Abstract
Background
Few studies have evaluated the association between being underweight and having cardiovascular disease in the general population. We investigated the incidence of stroke, myocardial infarction (MI), and all‐cause mortality according to detailed underweight categories in a large population cohort.
Methods
We included 4 164 364 individuals who underwent a health examination that was conducted as part of the Korean National Health Insurance Service between January 2009 and December 2012 and followed them up to determine the incidence of stroke, MI, and all‐cause mortality until 31 December 2016. Based on the body mass index, the study population was categorized into normal (18.50–22.99), mild (17.00–18.49), moderate (16.00–16.99), and severe underweight (<16.00) groups. Cox proportional hazards analyses were performed to calculate the hazard ratio for stroke, MI, and mortality according to the severity of underweight in reference to the normal weight. We adjusted for age, sex, lifestyle, economic status, co‐morbidity, blood pressure, glucose, lipid level, and waist circumference.
Results
The mean age of the 4 164 364 eligible subjects in this study cohort was 44.4 ± 14.3 years, and 46.1% of the participants were male; 46 728 strokes, 30 074 MIs, and 121 080 deaths occurred during 27 449 902 person‐years. The incidence of stroke, MI, and all‐cause mortality increased proportionally with the severity of underweight in the multivariate model. This proportional association became more evident when the waist circumference was additionally adjusted. The respective hazard ratios (95% confidence intervals) for mild, moderate, and severe underweight were 1.10 (1.06–1.15), 1.11 (1.02–1.20), and 1.38 (1.24–1.53) for stroke; 1.19 (1.14–1.25), 1.40 (1.27–1.53), and 1.86 (1.64–2.11) for MI; and 1.63 (1.60–1.67), 2.10 (2.02–2.17), and 2.98 (2.85–3.11) for all‐cause mortality. In stratified analyses based on waist circumference, the severity of underweight was consistently associated with a higher risk of stroke, MI, and death.
Conclusions
The severity of underweight was associated with a higher risk of stroke, MI, and all‐cause mortality.
Keywords: Underweight, Cardiovascular disease, Mortality, Claim data
Introduction
Being underweight is a well‐known risk factor for various chronic diseases, as it increases the risk of mortality, 1 , 2 osteoporosis and fracture, 2 and respiratory diseases, including asthma, 3 and poses an economic burden of up to 2.5–3.8% of the total gross domestic product in various countries (up to 49.7 billion dollars in China). 4 Being underweight could increase the risk of cardiovascular diseases (CVD) that is traditionally associated with obesity, in specific population groups such as patients with type I diabetes 5 and acute coronary syndrome, 6 those who undergo percutaneous coronary interventions, 7 , 8 and those with a stroke. 9 , 10 However, only a few studies have investigated the impact of being underweight on the incidence and prevalence of CVD in the general population. 11 , 12
In various studies, the degree of obesity has been categorized in detail as overweight and mild/moderate/severe obesity according to the body mass index (BMI). However, being underweight (leanness or thinness) is simply categorized into one group, with BMI less than 18.5, mainly due to its low prevalence (on average, 1–4% in developed countries). 11 This definition of leanness and the relatively small sample size of previous studies may have led to the controversial and inconsistent findings in the earlier studies. 13 , 14 , 15 , 16 Some studies showed no association between underweight and stroke or myocardial infarction (MI), 17 , 18 whereas other studies showed an increased risk of coronary artery disease in the underweight population. 15 , 19
Therefore, we aimed to investigate the incidence of stroke, MI, and all‐cause mortality according to detailed underweight categories according to the World Health Organization (WHO) classification, as mild (BMI 17.00–18.49), moderate (16.00–16.99), and severe underweight (<16.00), 20 in a large general population cohort.
Methods
Data source and study population
Our study used the entire South Korean population data from the Korean National Health Insurance Service (NHIS) database. The NHIS, a single insurer managed by Korean government, provides a mandatory universal health insurance. Furthermore, the NHIS provides regular health check‐ups every 1 or 2 years to insured adults older than 40 years and employees older than 20 years. These include anthropometric measurements [i.e. height, weight, and waist circumference (WC)]; blood pressure (BP); laboratory measurements (i.e. blood glucose, creatinine, total cholesterol, triglycerides, high‐density lipoprotein, and low‐density lipoprotein); lifestyle information [smoking, alcohol drinking, and physical activity (PA)]; and regular medications. The NHIS has been releasing its retrospective cohort database since 2015, and the database contains anonymized data on demographics, medical treatment, disease diagnosis according to the International Classification of Diseases, 10th Revision, Clinical Modification (ICD‐10‐CM) codes, and health examination for nearly the entire South Korean population.
