Abstract
Cancer cachexia is a complex multi‐organ catabolic syndrome that reduces mobility, increases fatigue, decreases the efficiency of therapeutic strategies, diminishes the quality of life, and increases the mortality of cancer patients. This review provides an exhaustive and comprehensive analysis of cancer cachexia‐related phenotypic changes in skeletal muscle at both the cellular and subcellular levels in human cancer patients, as well as in animal models of cancer cachexia. Cancer cachexia is characterized by a major decrease in skeletal muscle mass in human and animals that depends on the severity of the disease/model and the localization of the tumour. It affects both type 1 and type 2 muscle fibres, even if some animal studies suggest that type 2 muscle fibres would be more prone to atrophy. Animal studies indicate an impairment in mitochondrial oxidative metabolism resulting from a decrease in mitochondrial content, an alteration in mitochondria morphology, and a reduction in mitochondrial metabolic fluxes. Immuno‐histological analyses in human and animal models also suggest that a faulty mechanism of skeletal muscle repair would contribute to muscle mass loss. An increase in collagen deposit, an accumulation of fat depot outside and inside the muscle fibre, and a disrupted contractile machinery structure are also phenotypic features that have been consistently reported in cachectic skeletal muscle. Muscle function is also profoundly altered during cancer cachexia with a strong reduction in skeletal muscle force. Even though the loss of skeletal muscle mass largely contributes to the loss of muscle function, other factors such as muscle–nerve interaction and calcium handling are probably involved in the decrease in muscle force. Longitudinal analyses of skeletal muscle mass by imaging technics and skeletal muscle force in cancer patients, but also in animal models of cancer cachexia, are necessary to determine the respective kinetics and functional involvements of these factors. Our analysis also emphasizes that measuring skeletal muscle force through standardized tests could provide a simple and robust mean to early diagnose cachexia in cancer patients. That would be of great benefit to cancer patient's quality of life and health care systems.
Keywords: Cancer cachexia, Fibre type, Fibrosis, Force, Metabolism, Skeletal muscle, Regeneration
Introduction
Cancer cachexia is a complex catabolic syndrome characterized by an involuntary body mass loss essentially due to a severe depletion of skeletal muscle, with or without adipose tissue loss, while the non‐muscle protein compartment is relatively preserved. 1 Cancer cachexia cannot be reversed by increasing conventional nutritional intake, thus highlighting the fact that the hypercatabolic state of cachectic patients is a critical determinant of the syndrome. The prevalence of cachexia in cancer patients is quite variable, affecting 15–30% of prostate or breast cancer patients to 75% of pancreatic cancer patients, 2 with an increasing prevalence with the advanced stages of the disease. 2 It is thus estimated that the prevalence of cachexia can reach 90% in patients suffering from advanced colorectal cancer 100 days before death. 3 Furthermore, cancer cachexia can be also under‐recognized by some confounding factors, such as obesity 4 or weight gain in the form of ascites or peripheral oedema. 5 Overall, it has been recently estimated that 15.8 subjects per 10 000 of the total general population in European Union in 2013 suffered from cancer cachexia. 2
Cancer cachexia is one of the most debilitating and life‐threatening aspects of cancer, profoundly affecting the patient's quality of life. 6 , 7 , 8 , 9 , 10 , 11 , 12 Cachexia increases surgical risks 13 and the susceptibility to the adverse effects of chemotherapy. 11 , 14 , 15 It also induces a progressive reduction in the body's functional capacities 16 leading to an increase in sedentarization 9 , 17 and a loss of autonomy, ultimately requiring institutional care of patients. Since the pioneering works of Dewys et al. 18 it has been consistently reported that the extent of cachexia is inversely correlated with the survival of cancer patients. 8 , 9 , 10 , 19 , 20 , 21 , 22 , 23 , 24 , 25 Even if probably underestimated, it is generally assumed, according to Warren, 26 that cachexia will be responsible for the death of approximately 20% of cancer patients with death typically occurring when weight loss reaches 30%, thus making it the leading cause of death in cancer.
