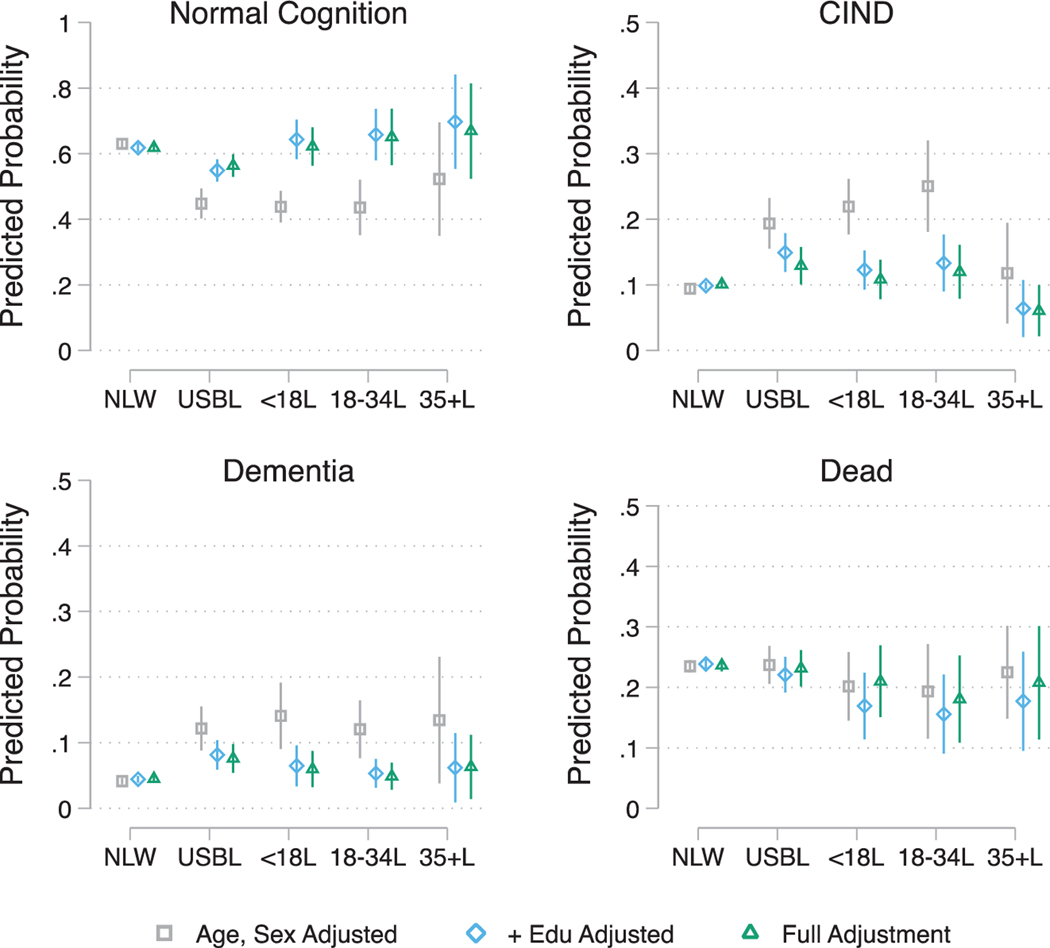
Fig. 2.
Estimated average marginal probabilities of longitudinal (2014) Langa-Weir cognitive status classification (Normal, CIND, and Dementia) and death by race/ethnicity, nativity, and age of migration. Note: NLW refers to US-Born (USB) non-Latino Whites; USBL refers to USB Latinos; <18L refers to Foreign Born (FB) Latinos who migrated prior to age 18; 18–34L refers to FB Latinos who migrated between the ages of 18–34 years; 35+L refers to FB Latinos who migrated at age 35 and older. Full Adjustment includes age, sex, education, marital status, insurance, census region, alcohol consumption, smoking, BMI, count of number of chronic conditions, and number of participated waves.
Finally, we examined sex modification in baseline global cognitive performance, baseline cognitive impairment and longitudinal cognitive status, and death. To do so, we refit the sequentially adjusted models described in steps 2, 3, and 5 above and included an interaction term between sex and the race/ethnicity and age of migration indicator. Sex specific marginal mean estimates for global cognition, and marginal probabilities of baseline and longitudinal dementia classifications are presented in Figs. 3 and 4. Detailed estimates of sex specific post-hoc ANOVA contrasts (relative to NLWs) for the marginal means and probabilities and their corresponding p-values and 95% confidence intervals are included in Supplementary Tables 3 and 4.
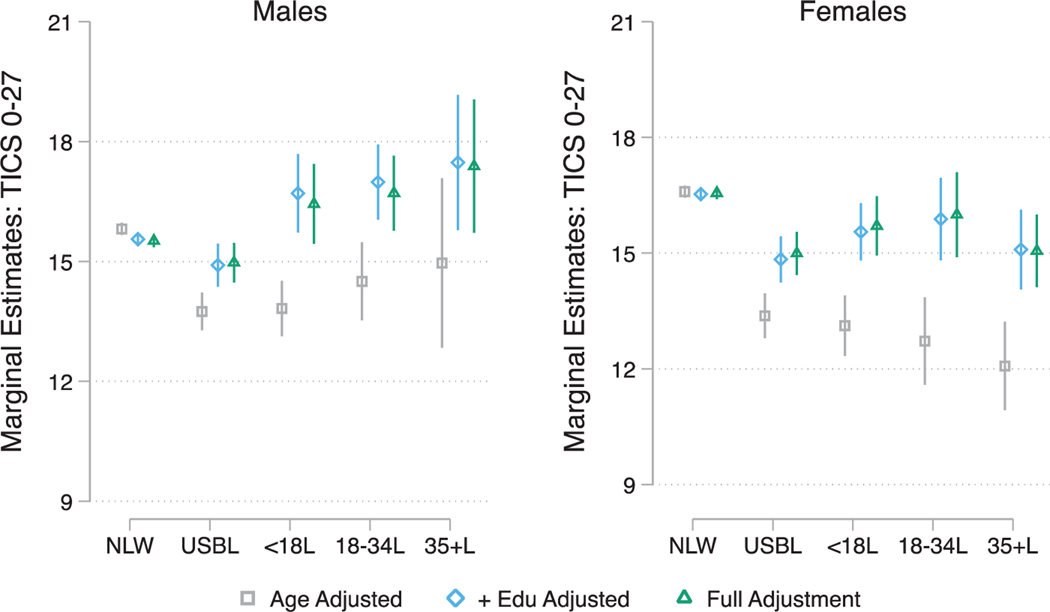
Fig. 3.
