Abstract
Background
Malnutrition and sarcopenia often occur simultaneously in cancer patients and are thought to have harmful effects on both surgical and oncological outcomes. Therefore, we want to evaluate the effects of sarcopenia and malnutrition on severe postoperative complications and overall survival in gynecologic cancer patients.
Methods
We assessed nutritional parameters and run a bioelectrical impedance analysis in 226 women. Extracellular mass to body cell mass index, phase angle alpha, muscle mass, and fat mass were evaluated. To determine if patients suffer from sarcopenia, we ran the Timed ‘Up and Go’ test, performed hand grip strength, and calculated a skeletal muscle index. Postoperative complications were categorized using Clavien–Dindo Classification. Utilizing ROC analysis and logistic regression, we determined predictive clinical factors for severe postoperative complications. Kaplan–Meier method and log‐rank test were used for overall survival analysis.
Results
Of the 226 female patients, 120 (53%) had a BMI ≥ 25 kg/m2, 56 (26%) had a phase angle < 4.75°, and 68 (32%) were sarcopenic according to skeletal muscle index < 27%. Within 30 days after surgery, 40 (18%) patients developed severe postoperative complications, and 4% had died. According to multivariable regression analysis, ECOG status > 1 (OR 4.56, 95% CI: 1.46–14.28, P = 0.009), BMI ≥ 25 kg/m2 (OR 8.22, 95% CI: 3.01–22.48, P < 0.001), phase angle < 4.75° (OR 3.95, 95% CI: 1.71–9.10, P = 0.001), and tumour stage ≥ III A (OR 3.65, 95% CI: 1.36–9.76, P = 0.01) were predictors of severe postoperative complications. During 59 months of follow‐up, 108 (48%) patients had died. According to multivariable Cox regression ECOG status > 1 (HR 2.51, 95% CI: 1.25–5.03, P = 0.01), hypoalbuminemia (HR 2.15, 95% CI: 1.28–3.59, P = 0.004), phase angle < 4.5° (HR 1.76, 95% CI 1.07–2.90, P = 0.03), tumour stage ≥ III A (HR 2.61, 95% CI: 1.53–4.45, P < 0.001), and severe postoperative complications (HR 2.82, 95% CI: 1.80–4.41, P < 0.001) were predictors of overall mortality.
Conclusions
We observed that preoperatively assessed ECOG status > 1, BMI > 25 kg, as well as phase angle alpha < 4.75° and FIGO stage ≥ III A are significantly associated with severe postoperative complications within the first month. Whereas ECOG status > 1, hypoalbuminemia, phase angle < 4.5° as well as FIGO stage ≥ III A and severe postoperative complications within 30 days correlate significantly with poor overall survival.
Keywords: Sarcopenia, Malnutrition, Postoperative complications, Gynecologic oncology, Overall survival
Introduction
Gynecologic cancer surgeries are often complex and include various multivisceral surgical procedures, which can be associated with life‐threatening complications. 1 Several studies have shown harmful effects of malnutrition and sarcopenia on both oncological and surgical outcomes, including higher rates of infections, longer hospitalization, and lower rates in progression‐free and overall survival. 2 , 3 , 4 , 5
Defined by the European working group on sarcopenia in older people (EWGSOP), sarcopenia is an acute or chronic muscle disease, and its key characteristic is impaired muscle strength accompanied by low muscle quantity and quality. 6 Whereas malnutrition is classified as a deficiency in energy and nutrient supply with adverse and measurable consequences. 7
Malnutrition and sarcopenia have analogies regarding their physiological mechanisms and often occur simultaneously, characterized by mutual features like changes in body composition and decreased functions. Moreover, sarcopenia can be caused by malnutrition. 8 The term ‘malnutrition‐sarcopenia syndrome’ was already proposed in research in order to integrate both conditions in one screening. 9 Both syndromes are associated with negative outcomes, involving decreased quality of life and functionality, increased hospitalization rates, morbidity, and mortality. 8 , 10 , 11 In cancer patients, undernourishment and weight loss occur due to many factors, including the tumour metabolism, the host response to the tumour, and anticancer treatments. 12 Malnutrition is proven to be a major problem in gynecologic cancer patients, particularly among those with ovarian cancer. 13
Former studies have shown that malnutrition and muscle attenuation are correlated with poor oncologic outcomes in gynecologic cancer patients. 14 , 15 , 16 , 17 , 18 , 19 Nevertheless, these studies were mostly performed retrospectively and have applied different definitions and measurements.
Therefore, we wanted to demonstrate the association of malnutrition and sarcopenia with severe postoperative complications as well as overall mortality in gynecologic cancer patients.
Methods
Patients, study design, and study measures
The data presented are part of the results of a prospective clinical cohort study (the RISC‐GYN trial by Inci et al.) on women undergoing gynecologic cancer surgery at a licensed gynecologic oncology centre of Charité – University Medicine of Berlin, Germany. From October 2015 to January 2017, we screened 237 women older than 18 years, who presented a gynecologic malignant tumour disease and were expected to undergo an elective surgery longer than 60 min. The Charité's Ethics Committee gave the ethical consent for this study with the approval ID EA2/122/15, and we obtained written consent from all women included.
The prospective screening included a detailed patient history, preoperative blood values, performance status (ECOG), quality of life parameters, and geriatric assessments. Intraoperatively information on the tumour dissemination and the operative procedure were noted. During ovarian cancer surgery, the Interoperative Mapping of Ovarian Cancer (IMO) was applied for precise tumour documentation. 20 Surgery was performed by qualified gynecologic oncology surgeons, and surgical complications were categorized using Clavien–Dindo Classification. 21 The Clavien–Dindo grading system ranks complications according to their required therapy, and it consists of seven grades. In addition, patients were subdivided into two groups, by severity of their postoperative complications [lower grade (Clavien–Dindo 0–III a) vs. higher grade complications (Clavien–Dindo III b–V)] to look for differences in patient characteristics in these cohorts. We only considered severe complications with grade III b or higher in statistical analyses.