During cancer cachexia, skeletal muscle represents the main site of protein loss. 27 , 28 The loss of muscle mass affects 5–89% of cancer patients depending on the cancer site and the method of measurement used. 29 Muscle mass loss is greater in weight‐losing cancer patients when compared with weight‐losing anorexia nervosa patients, 30 indicating that cancer‐specific factors independently of undernutrition contribute to decrease skeletal muscle mass. As described for body mass, skeletal muscle mass loss in cancer patients is an independent factor that increases the risk of surgical complications, 31 , 32 decreases surgical efficiency, 24 and increases chemotherapy toxicity. 33 , 34 , 35 , 36 Skeletal muscle mass loss has therefore been consistently associated with a decreased survival rate of cancer patients. 20 , 24 , 31 , 32 , 33 , 35 , 37 , 38 , 39 , 40
Cancer cachexia is a constantly developing area of great research interest. 41 Numerous clinical and experimental studies have thus been devoted to deciphering the molecular pathways involved in cancer cachexia, as well as developing strategies aimed at stopping or even reversing the loss of body mass and skeletal muscle mass loss (reviewed in literatures 5 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 ). However, what is less known are the effects of cancer cachexia on skeletal muscle structure and function. To fully understand the aetiology of cancer cachexia, it is essential to identify its effects on skeletal muscle phenotype. In this review, we will consider the effects of cancer cachexia on skeletal muscle contractile and metabolic phenotypes both in cancer patients and in animal models. Because muscle structure cannot be dissociated from muscle function, we will also consider how and to what extent cancer cachexia affects skeletal muscle function.
Skeletal muscle atrophy during cancer cachexia
Skeletal muscle atrophy in human cancer patients
When studying cancer cachexia in human patients, it is essential to determine the extent of skeletal muscle atrophy, because body mass loss in cancer patients does not strictly reflect skeletal muscle mass loss. 50 This is particularly true in obese cachectic patients where high body mass index may mask the extent of skeletal muscle depletion. 20 Different techniques have been used to quantify the atrophy of skeletal muscle mass during the time course of cancer cachexia (for review, see previous studies 51 , 52 ). Indirect assessments of skeletal muscle mass by the analysis of whole‐body composition using neutron activation, 27 dual‐energy X‐ray absorptiometry, 53 , 54 , 55 , 56 or bioelectric impedance 10 , 25 , 57 , 58 , 59 , 60 indicate that lean body mass is reduced in cachectic cancer patients compared with healthy controls or non‐cachectic cancer patients. When specifically looking at skeletal muscle by imaging techniques, studies also clearly evidence skeletal muscle atrophy. The quadriceps muscle area measured by magnetic resonance imaging is decreased by 10–33% in cachectic cancer patients when compared with healthy control subjects. 57 , 61 , 62 When compared with non‐cachectic cancer patients, the quadriceps muscle area of cachectic cancer patients is either stable (female) or decreased (male) but to a lesser extent (14%), 61 indicating that even if cachexia is not clinically diagnosed (based on body mass analysis), skeletal muscle catabolism has already started. Lately, the use of computerized tomography scans has spread widely as these images may be available in the medical records of patients. Computerized tomography scan analysis at the third lumbar vertebrae level allows the quantification of the areas of rectus abdominis, transversus abdominis, erector spinae, quadratus lumborum, psoas minor and major, and internal and external abdominal oblique muscles, 63 which gives a good estimate of the whole‐body muscle compartment. 64 By using this technique, the atrophy of skeletal muscle is also clearly evident and increases with body mass loss in cachectic cancer patients. 50 When skeletal muscle area is normalized to the height of patients (skeletal muscle index), similar results are obtained with a 4–13% decrease in skeletal muscle index in cachectic cancer patients compared with non‐cachectic cancer patients. 10 , 12 , 59 , 65 Once again, skeletal muscle mass loss increases with the severity of the disease.