Sex specific average marginal estimates of mean baseline global cognitive function by race/ethnicity, nativity, and age of migration. Note: NLW refers to US-Born (USB) non-Latino Whites; USBL refers to USB Latinos; <18L refers to Foreign Born (FB) Latinos who migrated prior to age 18; 18–34L refers to FB Latinos who migrated between the ages of 18–34 years; 35+L refers to FB Latinos who migrated at age 35 and older. Full Adjustment includes age, education, marital status, insurance, census region, alcohol consumption, smoking, BMI, count of number of chronic conditions, and number of participated waves.
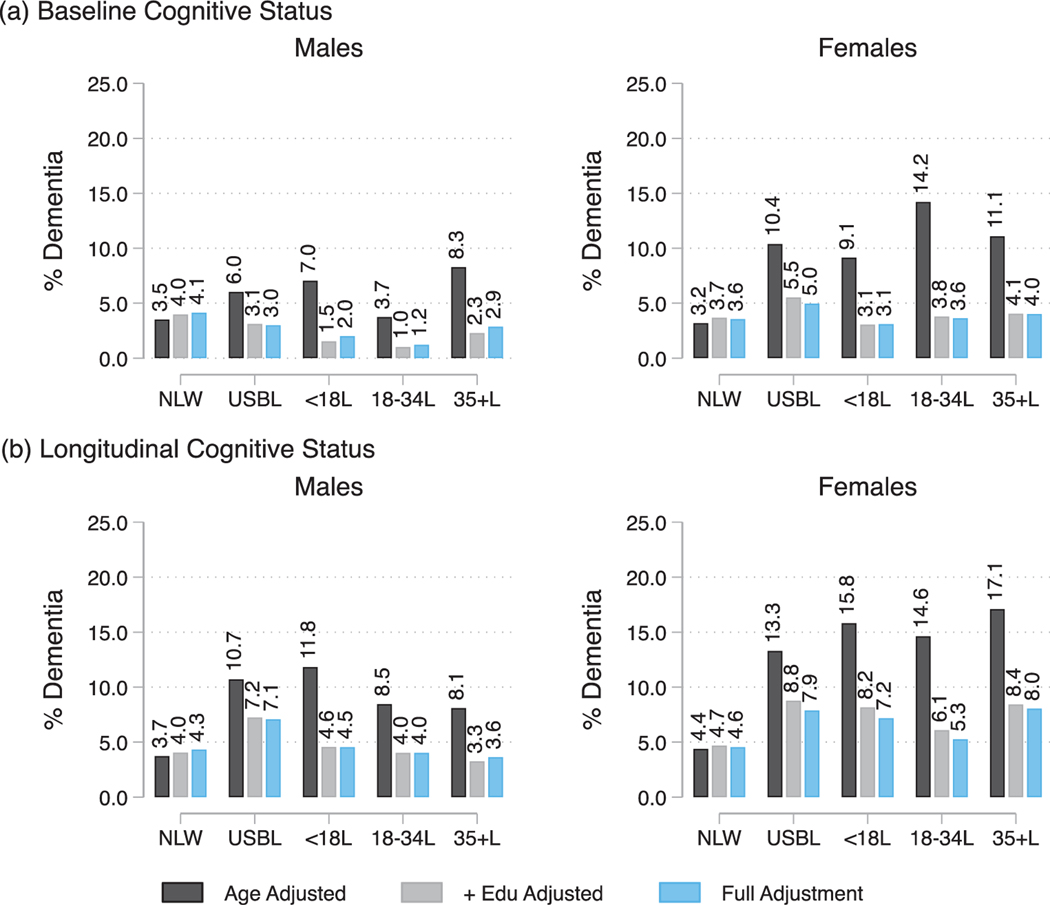
Fig. 4.
Sex specific average marginal estimates of (a) prevalence of baseline dementia, and (b) prevalence of longitudinal (2014) dementia classification by race/ethnicity, nativity, and age of migration. Note: NLW refers to US-Born (USB) non-Latino Whites; USBL refers to USB Latinos; <18L refers to Foreign Born (FB) Latinos who migrated prior to age 18; 18–34L refers to FB Latinos who migrated between the ages of 18–34 years; 35+L refers to FB Latinos who migrated at age 35 and older. Full Adjustment includes age, education, marital status, insurance, census region, alcohol consumption, smoking, BMI, count of number of chronic conditions, and number of participated waves.
All analyses used the data in wide form. The linear models focusing on baseline outcomes used the 2006 cross-section as the baseline. The multinomial logit models focusing on cognitive impairment outcomes at the end year (2014) and the longitudinal growth models used wide form data to link baseline measures to end wave outcomes; only individuals who are in the universe of the baseline target population (given the use of baseline weights) are considered in these analyses.
Sensitivity analyses
We conducted several sets of analyses to ensure that our results are robust. First, we split the sample into two age groups (<70 and 70+) and examined group invariance in effects of race/ethnicity and age of migration groupings on the intercept and change parameters in the longitudinal growth models. Invariance testing (model with freely estimated effects of race/ethnicity and migration groups on growth parameters versus model with effects constrained to equality across the two age stratas) showed no decrement in fit by restricting the effects to be equal within the two groups. Second, we re-estimated all models using generalized estimating equations models (population average models that focus on the fixed effects of groups) using the overall study population (see description above) as well as the subsample of participants 70 years and older using time since baseline as the time metric. Our results indicate that the group differences reported are consistent for both target populations. Importantly, as with the results from the latent growth models the group differences were restricted to baseline differences, and the slopes of change (decline in cognition) over time were statistically equivalent. Third, we re-estimated all models using generalized estimating equations and a group by age interaction (to specify the latter as the time metric). We did so for both the overall and 70+ subpopulation. As with the second set of sensitivity analyses detailed above and the primary findings reported in the manuscript, we found largely equivalent group differences and that the main effects were specific to intercept differences between groups rather than differences in change over age (i.e., no significant differences in estimated age slopes).