Our assessment was performed 1 to 3 days preoperatively by medically trained staff and took about 90 min per patient. Each woman was visited daily after surgery, and postoperative complications were noted within the first 14 days. After discharge from the hospital, a follow‐up call was made after 3 months in order to record later occurring postoperative complications. Follow‐up for overall mortality was carried out until September 2020. In order to display the course of the study, we included a consort diagram (Figure 1).
Figure 1.
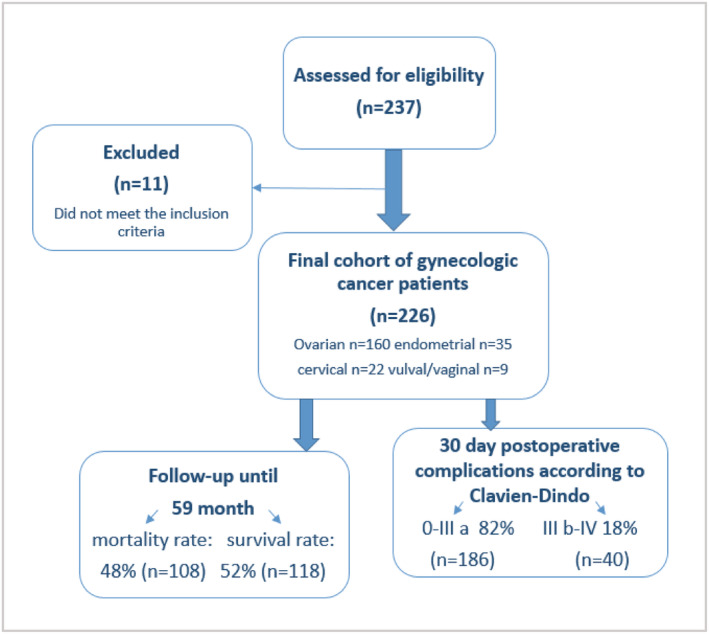
Study consort diagram.
Assessment of nutritional status
Preoperatively assessed nutritional parameters included albumin, BMI, and whether the patient had a weight loss ≥ 10% during the last 3 months. The Nutritional Risk Screening 2002 was used to identify women with a high risk of malnutrition. Additionally, we utilized parameters of bioelectrical impedance analysis such as phase angle alpha, extracellular mass to body cell mass index, fat mass, and muscle mass. Phase angle alpha displays the metabolic activity of the examined body, which is correlated with the training and nutritional state. A value of ≥ 5.0° is understood to be adequate for women. 22 The extracellular mass to body cell mass index is a parameter for assessing the nutritional state. In healthy, well‐nourished humans, the body cell mass is greater than extracellular mass, and the index is <1. 22 Bioelectrical impedance analysis was applied in a standardized procedure using BIACORPUS RX 4004M (Medical HealthCare GmbH, Karlsruhe Germany).
Assessment of sarcopenia
Sarcopenia was measured according to the European consensus definition of the EWGSOP as an analysis of strength, performance, and muscle mass. 6 To identify these parameters, we examined grip strength in both hands (SAEHAN Handdynamometer SH5001), ran the Timed ‘Up and Go’ test and calculated the skeletal muscle index. Because hand grip strength values are age‐related, we established age‐dependent cut‐offs and used values of the stronger hand. In order to obtain the skeletal muscle index, we calculated the amount of skeletal muscle mass (SMM) using the formula of Janssen et al. that includes a parameter from bioelectrical impedance analysis (BIA). 23
Height in centimetres; resistance in ohms; gender: women = 0; age in years
Ultimately, we calculated the skeletal muscle index: SMM/body mass × 100, where the patients' muscle mass was transformed to percentage muscle mass and adjusted for non‐skeletal muscle tissues, as originally introduced by Janssen et al. 24
Statistical analysis
This study was primary powered for postoperative complications. To achieve a high predictive value (ppW) with a high sensitivity, the proportion in the risk group had to correspond to the complication rate. For this study, a risk score that reaches a ppW of 80% was developed. The 95% confidence interval (95% CI) is between 70% and 88% in the target group with complications. The assumption was that the complication rate would be 40%, as in previous studies. The total number of cases, including 5% failure, should therefore be 237 patients.
To analyse comparisons between groups, we used the χ 2 test for normal variables, Fisher's exact test for dichotomous variables, Kendall's tau b for ordinal variables, and Kruskal–Wallis test or Mann–Whitney test for continuous variables. We evaluated the predictive accuracy of continuous variables by performing receiver‐operator characteristics (ROC) curve analyses to differentiate women with severe complications from those without and in order to define cut‐offs. Using logistic regression analysis, we obtained crude and adjusted odds ratios (ORs) with corresponding 95% CI. We performed a multivariable logistic regression analyses stepwise forward with P in = 0.05 and P out = 0.10. Kaplan–Meier method and log‐rank test were used for overall survival analysis. Applying multivariable Cox proportional hazards model, we obtained independent factors for overall survival, shown as hazard ratios (HRs) with corresponding 95% CI.
We excluded cases with missing data (<5%) from the multivariable analyses. IBM® SPSS® Statistics 25 (SPSS Inc. an IBM Company, Chicago, Illinois, USA) was utilized for statistical analysis, and statistical significance was obtained when P was <0.05.
Results
Initially, we enrolled 237 patients into our study. Subsequently, 11 patients were excluded, either due to a postoperatively histologically confirmed benign tumour entity or a surgery duration under 1 h, resulting in a final cohort of 226 gynecologic cancer patients. The study consort diagram is shown in Figure 1.
Patient demographics and clinical features
Fifty‐nine years was the median age of patients with a range of 18 to 87 years. Ovarian cancer was the most common entity, with 160 cases (71%); 146 (67%) patients had FIGO (International Federation of Gynaecology and Obstetrics) stage III A–IV A. Only 10 (4%) women received neoadjuvant chemotherapy. A severe risk of malnutrition was noted in 97 (43%) patients according to a Nutritional Risk Screening 2002 score ≥ 3. Twenty‐four (11%) women reported weight loss > 10% within the last 3 months. Twenty‐three (10%) had a BMI < 20 kg/m2. Hypoalbuminemia with <35.6 g/L was found in 26 (12%) patients and 56 (26%) had a phase angle < 4.75°. Sixty‐eight (32%) gynecologic cancer patients were sarcopenic according to a skeletal muscle index < 27%. In 47 (21%) cases, impaired hand grip strength was noted. Fifty‐five (25%) women presented a lower physical performance according to a Timed ‘Up and Go’ test > 9.5 s. Baseline characteristics, including nutritional and muscle status, are presented in Table 1 .
