Objective:
To study autonomic responses to postural changes in concussed adolescents. The influence of sex was also studied.
Design:
Longitudinal cohort observational study.
Participants:
Concussed adolescents (CONC; n = 65; 26 male adolescents; age 15 ± 1 years, range = 12-18 years) and a control (CTRL) group of nonconcussed adolescents of similar age and sport (CTRL; n = 54; 29 male adolescents; age 14 ± 1 years, range = 12-18 years).
Interventions:
Concussed participants were monitored through 6 weekly visits throughout usual physician care. Control participants underwent 2 visits separated by at least 1 week to account for intrapersonal variation in testing measures.
Main Outcome Measures:
Heart rate variability as the root mean square of successive differences in R–R intervals (RMSSD), heart rate (HR), and blood pressure [mean arterial pressure (MAP) and diastolic blood pressure (DBP)] were measured in supine, sitting, and standing postures.
Results:
A mixed analysis of variance revealed a group × sex × posture interaction (P = 0.04) where seated values of RMSSD were less in concussed female participants versus control female participants (42 ± 4 vs 61 ± 7 ms; P = 0.01; Mann–Whitney rank test). Compared with CTRL, CONC exhibited increased pretesting seated DBP (69 ± 1 vs 74 ± 1 mm Hg; P < 0.01), MAP (83 ± 1 vs 86 ± 1 mm Hg; P = 0.02), and baseline seated HR (72 ± 1 vs 77 ± 2 bpm; P = 0.03). Values of DBP (P = 0.03) and MAP (P < 0.01) improved at clinical discharge, whereas the RMSSD in female participants did not (P > 0.5). Data are mean ± SEM.
Conclusions:
A modest reduction in female cardiac autonomic regulation was observed during seated postures. Alterations in seated concussed DBP and MAP, but not RMSSD, resolved at clinical discharge (median = 37 days). The results indicate that, in adolescents, concussion may impair cardiovagal function in a sex- and posture-dependent manner. The findings also suggest that BP metrics, but not RMSSD, are associated with clinical concussion recovery.
Key Words: adolescent concussion, heart rate variability, blood pressure, sex differences, autonomic nervous system, sport-related concussion
INTRODUCTION
Concussion is the leading form of traumatic brain injury (TBI), with an estimated 1.2 to 3.6 million cases annually in the United States.1,2 The adolescent population is especially at risk of concussion, accounting for 3% to 8% of all sport-related emergency department visits.3,4 Although still in stages of frontal lobe and synaptic development,5 adolescents experience prolonged symptom profiles6 and functional irregularities,7,8 suggesting the adolescent brain is more susceptible to prolonged neurocognitive deficits than the fully developed adult brain.9–11
The ability to detect and project concussion recovery represents significant clinical challenges. For example, individuals who return to play within the first few days of a concussion have an increased risk of a secondary concussion.12 Whenever multiple concussive events occur, there is potential for cumulative neurophysiological effects.13,14 Despite these risks, subjective reporting forms the basis of many concussion symptoms. In this case, confidence in clinical decisions regarding return to play would be enhanced by an objective neurological measurement.
One method used to measure neurological outcomes from TBI is heart rate variability (HRV).15 Heart rate variability metrics examine beat-to-beat fluctuations in HR in the time and frequency domains16 and reflect autonomic neural contributions to HR regulation.17–19 Moderate and severe acute brain injuries reduce HRV.20–23 Over time, HRV measures can improve in these patients,15 demonstrating the recoverable nature of autonomic regulation, which could provide a feasible approach to track neurological resolution throughout clinical recovery.
In mild forms of TBI such as concussion, baseline HRV research has yielded mostly equivocal results; some studies indicate no effect,21,24,25 while others indicate a reduction in HRV after a concussion.22,26 One determinant of the varying conclusions may be the arousal state of the individual when the measures are made, where abnormalities have been reported when stressors of aerobic exercise21 and moderate-intensity isometric hand grips24 are used in adult cohorts. High-frequency power was decreased in the concussed group when a physiological stress was applied, while no difference occurred at rest.24 This suggests that a physiological stressor may be crucial to tease apart autonomic outcomes in concussion. Furthermore, no studies have reported HRV abnormalities in the developmental adolescent population. Sex can affect HRV outcomes,27,28 which may provide another explanation for the equivocal results in previous reports of HRV in mixed-sex concussion studies. In addition, concussed female participants are reported to have an increased risk of physiological impairment,29–32 as well as prolonged symptom profiles, such as continued anxiety and depression.33
An orthostatic challenge induces an autonomic reaction that is especially applicable to daily life. Normally, autonomic control of HR plays a critical role in rapid blood pressure (BP) control during postural stress.34 In adolescents with postconcussion syndrome, 70% experienced abnormal responses to a head-up-tilt test.35 These adolescents were categorized as isolated syncope or having postural tachycardia syndrome, suggesting impaired cardiovascular autonomic function. In adults who had experienced a concussion, a graded orthostatic challenge showed an increase in the low-frequency domain and a decrease in the high-frequency domain of HRV compared with controls, which did not return to baseline levels following return-to-play guidelines.36 This suggests incomplete recovery of autonomic function despite resolution of clinical measures. In concussed adolescents, cardiovascular-specific autonomic measurements to postural stress have not been reported.