RESULTS
Descriptive statistics (Table 1)
Participants reported a mean age of 66 years ±9.8 with an average education of 13 years ±2.8. Slightly more than half were female, two-thirds were married, and approximately 94% had health insurance. We found no group differences in sex or marital status. We found significant group variations in age, education, number of chronic conditions, and health behavior profiles among NLWs and Latino subgroups. Notably, FBLs35+ were older, had lower educational attainment, reported a higher number of chronic conditions, and exhibited lower levels of alcohol consumption and smoking behavior than NLWs and Latino subgroups. In addition, USBLs reported higher levels of educational attainment, alcohol consumption and smoking than their foreign-born counterparts. Detailed tests of differences (and their 95% confidence intervals) in characteristics among Latino groups relative to NLWs are presented in Supplementary Table 2.
Baseline cognitive function difference among NLWs and Latinos (Table 2A)
In age and sex adjusted models USBLs (β = −2.71; SE(0.22); p < 0.001), FBLs < 18 (β = −2.81; SE(0.28); p < 0.001), FBLs18–34 (β = −2.88; SE(0.46); p < 0.001), and FBLs35+ (β = −3.05; SE(0.59); p < 0.001) had lower cognitive scores relative to NLWs. Except for USBLs, (β = −1.23; SE (0.22); p < 0.001) group differences between all FBLs, irrespective of age of migration, were explained by adjusting for years of education. Additional adjustment for sociodemographic, health, and health behavior factors did not substantively attenuate the difference between USBLs and the other two groups (NLWs and FBLs). The estimated parameters for all foreign-born Latino groups remained statistically indistinguishable from NLWs. Similar results emerged when we modeled the memory and processing speed/attention domains separately. The effect sizes from these models were consistent with those derived for global cognition. Figure 1 provides a visualization of the estimated marginal means for each group and attenuation in the estimated means through adjustment for covariates.
Baseline cognitive impairment among NLWs and Latinos (Table 2B)
Similar to cognitive function, after adjusting for age and sex, USBLs (RRR = 3.57; 95% CI = [2.84;4.47]; p < 0.001), FBLs < 18 (RRR = 4.45; 95% CI = [3.50;5.66]; p < 0.001), FBLs18–34 (RRR = 3.51; 95% CI = [2.44; 5.03]; p < 0.001), and FBLs35+ (RRR = 3.52; 95% CI = [1.84;6.72]; p < 0.001) exhibited higher relative risk ratios for CIND in comparison to NLWs (see Fig. 1). Adjusting for education did not explain the difference between USBLs (RRR = 1.87; 95% CI = [1.41; 2.48]; p < 0.001) and NLWs. In contrast, years of education explained the difference between FBLs and NLWs, regardless of age of immigration. Additional adjustment for covariates did not reduce the estimated relative risk for USBLs (RRR = 1.78; 95% CI = [1.34;2.36]; p < 0.001). In age and sex adjusted models, all Latino groups were also at higher risk for dementia classification relative to NLWs; The estimated relative risk ratios were RRR = 4.23; 95% CI = [3.15;5.69]; p < 0.001, RRR = 4.52; 95% CI = [2.70; 7.57]; p < 0.001, RRR = 4.89; 95% CI = [2.69; 8.89]; p < 0.001, and RRR = 4.97; 95%CI = [2.73; 9.03]; p < 0.001 for USBLs, FBLs < 18, FBLs18–34, and FBLs35+, respectively. Adjusting for education completely attenuated the higher risk for dementia across all Latino groups relative to NLWs. Figure 1 provides a visualization of the estimated marginal probabilities for classification into (1) normal, (2) CIND, and (3) dementia for each group and attenuation in the estimated probabilities through adjustment for covariates.
Cognitive change (Table 3A)
The fixed effects estimates derived from the growth models were consistent with the results from the baseline regressions. The mean intercept was 16.0 (SE = 0.08; p < 0.001), and the average biennial decline (slope) estimate was −0.34 (SE = 0.01; p < 0.001). The random effects indicated significant and sizable variations around the mean estimates for the intercept and slope. The variances for the intercept and slope were 12.29 (SE = 0.33; p < 0.001) and 0.13 (SE = 0.02; p < 0.001), respectively. In age and sex adjusted models USBLs, FBLs < 18, FBLs18–34, and FBLs35+ had lower intercepts relative to NLWs. Apart from USBLs, group differences between all FBLs, irrespective of age of migration, were explained by adjusting for number of years of education. No additional attenuations in the estimates for USBLs were attained through additional covariates adjustment. The estimated slopes for all Latino groups were not significantly different from NLWs. This was independent of covariates adjustment. Latino ethnicity and age of migration explained 4% of the variance in the intercept. Adjusting for age and sex explained an additional 23.2% and education explained 16.1%. All other covariates explained an additional 5.5%. Age and sex adjustment explained 38.5% of the slope variance, but education did not contribute to slope variance explanation.
Longitudinal cognitive impairment (Table 3B)
In age and sex adjusted models USBLs (RRR = 3.30; 95% CI = [2.39; 4.56]; p < 0.001), FBLs < 18 (RRR = 3.80; 95% CI = [2.97; 4.87]; p < 0.001), FBLs18–34 (RRR = 4.32; 95% CI = [2.78; 6.72]; p < 0.001), but not FBLs35+, had higher relative risk ratios for CIND (versus normal cognition) relative to NLWs. After adjusting for education, there were no differences between NLWs and FBLs regardless of age of immigration. With the exception of USBLs (RRR = 1.85; 95% CI = [1.40; 2.44]; p < 0.001) adjusting for education explained the differences relative to NLWs. Additional adjustment for covariates slightly reduced the estimated relative risk for USBLs (RRR = 1.60; 95% CI = [1.17; 2.20]; p < 0.001). In age and sex adjusted models, all Latino groups were also at higher risk for dementia classification relative to NLWs; The estimated relative risk ratios were RRR = 5.31; 95% CI = [3.55; 7.94]; p < 0.001, RRR = 6.22; 95% CI = [3.82; 10.13]; p < 0.001, RRR = 5.23; 95% CI = [2.93; 9.34]; p < 0.001, and RRR = 4.70; 95% CI = [1.38; 16.05]; p < 0.05 for USBLs, FBLs < 18, FBLs18–34, and FBLs35+, respectively. Except for USBLs (RRR = 2.37; 95% CI = [1.62; 3.46]; p < 0.001), adjusting for education completely attenuated the higher risk for dementia across all Latino groups, relative to NLWs. Additional adjustment for covariates had minimal effects on the relative risk ratio estimates. Finally, in age and sex adjusted models USBLs and FBLs < 18 had higher relative risk of death (relative to survival with normal cognition). Again, these group differences were explained by adjusting for education. Figure 2 provides a visualization of the estimated marginal probabilities for classification into (a) normal cognition, (b) CIND, (b) dementia, and (d) death for each group and attenuation in the estimated probabilities through adjustment for covariates.