Table 1.
Baseline characteristics related to 30 day postoperative complications according to Clavien–Dindo
| Characteristics | Total n = 226 (range or %) | CDC 0‐III a n = 186 (range or %) | CDC III b‐V n = 40 (range or %) | P‐value |
|---|---|---|---|---|
| Age (years) | 59 a (18–87) | 59 a (18–86) | 63 a (31–87) | 0.26 |
| Age > 70 years | 58 (26) | 45 (24) | 13 (33) | 0.32 |
| Weight (kg) | 70 a (44–160) | 68 a | 81 a | <0.001 |
| ECOG PS > 1 | 19 (8) | 9 (5) | 10 (25) | <0.001 |
| Ascites | 72 (32) | 55 (31) | 17 (43) | 0.02 |
| Pleural effusion | 25 (11) | 18 (10) | 7 (18) | 0.08 |
| FIGO stage | 0.03 | |||
| FIGO stage I‐II B | 72 (33) | 65 (37) | 7 (18) | |
| FIGO III A‐IV A | 146 (67) | 113 (64) | 33 (83) | |
| Entities | 0.31 | |||
| Ovarian‐, fallopian tube‐, peritoneal cancer | 160 (71) | 126 (68) | 29 (73) | |
| Endometrial cancer | 35 (15) | 30 (16) | 5 (13) | |
| Cervical cancer | 22 (10) | 16 (9) | 6 (10) | |
| Vulval‐, vaginal cancer | 9 (4) | 9 (5) | 0 (0) | |
| Nutritional and Functional Parameters | ||||
| BMI (kg/m2) | 25 a (18–55) | 25 a (18–55) | 29 a (21–46) | <0.001 |
| Underweight (<20 kg/m2) | 23 (10) | 23 (12) | 0 (0) | |
| Normal (20–24.9 kg/m2) | 83 (37) | 77 (41) | 6 (15) | |
| Overweight (25 ≤ 30 kg/m2) | 60 (27) | 44 (24) | 16 (40) | |
| Obese (>30 kg/m2) | 60 (27) | 42 (23) | 18 (45) | |
| Phase angle alpha < 4.75 ° | 56 (26) | 38 (2) | 18 (49) | 0.002 |
| Extracellular mass to body cell mass index > 1.35 | 27 (13) | 16 (9) | 11 (31) | 0.001 |
| Nutritional Risk Screening 2002 ≥ 3 | 97 (43) | 76 (41) | 21 (53) | 0.22 |
| Weight loss > 10% last 3 months | 24 (11) | 20 (11) | 4 (10) | 1.00 |
| Albumin < 35.6 g/L | 26 (12) | 16 (9) | 10 (25) | 0.01 |
| Fat mass > 27.5 kg | 70 (33) | 47 (26) | 23 (62) | <0.001 |
| Muscle mass < 21.8 kg | 126 (58) | 113 (63) | 13 (35) | 0.003 |
| Skeletal muscle index < 27% | 68 (32) | 49 (27) | 19 (51) | 0.006 |
| Age‐dependent low hand grip strength | 47 (21) | 32 (17) | 15 (38) | 0.009 |
| Timed “Up and Go” test > 9.5 s | 55 (25) | 36 (20) | 19 (50) | <0.001 |
ASA, American Society of Anesthesiologists; BMI, body mass index; CDC, Clavien–Dindo classification; ECOG, Eastern Cooperative Oncology Group performance status; FIGO, International Federation of Gynecology and Obstetrics.
Numbers represent median values.
Forty (18%) women developed severe postoperative complications and 9 (4%) of these patients died within 30 days after surgery. Patients with severe postoperative complications had significantly lower preoperative albumin levels and a significantly higher median weight as well as a higher BMI than the cohort with low grade postoperative complications. Moreover, patients with severe postoperative complications had significantly lower phase angle values, a lower skeletal muscle index, a higher fat mass, and a higher extracellular mass to body cell mass index. Their ECOG score and American Society of Anaesthesiologists physical status (ASA PS) classification system score were considerably higher as well (Table 1 ).
Univariable analysis of nutritional and muscle status for postoperative complications
Hypoalbuminemia (≤35.6 g/L) was a predictive indicator for severe postoperative complications (OR 5.28, 95% CI: 1.85–15.08, P = 0.002). A phase angle < 4.75° was highly associated with severe postoperative complications (OR 3.52, 95% CI: 1.68–7.35, P = 0.001), and the extracellular mass to body cell mass index > 1.35 was significant as well (OR 4.46, 95% CI: 1.86–10.69, P = 0.001). Overweight (BMI > 25 kg/m2) was a predictive factor for severe complications (OR 6.59, 95% CI: 2.64–16.44, P < 0.001). Also, fat mass > 27.5 kg (OR 7.63, 95% CI: 2.72–21.44, P < 0.001) and muscle mass < 21.8 kg (OR 3.16, 95% CI: 1.51–6.63, P = 0.002) were both significant parameters in predicting severe postoperative complications. At a cut‐off of <27%, the skeletal muscle index was notably associated (OR 2.80, 95% CI: 1.36–5.77, P = 0.005) with severe postoperative complications. Low age‐dependent hand grip strength had an impact on postoperative complications (OR 2.85, 95% CI: 1.35–6.00, P = 0.006) and the Timed ‘Up and Go’ test > 9.5 s was closely associated with severe complications (OR 8.18, 95% CI: 2.58–25.93, P < 0.001) as well. The univariable analysis of nutritional and muscle status associated with severe postoperative complications is presented in Table 2 .
Table 2.