In our study, we included the data of people who underwent health examination through the NHIS between 1 January 2009 and 31 December 2012. We excluded individuals with a prior diagnosis of MI or stroke during the 5 years before enrolment (n = 430 621), those with any missing variables (n = 510 925), and those who were overweight or obese (n = 5 384 581). The remaining 4 164 364 eligible subjects were included in the analyses and followed up until the date of death or until 31 December 2016 (Figure 1). The Seoul National University Hospital (Seoul, South Korea) institutional review board approved this study (IRB no. E‐1809‐073‐973). The requirement for written informed consent was waived by the review board because anonymized, de‐identified information was used for analysis.
Figure 1.
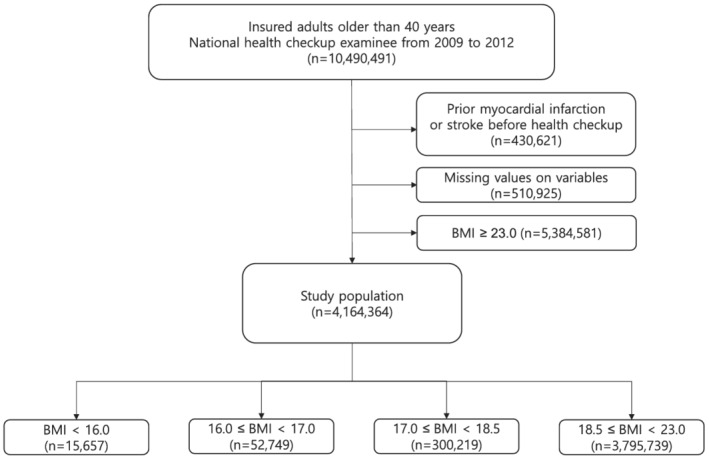
Flow chart of study population. BMI denotes body mass index.
Outcomes
The primary outcomes of the study were newly diagnosed stroke, MI, and all‐cause mortality. MI was defined as ICD‐10‐CM code I21 or I22 during hospitalization. The ICD‐10‐CM code for stroke was I63 or I64 during hospitalization with brain magnetic resonance imaging or computerized tomography claims.
Measurements and definitions
Study subjects provided answers to standardized questions regarding their past medical history and lifestyle such as smoking, alcohol consumption, and PAs. Smoking status was classified into non‐smoker or current smoker. Individuals who consumed more than 30 g of alcohol daily were defined as heavy alcohol consumers. 21 The questionnaire for PA comprised queries on the frequencies of at least 30 min of moderate to intensity activity and 20 min of vigorous activity. Based on the PA level, we categorized subjects into two groups, as ‘none’ or ‘at least once’ according to the frequency of moderate or vigorous activity per week. Baseline co‐morbidities included hypertension, diabetes mellitus, and dyslipidaemia. Hypertension was defined as systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg, or the use of antihypertensive drugs with a prior diagnosis (ICD‐10‐CM; I10‐13, I15). Similarly, diabetes mellitus was defined as a prior diagnosis (ICD‐10‐CM; E11‐14) and treatment with glucose‐lowering agents or as overnight fasting plasma glucose ≥ 126 mg/dL. Dyslipidaemia was defined as a prior diagnosis (ICD‐10‐CM; E78) and treatment with statins or as a total cholesterol ≥ 240 mg/dL. The premiums were determined according to the income level, and we accordingly defined the low‐income group as subjects whose premiums were less than the lowest quintile in the insured group.
During the health examination, height, weight, and WC were measured, and BMI was calculated by dividing weight (kg) by height (m) squared. Subjects were categorized according to BMI using the WHO Western Pacific Region guideline 22 strata of underweight (<18.5) and normal weight (18.5–22.9). The underweight population was further categorized into mild (BMI 17.00–18.49), moderate (16.00–16.99), and severe underweight (<16.00). 20 BP was measured after the subjects had been seated for 5 min with the arm in the appropriate position. Blood samples for the measurement of serum glucose, total cholesterol, and creatinine levels were drawn after overnight fasting. The estimated glomerular filtration rate was calculated using the equation from the Modification of Diet in Renal Disease study, estimated glomerular filtration rate = 175 × serum creatinine−1.154 × age−0.203, and further multiplied by 0.742 for women. 23
Statistical analysis
The baseline characteristics of the study subjects according to BMI categories are presented as the mean (standard deviation) or n (%), as appropriate. Values were compared using one‐way analysis of variance test for continuous variables and the χ 2 test for categorical variables. Incidence rates of MI, stroke, and all‐cause mortality were calculated by dividing the number of events by 1000 person‐years. Cox proportional hazards analyses were performed to evaluate the association of BMI categories with incident MI, stroke, and all‐cause mortality, and hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. Model 1 was adjusted for demographic factors (age and sex), lifestyle (smoking status, alcohol consumption, and PA), and economic status (household income). Model 2 was additionally adjusted for non‐communicable disease‐related clinical factors, such as BP, fasting blood sugar, total cholesterol, and medications (for hypertension, diabetes, and dyslipidaemia). Model 3 was further adjusted for the WC. In addition, stratified analysis according to sex, age (<65 or ≥65 years), smoking status, and presence of diabetes was performed because these factors have a significant impact on both weight and CVD. Furthermore, we performed stratified analysis according to WC to minimize the effect of fat on the association of underweight with CVD and death. Statistical analyses were conducted using SAS software (Version 9.4; SAS Institute, Cary, NC, USA), and a P‐value < 0.05 was considered statistically significant.