Sex modifications
We found consistent evidence supporting sex modifications in the associations between our primary exposure and baseline cognitive performance and impairment classification (Supplementary Tables 3A and 3B). Adjusting for covariates, Latinas, particularly USBLs, FBLs < 18, and FBLs35+ had more disadvantageous cognitive scores profiles relative to NLW females; whereas only USBL males exhibited poorer baseline global functioning compared to NLW males (Supplementary Table 3A, and Fig. 3). The higher rates of baseline dementia but not CIND among USBLs, and FBLs < 18 Latinas were explained by covariates adjustment (Supplementary Table 3B, also see Fig. 4a for prevalence estimates of baseline dementia classification). Sex did not significantly modify (insignificant test of interaction) the association between the primary exposure and longitudinal CIND and dementia classifications. Group contrasts tests for longitudinal classifications are presented in Supplementary Table 4, and dementia classification estimates in Fig. 4b.
DISCUSSION
The current study adds to the growing literature on Latino cognitive health by using a life course perspective to examine how race/ethnicity, nativity status, age of migration, and sex influence cognitive function and cognitive impairment using a nationally representative sample of older USB non-Latino Whites and Latinos residing in the US. Our findings highlight that cognitive disparities vary by nativity and time of migration and distinctively so among Latinas but are largely due to baseline differences, rather than trajectories of cognitive change, likely as a result of varying cognitive reserve levels. Educational attainment, assessed using number of school years completed, fully explained the disadvantageous cognitive performance and higher rates of cognitive impairment (both CIND and dementia) among foreign-born, but not US-born Latinos relative to USB non-Latino Whites. Education also played an important role in explaining overall variations in baseline cognitive performance but had a minimal role in explaining variations in trajectories of decline. Health conditions and health behaviors played a negligible role in explaining differences between Latinos and USB non-Latino Whites and had minimal influence on the explained variances in baseline performance and cognitive decline. Taken together these findings suggest that modifying baseline risk can yield substantive population level gains and potentially decrease cognitive disease prevalence and incidence among Latinos.
First, we reported that Latinos had lower baseline cognitive scores and higher prevalence of CIND and dementia at both baseline and on average 8-years later relative to non-Latino Whites. These results mirror existing evidence that suggest higher risk for cognitive impairment and Alzheimer’s disease and related dementias (ADRDs) among Latinos [6, 9, 15, 17–19]. Our results extend these findings by showing that this elevated risk is potentially uniform for both US-born and foreign-born Latinos and remains largely consistent independent of age of migration. Our results also showed that Latinos, independent of nativity status and age of migration, had similar trajectories of cognitive decline in TICS-M scores compared to non-Latino Whites. The evidence of equivalent slopes of cognitive decline over time suggest that group differences in CIND and dementia prevalence, 8-years later, are extensions of baseline differences resulting from lifecourse risk accumulation rather than acceleration in cognitive disease morbidity.
Second, we found that educational attainment, particularly for foreign-born Latinos, plays a critical role in tempering cognitive group differences. Our analyses indicated that US-born Latinos had a higher risk for cognitive impairment even after accounting for educational attainment differences. These findings are consistent with existing literature highlighting the protective role of foreign-born status among some Latino groups with respect to several health and aging outcomes [30, 65, 66]. Educational attainment, assessed using self-reported number of school years completed, fully explained the disadvantageous cognitive performance and higher rates of cognitive impairment (both CIND and dementia) among all foreign-born Latino subgroups relative to non-Latino Whites; however, this was not the case for US-born Latinos. These findings suggest that US-born Latinos are likely more vulnerable to dementia risk than their foreign-born counterparts. Although, education attainment played an important role in explaining overall variations around baseline cognitive performance, education had a minimal role in explaining variations in cognitive trajectories of decline.
Two concurrent explanations can be offered regarding the role of education. One, performance on cognitive tests is intrinsically linked to the level and quality of education [67–69]. As such, the group differences derived through modeling cognition based on standard measures such as the TICS-M are potentially attributable to the confounding effect of education and to the bias that this confounding introduces when testing groups with systematically different educational characteristics, rather than due to race/ethnicity, nativity, or age of migration. The difference in educational quality reflects, to some extent, differences in socioeconomic status, although differences may also reflect disparities in quality of educational attainment and differential opportunities for educational advancement by sex. For the current cohort of US Latinos in mid- to late age, it is important to consider not just the number of years formal schooling, but also the less-than-optimal quality of education they experienced, and the country where education was obtained. Two, cognitive reserve conferred through higher levels and quality of education may potentially delay the onset of ADRD [70–74]. Although, large differentials in educational attainment among Latinos relative to non-Latino Whites may render Latino subgroups more susceptible to cognitive dysfunction due to lowered cognitive reserve. However, our results remained consistent when we considered subsets of the TICS-M, assessing memory performance, that may be less sensitive to educational bias.
Third, our results indicated that female sex modified the association between race/ethnicity, nativity status, age of migration and cognitive function and cognitive impairment among Latinos 50 years and older. US-born and younger age migrating Latinas were more vulnerable to cognitive deficits and dysfunctions relative to non-Latino White females. Prior research shows the process of migration varies by sex with men more likely to migrate to seek employment while women are more likely to migrate for family reunification [75, 76]. Cultural conflicts between the country of origin and new culture have been proposed to add constant stress to the lives of immigrant women [65, 77]. Chronic stress exposure and the continual activation of the physiological stress response in turn may disrupt proper hippocampus functioning and accelerate cognitive decline [78]. Differences between Latino males and non-Latino White males in cognitive function and cognitive impairment were less pronounced, suggesting Latino males (particularly the foreign-born) may be more positively selected on health in regard to cognitive functioning and cognitive decline than their female counterparts. Recent work suggests similarly disadvantageous positions for Latinas relative to Latinos [17, 18, 23]. Our findings extend this work by demonstrating that the lower cognitive performance and higher rates of cognitive impairment may be concentrated in US-born Latinas and those immigrating to the US in formative pre-adulthood years. These groups are arguably more exposed to social stressors and acculturative stress relative to more recent immigrants [23]. Further research is required to better understand the complex biopsychosocial mechanisms underlying sex differences in cognitive function and impairment.