Skeletal muscle atrophy in animal models of cancer cachexia
Studies using different animal models of cancer cachexia also consistently report, when compared with control animals, a decrease in lean body mass, 66 , 67 , 68 , 69 as well as in the mass of skeletal muscles with different metabolic and contractile properties such as of gastrocnemius, 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 soleus, 69 , 73 , 77 , 78 , 80 , 81 , 82 , 86 , 88 , 90 , 93 , 96 , 97 , 98 extensor digitorum longus, 69 , 79 , 80 , 85 , 88 , 89 , 93 , 97 , 98 , 99 , 100 , 101 tibialis anterior, 69 , 70 , 73 , 76 , 79 , 80 , 81 , 84 , 85 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 97 , 98 , 99 , 101 , 102 plantaris, 80 , 82 , 85 , 86 , 99 quadriceps, 66 , 68 , 70 , 76 , 79 , 80 , 85 , 91 , 92 , 94 , 99 triceps, 66 epitrochlearis, 89 and diaphragm 86 , 96 muscles. When compared with non‐cachectic cancer animals, the muscle mass of cachectic cancer animals is also lower. 71 , 77 , 79 , 102 , 103 , 104 , 105 , 106 Similarly to human cancer patients, the extent of skeletal muscle mass loss increases with the severity of the experimental model. 71 , 104 , 105 , 107 When normalized to body mass, skeletal muscle mass still remains lower in cachectic cancer animals, 72 , 79 , 80 , 85 , 103 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 indicating that skeletal muscle mass loss exceeds that of all other tissues and organs. Interestingly, many studies reported no difference in soleus muscle mass between cachectic cancer animals and healthy animals. 79 , 85 , 89 , 99 , 104 , 106 , 112 , 113 The postural/anti‐gravitational function of the soleus muscle (enriched in type 1 muscle fibres 119 ), which involves a tonic motor nerve activity, may explain its resistance to cancer cachexia, also suggesting that skeletal muscle phenotype, function, and pattern of neuronal innervation are critical in determining skeletal muscle sensitivity to cancer cachexia.
Skeletal muscle atrophy and metastasis
A higher incidence of metastasis (the dissemination of tumour cells from the primary site in distant organs) has been reported in pancreatic cachectic cancer patients compared with non‐cachectic patients. 120 Similarly, lung cancer patients with metastases developed cachexia more often than patients without metastases. 121 It is thus generally considered that cachexia is a hallmark of metastatic cancer and the main feature of terminal metastatic cancer. However, if the presence of metastasis is associated with a higher incidence of cancer cachexia, it is not clear whether the extent of skeletal muscle depletion is increased in metastatic cancer patients. Animal studies provide important complementary information. Although largely underestimated (the vast majority of cancer cachexia studies in animals are not conducted in a metastatic context 122 ), some recent studies covering this topic showed that skeletal muscle mass loss is exacerbated in metastatic compared with non‐metastatic C26 123 or HCT116 124 tumor‐bearing mice. Therefore, metastasis would aggravate the cachectic phenotype in animal models of cancer cachexia. This also illustrates the need to use in vivo metastatic models for the study of cancer cachexia to more closely mimic the diversity and complexity of cancer cachexia observed in human patients.
Skeletal muscle atrophy and chemotherapy
The depletion of the skeletal muscle compartment may also have important implications for anticancer drug toxicity. It has thus been shown that patients with depletion of skeletal muscle mass presented higher chemotherapy‐related toxicity compared with patients with larger amounts of lean body mass. 11 , 14 , 15 , 33 , 36 , 125 , 126 Those patients are forced to reduce dosage or to delay the cycles of administration and ultimately have worsened outcomes. 125 Chemotherapy also triggers very debilitating side effects, including unintentional body weight loss that primarily affects skeletal muscle. Therefore, understanding the complex interplay between chemotherapy, skeletal muscle toxicities, and cachexia is an important issue. Some recent studies addressed this question by determining the effects of chemotherapy itself on skeletal muscle or by studying the combined effects of tumour growth and chemotherapy on skeletal muscle. Administration of FOLFOX (5‐fluorouracil, leucovorin, oxaliplatin) or FOLFIRI (5‐fluorouracil, leucovorin, CPT‐11) in originally healthy mice triggers a loss of muscle mass and an increase in muscle weakness. 127 , 128 , 129 Similar observations have been reported using carboplatin, 130 , 131 cisplatin, 132 , 133 , 134 doxorubicin, 135 , 136 , 137 and 5‐fluorouracil. 138 Injection of gemcitabine and cisplatin in mice induced an even greater decrease in skeletal muscle mass than an orthotopic injection of tumour cells alone. 139 One should note however that if some studies used similar dosages to that encountered in human patients, 127 , 128 , 129 , 132 , 133 , 134 others used higher dosages. 130 , 131 , 135 , 136 , 138 Furthermore, the drug is generally more frequently administered in animal studies compared with human patients leading to cumulative higher dosages. Overall, this may contribute to exacerbate the extent of skeletal muscle mass loss. In tumour‐bearing mice, the combination of cancer cachexia and FOLFIRI administration resulted in a more severe depletion of quadriceps muscle mass compared with cancer cachexia or FOLFIRI alone. 94 Similar observations were also reported in tumour‐bearing mice with the use of cisplatin 140 and doxorubicin. 141 Therefore, and while chemotherapy is obviously dispensable to combat tumour growth, these data from animal studies strongly suggest that chemotherapy potentially also plays a causative role in the occurrence of skeletal muscle loss and weakness during cancer cachexia. Developing strategies that preserve skeletal muscle mass would therefore counteract both chemotherapy‐related toxicity and deleterious effects of chemotherapy on skeletal muscle.