Results
General characteristics of study population
In 4 164 364 eligible subjects, 1 918 963 (46.1%) were men. The mean age in the whole study population was 44.4 (standard deviation, 14.3) years. Subjects with normal weight were more likely to be male (47.4% vs. 32.9%), older (44.8 vs. 40.0 years), smokers (25.1% vs. 22.9%), and engaged in regular PAs (49.8% vs. 40.4%) and had a higher prevalence of diabetes, hypertension, and dyslipidaemia (Supporting Information, Table S1). In addition, they were more likely to have higher WC (74.3 vs. 66.2 cm), systolic BP (118.5 vs. 113.5 mmHg), total cholesterol (188.7 vs. 177.6 mg/dL), and serum glucose level (93.7 vs. 90.5 mg/dL). Among underweight subjects, individuals in the severe underweight group were older and had a higher prevalence of diabetes, hypertension, and dyslipidaemia than those in the mild underweight group (Table 1).
Table 1.
Baseline characteristics of the study population
| Severe underweight (BMI < 16.0) | Moderate underweight (16.0 ≤ BMI < 17.0) | Mild underweight (17.0 ≤ BMI < 18.5) | Normal weight (18.5 ≤ BMI < 23.0) | P‐value | |
|---|---|---|---|---|---|
| Counts | 15 657 | 52 749 | 300 219 | 3 795 739 | |
| Male—no. (%) | 5147 (32.87) | 16 586 (31.44) | 99 594 (33.17) | 1 797 636 (47.36) | <0.001 |
| Age (years) | 45.68 ± 20.36 | 40.45 ± 17.63 | 39.58 ± 15.64 | 44.78 ± 14.11 | <0.001 |
| Lifestyle | |||||
| Current smoker—no. (%) | 3554 (22.7) | 11 916 (22.59) | 68 759 (22.9) | 952 087 (25.08) | <0.001 |
| Heavy alcohol drinker—no. (%) | 610 (3.9) | 1912 (3.62) | 11 750 (3.91) | 208 187 (5.48) | <0.001 |
| Regular physical activity—no. (%) | 5270 (33.66) | 19 493 (36.95) | 124 248 (41.39) | 1 891 378 (49.83) | <0.001 |
| Low‐income group—no. (%) | 3743 (23.91) | 11 881 (22.52) | 64 159 (21.37) | 814 190 (21.45) | <0.001 |
| Co‐morbidities | |||||
| With hypertension—no. (%) | 2020 (12.9) | 4705 (8.92) | 24 383 (8.12) | 574 429 (15.13) | <0.001 |
| With diabetes—no. (%) | 761 (4.86) | 1775 (3.36) | 8832 (2.94) | 195 832 (5.16) | <0.001 |
| With dyslipidaemia—no. (%) | 1013 (6.47) | 2484 (4.71) | 15 142 (5.04) | 427 254 (11.26) | <0.001 |
| Measurements | |||||
| Systolic blood pressure (mmHg) | 113.68 ± 15.51 | 112.91 ± 14.21 | 113.58 ± 13.69 | 118.49 ± 14.24 | <0.001 |
| Total cholesterol (mg/dL) | 177.35 ± 34.33 | 176.08 ± 31.79 | 177.83 ± 31.7 | 188.67 ± 34.68 | <0.001 |
| Fasting blood glucose (mg/dL) | 92.21 ± 22.44 | 90.57 ± 19.23 | 90.42 ± 17.87 | 93.7 ± 19.97 | <0.001 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 93.12 ± 43.88 | 94.18 ± 48.86 | 93.68 ± 48.41 | 90.15 ± 44.23 | <0.001 |
| Waist circumference (cm) | 63.5 ± 6.59 | 64.42 ± 5.67 | 66.6 ± 5.5 | 74.25 ± 6.28 | <0.001 |
Values with ± symbols denote mean ± standard deviation. BMI denotes body mass index.