Finally, additional influence of health conditions and health behaviors after controlling for age, sex, and education were negligible for explaining differences between Latinos and non-Latino Whites and had minimal influence on the explained variances in baseline performance and cognitive decline. Lower cognitive functioning and higher rates of baseline and longitudinal cognitive impairment and dementia among US-born Latinos was not explained by differences in comorbid health conditions or health behaviors. Measures of comorbidities and health behaviors available in HRS are crude and as such can potentially underestimate the true explanatory effects derived from more precise measurements of cardiovascular risk factors (e.g., sphygmomanometer assessed hypertension) or objective measures of health behaviors (e.g., frequency or intensity of physical activity). In addition, evidence on the influence of psychosocial factors (e.g., acculturation) on cognitive decline are mixed. Acculturative risks, what some researchers term as “negative acculturation”, accumulate as individuals integrate within US culture, are exposed to social, cultural, and economic stressors subsequently adopting habits that reverse potentially advantageous health characteristics. Our findings suggest that immigrants who arrive at younger ages may be less likely than individuals who migrate in adulthood to preserve customs from their country of origin which may be protective for cognitive health outcomes. Conversely, some work suggests that the effects of social risks are primarily predisposing to lower baseline cognitive functioning [79], which is also evidenced in this study, and that downstream cascades are largely reflections of these initial inductions. Other studies indicate more intricate pathways for social risks that affect individuals throughout the life course and compound health risks and the trajectories of disease evolution and severity as individuals age [80, 81]. While these pathways are plausible, we did not find evidence to corroborate a direct effect for Latino ethnicity, nativity status, or age of migration on the slopes of change in this study. Differences between the current analyses and prior research examining the association between nativity status, age of migration, sex and cognitive functioning among Latinos may be largely due to variations in study design, sample size, socio-demographic characteristics, and cognitive measures across studies. Beyond the scope of the current analyses, future research should develop complex models, inclusive of longer follow-up periods, and other instruments to examine such pathways. Biological markers that can influence group differences should be considered in future research as both risk indices (e.g., Framingham Risk Scores) and individual risk factors (e.g., A1c based assessment of diabetes) would offer valuable information on causal biological mechanisms underlying differences between US Latinos and non-Latino Whites.
Limitations
Our findings should be considered in the context of several limitations. First, the cognitive instruments used in HRS are brief and limited with regard to the level of detail and precision in measurement of cognitive function. These instruments may be impacted by bias due to demographic and sociocultural factors [82, 83], and dementia classification with these data have been shown to be less accurate for Latinos relative to non-Latino Whites [84]. Importantly, we were unable to account for direct measures of literacy and numeracy in this study. Recent findings [85] indicate that illiteracy is associated with an approximately three times higher risk of dementia. These limitations make it necessary for our findings to be replicated using detailed data that more precisely assess both fluid and crystallized knowledge, account for culturally appropriate cognitive measures, and include a broader assessment of cognitive domains. Despite this, the TICS-M and associated cognitive impairment classifications are used extensively in epidemiological studies to ascertain impairment and dementia prevalence and risks in the US [9, 19, 36, 84]. Second, the HRS is a biennial survey. More frequent interviews at smaller time intervals may capture more specific variations and nuances in cognitive change and possibly enhance precision of estimations. Third, the variables used to assess risk in our models are self-reported and in some instances crude and as such are subject to errors known to exist with self-reported data (e.g., recall bias, social desirability among others) [83, 86]. For example, evidence indicates that alcohol dosage may have differential effect on the risk of dementia, wherein low to moderate alcohol consumption may be protective but excessive alcohol consumption may elevate dementia risk. We used a crude measure for alcohol consumption which could have biased the estimates [87]. Future work, with more precise measures of behavioral risks, including alcohol, is needed to better clarify potential attenuations in effects. Fourth, we only focused on baseline risk factors in our longitudinal models. More complex models that account for time varying covariates can provide higher levels of precision in assessing the influence of some of the risk factors (e.g., comorbid conditions). Fifth, we did not include a measure capturing language of interview or respondent bilingualism because of confounding with the non-Latino White referent. Sixth, we were unable to consider participants’ country of origin (e.g., Mexicans and Puerto Ricans) and as such assume a homogenous risk profile across Latino subgroups. Existing studies suggest important variations in cognitive risks, comorbidities (e.g., higher incidence of diabetes among Mexican-origin Latinos) [14] and health behaviors profiles (e.g., higher rates of smoking among Puerto Rican-origin Latinos) [88, 89]. Disaggregating Latino subgroups offer an important future line of research on differential risks within Latinos relative to non-Latino Whites. Finally, given the scope of the work we did not model discrete switching in cognitive status over time and focused only on baseline and endpoint estimates of CIND and dementia classification. Examining the correlates of maintenance of pre-clinical cognitive status (e.g., CIND), reversion to normal function, and mixed switching (e.g., CIND to normal and back) is critical to clarify the pathways from pre-clinical to clinical status. Future work should consider using longitudinal Latent Class Analyses or Latent Transition Models to better characterize these processes and their correlates.
Conclusion
US-born Latinos 50 years and older, regardless of sex exhibit poorer cognitive function than older non-Latino Whites and their foreign-born Latino counterparts. Social determinants (e.g., education) that affect cognitive reserve, particularly those that compensate for education as well as sex specific stressors, warrant further investigation. These factors may help to decrease the burden of cognitive dysfunction and ADRD among Latinos in the US and yield important population health benefits.
Supplementary Material
ACKNOWLEDGMENTS
Dr. Tarraf is supported by R01AG48642 (National Institute on Aging). Drs. Garcia, Vega, and Diminich are supported by P30AG059300 (National Institute on Aging). Dr. Garcia is also supported by the Nebraska Tobacco Settlement Biomedical Research Development Funds. Mr. Ortiz is supported by R01AG054466-0S1(National Institute on Aging), U54MD004811-0S1 (National Institute of Minority Health & Health Disparities) and R01NR015241-0S1 (National Institute of Nursing Research). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the views of NIH or other funding organizations.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-1296r2).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-191296.