Skeletal muscle fibre typology, size, and number during cancer cachexia
Skeletal muscle fibre type
Skeletal muscle fibres are heterogeneous with respect to their expression of myosin heavy chain isoforms conferring them distinct contractile properties. 142 Based on myosin heavy chain isoform expression, it is possible to distinguish type 1, 2A, 2X, and 2B (absent in human) fibres. The relative proportion of each fibre type inside a muscle differs according to species, muscle function, and innervation but also reflects dynamic adaptations to whole‐body energy metabolism, neuromuscular activity, and muscle injuries. 142
If one study reported a shift towards an increase in the proportion of fast myosin heavy chain isoform in cachectic cancer patients, 143 all other studies indicated that the distribution between type 1 and type 2 skeletal muscle fibres remained unchanged in cachectic cancer patients when compared with either non‐cachectic cancer patients 54 , 144 , 145 or healthy control subjects 62 , 146 (Table 1). Similarly, in animal models of cancer cachexia, a large majority of studies did not find any difference in fibre type distribution in the gastrocnemius, 77 , 82 , 111 , 118 , 147 , 148 , 149 plantaris, 82 tibialis anterior, 80 , 99 , 111 , 150 diaphragm, 96 , 99 , 118 , 147 , 148 , 149 extensor digitorum longus, 111 quadriceps, 151 and soleus 111 muscles (Table 2). However, some animal studies reported an increase in the proportion of type 2A and 2B muscle fibres together with a concomitant decrease in the proportion of type 1 fibres in the slow‐twitch oxidative soleus muscle, in cachectic cancer mice compared with control mice. 78 , 82 , 100 A transition from type 2A towards type 2B has also been reported in the soleus 100 , 152 and tibialis anterior 153 muscles of cachectic cancer mice. Differences between studies could be explained by the physiological function of the muscle (postural/anti‐gravitational such as soleus vs. locomotor such as gastrocnemius) and the extent of cachexia. From a teleological point of view, a shift from slow to fast fibre type may lead to higher and faster force production (at the expense of greater fatigability), which may tentatively compensate for the whole decrease in muscle force due to muscle mass loss (see succeeding text). If the existence of a slow‐to‐fast fibre type shift needs to be further strengthened, such a shift could reflect an altered neuromuscular control, as the innervation pattern and neuromuscular junction integrity are essential for conferring contractile and metabolic characteristics to the muscle fibre. 154 This hypothesis is supported by observations showing that expression of denervation markers was increased in skeletal muscle of C26 tumour‐bearing mice, which is consistent with the notion that muscle fibre denervation may contribute to muscle wasting during cachexia. 155 Although less convincing, fairly similar observations were also reported in skeletal muscle of cancer cachectic patients compared with non‐weight‐losing cancer patients and control subjects. 155 However, a recent study showed that neuromuscular junction morphology and structural integrity are conserved between control subjects, weight‐stable cancer patients, and cachectic cancer patients. 156 Although a molecular characterization of denervation markers was not performed in this study, the absence of neuromuscular junction pathology is in contrast to what is found in the aforementioned study in cachectic C26 tumour‐bearing mice. 155 Electromyographic studies would be helpful to provide complementary information about the functionality of motoneurons. Therefore, whether cancer cachexia is associated with alteration in the innervation pattern and neuromuscular junction fragmentation of the postsynaptic membrane remains to be further explored.
Table 1.