Outcomes according to underweight categories
In total, 46 728, 30 074, and 121 080 occurrences of stroke, MI, and mortality occurred during the 27 449 902 person‐years of follow‐up. When we examined the association between level of underweight (according to WHO classification) and clinical outcomes, the severity of underweight was significantly associated with the risk of MI and all‐cause mortality (Table 2). This association became more evident after adjusting for demographic factors and non‐communicable disease‐related factors (Model 2); multivariate adjusted HR and 95% CI for mild, moderate, and severe underweight were 1.03 (0.99–1.07), 1.02 (0.94–1.10), and 1.24 (1.12–1.38) for stroke; 1.10 (1.05–1.15), 1.25 (1.14–1.38), and 1.65 (1.45–1.86) for MI; and 1.63 (1.60–1.66), 2.09 (2.02–2.16), and 2.97 (2.85–3.10) for all‐cause mortality, respectively. We further adjusted for WC to minimize the effect of adiposity on the association of underweight and CVD and mortality. The severity of underweight was associated with a higher risk of CVD and death. The adjusted HRs (95% CI) for mild, moderate, and severe underweight were as follows: 1.10 (1.06–1.15), 1.11 (1.02–1.20), and 1.38 (1.24–1.53), respectively, for stroke; 1.19 (1.14–1.25), 1.40 (1.27–1.53), and 1.86 (1.64–2.11), respectively, for MI; and 1.63 (1.60–1.67), 2.10 (2.02–2.17), and 2.98 (2.85–3.11), respectively, for death.
Table 2.
Incidence of cardiovascular disease and all‐cause mortality according to the severity of underweight
| Outcomes | Body mass index | Events | Person‐years | Incidence rate (per 1000 person‐years) | Estimated hazard ratio (95% confidence interval) | ||
|---|---|---|---|---|---|---|---|
| Model 1 a | Model 2 b | Model 3 c | |||||
| Stroke | <16.0 | 348 | 106 440.4 | 3.3 | 1.10 (0.99–1.23) | 1.24 (1.12–1.38) | 1.38 (1.24–1.53) |
| 16.00–16.99 | 648 | 374 221.5 | 1.7 | 0.91 (0.84–0.98) | 1.02 (0.94–1.10) | 1.11 (1.02–1.20) | |
| 17.00–18.49 | 2938 | 2 160 165.5 | 1.4 | 0.94 (0.90–0.97) | 1.03 (0.99–1.07) | 1.10 (1.06–1.15) | |
| 18.50–22.99 | 42 794 | 27 522 854.5 | 1.6 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Myocardial infarction | <16.00 | 255 | 106 808.0 | 2.4 | 1.46 (1.29–1.66) | 1.65 (1.45–1.86) | 1.86 (1.64–2.11) |
| 16.00–16.99 | 463 | 374 771.0 | 1.2 | 1.120 (1.02–1.23) | 1.25 (1.14–1.38) | 1.40 (1.27–1.53) | |
| 17.00–18.49 | 1932 | 2 163 290.9 | 0.9 | 1.00 (0.96–1.05) | 1.10 (1.05–1.15) | 1.19 (1.14–1.25) | |
| 18.50–22.99 | 27 424 | 27 567 781.9 | 1.0 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| All‐cause death | <16.00 | 2208 | 107 278.9 | 20.6 | 2.82 (2.70–2.94) | 2.97 (2.85–3.10) | 2.98 (2.85–3.11) |
| 16.00–16.99 | 3565 | 375 877.0 | 9.5 | 1.99 (1.94–2.06) | 2.09 (2.02–2.16) | 2.10 (2.02–2.17) | |
| 17.00–18.49 | 12 136 | 2 168 088.5 | 5.6 | 1.56 (1.53–1.59) | 1.63 (1.60–1.66) | 1.63 (1.60–1.67) | |
| 18.50–22.99 | 103 171 | 27 645 996.2 | 3.7 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
Model 1: adjusted for age, sex, smoking status, alcohol drinking, physical activity, and income.
Model 2: adjusted for Model 1 + fasting blood glucose, total cholesterol, systolic blood pressure, estimated glomerular filtration rate, hypertension, diabetes mellitus, and dyslipidaemia.
Model 3: adjusted for Model 2 + waist circumference.
When we performed stratified analysis according to age (≥65 or <65 years), sex, smoking status (current vs. never or ever), and presence of diabetes, only age was found to be an effect modifier, and the trend in the negative association of BMI‐defined underweight and composite outcome remained unchanged (Table 3). In the stratification analysis by WC, the severity of underweight was associated with a higher risk of MI and death in every tertile (Table 4).
Table 3.