REFERENCES
- [1].Alzheimer’s Association (2020) 2020 Alzheimer’s disease facts and figures. Alzheimers Dement 16, 391–460. [DOI] [PubMed] [Google Scholar]
- [2].Vespa J, Armstrong DM, Medina L (2018) Demographic turning points for the United States: Population projections for 2020 to 2060. United States Census Bureau, https://www.census.gov/content/dam/Census/library/publications/2018/demo/P251144.pdf. [Google Scholar]
- [3].U.S. Census Bureau (2019) Population Estimates Show Aging Across Race Groups Differs. U.S. Department of Commerce, Washington, D.C. https://www.census.gov/newsroom/press-kits/2019/detailed-estimates.html [Google Scholar]
- [4].Matthews KA, Xu W, Gaglioti AH, Holt JB, Croft JB, Mack D, McGuire LC (2019) Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimers Dement 15, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mayeda ER, Glymour MM, Quesenberry CP, Johnson JK, Pérez-Stable EJ, Whitmer RA (2017) Survival after dementia diagnosis in five racial/ethnic groups. Alzheimers Dement 13, 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wu S, Vega W, Resendez J, Jin H (2016) Latinos & Alzheimer’s disease: New numbers behind the crisis. Projection of the costs for US Latinos living with Alzheimer’s disease through 2060. USC Edward R. Roybal Institute on Aging and the Latinos Against Alzheimer’s Network. [Google Scholar]
- [7].Babulal GM, Quiroz YT, Albensi BC, Arenaza-Urquijo E, Astell AJ, Babiloni C, Bahar-Fuchs A, Bell J, Bowman GL, Brickman AM, Chételat G, Ciro C, Cohen AD, Dilworth-Anderson P, Dodge HH, Dreux S, Edland S, Esbensen A, Evered L, Ewers M, Fargo KN, Fortea J, Gonzalez H, Gustafson DR, Head E, Hendrix JA, Hofer SM, Johnson LA, Jutten R, Kilborn K, Lanctôt KL, Manly JJ, Martins RN, Mielke MM, Morris MC, Murray ME, Oh ES, Parra MA, Rissman RA, Roe CM, Santos OA, Scarmeas N, Schneider LS, Schupf N, Sikkes S, Snyder HM, Sohrabi HR, Stern Y, Strydom A, Tang Y, Terrera GM, Teunissen C, Melo van Lent D, Weinborn M, Wesselman L, Wilcock DM, Zetterberg H, O’Bryant SE (2019) Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: Update and areas of immediate need. Alzheimers Dement 15, 292–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brewster P, Barnes L, Haan M, Johnson JK, Manly JJ, Nápoles AM, Whitmer RA, Carvajal-Carmona L, Early D, Farias S (2019) Progress and future challenges in aging and diversity research in the United States. Alzheimers Dement 15, 995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Díaz-Venegas C, Downer B, Langa KM, Wong R (2016) Racial and ethnic differences in cognitive function among older adults in the USA. Int J Geriatr Psychiatry 31, 1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Masel MC, Peek MK (2009) Ethnic differences in cognitive function over time. Ann Epidemiol 19, 778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schwartz BS, Glass TA, Bolla KI, Stewart WF, Glass G, Rasmussen M, Bressler J, Shi W, Bandeen-Roche K (2004) Disparities in cognitive functioning by race/ethnicity in the Baltimore memory study. Environ Health Perspect 112, 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang Z, Hayward MD, Yu YL (2016) Life course pathways to racial disparities in cognitive impairment among older Americans. J Health Soc Behav 57, 184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Xu H, Zhang Y, Wu B (2017) Association between migration and cognitive status among middle-aged and older adults: A systematic review. BMC Geriatr 17, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Garcia C, Garcia MA, Ailshire JA (2018) Sociocultural variability in the Latino population: Age patterns and differences in morbidity among older US adults. Demogr Res 38, 1605–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Garcia MA, Saenz J, Downer B, Wong R (2018) The role of education in the association between race/ethnicity/nativity, cognitive impairment, and dementia among older adults in the United States. Demogr Res 38, 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Garcia MA, Downer B, Chiu C-T, Saenz JL, Rote S, Wong R (2019) Racial/ethnic and nativity differences in cognitive life expectancies among older adults in the United States. Gerontologist 59, 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Casanova M, Aguila E (2019) Gender differences in cognitive function among older Mexican immigrants. J Econ Ageing doi: 10.1016/j.jeoa.2019.100226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Avila JF, Vonk JM, Verney SP, Witkiewitz K, Rentería MA, Schupf N, Mayeux R, Manly JJ (2019) Sex/gender differences in cognitive trajectories vary as a function of race/ethnicity. Alzheimers Dement 15, 1516–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA, Kabeto MU, Weir DR (2017) A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med 177, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen C, Zissimopoulos JM (2018) Racial and ethnic differences in trends in dementia prevalence and risk factors in the United States. Alzheimers Dement (N Y) 4, 510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Noe-Bustamante L, Flores A (2019) Facts on Latinos in the US. Pew Research Center. [Google Scholar]
- [22].Downer B, Garcia MA, Raji M, Markides KS (2018) Cohort differences in cognitive impairment and cognitive decline among Mexican-Americans aged 75 and older. Am J Epidemiol 188, 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hill TD, Angel JL, Balistreri KS, Herrera AP (2012) Immigrant status and cognitive functioning in late-life: An examination of gender variations in the healthy immigrant effect. Soc Sci Med 75, 2076–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mungas D, Early DR, Glymour MM, Zeki Al Hazzouri A, Haan MN (2018) Education, bilingualism, and cognitive trajectories: Sacramento Area Latino Aging Study (SALSA). Neuropsychology 32, 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dannefer D (2003) Cumulative advantage/disadvantage and the life course: Cross-fertilizing age and social science theory. J Gerontol B Psychol Sci Soc Sci 58, S327–S337. [DOI] [PubMed] [Google Scholar]
- [26].Elder GH Jr, Johnson MK, Crosnoe R (2003) The emergence and development of life course theory. In Handbook of the Life Course Springer, pp. 3–19. [Google Scholar]