Our study tested the primary hypothesis that concussion in adolescents impairs autonomic regulation. Additional analysis of a sex effect was conducted as well. This study characterized the impact of supine, seated, and upright postures on HRV and BP patterns in male and female cohorts of concussed and nonconcussed adolescents.
METHODS AND MATERIALS
Participants
Participant characteristics are outlined in Table 1. The concussed adolescents were diagnosed by a physician from the Fowler Kennedy Sport Medicine Clinic (FKSMC) and participated in a variety of sports (soccer n = 14, hockey n = 12, rugby n = 8, basketball n = 6, football n = 4, and other n = 21) at a recreational and competitive level. After diagnosis, eligible patients were interviewed for involvement in this study by the research coordinator. The control population consisted of age- and activity-level matched healthy adolescents and was recruited from local adolescent sporting organizations. Nineteen participants (control, n = 7; concussed, n = 12) were medicated for asthma and exercise-induced asthma (salbutamol, n = 9), anxiety (sertraline HCL, n = 3; lorazepam, n = 1), depression (escitalopram, n = 1), migraines (amitriptyline, n = 1; gabapentin, n = 1), acne (minocycline, n = 2), and attention-deficit hyperactivity disorder (methylphenidate, n = 1; atomoxetine, n = 1). Twelve female participants (control, n = 3; concussed, n = 9) were taking oral contraceptives. Control participants who had a previously diagnosed concussion were only included if they had not experienced symptoms in the 6-month period before testing (n = 16). All participants provided written informed consent for the study procedures. This study was approved by the Health Sciences Research Ethics Board at Western University and performed to the standards of the Declaration of Helsinki.
TABLE 1.
| Participant Characteristics | CTRL | CONC |
| n, males/females | 54, 29/25 | 65, 26/39 |
| Age (yrs) | 14 ± 1 | 15 ± 1 |
| Height (cm) | 169 ± 1 | 171 ± 1 |
| Weight (kg) | 64 ± 2 | 64 ± 2 |
| BMI (kg/m2) | 22 ± 1 | 22 ± 1 |
| Previous concussions | 0.4 ± 0.1 | 0.8 ± 0.1* |
| Days after concussion | N/A | 15 ± 2 |
| MAP (mm Hg) | 83 ± 1 | 86 ± 1* |
| SBP (mm Hg) | 113 ± 1 | 114 ± 2 |
| DBP (mm Hg) | 69 ± 1 | 74 ± 1* |
| GAD-7 score | 3.9 ± 0.4 | 6.1 ± 0.6* |
| SCAT3 symptom score | 5 ± 0.6 | 11 ± 0.8* |
| SCAT3 severity score | 8 ± 1 | 28 ± 3* |
Baseline participant characteristics. Values are reported as mean ± SEM.
Significantly different from CTRL P < 0.05.
BMI, body mass index; CONC, concussed; CTRL, control; DBP, diastolic blood pressure; MAP, mean arterial pressure; SBP, systolic blood pressure; SCAT3, Sport concussion assessment tool 3.
Experimental Protocols and Measurements
To establish the reproducibility of measures, control participants participated in 2 laboratory sessions separated by at least 1 week. To study the effect of concussion and recovery, concussed adolescents completed 6 weekly laboratory sessions, or until granted medical clearance by a physician at FKSMC (2-6 weeks), upon which time one final testing session was completed. The final testing session marked the clinical endpoint for each participant. The laboratory testing protocols remained consistent each week, for the entire length of the study.
At the beginning of the initial testing, participants provided a detailed medical history. Concussed participants provided their symptomology and etiology of the inciting event. Participants with a previous history of concussion provided specific details on previous symptomology and length of recovery from concussion. At the beginning of each visit, a medical update form, including changes to medications, was completed. However, no participant was prescribed medication for their concussion during the study. For the concussed adolescents, clinical progression and rehabilitation details were documented each visit. The Sport Concussion Assessment Tool (SCAT3) was completed by all participants to outline their symptom profiles at the time of testing. The Child SCAT3 was completed for participants aged 12 years (control, n = 7; concussed, n = 5).37 Clinical discharge was achieved with a combination of return to baseline SCAT3 score and/or physician appraisal. The Generalized Anxiety Disorder 7-Item Scale (GAD-7) assessed general anxiety at the beginning of each testing session. Anthropometric data were recorded, and seated manual sphygmomanometer BPs were measured in triplicate.
Cardiac R–R interval data were measured with 3-lead electrocardiography (ECG; Bioamp, Powerlab, AD Instruments, Colorado Springs, CO). Continuous arterial BP measures were made by finger photoplethysmography (Finometer; Finapres Medical Systems BV, Amersterdam, the Netherlands). A respiratory strain gauge provided breathing frequency information.
Measures were made for 5 minutes while supine, 3 minutes while seated, and then an unassisted sit-to-stand protocol that included 2 sets of seated (3 minutes) and standing (2 minutes) positions.
Short-Term Heart Rate Variability
Most measures of HRV require a minimum recording length of 3 to 5 minutes for accurate and reliable clinical measurements.16 However, the root mean square of successive differences in R–R interval (RMSSD) provides reliable measures in recordings of 10 to 60 seconds,38 or even less.39–41 Therefore, the RMSSD method enabled the study of HRV during the steady-state time durations of sitting and standing in our protocol.