Effect of cancer cachexia on muscle fibre cross‐sectional area and muscle fibre type distribution in human cancer patients
| Ref. | Skeletal muscle | Cancer type | Definition of cachexia | Control subjects | Fibre cross‐sectional area | Fibre type distribution | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2A | 2X | 1 | 2A | 2X | |||||
| Johns et al. 144 | Rectus abdominis | Gastrointestinal or pancreatic (n = 17) | Stature adjusted SMI consistent with low muscularity and weight loss >2% over past last 6 months | Gastrointestinal or pancreatic cancer age‐paired patients without cachexia (n = 24) | −26% | −26% | = | = | ||
| Weber et al. 62 | Vastus lateralis | Gastric, pancreatic, colon, bronchogenic carcinoma, chronic lymphatic leukaemia (n = 11) | Weight loss >10% over past last 6 months | Age‐paired, gender‐paired, and body weight‐paired healthy controls (n = 15) | = | = | −44% | = | = | = |
| Weber et al. 57 | Vastus lateralis | Gastrointestinal (n = 19) | Weight loss >10% over past last 6 months | Age‐paired, gender‐paired, and body height‐paired healthy controls (n = 19) | −32% | Not reported | ||||
| Schmitt et al. 145 | Rectus abdominis | Pancreatic carcinoma (n = 8) | Weight loss >10% over past last 6 months before surgery | Pancreatic carcinoma or chronic pancreatitis age‐paired patients without cachexia (n = 8) | Not reported | = | = | |||
| Zhang et al. 157 | Rectus abdominis | Gastric (n = 13) | Weight loss >5% over past last 6 months before surgery + SMI < cut‐off values | Gastric cancer patients with weight loss <5% over past last 6 months before surgery + SMI > cut‐off values (n = 10) | −72% | Not reported | ||||
| Op den Kamp et al. 54 | Quadriceps | Non‐small cell lung (n = 16) | International consensus 1 | Non‐small cell lung cancer patients without cachexia (n = 10) | −20% | = | = | = | ||
| Healthy age‐paired and gender‐paired controls (n = 22) | −17% | −35% | = | = | ||||||
| Zampieri et al. 220 | Rectus abdominis | Colorectal (n = 10) | Cachectic patients even if the clinical criteria used for cachexia are not presented | Patients without neoplasia (n = 7) | = | Not reported | ||||
| Puig‐Vilanova et al. 146 | Vastus lateralis | Lung cancer (n = 10) | International consensus | Healthy sedentary controls (n = 10) | = | −24% | = | = | ||
Muscle fibre types were classified as type 1, type 2A, and type 2X according to the expression of myosin heavy chain isoforms. Ref., reference; SMI, skeletal muscle index.
Table 2.
Cancer cachexia model
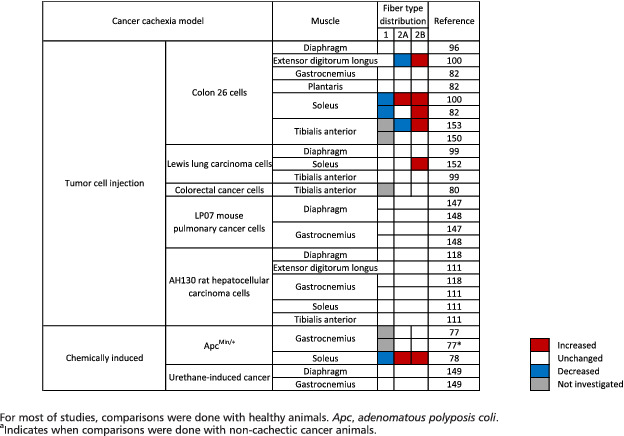
Skeletal muscle fibre size
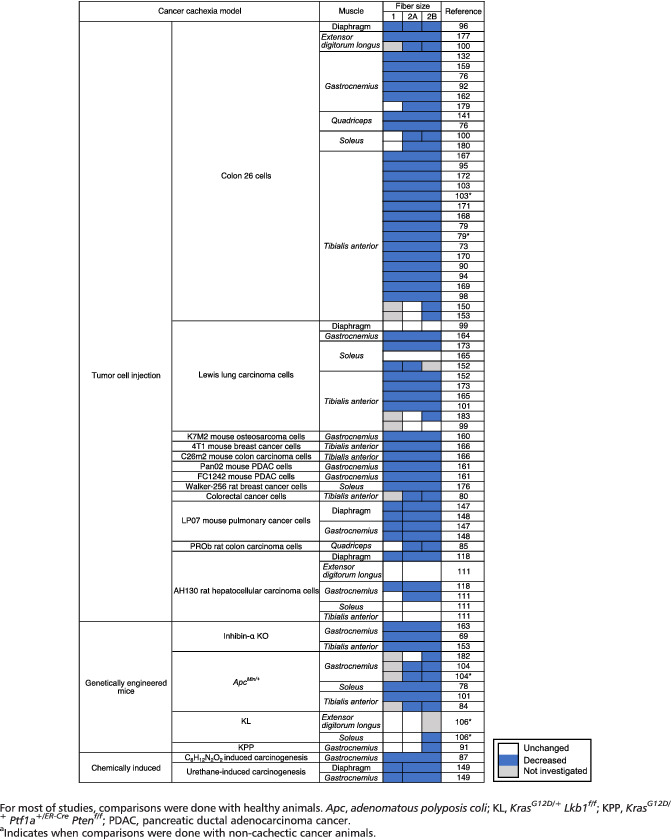
Skeletal muscle fibre cross‐sectional area is decreased in cachectic cancer patients, when compared with healthy subjects, 55 , 57 non‐cachectic cancer patients, 55 , 157 or atherosclerotic patients 158 (Table 1). A reduction in fibre cross‐sectional area has also been consistently reported in cachectic cancer mice compared with control mice in the quadriceps, 76 , 141 gastrocnemius (−28% to −62%), 69 , 76 , 92 , 132 , 159 , 160 , 161 , 162 , 163 , 164 tibialis anterior (−22% to −40%), 79 , 90 , 94 , 95 , 97 , 98 , 101 , 114 , 117 , 150 , 152 , 153 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 soleus (−24% to −40%), 78 , 152 , 173 , 176 and extensor digitorum longus (−28%) 112 , 177 muscles (Table 3). Similar results have also been reported in cachectic cancer rats. 87 , 112
Table 3.
Muscle fibre cross‐sectional area in mice and rat models of cancer cachexia