- [27].Downer B, Garcia MA, Saenz J, Markides KS, Wong R (2018) The role of education in the relationship between age of migration to the United States and risk of cognitive impairment among older Mexican Americans. Res Aging 40, 411–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Garcia MA, Reyes AM, Downer B, Saenz JL, Samper-Ternent RA, Raji M (2018) Age of migration and the incidence of cognitive impairment: A cohort study of elder Mexican-Americans. Innov Aging 1, igx037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Garcia MA, Saenz JL, Downer B, Chiu C-T, Rote S, Wong R (2018) Age of migration differentials in life expectancy with cognitive impairment: 20-year findings from the Hispanic-EPESE. Gerontologist 58, 894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Reyes AM, Garcia MA (2019) Gender and age of migration differences in mortality among older Mexican Americans. J Gerontol B Psychol Sci Soc Sci doi: 10.1093/geronb/gbz038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Holmes JS, Driscoll AK, Heron M (2015) Mortality among US-born and immigrant Hispanics in the US: Effects of nativity, duration of residence, and age at immigration. Int J Public Health 60, 609–617. [DOI] [PubMed] [Google Scholar]
- [32].Angel RJ, Angel JL, Díaz Venegas C, Bonazzo C (2010) Shorter stay, longer life: Age at migration and mortality among the older Mexican-origin population. J Aging Health 22, 914–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gubernskaya Z, Bean FD, Van Hook J (2013) (Un) Healthy immigrant citizens: Naturalization and activity limitations in older age. J Health Soc Behav 54, 427–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gubernskaya Z (2014) Age at migration and self-rated health trajectories after age 50: Understanding the older immigrant health paradox. J Gerontol B Psychol Sci Soc Sci 70, 279–290. [DOI] [PubMed] [Google Scholar]
- [35].Tarraf W, Jensen GA, Dillaway HE, Vásquez PM, González HM (2018) Trajectories of aging among U.S. older adults: Mixed evidence for a Hispanic paradox. J Gerontol B Psychol Sci Soc Sci 75, 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Weden MM, Miles JN, Friedman E, Escarce JJ, Peterson C, Langa KM, Shih RA (2017) The Hispanic paradox: Race/ethnicity and nativity, immigrant enclave residence and cognitive impairment among older US adults. J Am Geriatr Soc 65, 1085–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bostean G (2013) Does selective migration explain the Hispanic paradox? A comparative analysis of Mexicans in the U.S. and Mexico. J Immigr Minor Health 15, 624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cantu P, Hayward M, Hummer R, Chiu C-T (2013) New estimates of racial/ethnic differences in life expectancy with chronic morbidity and functional loss: Evidence from the national health interview survey. J Cross Cult Gerontol 28, 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Antecol H, Bedard K (2006) Unhealthy assimilation: Why do immigrants converge to American health status levels? Demography 43, 337–360. [DOI] [PubMed] [Google Scholar]
- [40].Riosmena F, Wong R, Palloni A (2013) Migration selection, protection, and acculturation in health: A binational perspective on older adults. Demography 50, 1039–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Garcia MA, Reyes AM, Rote S (2019) Disability and the Immigrant Health Paradox: Gender and Timing of Migration. In Contextualizing Health and Aging in the Americas: Effects of Space, Time and Place Vega WA, Angel JL, Gutiérrez Robledo LMF, Markides KS, eds. Springer International Publishing, Cham, pp. 249–269. [Google Scholar]
- [42].Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR (2014) Cohort profile: The health and retirement study (HRS). Int J Epidemiol 43, 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Heeringa SG, Connor JH (1995) Sample design and methods for the Health and Retirement Survey. Statistical Design Group, Survey Research Center, University of Michigan, Ann Arbor. [Google Scholar]
- [44].Brodaty H, Seeher K, Gibson L (2012) Dementia time to death: A systematic literature review on survival time and years of life lost in people with dementia. Int Psychogeriatr 24, 1034–1045. [DOI] [PubMed] [Google Scholar]
- [45].Rand H (2019) Longitudinal File 2014 (V2). Produced by the RAND Center for the Study of Aging, with funding from the National Institute on Aging and the Social Security Administration Santa Monica, CA (February 2018). [Google Scholar]
- [46].Knopman DS, Roberts RO, Geda YE, Pankratz VS, Christianson TJ, Petersen RC, Rocca WA (2010) Validation of the telephone interview for cognitive status-modified in subjects with normal cognition, mild cognitive impairment, or dementia. Neuroepidemiology 34, 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cook SE, Marsiske M, McCoy KJ (2009) The use of the Modified Telephone Interview for Cognitive Status (TICS-M) in the detection of amnestic mild cognitive impairment. J Geriatr Psychiatry Neurol 22, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Plassman BL, Newman TT, Welsh KA, Helms M (1994) Properties of the telephone interview for cognitive status: Application in epidemiological and longitudinal studies. Neuropsychiatry Neuropsychol Behav Neurol 7, 235–241. [Google Scholar]
- [49].Brandt J, Spencer M, Folstein M (1988) The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol 1, 111–117. [Google Scholar]
- [50].Crimmins EM, Kim JK, Langa KM, Weir DR (2011) Assessment of cognition using surveys and neuropsychological assessment: The health and retirement study and the aging, demographics, and memory study. J Gerontol B Psychol Sci Soc Sci, 66 i162–i171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Rocca WA, Petersen RC, Knopman DS, Hebert LE, Evans DA, Hall KS, Gao S, Unverzagt FW, Langa KM, Larson EB, White LR (2011) Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement, 7 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Langa KM, Larson EB, Karlawish JH, Cutler DM, Kabeto MU, Kim SY, Rosen AB (2008) Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity? Alzheimers Dement 4, 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, Wallace RB (2007) Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology 29, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zajacova A, Ailshire J (2014) Body mass trajectories and mortality among older adults: A joint growth mixture–discrete-time survival analysis. Gerontologist 54, 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Raghupathi W, Raghupathi V (2018) An empirical study of chronic diseases in the United States: A visual analytics approach to public health. Int J Environ Res Public Health 15, 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Arvanitakis Z, Capuano AW, Bennett DA, Barnes LL (2017) Body mass index and decline in cognitive function in older black and white persons. J Gerontol A Biol Sci Med Sci 73, 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Michaud TL, Siahpush M, Farazi PA, Kim J, Yu F, Su D, Murman DL (2018) The association between body mass index, and cognitive, functional, and behavioral declines for incident dementia. J Alzheimers Dis 66, 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sabia S, Nabi H, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A (2009) Health behaviors from early to late midlife as predictors of cognitive function: The Whitehall II study. Am J Epidemiol 170, 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Finch WH, French BF (2015) Latent variable modeling with R Routledge. [Google Scholar]