Stratified analyses according to age, sex, diabetes mellitus, and smoking status
| Stratified variable | Outcomes | Category | Estimated hazard ratio (95% confidence interval) a | P for interaction | ||
|---|---|---|---|---|---|---|
| Severe underweight | Moderate underweight | Mild underweight | ||||
| Age | Stroke | Less than 65 | 1.19 (1.01–1.39) | 0.98 (0.84–1.15) | 1.00 (0.85–1.17) | 0.05 |
| 65 and more | 1.09 (0.98–1.22) | 1.08 (0.96–1.21) | 1.11 (0.99–1.25) | |||
| Myocardial infarction | Less than 65 | 1.13 (0.94–1.35) | 1.05 (0.87–1.25) | 1.03 (0.86–1.23) | 0.15 | |
| 65 and more | 1.39 (1.20–1.59) | 1.15 (0.99–1.34) | 1.09 (0.94–1.28) | |||
| All‐cause death | Less than 65 | 1.77 (1.63–1.92) | 1.22 (1.12–1.33) | 1.05 (0.96–1.14) | <0.001 | |
| 65 and more | 1.54 (1.46–1.62) | 1.21 (1.14–1.28) | 1.13 (1.07–1.20) | |||
| Sex | Stroke | Male | 1.05 (0.94–1.19) | 1.05 (0.93–1.18) | 1.07 (0.95–1.21) | 0.30 |
| Female | 1.16 (1.01–1.33) | 1.04 (0.89–1.20) | 1.06 (0.92–1.23) | |||
| Myocardial infarction | Male | 1.23 (1.06–1.42) | 1.12 (0.97–1.30) | 1.04 (0.89–1.21) | 0.46 | |
| Female | 1.36 (1.15–1.62) | 1.07 (0.88–1.28) | 1.10 (0.92–1.33) | |||
| All‐cause death | Male | 1.64 (1.56–1.73) | 1.25 (1.18–1.32) | 1.14 (1.08–1.21) | 0.37 | |
| Female | 1.51 (1.40–1.63) | 1.15 (1.06–1.25) | 1.05 (0.96–1.14) | |||
| Diabetes mellitus | Stroke | No | 1.12 (1.02–1.24) | 1.05 (0.95–1.17) | 1.08 (0.97–1.19) | 0.37 |
| Yes | 1.04 (0.83–1.29) | 1.01 (0.80–1.27) | 1.07 (0.85–1.34) | |||
| Myocardial infarction | No | 1.30 (1.16–1.46) | 1.06 (0.94–1.21) | 1.03 (0.91–1.17) | 0.06 | |
| Yes | 1.24 (0.93–1.66) | 1.34 (1.00–1.80) | 1.27 (0.94–1.71) | |||
| All‐cause death | No | 1.60 (1.53–1.68) | 1.22 (1.16–1.29) | 1.10 (1.05–1.16) | 0.58 | |
| Yes | 1.69 (1.52–1.88) | 1.21 (1.08–1.37) | 1.18 (1.05–1.33) | |||
| Smoking status | Stroke | None or past | 1.12 (1.00–1.25) | 1.04 (0.93–1.17) | 1.05 (0.93–1.18) | 0.65 |
| Current | 1.06 (0.91–1.23) | 1.03 (0.89–1.20) | 1.10 (0.94–1.28) | |||
| Myocardial infarction | None or past | 1.37 (1.19–1.57) | 1.08 (0.93–1.25) | 1.08 (0.93–1.26) | 0.07 | |
| Current | 1.15 (0.96–1.37) | 1.14 (0.95–1.37) | 1.03 (0.86–1.25) | |||
| All‐cause death | None or past | 1.58 (1.49–1.66) | 1.18 (1.11–1.25) | 1.09 (1.02–1.16) | 0.09 | |
| Current | 1.63 (1.51–1.75) | 1.28 (1.18–1.38) | 1.14 (1.06–1.24) | |||
Adjusted for age, sex, smoking status, alcohol drinking, physical activity and income, fasting blood glucose, total cholesterol, systolic blood pressure, estimated glomerular filtration rate, waist circumference, hypertension, diabetes mellitus, and dyslipidaemia.
Comparison with the normal‐weight group as the reference group.
Table 4.
Stratified analyses according to waist circumference
| Outcomes | Category of waist circumference | Estimated hazard ratio (95% confidence interval) a | P for interaction | ||
|---|---|---|---|---|---|
| Severe underweight | Moderate underweight | Mild underweight | |||
| Stroke | 1st tertile b | 1.45 (1.27–1.64) | 1.08 (0.97–1.19) | 1.08 (1.02–1.14) | 0.048 |
| 2nd tertile c | 0.78 (0.53–1.11) | 1.14 (0.95–1.37) | 1.07 (0.99–1.16) | ||
| 3rd tertile d | 1.31 (0.99–1.74) | 1.06 (0.85–1.32) | 1.18 (1.08–1.29) | ||
| Myocardial infarction | 1st tertile | 1.90 (1.63–2.21) | 1.37 (1.21–1.54) | 1.19 (1.11–1.27) | 0.882 |
| 2nd tertile | 1.66 (1.15–2.39) | 1.42 (1.13–1.79) | 1.20 (1.09–1.32) | ||
| 3rd tertile | 1.59 (1.11–2.27) | 1.48 (1.15–1.92) | 1.24 (1.10–1.40) | ||
| All‐cause death | 1st tertile | 2.91 (2.76–3.08) | 1.95 (1.87–2.04) | 1.46 (1.42–1.50) | <0.001 |
| 2nd tertile | 2.95 (2.61–3.34) | 2.37 (2.18–2.57) | 1.74 (1.67–1.81) | ||
| 3rd tertile | 2.50 (2.19–2.84) | 1.97 (1.78–2.17) | 1.88 (1.79–1.97) | ||
Adjusted for age, sex, smoking status, alcohol consumption, physical activity and income, fasting blood glucose, total cholesterol, systolic blood pressure, estimated glomerular filtration rate, hypertension, diabetes mellitus, and dyslipidaemia.