Data Analysis
Mean arterial pressure (MAP) was calculated from manual recordings of systolic BP (SBP) and diastolic BP (DBP) using the formula: . Measures of HR and RMSSD were collected for the entire 5-minute supine baseline state. In the sit-to-stand task, recordings were taken in the middle minute of sitting and the last minute of steady-state standing.
Statistical Analysis
Data are presented as mean ± SEM, unless otherwise indicated. SigmaPlot 12.5 and SPSS Statistics 23 were used for statistical analysis. Participant characteristics were compared between the 2 groups using an independent t test. Supine measures of hemodynamics and respiration between control and concussed adolescents were evaluated through independent t tests. Linear regressions (r2 = 0.75, P < 0.001) and a Bland–Altman (Limits of Agreement = −55.7, 34.4 ms) test established the repeatability of the sit-to-stand tasks between the 2 sessions performed by the control group. A mixed-model repeated-measures analysis of variance assessed the effects of group, sex, and posture on RMSSD and BP variables. The effect of recovery on BP and HRV in the concussion group was assessed by paired t tests between the final laboratory sessions at clinical discharge. Statistical probability was set at P < 0.05 for all analyses without any modification for multiple statistical tests.
RESULTS
Participant Characteristic and Baseline Measures
Table 1 displays demographics, anthropometrics, and baseline hemodynamics for participant groups. Control and concussed groups were similar in all anthropometric measures. Compared with controls, DBP (P < 0.01) and MAP (P = 0.02) were greater in concussed adolescents. Also, GAD-7 (P < 0.01), SCAT3 symptom score (P < 0.01), and SCAT3 severity score (P < 0.01) were greater in the concussed versus the control group.
Pretesting seated SBP (115 ± 1 vs 112 ± 2 mm Hg, P = 0.31), DBP (71 ± 1 vs 72 ± 1 mm Hg; P > 0.5), and MAP (85 ± 1 vs 85 ± 1 mm Hg; P > 0.5) were not different between male participants and female participants. At the initial visit, no sex differences in GAD-7 scores were observed (4.5 ± 0.6 vs 5.7 ± 0.5; P = 0.11).
Compared with the first study visit, pretesting seated BP (DBP and MAP) improved (DBP, P = 0.02; MAP, P = 0.009) at the time of clinical discharge (Figure 1). Also, compared with the first visit, GAD-7 scores were reduced at the time of clinical discharge (6.1 ± 0.6 vs 2.7 ± 0.4; P < 0.01).
Figure 1.
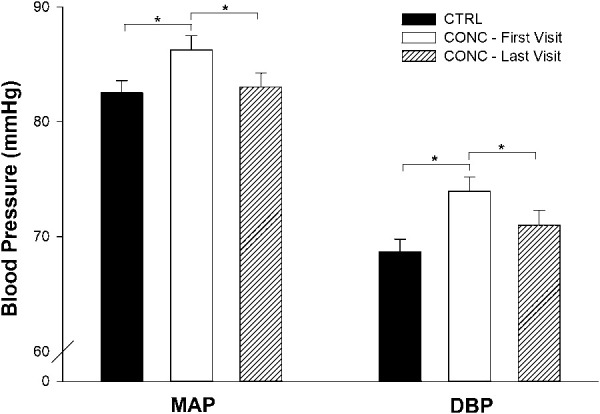
Temporal resolution of pretesting BP. Values are reported as mean ± SEM. CTRL, control; CONC, concussed. *There were significant increases in MAP (P = 0.009) and DBP (P = 0.02) in CONC—first visit. By the time of clinical discharge, BPs decreased to the levels of the control population.
Heart Rate and Heart Rate Variability Measures
Compared with controls, HR was greater in the concussed group (main effect of group; P = 0.02) but no group × sex (P = 0.23) or group × posture × sex (P > 0.5) interactions were observed. Compared with controls, HR in concussed adolescents was increased in each posture (Figure 2; supine, P = 0.03; seated, P = 0.006; standing, P = 0.009).
Figure 2.
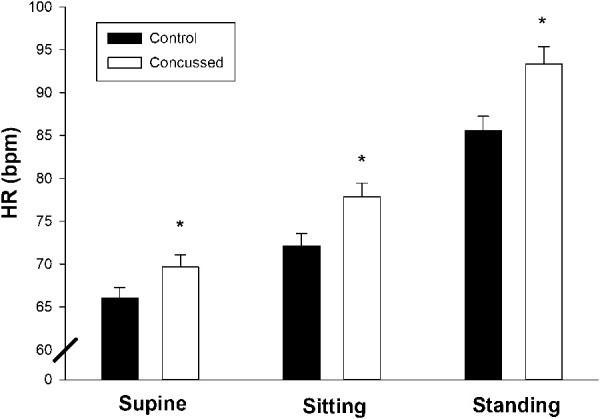
Effect of postural change on heart rate (HR) in adolescent concussion at the initial visit. Values are reported as mean ± SEM. *HR was increased in the concussed group in all postures (supine, P = 0.03; seated, P = 0.006; standing, P = 0.009).