Analysis of preoperative factors related to severe postoperative complications according to Clavien–Dindo ≥ III b
| Variable | Univariable analysis | P‐value | Multivariable analysis a | P‐value |
|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |||
| Age ≥ 70 years | 1.54 (0.73–3.31) | 0.27 | ||
| ECOG PS > 1 | 10.81 (3.62–32.26) | <0.001 | 4.56 (1.46–14.28) | 0.009 |
| FIGO ≥ III A | 2.71 (1.14–6.48) | 0.03 | 3.65 (1.36–9.76) | 0.01 |
| BMI ≥ 25 kg/m2 | 6.59 (2.64–16.44) | <0.001 | 8.22 (3.01–22.48) | <0.001 |
| Phase angle alpha < 4.75° | 3.52 (1.68–7.35) | 0.001 | 3.95 (1.71–9.10) | 0.001 |
| Nutritional Risk Screening 2002 ≥ 3 | 1.60 (0.81–3.18) | 0.89 | ||
| Weight loss > 10% last 3 months | 0.92 (0.30–2.86) | 0.90 | ||
| Albumin < 35.6 g/L | 5.28 (1.85–15.08) | 0.002 | ||
| Extracellular mass to body cell mass index > 1.35 | 4.46 (1.86–10.69) | 0.001 | ||
| Fat mass > 27.5 kg | 7.63 (2.72–21.44) | <0.001 | ||
| Age‐dependent low hand grip strength | 2.85 (1.35–6.00) | 0.006 | ||
| Timed ‘Up and Go’ test > 9.5 s | 8.18 (2.58–25.93) | <0.001 | ||
| Muscle mass > 21.8 kg | 3.16 (1.51–6.63) | 0.002 | ||
| Skeletal muscle index < 27% | 2.80 (1.36–5.77) | 0.005 |
BMI, body mass index; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; FIGO, International Federation of Gynecology and Obstetrics; OR, odds ratio.
Multivariable logistic regression analyses stepwise forward.
Multivariable analysis of nutritional and muscle status for postoperative complications
In stepwise logistic regression including FIGO stage, BMI, ECOG status, ASA PS, Charlson comorbidity index, Nutritional Risk Screening 2002, weight loss, albumin, phase angle alpha, fat mass, muscle mass, skeletal muscle index, Timed ‘Up and Go’ test, and hand grip strength, we found BMI > 25 kg/m2 (OR 8.22, 95% CI: 3.01–22.48, P < 0.001) as well as ECOG > 1 (OR 4.56, 95% CI: 1.46–14.28, P = 0.009) as significant predictors of severe postoperative complications. Moreover, severe complications were predicted by phase angle < 4.75° (OR 3.95, 95% CI: 1.71–9.10, P = 0.001) and FIGO ≥ III A (OR 3.65, 95% CI: 1.36–9.76, P = 0.01). The significant results of multivariable analysis are presented in Table 2 . The conducted ROC analysis for significant nutritional parameters, including AUC (area under the curve), specific cut‐off, sensitivity, specificity, and P‐value, is shown in Figure 2.
Figure 2.
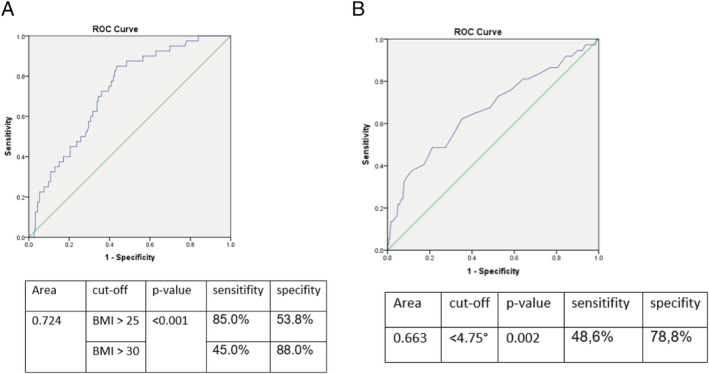
ROC‐analysis for (A) body mass index and (B) phase angle for predicting severe postoperative complications including AUC, specific cut‐offs, sensitivity, specificity, and P‐value.
Association between nutritional and muscle status and overall survival
Within 59 months, 108 (48%) patients died. The median observation period was 37.6 months (range: 0 to 59 months), and the median overall survival was 49.1 months.
In patients with albumin <35.5 g/L, the median overall survival was 9.7 months, whereas in women with higher albumin levels, the median survival was 53.5 months (P < 0.001). Patients with a phase angle smaller than 4.5° had a median overall survival of 9.7 month and those with a higher angle of 53.5 months (P < 0.001). Women with an extracellular mass to body cell mass index higher than 1.3 showed a significant decreased median survival of 18.6 months compared with 52.9 months in women with a smaller index (P < 0.001). Moreover, women with a Timed ‘Up and Go’ test slower than 11 s presented a reduced median survival of 20.5 months compared with 52.9 months, of those with faster gait speed (P = 0.02). Overweight and underweight measured by BMI were not statistically significant for poor survival (P = 0.98). The results of the overall survival analysis are shown in Table 3 . Unadjusted HRs with corresponding 95% CI are presented in Table 4 .
Table 3.
Preoperative factors with cut‐offs, obtained from log‐rank test, related to overall survival in months
| Variable | Median OS | P‐value |
|---|---|---|
| Age ≥ 65 years | 38.8 vs. 53.5 | 0.08 |
| Nutritional Risk Screening 2002 ≥ 3 | 36.4 vs. 53.5 | 0.07 |
| BMI ≥ 25–30 kg/m2 | 44.6 vs. 50.0 | 0.76 |
| Weight loss ≥ 10% last 3 months | 27.9 vs. 50.0 | 0.39 |
| Albumin < 35.5 g/L | 9.7 vs. 53.5 | <0.001 |
| Phase angle alpha < 4.5° | 9.7 vs. 53.5 | <0.001 |
| Extracellular mass to body cell mass index > 1.3 | 18.6 vs. 52.9 | <0.001 |
| Fat mass > 39 kg | 19.2 vs. 50.0 | 0.20 |
| Age‐dependent low hand grip strength | 37.9 vs. 52.3 | 0.20 |
| Timed ‘Up and Go’ test > 11 s | 20.5 vs. 52.9 | 0.02 |
| Muscle mass < 17 kg | 37.9 vs. 50.0 | 0.35 |
| Skeletal muscle index ≤ 24.5% | 23.6 vs. 52.3 | 0.12 |
BMI, body mass index; OS, overall survival.