Comparison with the normal‐weight group as the reference group.
<75 cm in men, <68 cm in women.
75–79 cm in men, 68–72 cm in women.
≥80 cm in men, ≥73 cm in women.
Discussion
In this large‐scale, population‐based, longitudinal study, we found that being underweight was associated with a higher risk of stroke, MI, and all‐cause mortality. The severity of underweight was associated with a higher risk of stroke, MI, and all‐cause mortality, and this association became more evident in the WC‐adjusted model.
Comparison with previous studies
Previous studies on the association between underweight and stroke, MI, and all‐cause mortality have shown inconsistent results. Those inconsistencies might be due to differences in study population, relatively low outcome cases, and differences in the covariates considered. Previous longitudinal studies among patients with CVD showed that being underweight increased the risk of MI. Lamelas et al. conducted a meta‐analysis that included 81 553 patients with acute coronary syndrome and showed that being underweight (BMI < 18.5 kg/m2) increased the risk of MI and all‐cause mortality. 6 Similarly, Faggioni et al. performed a pooled analysis including female patients who underwent percutaneous coronary intervention and showed that being underweight increased cardiovascular mortality but not MI. 8
The results of studies conducted among the general population were inconsistent with regard to the association of underweight and incidence of stroke or MI. 15 , 17 , 18 , 19 These discrepancies might be due to the relatively small number of underweight population (<5000 individuals) and incident cases (<400 cases). This study had a sufficiently large number of underweight population (368 625 individuals) and cases (3934 stroke and 2650 MI). Furthermore, most previous studies did not consider WC, which is a strong risk factor for CVD, as a potential covariate.
Possible explanations and implications
Generally, CVD is largely attributable to the detrimental effects of adiposity and other modifiable risk factors associated with obesity, while underweight was not regarded as a risk factor for CVD. However, in our study, with WC added to the fully adjusted model (to adjust for the effect of adiposity), the risk of stroke, MI, and mortality increased gradually with the severity of underweight. Lean mass is generally proportional to the muscle mass, and loss of muscle mass is known to be associated with a hyperinflammatory status, 24 insulin resistance, and multiple metabolic disorders, 25 which could lead to MI and stroke. Greater muscle mass is associated with better exercise capacity and cardiorespiratory fitness and could in turn lead to decreased CVD risk. 26 , 27 Recent studies indicated that lower lean body mass increased the risk of mortality in health professionals 28 and risk of major adverse cardiac events in patients with coronary artery disease. 29 Our finding of a linear increase in the risk of MI, stroke, and mortality with underweight severity after adjustment for WC supports this hypothesis.
Being underweight can be a surrogate marker for patients' frail conditions including malnutrition and combined non‐cardiovascular co‐morbidities, and frailty itself can also increase the risk of CVD and mortality. 30 , 31 A substantial portion of our study population could possibly have frailty, and our findings could have been a result of frailty.
Strengths and limitations
There are several limitations to our study. Firstly, a cause–effect relationship is not determined because of the retrospective nature of our study. We attempted to statistically control possible confounders; however, there is a possibility of residual confounders. Secondly, we could not consider weight change. Intentional weight loss, especially among overweight/obese population, generally leads to improvement in CVD risk factors. However, its effect on total and CVD‐associated mortality is inconsistent. 32 , 33 , 34 A previous study even reported that observed (intentional or unintentional) weight loss increased CVD risk. 35 Therefore, there is need for large‐scale longitudinal study in the general population to determine the effect of weight change on long‐term CVD outcome and all‐cause mortality. Thirdly, we could not differentiate between the effect of body composition and CVD and mortality risks. Lean mass and fat mass have different effects on CVD and mortality 26 , 28 ; therefore, identifying these differences in the effect of lean mass and fat mass on CVD and mortality could have clarified about the effect of lean mass and fat mass on the outcomes. However, we adjusted for the WC and performed a stratified analysis according to the WC to minimize the effect of adiposity on the outcome.
Despite these limitations, our study has several strengths. To our knowledge, this is the first study to show the association of WHO‐defined underweight categories and stroke, MI, and all‐cause mortality. Moreover, our study findings can be generalized to the population of Korea because our study population was very large and we considered most risk factors of CVD and mortality. Our study population also included a wide age range, which enabled us to perform sensitivity analysis. No substantial difference was found in the association between underweight severity and outcomes according to age group. We also performed extensive stratified analyses according to other covariates such as smoking and history of diabetes, which enhanced the reliability of our results. Finally, we adjusted for WC, unlike in previous studies, to minimize the effect of adiposity on the association of underweight and CVD and mortality.