Figure 3 depicts the RMSSD in the control and the concussed groups throughout various degrees of postural change. A group × sex × posture interaction (P = 0.04) was observed for RMSSD. Based on previous findings regarding sex28 and physiological stressors inducing autonomic differences in concussion,21,24 a specific contrast was performed of the seated posture in female participants between the concussed and control groups: These data were not normally distributed, so a Mann–Whitney rank sum test was executed. This approach exposed a smaller RMSSD value in the concussed female cohort during the seated posture when compared with controls (42 ± 4 vs 61 ± 7 ms; P = 0.01). SCAT severity score was correlated with seated RMSSD (Figure 4, r2 = 0.07, P = 0.005). Regardless of concussion status, age was not correlated with RMSSD (P = 0.15).
Figure 3.
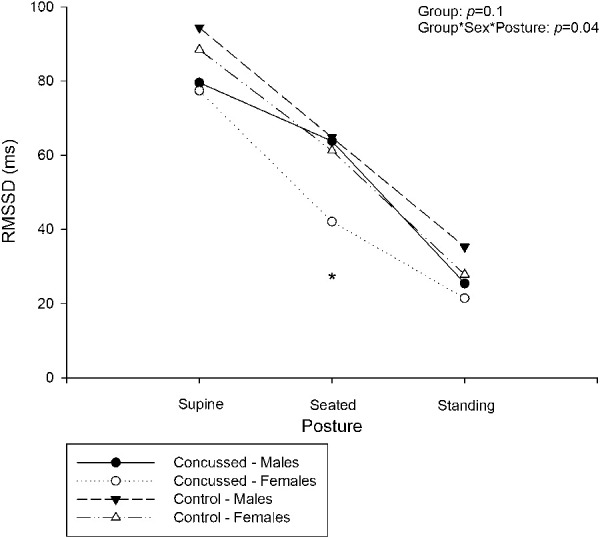
Effect of postural change on RMSSD in adolescent concussion, separated by sex. Values are reported as mean. *Significantly different from control < 0.05. There was no group interaction between concussed and controls (P = 0.1). A group × sex × posture interaction was identified (P = 0.04). A measure of simple effects revealed a reduction of RMSSD in the female seated posture, compared with controls.
Figure 4.
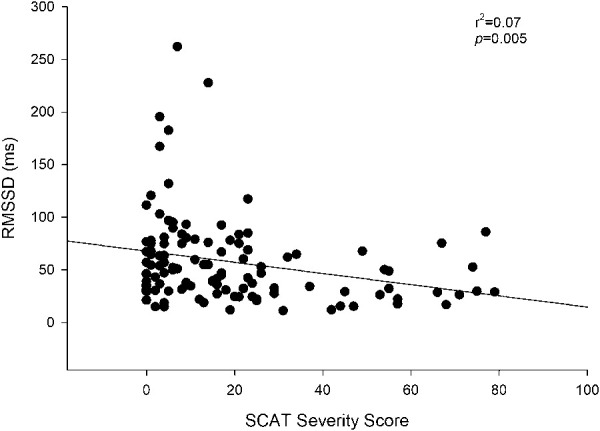
SCAT severity score and RMSSD values of concussed and control adolescents at the initial visit. SCAT, Sport Concussion Assessment Tool 3. SCAT severity score was weakly correlated (r2 = 0.07; P < 0.05) with RMSSD.
At discharge (median = 37 days), concussed female seated RMSSD did not increase (Figure 5, P > 0.5) compared with their first visit. In addition, the elevated seated HR levels in concussion were not decreased at the time of clinical discharge (Figure 6, P > 0.5).
Figure 5.
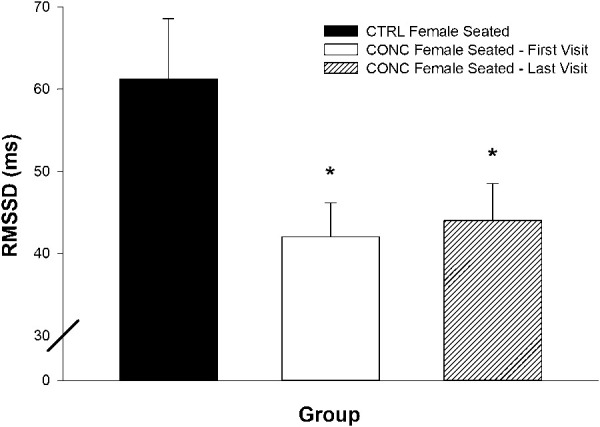
Female seated RMSSD at clinical endpoints of concussion. Values are reported as mean ± SEM. CTRL, control; CONC, concussed; *RMSSD was decreased in CONC, first visit (P = 0.01), and did not increase by the time of clinical discharge.
Figure 6.
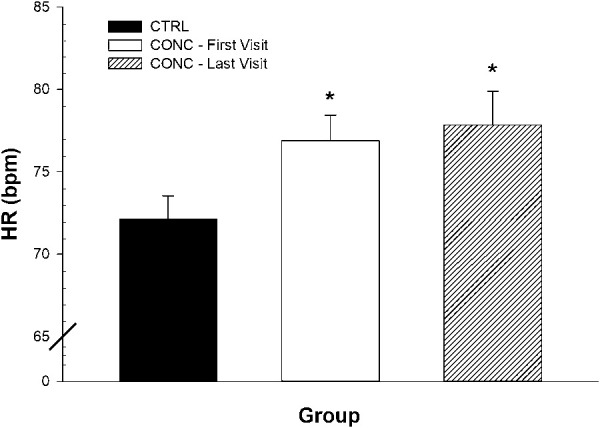
Seated HR at clinical endpoints of concussion. Values are reported as mean ± SEM. CTRL, control; CONC, concussed. *HR was increased in CONC, first visit (P = 0.006), and did decrease by the time of clinical discharge.