Table 4.
Univariable and multivariable analysis of predictive factors related to overall survival
| Variable | Univariable | P‐value | Multivariable a | P‐value |
|---|---|---|---|---|
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |||
| Age ≥ 65 years | 1.40 (0.95–2.04) | 0.86 | ||
| Severe 30 day postoperative complications Clavien–Dindo ≥ III b | 3.78 (2.48–5.75) | <0.001 | 2.82 (1.80–4.41) | <0.001 |
| ECOG > 1 | 3.38 (1.78–6.41) | <0.001 | 2.51 (1.25–5.03) | 0.01 |
| FIGO stage ≥ III A | 3.39 (2.04–5.63) | <0.001 | 2.61 (1.53–4.45) | <0.001 |
| Albumin < 35.5 g/L | 2.80 (1.71–4.58) | <0.001 | 2.15 (1.28–3.59) | 0.004 |
| Phase angle alpha < 4.5° | 3.10 (1.96–4.90) | <0.001 | 1.76 (1.07–2.90) | 0.03 |
| Nutritional Risk Screening 2002 ≥ 3 | 1.42 (0.97–2.07) | 0.07 | ||
| BMI ≥ 25–30 kg/m2 | 1.11 (0.57–2.17) | 0.76 | ||
| BMI > 30 kg/m2 | 1.01 (0.51–2.01) | 0.97 | ||
| Weight loss ≥ 10% last 3 months | 1.30 (0.72–2.31) | 0.39 | ||
| Extracellular mass to body cell mass index > 1.3 | 2.71 (1.74–4.24) | <0.001 | ||
| Fat mass > 39 kg | 1.45 (0.82–2.54) | 0.20 | ||
| Low hand grip strength | 1.30 (0.87–1.93) | 0.20 | ||
| Timed ‘Up and Go’ test > 11 s | 1.92 (1.09–3.37) | 0.02 | ||
| Muscle mass < 17 kg | 1.36 (0.71–2.62) | 0.35 | ||
| Skeletal muscle index ≤ 24.5% | 1.45 (0.90–2.32) | 0.13 |
BMI, body mass index; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio.
Multivariable logistic regression analyses stepwise forward.
According to multivariable Cox regression ECOG status > 1 (HR 2.51, 95% CI: 1.25–5.03, P = 0.01), hypoalbuminemia (HR 2.15, 95% CI: 1.28–3.59, P = 0.004), phase angle < 4.5° (HR 1.76, 95% CI 1.07–2.90, P = 0.03) as well as FIGO stage ≥ III A (HR 2.61, 95% CI: 1.53–4.45, P < 0.001), and severe postoperative complications (HR 2.82, 95% CI: 1.80–4.41, p < 0.001) resulted as significant parameters for poor overall survival. Results of multivariable Cox regression are presented in Table 4 . The conducted Kaplan–Meier curves of significant factors for poor survival are shown in Figure 3.
Figure 3.
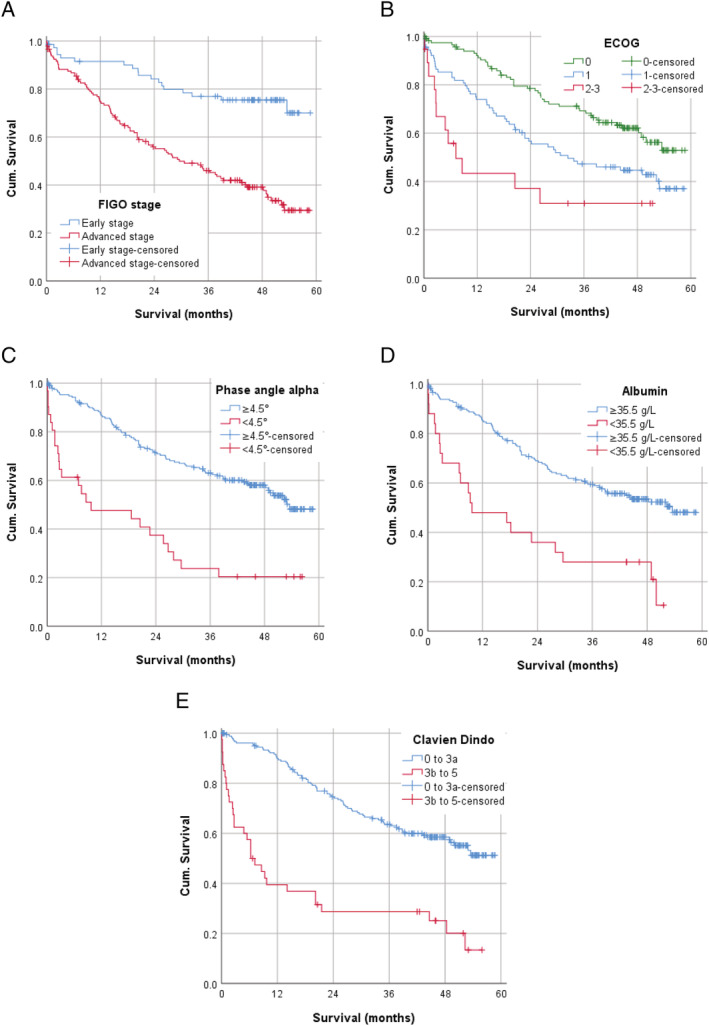
Kaplan–Meier curves for (A) FIGO stage ≥ III A versus I–II B, (B) for ECOG status, (C) for phase angle < 4.5° versus ≥4.5°, (D) for albumin < 35.5 g/L versus ≥35.5 g/L, and (E) for severe postoperative complications Clavien–Dindo ≥ III b versus Clavien–Dindo 0–III a.