Conclusions
As underweight categories became more severe, the risk of stroke, MI, and all‐cause mortality increased proportionally. The underlying mechanism should be investigated in further well‐designed prospective cohort studies that include information on the body composition.
Conflict of interest
None declared.
Ethical approval
The study procedures were approved by the Seoul National University Hospital (Seoul, South Korea) institutional review board (IRB no. E‐1809‐073‐973) and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The requirement for written informed consent was waived by the review board because anonymous and de‐identified information was used for analysis.
Supporting information
Table S1. Comparison of baseline characteristics between underweight and normal weight group.
Acknowledgements
The authors received no financial support for the research, authorship, and/or publication of this article. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 36
Kwon H., Yun J. M., Park J. H., Cho B. L., Han K., Joh H.‐K., Son K. Y., and Cho S. H. (2021) Incidence of cardiovascular disease and mortality in underweight individuals, Journal of Cachexia, Sarcopenia and Muscle, 12, 331–338, 10.1002/jcsm.12682
Contributor Information
Jin Ho Park, Email: pjhn@snu.ac.kr.
Be Long Cho, Email: belong@snu.ac.kr.
References
- 1. Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, de Gonzalez AB, et al. Global BMI Mortality Collaboration. Body‐mass index and all‐cause mortality: individual‐participant‐data meta‐analysis of 239 prospective studies in four continents. Lancet 2016;388:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kvamme JM, Wilsgaard T, Florholmen J, Jacobsen BK. Body mass index and disease burden in elderly men and women: the Tromsø Study. Eur J Epidemiol 2010;25:183–193. [DOI] [PubMed] [Google Scholar]
- 3. Patra J, Maher YI, Mishra S, Bhatia M, Alam D, Malini DS, et al. Effects of body mass index, tobacco smoking, alcohol drinking and solid fuel use on the risk of asthma: Individual Participant Data (IPD) meta‐analysis of 175 000 individuals from 51 nationally representative surveys. BMJ Open Respir Res 2016;3:e000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoque ME, Mannan M, Long KZ, Al Mamun A. Economic burden of underweight and overweight among adults in the Asia‐Pacific region: a systematic review. Trop Med Int Health 2016;21:458–469. [DOI] [PubMed] [Google Scholar]
- 5. Vestberg D, Rosengren A, Eeg‐Olofsson K, Miftaraj M, Franzen S, Svensson AM, et al. Body mass index as a risk factor for coronary events and mortality in patients with type 1 diabetes. Open Heart 2018;5:e000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lamelas P, Schwalm JD, Quazi I, Mehta S, Devereaux PJ, Jolly S, et al. Effect of body mass index on clinical events after acute coronary syndromes. Am J Cardiol 2017;120:1453–1459. [DOI] [PubMed] [Google Scholar]
- 7. Kim BG, Hong SJ, Kim BK, Ahn CM, Shin DH, Kim JS, et al. Association between body mass index and clinical outcomes after new‐generation drug‐eluting stent implantation: Korean multi‐center registry data. Atherosclerosis 2018;277:155–162. [DOI] [PubMed] [Google Scholar]
- 8. Faggioni M, Baber U, Afshar AE, Giustino G, Sartori S, Sorrentino S, et al. Effects of body mass index on clinical outcomes in female patients undergoing percutaneous coronary intervention with drug‐eluting stents: results from a patient‐level pooled analysis of randomized controlled trials. JACC Cardiovasc Interv 2018;11:68–76. [DOI] [PubMed] [Google Scholar]
- 9. Olsen TS, Dehlendorff C, Petersen HG, Andersen KK. Body mass index and poststroke mortality. Neuroepidemiology 2008;30:93–100. [DOI] [PubMed] [Google Scholar]
- 10. Kim BJ, Lee SH, Ryu WS, Kim CK, Lee J, Yoon BW. Paradoxical longevity in obese patients with intracerebral hemorrhage. Neurology 2011;76:567–573. [DOI] [PubMed] [Google Scholar]
- 11. Park D, Lee JH, Han S. Underweight: another risk factor for cardiovascular disease? A cross‐sectional 2013 Behavioral Risk Factor Surveillance System (BRFSS) study of 491,773 individuals in the USA. Medicine (Baltimore) 2017;96:e8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suastika K, Dwipayana P, Saraswati MR, Gotera W, Budhiarta AA, Sutanegara ND, et al. Underweight is an important risk factor for coronary heart disease in the population of Ceningan Island. Bali Diab Vasc Dis Res 2012;9:75–77. [DOI] [PubMed] [Google Scholar]
- 13. Cui R, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, et al. Body mass index and mortality from cardiovascular disease among Japanese men and women: the JACC study. Stroke 2005;36:1377–1382. [DOI] [PubMed] [Google Scholar]
- 14. Hu G, Tuomilehto J, Silventoinen K, Sarti C, Männistö S, Jousilahti P. Body mass index, waist circumference, and waist‐hip ratio on the risk of total and type‐specific stroke. Arch Intern Med 2007;167:1420–1427. [DOI] [PubMed] [Google Scholar]