- [60].Duncan TE, Duncan SC, Strycker LA (2013) An introduction to latent variable growth curve modeling: Concepts, issues, and application Routledge. [Google Scholar]
- [61].Newsom JT (2015) Longitudinal structural equation modeling: A comprehensive introduction Routledge. [Google Scholar]
- [62].Grimm KJ, Ram N, Estabrook R (2016) Growth modeling: Structural equation and multilevel modeling approaches Guilford Publications. [Google Scholar]
- [63].Little TD (2013) Longitudinal structural equation modeling Guilford Press. [Google Scholar]
- [64].Muthén L, Muthén B (2019) Mplus. The comprehensive modelling program for applied researchers: User’s guide 5. [Google Scholar]
- [65].García C, Garcia MA, Chiu C-T, Rivera FI, Raji M (2019) Life expectancies with depression by age of migration and gender among older Mexican Americans. Gerontologist 59, 877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Garcia MA, Garcia C, Chiu C-T, Raji M, Markides KS (2018) A comprehensive analysis of morbidity life expectancies among older Hispanic subgroups in the United States: Variation by nativity and country of origin. Innov Aging 2, igy014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Manly JJ, Schupf N, Tang M-X, Stern Y (2005) Cognitive decline and literacy among ethnically diverse elders. J Geriatr Psychiatry Neurol 18, 213–217. [DOI] [PubMed] [Google Scholar]
- [68].Arévalo SP, Kress J, Rodriguez FS (2019) Validity of cognitive assessment tools for older adult Hispanics: A systematic review. J Am Geriatr Soc 68, 882–888. [DOI] [PubMed] [Google Scholar]
- [69].Lezak M, Howieson D, Loring D (2012) Neuropsychological assessment 5th edn. Oxford University Press, Oxford, New York. [Google Scholar]
- [70].Soldan A, Pettigrew C, Cai Q, Wang J, Wang M-C, Moghekar A, Miller MI, Albert M, Team BR (2017) Cognitive reserve and long-term change in cognition in aging and preclinical Alzheimer’s disease. Neurobiol Aging 60, 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Stern Y (2012) Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11, 1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Stern Y (2006) Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord 20, 112–117. [DOI] [PubMed] [Google Scholar]
- [73].Zahodne LB, Schupf N, Brickman AM, Mayeux R, Wall MM, Stern Y, Manly JJ (2016) Dementia risk and protective factors differ in the context of memory trajectory groups. J Alzheimers Dis 52, 1013–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Zahodne LB, Stern Y, Manly JJ (2015) Differing effects of education on cognitive decline in diverse elders with low versus high educational attainment. Neuropsychology 29, 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Angel RJ, Angel JL, Díaz Venegas C, Bonazzo C (2010) Shorter stay, longer life: Age at migration and mortality among the older Mexican-origin population. J Aging Health 22, 914–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Treas J, Gubernskaya Z (2016) Immigration, aging, and the life course. In Handbook of Aging and the Social Sciences (Eighth Edition). Elsevier, pp. 143–161. [Google Scholar]
- [77].Vega WA, Amaro H (1994) Latino outlook: Good health, uncertain prognosis. Annu Rev Public Health 15, 39–67. [DOI] [PubMed] [Google Scholar]
- [78].McEwen BS, Sapolsky RM (1995) Stress and cognitive function. Curr Opin Neurobiol 5, 205–216. [DOI] [PubMed] [Google Scholar]
- [79].Oi K, Haas S (2019) Cardiometabolic risk and cognitive decline: The role of socioeconomic status in childhood and adulthood. J Health Soc Behav 60, 326–343. [DOI] [PubMed] [Google Scholar]
- [80].Zeki Al Hazzouri A, Haan MN, Kalbfleisch JD, Galea S, Lisabeth LD, Aiello AE (2011) Life-course socioeconomic position and incidence of dementia and cognitive impairment without dementia in older Mexican Americans: Results from the Sacramento area Latino study on aging. Am J Epidemiol 173, 1148–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Haan MN, Zeki Al-Hazzouri A, Aiello AE (2011) Life-span socioeconomic trajectory, nativity, and cognitive aging in Mexican Americans: The Sacramento Area Latino Study on Aging. J Gerontol B Psychol Sci Soc Sci 66, i102–i110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Schneider S (2016) Extracting response style bias from measures of positive and negative affect in aging research. J Gerontol B Psychol Sci Soc Sci 73, 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Weir D, Faul J, Langa K (2011) Proxy interviews and bias in the distribution of cognitive abilities due to non-response in longitudinal studies: A comparison of HRS and ELSA. Longit Life Course Stud 2, 170–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Gianattasio KZ, Wu Q, Glymour MM, Power MC (2019) Comparison of methods for algorithmic classification of dementia status in the Health and Retirement Study. Epidemiology 30, 291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Rentería MA, Vonk JM, Felix G, Avila JF, Zahodne LB, Dalchand E, Frazer KM, Martinez MN, Shouel HL, Manly JJ (2019) Illiteracy, dementia risk, and cognitive trajectories among older adults with low education. Neurology 93, e2247–e2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Wu Q, Tchetgen EJT, Osypuk TL, White K, Mujahid M, Glymour MM (2013) Combining direct and proxy assessments to reduce attrition bias in a longitudinal study. Alzheimer Dis Assoc Disord 27, 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Xu W, Wang H, Wan Y, Tan C, Li J, Tan L, Yu JT (2017) Alcohol consumption and dementia risk: A dose-response meta-analysis of prospective studies. Eur J Epidemiol 32, 31–42. [DOI] [PubMed] [Google Scholar]
- [88].González HM, Tarraf W, Rodríguez CJ, Gallo LC, Sacco RL, Talavera GA, Heiss G, Kizer JR, Hernandez R, Davis S (2016) Cardiovascular health among diverse Hispanics/Latinos: Hispanic Community Health Study/Study of Latinos (HCHS/SOL) results. Am Heart J 176, 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Gonzalez HM, Tarraf W, Gouskova N, Gallo LC, Penedo FJ, Davis SM, Lipton RB, Arguelles W, Choca JP, Catellier DJ, Mosley TH (2015) Neurocognitive function among middle-aged and older Hispanic/Latinos: Results from the Hispanic Community Health Study/Study of Latinos. Arch Clin Neuropsychol 30, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.