Discussion
Our work is one of few prospective studies evaluating the impact of malnutrition and sarcopenia on severe postoperative complications and overall survival in gynecologic malignancies systematically and multidimensionally. We observed that preoperatively assessed ECOG status > 1, BMI > 25 kg, as well as phase angle alpha < 4.75° and FIGO stage ≥ III A are significantly associated with severe postoperative complications within the first month. Whereas ECOG status > 1, hypoalbuminemia, phase angle < 4.5° as well as FIGO stage ≥ III A and severe postoperative complications within 30 days correlate significantly with poor overall survival.
Referring the study on 154 women with gynecologic tumours, the chance of malnutrition in ovarian cancer patients is 19 times higher than in benign gynecologic tumour patients. 13 Forty‐three per cent of our patients were at a considerable risk of malnutrition, according to a Nutritional Risk Screening 2002 score of three and more. Diagnosing malnutrition with conventional criteria, such as a low BMI or weight loss, is insufficient and can be deceptive, as an accumulation of ascites increases the body weight, while body cell mass is truly decreasing. Consequently, a reasonable explanation why measures like weight loss > 10%, Nutritional Risk Screening 2002 ≥ 3, and BMI < 20 kg/m2 did not correlate with severe postoperative complications or poor overall survival in our study is that cachectic patients were only partially detected. Malnutrition is a complex and multifactorial syndrome, which can be assessed using several anthropometric measures and serum protein markers, including albumin. 25 Many published studies demonstrate the predictive value of hypoalbuminemia on surgical outcomes. 26 , 27 , 28 , 29 , 30 Moreover, surgical complications, such as septicaemia and disturbed wound healing, are associated with a preoperative serum albumin concentration ≤ 30 g/L in ovarian cancer patients. 28 According to our outcomes, hypoalbuminemia was not predictive for severe postoperative complications within 30 days but emerged as a significant factor for poor overall survival. This was also shown in large a meta‐analysis on 3884 ovarian cancer patients, where the authors determined hypoalbuminemia as an important predictor for reduced overall survival. 29
Furthermore, our results indicate that overweight and obesity are associated with severe postoperative complications. The median body weight as well as the median BMI of women with serious complications in our study are notably high (81 kg, 29 kg/m2). Obese ovarian cancer patients have a higher incidence of postoperative wound complications and longer hospitalization after surgery. 31 Corresponding to a retrospective analysis of more than 500 patients with ovarian cancer, patients with a BMI ≥ 40 kg/m2 have increased rates of postoperative complications, develop higher 90‐day postsurgical mortality, and have an ASA score ≥ 3 more frequently. 32 In uterine cancer, obesity is correlated with higher rates of surgical complications such as thrombophlebitis, infections, wound complications, and longer clinic stay. 33 , 34
Moreover, a phase angle smaller than 4.75° is prognostic for severe postoperative complications and an angle smaller than 4.5° is predictive for poor overall survival in our investigation. Phase angle alpha displays the metabolic activity of the examined body, which is correlated with the training and nutritional state. 22 Research on the prognostic capability of phase angle on oncological outcomes has been increasing lately. A broad review, that includes 27 research articles about the value of bioelectrical impedance analysis in cancer patients, indicates that the usage of BIA measures and phase angle alpha can be beneficial for tumour patients in prevention, prognosis, and outcomes. 35 The first prospective investigation, that included ovarian cancer patients, was performed in 2016 by our working group; here, an angle smaller than 4.5° was associated with poor overall survival as well. 36 Additionally, a prospective study in patients with advanced ovarian malignancy determined phase angle alpha as a prognostic forecaster for perioperative complications and residual tumour disease after cytoreductive surgery. 37 More studies are consistent with our results and introduce phase angle alpha as a predictor for adverse outcomes in various patient cohorts. 38 , 39 , 40 , 41
Previous studies determined sarcopenia as an indicator of poor outcomes, particularly of decreased overall survival, in surgical oncology 5 and gynecologic oncology. 18 , 19 , 42
According to our results, other factors, including ECOG performance status, were more important in predicting severe postoperative complications and poor overall survival than the measured sarcopenia parameters. The ECOG performance status refers to fitness and functioning in daily life of oncological patients and can be utilized as a complementary tool in assessing the patients' functional health status. 43 In a prospective study on gynecologic cancer patients, our study group could reveal that ECOG status can predict severe postoperative complications more precisely than Fried Frailty Score. 44
Our investigation has some limitations. The enrolled patients had different types of gynecologic cancers in varying stages of disease (FIGO I to FIGO IV A). More studies in the mentioned subgroups should be performed to confirm our results. Nevertheless, the inclusion of different tumour entities and stages make our outcomes suitable to a wider patient range. Furthermore, bioelectrical impedance analysis calculates the body composition indirectly with an equation, which is a possible source of error. However, phase angle alpha is a raw value and independent from calculations, making it a reliable and promising parameter. Strengths of our analysis include its prospective design and the exploration of multiple variables, in a preoperative assessment lasting around 90 min. Additionally, each woman was visited daily after surgery with systematical application of Clavien–Dindo criteria. Our study was the first to combine a multidimensional analysis of malnutrition with the analysis of sarcopenia, including bioelectrical impedance analysis, in a large cohort of gynecologic cancer patients.
Conclusion
The prevalence of malnutrition and sarcopenia in gynecologic cancer patients is significantly high. Besides FIGO stage and ECOG status, the preoperative nutritional status, including BMI and phase angle, is an important indicator for severe postoperative complications. Additional to these factors, hypoalbuminemia and severe postoperative complication are essential predictors of poor overall survival. These findings express the need to integrate a nutritional assessment, involving bioelectrical impedance analysis, into preoperative counselling to effectively determine high‐risk patients as a mandatory procedure. Physicians can intervene on that basis and improve patient outcomes by optimizing the nutritional as well as functional status prior to surgery.
Conflict of interest
Dr. Anker reports personal fees from Servier, outside the submitted work. All other authors declare no conflict of interest.