DISCUSSION
The novel findings of the current investigation are (1) concussed female adolescents exhibited decreased RMSSD versus controls in the seated position but not in the supine or upright postures, (2) seated DBP and MAP were increased in concussed adolescents when compared with control counterparts at the time of first visit (∼15 days after day of injury); and (3) seated DBP and MAP were similar to the control group at the time of clinical discharge (median = 37 days after injury). The results support the hypothesis that concussion impairs autonomic function in adolescents. However, the impact of concussion on HRV was only evident in female participants in the seated position, suggesting a sex difference in susceptibility toward autonomic impairment after concussion.
Heart Rate Variability in Adolescent Concussion
Early work in the examination of autonomic function and acute severe brain injury demonstrated that HRV declines after injury.21–23,42 Importantly, the level of autonomic nervous system impairment was proportional to the level of traumatic neurological insult patients had experienced.22 This impairment was also found in adolescents who had experienced a moderate to severe TBI.20 To the best of our knowledge, this is the first observation of dysregulated cardiovagal control in adolescent concussion. However, in our sample, the reduction in seated HRV was observed only in female participants. These results also extend an earlier study of a cohort of young adults that reported cardiac autonomic dysregulation after a concussion, with female participants providing a major influence on the overall outcome.36 Consistent with our findings, the cardiovagal impairment persisted beyond clinical discharge in this previous study sample.36 In this regard, available data suggest that impaired cardiovagal function after concussion largely affects female participants more than male participants and exhibits a slower trajectory of recovery than clinical symptoms.
In the context of concussion, a physiological challenge may be needed to uncover differences in autonomic regulation. For example, previous studies reporting an impact of concussion on neurocardiovascular control have examined HR, HRV, and BP during aerobic exercise,21 squat-stand paradigms,43 and isometric hand grip24 models. The current data indicated that the effect of concussion on cardiovagal function was exposed by the seated posture, a mild form of postural stress that induces a reduction in the dominance of parasympathetic cardiac control.44,45 Because RMSSD and high-frequency spectral domain metrics reflect short-term oscillations in HR, they typically are interpreted to reflect parasympathetic influences.19 Therefore, we interpret the current results as a sex-dependent decrease in cardiovagal drive, even in mild forms of TBI, such as concussion. Caution must be taken, however, because the capacity of time-domain analysis of HRV is limited during periods of increased stressors, where a “basement effect” can occur, possibly obscuring further reductions on moving from the seated to the upright position.
Blood Pressure Changes
In the current study, adolescent concussion increased pretesting seated DBP and MAP. The mechanisms of these changes in BP were not explored. However, they may include elevated sympathetic outflow in the presence of concussion that would correspond with impaired cardiovagal control. This conjecture receives some support from observations of paroxysmal sympathetic outcomes after moderate to severe TBI.46 However, direct sympathetic nerve recordings or measures of circulating catecholamines are needed to support the sympathetic nervous system activation inference.
Symptoms and Markers at Clinical Discharge
Previously, deficits in parasympathetic activity predicted recovery from traumatic events,47 while also showing a recoverable nature in longitudinal moderate and severe TBI studies in adults.15 Our results indicate that this deficit and recovery model cannot be applied to adolescent concussion as a whole, as RMSSD was only decreased in female participants undergoing a mild orthostatic stressor (Figure 3), and these did not recover during the 6-week follow-up period. Alternatively, DBP and MAP improved in the concussed groups by the time of clinical discharge (Figure 1). In the context of adolescent concussion, BP, but not RMSSD, seems to support clinical decisions regarding neurological impairment.
The reduction in female seated RMSSD was not linked with concussion recovery symptoms. In other words, even when clinical symptoms resolved in female participants, RMSSD was impaired. Therefore, RMSSD may reflect a persistent state of postconcussion syndrome. Of note, entry into this study was not at the point of injury, but later when they pursued treatment through the clinic. Current thought suggests 80% to 90% of concussion symptoms are remedied within the first 7 to 10 days.37,48 This 7- to 10-day figure aligns with neurological cascade profiles demonstrated in animal models.49 However, studies have shown that 29% of concussed adolescents present with postconcussion syndrome symptoms after a 3-month follow-up visit.7,8 The average time from concussion to clinical discharge in our sample was 44 days (median = 37 days) suggesting that these patients largely comprised the ∼20% who advance into a postconcussion syndrome phase.
There is some uncertainty regarding the root cause for diminished HRV and elevated BP in adolescent concussion. The concussed participants did exhibit increased anxiety over the control group, and there was a weak correlation between RMSSD and GAD-7 results (r2 = 0.06; P = 0.01). Therefore, anxiety might play a small role in the reduction of RMSSD in these patients. This association, however, cannot inform us about whether the reduced HRV reflected concussion-induced neurological damage or an indirect reaction to other emotional factors.