The size of muscle fibres expressing type 1 myosin heavy chain (the slow contractile isoform) is decreased by about 26% in skeletal muscle of cachectic compared with non‐cachectic cancer patients, 144 as well as between cancer patients and healthy control subjects. 54 , 178 Similarly, the cross‐sectional area of type 1 fibres is also decreased by about 30% in the gastrocnemius, 118 , 147 , 148 , 149 diaphragm, 96 , 118 , 147 , 148 , 149 and soleus 152 muscles of cachectic cancer mice or rats compared with control animals. However, some studies did not report any change in type 1 fibre size in cachectic cancer patients when compared with either non‐cachectic cancer patients 54 or healthy subjects. 62 , 146 Similar results have also been reported in cachectic cancer animals vs. control animals. 85 , 91 , 99 , 100 , 106 , 111 , 179 , 180 These discrepancies between studies regarding the extent of the decrease in type 1 fibre cross‐sectional area could be explained by the tumour localization, the severity of cachexia, the typology and function of the analysed skeletal muscles, and also the criterion used to define cachexia. For instance, Johns et al. 144 showed that type 1 fibre cross‐sectional area was significantly lower in cancer patients with cachexia compared with non‐cachectic cancer patients only when considering low muscularity plus body mass loss as a criterion for cachexia, while no difference was observed when using either body mass loss only or low muscularity as a criterion of cachexia.
The cross‐sectional area of type 2 muscle fibres (expressing either type 2A or type 2X myosin heavy chain isoforms) is smaller in the quadriceps muscle of cachectic cancer patients. 54 Similar observations have been also reported in the diaphragm 147 , 148 , 149 and gastrocnemius 147 , 148 , 149 , 179 muscles of cachectic cancer mice, as well as in the extensor digitorum longus, 112 diaphragm, 118 and gastrocnemius 111 , 118 muscles of cachectic cancer rats. If some studies show that muscle fibre atrophy is not fibre type dependent in both patients 54 , 144 , 178 and animals, 96 , 118 , 147 , 148 , 149 , 152 , 174 , 175 a preferential atrophy of type 2 muscle fibres has been reported in a biceps muscle biopsy from a cachectic cancer patient 181 and more recently in the quadriceps muscle of cachectic cancer patients that were compared with healthy patients. 146 Similarly, animal studies also indicate that type 2 muscle fibres are more prone to atrophy than type 1 muscle fibres. 85 , 100 , 111 , 179 , 180 Therefore, despite controversies, preferential atrophy of type 2 muscle fibres could occur during cancer cachexia, suggesting that the contractile and metabolic phenotypes of skeletal muscle fibre types may affect their sensibility to cancer cachexia. When specifically looking at the effect of cancer cachexia on type 2A and type 2X muscle fibres, human studies indicate that the decrease in the cross‐sectional area of type 2 muscle fibres is similar in type 2A 144 , 178 and type 2X 62 muscle fibres of cachectic cancer patients compared with healthy controls. Animal studies also show a similar reduction in the cross‐sectional area of type 2A muscle fibres 80 , 84 , 96 , 100 , 104 , 106 , 152 and type 2B 80 , 84 , 91 , 96 , 100 , 104 , 182 in cachectic cancer mice. Similar results have also been reported in skeletal muscle of cachectic cancer rats. 85 However, it should be noted though that some animal studies reported a greater decrease in fibre cross‐sectional area of type 2B compared with type 2A muscle fibres, 91 , 104 , 106 , 150 , 153 , 182 , 183 suggesting greater sensitivity of type 2B muscle fibres to cachexia.