Acknowledgements
This study was funded by the ALLIMOGI Foundation. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 45
Sehouli J., Mueller K., Richter R., Anker M., Woopen H., Rasch J., Grabowski J. P., Prinz‐Theissing E., and Inci M. G. (2021) Effects of sarcopenia and malnutrition on morbidity and mortality in gynecologic cancer surgery: results of a prospective study, Journal of Cachexia, Sarcopenia and Muscle, 12, 393–402, doi: 10.1002/jcsm.12676.
These results have been presented partially at the 12th International Conference on Cachexia, Sarcopenia and Muscle Wasting in Berlin, Germany.
References
- 1. Romeo A, Gonzalez MI, Jaunarena J, Zubieta ME, Favre G, Tejerizo JC. Pelvic exenteration for gynecologic malignancies: postoperative complications and oncologic outcomes. Actas Urol Esp 2018;42:121–125. [DOI] [PubMed] [Google Scholar]
- 2. Su H, Ruan J, Chen T, Lin E, Shi L. CT‐assessed sarcopenia is a predictive factor for both long‐term and short‐term outcomes in gastrointestinal oncology patients: a systematic review and meta‐analysis. Cancer Imaging 2019;19:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Otten L, Stobäus N, Franz K, Genton L, Müller‐Werdan U, Wirth R, et al. Impact of sarcopenia on 1‐year mortality in older patients with cancer. Age Ageing 2019;48:413–418. [DOI] [PubMed] [Google Scholar]
- 4. Reisinger KW, van Vugt JLA, Tegels JJW, Snijders C, Hulsewé KW, Hoofwijk AG, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg 2015;261:345–352. [DOI] [PubMed] [Google Scholar]
- 5. Joglekar S, Nau PN, Mezhir JJ. The impact of sarcopenia on survival and complications in surgical oncology: a review of the current literature. J Surg Oncol 2015;112:503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sarcopenia: revised European consensus on definition and diagnosis. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6322506/. Accessed December 12, 2019. [DOI] [PMC free article] [PubMed]
- 7. Bauer JM, Volkert D, Wirth R, Vellas B, Thomas D, Kondrup J, et al. Diagnostik der Mangelernährung des älteren Menschen. DMW ‐Dtsch Med Wochenschr 2006;131:223–227. [DOI] [PubMed] [Google Scholar]
- 8. Lardiés‐Sánchez B, Sanz‐París A Sarcopenia and malnutrition in the elderly. Frailty Sarcopenia ‐ Onset Dev Clin Chall. August 2017. 10.5772/intechopen.68426 [DOI]
- 9. Vandewoude MFJ, Alish CJ, Sauer AC, Hegazi RA. Malnutrition‐sarcopenia syndrome: is this the future of nutrition screening and assessment for older adults?. In Cox C, ed. Clinical Nutrition and Aging. Apple Academic Press; 2016. 16. 10.1201/9781315364971-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saka B, Ozkaya H, Karisik E, Akin S, Akpinar TS, Tufan F. Malnutrition and sarcopenia are associated with increased mortality rate in nursing home residents: a prospective study. Eur Geriatr Med 2016;7:232–238. [Google Scholar]
- 11. Beaudart C, Zaaria M, Pasleau F, Reginster J‐Y, Bruyère O. Health outcomes of sarcopenia: a systematic review and meta‐analysis. PLoS ONE 2017;12:e0169548, 10.1371/journal.pone.0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. von Meyenfeldt M. Cancer‐associated malnutrition: an introduction. Eur J Oncol Nurs Off J Eur Oncol Nurs Soc 2005;9:S35–S38. [DOI] [PubMed] [Google Scholar]
- 13. Laky B, Janda M, Bauer J, Vavra C, Cleghorn G, Obermair A. Malnutrition among gynaecological cancer patients. Eur J Clin Nutr 2007;61:642–646. [DOI] [PubMed] [Google Scholar]
- 14. Kathiresan ASQ, Brookfield KF, Schuman SI, Lucci JA. Malnutrition as a predictor of poor postoperative outcomes in gynecologic cancer patients. Arch Gynecol Obstet 2011;284:445–451. [DOI] [PubMed] [Google Scholar]
- 15. Ubachs J, Ziemons J, Minis‐Rutten IJG, Kruitwagen RFPM, Kleijnen J, Lambrechts S. Sarcopenia and ovarian cancer survival: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle 2019;10:1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uppal S, Al‐Niaimi A, Rice LW, Rose SL, Kushner DM, Spencer RJ, et al. Preoperative hypoalbuminemia is an independent predictor of poor perioperative outcomes in women undergoing open surgery for gynecologic malignancies. Gynecol Oncol 2013;131:416–422. [DOI] [PubMed] [Google Scholar]
- 17. Kumar A, Torres ML, Cliby WA, Kalli KR, Bogani G, Aletti G, et al. Inflammatory and nutritional serum markers as predictors of peri‐operative morbidity and survival in ovarian cancer. Anticancer Res 2017;37:3673–3677. [DOI] [PubMed] [Google Scholar]
- 18. Aust S, Knogler T, Pils D, Obermayr E, Reinthaller A, Zahn L, et al. Skeletal muscle depletion and markers for cancer cachexia are strong prognostic factors in epithelial ovarian cancer. PLoS ONE 2015;10:e0140403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kumar A, Moynagh MR, Multinu F, Cliby WA, McGree ME, Weaver AL, et al. Muscle composition measured by CT scan is a measurable predictor of overall survival in advanced ovarian cancer. Gynecol Oncol 2016;142:311–316. [DOI] [PubMed] [Google Scholar]
- 20. Sehouli J, Könsgen D, Mustea A, Oskay‐Özcelik G, Katsares I, Weidemann H, et al. “IMO”—intraoperative mapping of ovarian cancer. Zentralbl Gynakol 2003;125:129–135. [DOI] [PubMed] [Google Scholar]
- 21. Dindo D, Demartines N, Clavien P‐A. Classification of surgical complications. Ann Surg 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stobäus N, Norman K, Pirlich M. Phasenwinkel und Bioelektrische Impedanzvektoranalyse – Klinische Anwendbarkeit der Impedanzparameter. Aktuelle Ernährungsmedizin 2010;35:124–130. [Google Scholar]