In addition to contributions of anxiety to higher BP in the concussed individuals, the consequences of detraining coincident with the reduced physical activity (generally prescribed following concussion) may include reduced HRV and increased baseline HR and BP. However, after 4 weeks, HRV and BP are not affected by detraining, while HR can be elevated.50–53 These findings may explain why BP in concussed adolescents return to levels observed in controls, while HR stayed elevated at the time of clinical discharge. Furthermore, the roles of neurological damage due to concussion, or physical inactivity in the recovery phase on HRV and BP, remain to be explored.
Sex Differences in Autonomic Outcomes
As mentioned, an important observation of the current study was the sex-based difference in HRV changes in concussion. Previous work suggests that female participants are at greater risk of impairments in neuropsychological factors,29 neurocognitive factors,30 symptom frequency,31 and vestibular/ocular factors,32 while others report no differences between the sexes after concussion.54 Also, female participants may exhibit a greater risk of prolonged symptoms, as well as negative psychological outcomes such as anxiety and depression.33 Our current study showed no difference in regard to changes in BP, days to medical discharge, or anxiety scores between the sexes. The current data add to the growing understanding that sex differences may form a critical concept in the presentation of concussion neurological outcomes—specifically those related to the autonomic nervous system.
Limitations
There are some limitations to this study. First, we did not assess pubertal stage of the participants. Adolescents are in a stage of life marked by heterogeneous developmental, physical, and psychological changes. The diverse range in age may play a factor in recordings of HR,55 HRV,56 and BP,57 as participants of all ages may be undergoing different stages of puberty and neurological development. However, we did not observe any relationship between participant age and RMSSD (P = 0.15).
Second, the sex-dependent differences in RMSSD may offer insight into the different ways adolescents are affected by concussion by further exploration into the effect of sex hormones58 and/or menstrual phase,59,60 which may have an effect on HRV. The use of oral contraceptives was larger in the concussed versus control group (23% vs 12%). However, oral contraceptive use does not alter HRV throughout the menstrual cycle.61
Third, the RMSSD metric was selected among several indices of HRV for the reasons established above. However, the baseline HR and BP data suggest a broader effect of autonomic impairment may be occurring that incorporates sympathetic and parasympathetic outcomes. Additional studies are required to discover these complex interactions.
CONCLUSIONS
The current study provides insight into autonomic regulation after a concussion in the developing adolescent brain. In the adolescents studied in this report, concussion was associated with elevations in BP (DBP and MAP) in male adolescents and female adolescents, as well as cardiac autonomic dysregulation in female adolescents, which manifested as reduced seated HRV (RMSSD). The restoration of BP aligned with resolution of clinical symptoms, whereas cardiovagal function persisted beyond clinical symptom resolution. Therefore, although the current data support the hypothesis that adolescent concussion impairs autonomic function, the persistent reduction in seated RMSSD beyond clinical discharge, indicates that cardiovagal impairment, for those individuals in whom it is affected by concussion, remains a subtle marker of continued, subclinical, neurological damage.
ACKNOWLEDGMENTS
The authors acknowledge Dave Humphreys and the Fowler Kennedy Sports Medicine Clinic for participant recruitment, as well as the participants and their families for their contribution and continuing interest in concussion research.
Footnotes
This research was funded by the Children's Health Foundation, London, ON, Canada.
The authors report no conflicts of interest.
References
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury a brief overview. J Head Trauma Rehabil. 2006;21:375–378. [DOI] [PubMed] [Google Scholar]
- 2.Choe MC, Babikian T, DiFiori J, et al. A pediatric perspective on concussion pathophysiology. Curr Opin Pediatr. 2012;24:689–695. [DOI] [PubMed] [Google Scholar]
- 3.Kelly KD, Lissel HL, Rowe BH, et al. Sport and recreation-related head injuries treated in the emergency department. Clin J Sport Med. 2001;11:77–81. [DOI] [PubMed] [Google Scholar]
- 4.Boyce SH, Quigley MA. An audit of sports injuries in children attending an accident & emergency department. Scott Med J. 2003;48:88–90. [DOI] [PubMed] [Google Scholar]
- 5.Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry. 2006;47:296–312. [DOI] [PubMed] [Google Scholar]