- 15. Choi S, Kim K, Kim SM, Lee G, Jeong SM, Park SY, et al. Association of obesity or weight change with coronary heart disease among young adults in South Korea. JAMA Intern Med 2018;178:1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kong KA, Park J, Hong SH, Hong YS, Sung YA, Lee H. Associations between body mass index and mortality or cardiovascular events in a general Korean population. PLoS ONE 2017;12:e0185024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Song YM, Sung J, Davey Smith G, Ebrahim S. Body mass index and ischemic and hemorrhagic stroke: a prospective study in Korean men. Stroke 2004;35:831–836. [DOI] [PubMed] [Google Scholar]
- 18. Chei CL, Iso H, Yamagishi K, Inoue M, Tsugane S. Body mass index and weight change since 20 years of age and risk of coronary heart disease among Japanese: the Japan Public Health Center‐Based Study. Int J Obes (Lond) 2008;32:144–151. [DOI] [PubMed] [Google Scholar]
- 19. Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, et al. Separate and combined associations of body‐mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet 2011;377:1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization . Obesity: Preventing and Managing the Global Epidemic. Geneva: WHO; 2000. [PubMed] [Google Scholar]
- 21. Choi YJ, Lee DH, Han KD, Kim HS, Yoon H, Shin CM, et al. The relationship between drinking alcohol and esophageal, gastric or colorectal cancer: a nationwide population‐based cohort study of South Korea. PLoS ONE 2017;12:e0185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Health Organization . The Asia‐Pacific perspective: redefining obesity and its treatment. 2000.
- 23. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247–254. [DOI] [PubMed] [Google Scholar]
- 24. Li CW, Yu K, Shyh‐Chang N, Li GX, Jiang LJ, Yu SL, et al. Circulating factors associated with sarcopenia during ageing and after intensive lifestyle intervention. J Cachexia Sarcopenia Muscle 2019;10:586–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Du Y, Oh C, No J. Associations between sarcopenia and metabolic risk factors: a systematic review and meta‐analysis. J Obes Metab Syndr 2018;27:175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O'Donovan G, Owen A, Kearney EM, Jones DW, Nevill AM, Woolf‐May K, et al. Cardiovascular disease risk factors in habitual exercisers, lean sedentary men and abdominally obese sedentary men. Int J Obes (Lond) 2005;29:1063–1069. [DOI] [PubMed] [Google Scholar]
- 27. Lee DC, Sui X, Artero EG, Lee IM, Church TS, McAuley PA, et al. Long‐term effects of changes in cardiorespiratory fitness and body mass index on all‐cause and cardiovascular disease mortality in men: the Aerobics Center Longitudinal Study. Circulation 2011;124:2483–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee DH, Keum N, Hu FB, Orav EJ, Rimm EB, Willett WC, et al. Predicted lean body mass, fat mass, and all cause and cause specific mortality in men: prospective US cohort study. BMJ 2018;362:k2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hioki H, Miura T, Motoki H, Kobayashi H, Kobayashi M, Nakajima H, et al. Lean body mass index prognostic value for cardiovascular events in patients with coronary artery disease. Heart Asia 2015;7:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Adabag S, Vo TN, Langsetmo L, Schousboe JT, Cawthon PM, Stone KL, et al. Frailty as a risk factor for cardiovascular versus noncardiovascular mortality in older men: results from the MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study. J Am Heart Assoc 2018;7:e008974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Veronese N, Sigeirsdottir K, Eiriksdottir G, Marques EA, Chalhoub D, Phillips CL, et al. Frailty and risk of cardiovascular diseases in older persons: the age, gene/environment susceptibility‐Reykjavik study. Rejuvenation Res 2017;20:517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Williamson DF, Pamuk E, Thun M, Flanders D, Byers T, Heath C. Prospective study of intentional weight loss and mortality in overweight white men aged 40–64 years. Am J Epidemiol 1999;149:491–503. [DOI] [PubMed] [Google Scholar]
- 33. Diaz VA, Mainous AG, Everett CJ. The association between weight fluctuation and mortality: results from a population‐based cohort study. J Community Health 2005;30:153–165. [DOI] [PubMed] [Google Scholar]
- 34. Sørensen TI, Rissanen A, Korkeila M, Kaprio J. Intention to lose weight, weight changes, and 18‐y mortality in overweight individuals without co‐morbidities. PLoS Med 2005;2:e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bowman TS, Kurth T, Sesso HD, Manson JE, Gaziano JM. Eight‐year change in body mass index and subsequent risk of cardiovascular disease among healthy non‐smoking men. Prev Med 2007;45:436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of baseline characteristics between underweight and normal weight group.