- 23. Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol 2000;89:465–471. [DOI] [PubMed] [Google Scholar]
- 24. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50:889–896. [DOI] [PubMed] [Google Scholar]
- 25. Bharadwaj S, Ginoya S, Tandon P, Gohel TD, Guirguis J, Vallabh H, et al. Malnutrition: laboratory markers vs nutritional assessment. Gastroenterol Rep 2016;4:272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim J, Shim S‐H, Oh I‐K, Yoon SH, Lee SJ, Kim SN, et al. Preoperative hypoalbuminemia is a risk factor for 30‐day morbidity after gynecological malignancy surgery. Obstet Gynecol Sci 2015;58:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ataseven B, du Bois A, Reinthaller A, Traut A, Heitz F, Aust S, et al. Pre‐operative serum albumin is associated with post‐operative complication rate and overall survival in patients with epithelial ovarian cancer undergoing cytoreductive surgery. Gynecol Oncol 2015;138:560–565. [DOI] [PubMed] [Google Scholar]
- 28. Obermair A, Hagenauer S, Tamandl D, Clayton RD, Nicklin JL, Perrin LC. Safety and efficacy of low anterior en bloc resection as part of cytoreductive surgery for patients with ovarian cancer. Gynecol Oncol 2001;83:115–120. [DOI] [PubMed] [Google Scholar]
- 29. Ge L‐N, Wang F. Prognostic significance of preoperative serum albumin in epithelial ovarian cancer patients: a systematic review and dose–response meta‐analysis of observational studies. Cancer Manag Res 2018;10:815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aletti GD, Eisenhauer EL, Santillan A, Axtell A, Aletti G, Holschneider C, et al. Identification of patient groups at highest risk from traditional approach to ovarian cancer treatment. Gynecol Oncol 2011;120:23–28. [DOI] [PubMed] [Google Scholar]
- 31. Smits A, Lopes A, Das N, Kumar A, Cliby W, Smits E, et al. Surgical morbidity and clinical outcomes in ovarian cancer—the role of obesity. BJOG 2016;123:300–308. [DOI] [PubMed] [Google Scholar]
- 32. Kumar A, Bakkum‐Gamez JN, Weaver AL, McGree ME, Cliby WA. Impact of obesity on surgical and oncologic outcomes in ovarian cancer. Gynecol Oncol 2014;135:19–24. [DOI] [PubMed] [Google Scholar]
- 33. Gunderson CC, Java J, Moore KN, Walker JL. The impact of obesity on surgical staging, complications, and survival with uterine cancer: a Gynecologic Oncology Group LAP2 ancillary data study. Gynecol Oncol 2014;133:23–27. [DOI] [PubMed] [Google Scholar]
- 34. Mahdi H, Jernigan AM, Aljebori Q, Lockhart D, Moslemi‐Kebria M. The impact of obesity on the 30‐day morbidity and mortality after surgery for endometrial cancer. J Minim Invasive Gynecol 2015;22:94–102. [DOI] [PubMed] [Google Scholar]
- 35. Grundmann O, Yoon SL, Williams JJ. The value of bioelectrical impedance analysis and phase angle in the evaluation of malnutrition and quality of life in cancer patients—a comprehensive review. Eur J Clin Nutr 2015;69:1290–1297. [DOI] [PubMed] [Google Scholar]
- 36. Sehouli J, Ali P, Braicu EI, Chekerov R, Grabowski JP. The impact of preoperative malnutrition on surgery outcome and overall survival in ovarian or peritoneal cancer patients: a prospective study. J Clin Oncol 2016;34:5574–5574. [Google Scholar]
- 37. Uccella S, Mele MC, Quagliozzi L, Rinninella E, Nero C, Cappuccio S, et al. Assessment of preoperative nutritional status using BIA‐derived phase angle (PhA) in patients with advanced ovarian cancer: correlation with the extent of cytoreduction and complications. Gynecol Oncol 2018;149:263–269. [DOI] [PubMed] [Google Scholar]
- 38. Barbosa‐Silva MCG, Barros AJD. Bioelectric impedance and individual characteristics as prognostic factors for post‐operative complications. Clin Nutr Edinb Scotl 2005;24:830–838. [DOI] [PubMed] [Google Scholar]
- 39. Hui D, Bansal S, Morgado M, Dev R, Chisholm G, Bruera E. Phase angle for prognostication of survival in patients with advanced cancer: preliminary findings. Cancer 2014;120:2207–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Toso S, Piccoli A, Gusella M, Menon D, Bononi A, Crepaldi G, et al. Altered tissue electric properties in lung cancer patients as detected by bioelectric impedance vector analysis. Nutrition 2000;16:120–124. [DOI] [PubMed] [Google Scholar]
- 41. Gupta D, Lammersfeld CA, Burrows JL, Dahlk SL, Vashi PG, Grutsch JF, et al. Bioelectrical impedance phase angle in clinical practice: implications for prognosis in advanced colorectal cancer. Am J Clin Nutr 2004;80:1634–1638. [DOI] [PubMed] [Google Scholar]
- 42. Ataseven B, Luengo TG, du Bois A, Waltering KU, Traut A, Heitz F, et al. Skeletal muscle attenuation (sarcopenia) predicts reduced overall survival in patients with advanced epithelial ovarian cancer undergoing primary debulking surgery. Ann Surg Oncol 2018;25:3372–3379. [DOI] [PubMed] [Google Scholar]
- 43. ECOG Performance Status . ECOG‐ACRIN. https://ecog-acrin.org/resources/ecog-performance-status. Accessed March 12, 2020.
- 44. Inci MG, Anders L, Heise K, Richter R, Woopen H, Sehouli J. Can Fried frailty score predict postoperative morbidity and mortality in gynecologic cancer surgery? Results of a prospective study. J Geriatr Oncol 2020; 10.1016/j.jgo.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 45. Von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]