- 6.Eisenberg MA, Meehan WP, Mannix R. Duration and course of post-concussive symptoms. Pediatrics. 2014;133:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babcock L, Byczkowski T, Wade SL, et al. Predicting postconcussion syndrome after mild traumatic brain injury in children and adolescents who present to the emergency department. JAMA Pediatr. 2013;167:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barlow KM, Crawford S, Stevenson A, et al. Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics. 2010;126:e374–81. [DOI] [PubMed] [Google Scholar]
- 9.Field M, Collins MW, Lovell MR, et al. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatr. 2003;142:546–553. [DOI] [PubMed] [Google Scholar]
- 10.Guskiewicz KM, Valovich McLeod TC. Pediatric sports-related concussion. PM R. 2011;3:353–364. [DOI] [PubMed] [Google Scholar]
- 11.Levin HS, Eisenberg HM, Wigg NR, et al. Memory and intellectual ability after head injury in children and adolescents. Neurosurgery. 1982;11:668–673. [DOI] [PubMed] [Google Scholar]
- 12.Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football Players: the NCAA Concussion Study. J Am Med Assoc. 2003;290:2549–2555. [DOI] [PubMed] [Google Scholar]
- 13.Iverson GL, Gaetz M, Lovell MR, et al. Cumulative effects of concussion in amateur athletes. Brain Inj. 2004;18:433–443. [DOI] [PubMed] [Google Scholar]
- 14.Collins MW, Lovell MR, Iverson GL, et al. Cumulative effects of concussion in high school athletes. Neurosurgery. 2002;51:1175–1181. [DOI] [PubMed] [Google Scholar]
- 15.Keren O, Yupatov S, Radai MM, et al. Heart rate variability (HRV) of patients with traumatic brain injury (TBI) during the post-insult sub-acute period. Brain Inj. 2005;19:605–611. [DOI] [PubMed] [Google Scholar]
- 16.Task Force. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society for Pacing and Electrophysiology. Eur Hear J. 1996;17:354–381. [PubMed] [Google Scholar]
- 17.Saboul D, Pialoux V, Hautier C. The breathing effect of the LF/HF ratio in the heart rate variability measurements of athletes. Eur J Sport Sci. 2014;14:S282–S288. [DOI] [PubMed] [Google Scholar]
- 18.Goldberger JJ, Challapalli S, Tung R, et al. Relationship of heart rate variability to parasympathetic effect. Circulation. 2001;103:1977–1983. [DOI] [PubMed] [Google Scholar]
- 19.Katona PG, Jih F. Respiratory sinus arrhythmia: noninvasive measure of parasympathetic cardiac control. J Appl Physiol. 1975;39:801–805. [DOI] [PubMed] [Google Scholar]
- 20.Biswas AK, Scott WA, Sommerauer JF, et al. Heart rate variability after acute traumatic brain injury in children. Crit Care Med. 2000;28:3907–3912. [DOI] [PubMed] [Google Scholar]
- 21.Gall B, Parkhouse W, Goodman D. Heart rate variability of recently concussed athletes at rest and exercise. Med Sci Sports Exerc. 2004;36:1269–1274. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein B, Toweill D, Lai S, et al. Uncoupling of the autonomic and cardiovascular systems in acute brain injury. Am J Physiol. 1998;275:R1287–R1292. [DOI] [PubMed] [Google Scholar]
- 23.Goodman B, Vargas B, Dodick D. Autonomic nervous system dysfunction in concussion. Neurology. 2013;80:P01–265. [Google Scholar]
- 24.Abaji JP, Curnier D, Moore RD, et al. Persisting effects of concussion on heart rate variability during physical exertion. J Neurotrauma. 2015;33:811–817. [DOI] [PubMed] [Google Scholar]
- 25.La Fountaine MF, Heffernan KS, Gossett JD, et al. Transient suppression of heart rate complexity in concussed athletes. Auton Neurosci. 2009;148:101–103. [DOI] [PubMed] [Google Scholar]
- 26.Hilz MJ, Defina PA, Anders S, et al. Frequency analysis unveils cardiac autonomic dysfunction after mild traumatic brain injury. J Neurotrauma. 2011;1738:1727–1738. [DOI] [PubMed] [Google Scholar]
- 27.Stein PK, Kleiger RE, Rottman JN. Differing effects of age on heart rate variability in men and women. Am J Cardiol. 1997;80:302–305. [DOI] [PubMed] [Google Scholar]
- 28.Faulkner MS, Hathaway D, Tolley B. Cardiovascular autonomic function in healthy adolescents. Heart Lung. 2003;32:10–22. [DOI] [PubMed] [Google Scholar]
- 29.Covassin T, Schatz P, Swanik CB. Sex differences in neuropsychological function and post-concussion symptoms of concussed collegiate athletes. Neurosurgery. 2007;61:345–350. [DOI] [PubMed] [Google Scholar]
- 30.Broshek DK, Kaushik T, Freeman JR, et al. Sex differences in outcome following sports-related concussion. J Neurosurg. 2005;102:856–863. [DOI] [PubMed] [Google Scholar]
- 31.Ono KE, Burns TG, Bearden DJ, et al. Sex-based differences as a predictor of recovery trajectories in young athletes after a sports-related concussion. Am J Sports Med. 2015;44:748–752. [DOI] [PubMed] [Google Scholar]
- 32.Sufrinko AM, Mucha A, Covassin T, et al. Sex differences in vestibular/ocular and neurocognitive outcomes after sport-related concussion. Clin J Sport Med. 2017;27:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farace E, Alves WM. Do women fare worse: a metaanalysis of gender differences in traumatic brain injury outcome. J Neurosurg. 2000;93:539–545. [DOI] [PubMed] [Google Scholar]
- 34.Benarroch EE. The arterial baroreflex: functional organization and involvement in neurologic disease. Neurology. 2009;71:1733–1738. [DOI] [PubMed] [Google Scholar]
- 35.Heyer GL, Fischer A, Wilson J, et al. Orthostatic intolerance and autonomic dysfunction in youth with persistent postconcussion symptoms: a head-upright tilt table study. Clin J Sport Med. 2016;26:40–45. [DOI] [PubMed] [Google Scholar]
- 36.Hutchison MG, Mainwaring L, Senthinathan A, et al. Psychological and physiological markers of stress in concussed athletes across recovery milestones. J Head Trauma Rehabil. 2017;32:E38–E48. [DOI] [PubMed] [Google Scholar]
- 37.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurick, November 2012. Br J Sports Med. 2013;47:250–258. [DOI] [PubMed] [Google Scholar]
- 38.Nussinovitch U, Elishkevitz KP, Katz K, et al. Reliability of ultra-short ECG indices for heart rate variability. Ann Noninvasive Electrocardiol. 2011;16:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munoz ML, Van Roon A, Riese H, et al. Validity of (ultra-)short recordings for heart rate variability measurements. PLoS One. 2015;10:e0138921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNames J, Aboy M. Reliability and accuracy of heart rate variability metrics versus ECG segment duration. Med Biol Eng Comput. 2006;44:747–756. [DOI] [PubMed] [Google Scholar]
- 41.Thong T, Li K, McNames J, et al. Accuracy of ultra-short heart rate variability measures. Proc 25th Annu Int Conf IEEE Eng Med Biol Soc (IEEE Cat No03CH37439). 2003;3:2424–2427. doi: 10.1109/IEMBS.2003.1280405. [DOI]
- 42.Baguley IJ, Heriseanu RE, Felmingham KL, et al. Dysautonomia and heart rate variability following severe traumatic brain injury. Brain Inj. 2006;20:437–444. [DOI] [PubMed] [Google Scholar]
- 43.Bishop S, Dech R, Baker T, et al. Parasympathetic baroreflexes and heart rate variability during acute stage of sport concussion recovery. Brain Inj. 2017;31:247–259. [DOI] [PubMed] [Google Scholar]
- 44.Mullen TJ, Appel ML, Mukkamala R, et al. System identification of closed-loop cardiovascular control: effects of posture and autonomic blockade. Am J Physiol. 1997;272:H448–H461. [DOI] [PubMed] [Google Scholar]
- 45.Pomeranz B, Macaulay RJ, Caudill MA, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985;248:H151–H153. [DOI] [PubMed] [Google Scholar]
- 46.Perkes I, Baguley IJ, Nott MT, et al. A review of paroxysmal sympathetic hyperactivity after acquired brain injury. Ann Neurol. 2010;68:126–135. [DOI] [PubMed] [Google Scholar]
- 47.King DR, Ogilvie MP, Pereira BMT, et al. Heart rate variability as a triage tool in patients with trauma during prehospital helicopter transport. J Trauma. 2009;67:436–440. [DOI] [PubMed] [Google Scholar]
- 48.Bleiberg J, Cernich AN, Cameron K, et al. Duration of cognitive impairment after sports concussion. Neurosurgery. 2004;54:1073–1080. [DOI] [PubMed] [Google Scholar]
- 49.Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75:S24–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansen AL, Johnsen BH, Sollers JJ, et al. Heart rate variability and its relation to prefrontal cognitive function: the effects of training and detraining. Eur J Appl Physiol. 2004;93:263–272. [DOI] [PubMed] [Google Scholar]
- 51.Gamelin FX, Berthoin S, Sayah H, et al. Effect of training and detraining on heart rate variability in healthy young men. Int J Sports Med. 2007;28:564–570. [DOI] [PubMed] [Google Scholar]
- 52.Sugawara J, Murakami H, Maeda S, et al. Change in post-exercise vagal reactivation with exercise training and detraining in young men. Eur J Appl Physiol. 2001;85:259–263. [DOI] [PubMed] [Google Scholar]
- 53.Coyle EF, Hemmert MK, Coggan AR. Effects of detraining on cardiovascular responses to exercise: role of blood volume. J Appl Physiol. 1986;60:95–99. [DOI] [PubMed] [Google Scholar]
- 54.Brooks BL, Mrazik M, Barlow KM, et al. Absence of differences between male and female adolescents with prior sport concussion. J Head Trauma Rehabil. 2014;29:257–264. [DOI] [PubMed] [Google Scholar]
- 55.Silvetti MS, Drago F, Ragonese P. Heart rate variability in healthy children and adolescents is partially related to age and gender. Int J Cardiol. 2001;81:169–174. [DOI] [PubMed] [Google Scholar]
- 56.Pagani M, Lombardi F, Guzzetti S, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178–193. [DOI] [PubMed] [Google Scholar]
- 57.Kotchen JM, McKean HE, Kotchen TA. Blood pressure trends with aging. Hypertension. 1982;4(5 pt 2):III-128–III-134. [DOI] [PubMed] [Google Scholar]
- 58.Leicht AS, Hirning DA, Allen GD. Heart rate variability and endogenous sex hormones during the menstrual cycle in young women. Exp Physiol. 2003;88:441–446. [DOI] [PubMed] [Google Scholar]
- 59.Sato N, Miyake S, Akatsu J, et al. Power spectral analysis of heart rate variability in healthy young women during the normal menstrual cycle. Psychosom Med. 1995;57:331–335. [DOI] [PubMed] [Google Scholar]
- 60.Tenan MS, Brothers RM, Tweedell AJ, et al. Changes in resting heart rate variability across the menstrual cycle. Psychophysiology. 2014;51:996–1004. [DOI] [PubMed] [Google Scholar]
- 61.Teixeira AL, Ramos PS, Vianna LC, et al. Heart rate variability across the menstrual cycle in young women taking oral contraceptives. Psychophysiology. 2015;52:1451–1455. [DOI] [PubMed] [Google Scholar]