Skeletal muscle fibre number
Skeletal muscle mass also depends on the number of muscle fibres. However, cancer cachexia does not seem to involve a decrease in muscle fibre number. The number of muscle fibres is similar in the vastus lateralis of cachectic cancer patients and healthy control subjects, 57 as well as the number of type 1 and type 2A muscle fibres in the rectus abdominis muscle of cachectic and non‐cachectic cancer patients. 144 However, the count of muscle fibres was expressed per square millimetre, 57 , 144 which obviously did not allow the determination of the absolute number of fibres in the muscle. In a mouse model of cancer cachexia, it has also been reported that the whole number of tibialis anterior muscle fibres was unchanged between control and cachectic cancer mice. 99 Therefore, the decrease in muscle mass during cancer cachexia would be mainly due to a decrease in muscle fibre volume rather than a decrease in muscle fibre number. If the number of muscle fibres seems to be unchanged during cancer cachexia, nuclear death by apoptosis may occur locally along the muscle fibre. Although not systematically observed, 184 the presence of extrafibre and intrafibre apoptotic nuclei has been reported in the muscle of cachectic cancer patients, 185 , 186 as well as in muscles of cachectic cancer mice and rats. 99 , 104 , 115 , 116 , 118 , 147 , 148 , 149 However, apoptosis does not seem to be high enough to decrease the whole number of muscle fibres, so that these apoptotic events may rather weaken the muscle fibre locally, rendering it more sensitive to micro‐injuries (see succeeding text).
Skeletal muscle metabolic phenotype during cancer cachexia
Skeletal muscle metabolism in human cancer patients
The diversity between muscle fibres is not restricted to the expression of myofibrillar proteins but also extends to the energetic metabolic characteristics of the fibre. Only a limited number of studies have explored the metabolic phenotype of skeletal muscle in cancer patients. The balance between oxidative and glycolytic metabolisms seems to be maintained in skeletal muscle of cachectic cancer patients as indicated by a similar ratio of oxidative‐to‐glycolytic enzyme activities in the quadriceps muscle of cachectic cancer patients compared with that of patients without cachexia and healthy subjects. 54 Regarding mitochondrial content, studies provide contrasted results. Electron microscopy analyses showed unchanged mitochondrial density and diameter in skeletal muscle of cachectic cancer patients compared with healthy controls, 146 or increased intermyofibrillar mitochondrial area in skeletal muscle of cachectic cancer patients compared with non‐cachectic patients. 186 This latter result may be explained by mitochondrial swelling and/or by a relative increase in mitochondrial density due to a faster and greater reduction in myofibrillar protein loss. In contrast, a pioneering case study 181 reported a decrease in the number of skeletal muscle mitochondria in a cachectic cancer patient. Finally, a biomolecular analysis indicated that the mitochondrial DNA copy number is unchanged in skeletal muscle of cachectic and non‐cachectic cancer patients. 186 Regarding mitochondrial morphology, the presence of mitochondria with swollen appearance and the absence of cristae 157 have been reported, suggesting an impairment in mitochondrial function. Clearly, the number of studies aimed at exploring energetic metabolism and mitochondrial structure and function in skeletal muscle of human cachectic cancer patients is too limited to draw an accurate picture of the metabolic phenotypic alterations that occurs during cancer cachexia. An in‐depth analysis of metabolism in skeletal muscle of cachectic cancer patients is therefore necessary.
Skeletal muscle metabolism in animal models of cancer cachexia
Animal studies provide important complementary information and allow us to draw a more precise picture of the regulation of energetic metabolism in skeletal muscle during cancer cachexia (Figure 1). The mitochondrial DNA‐to‐nuclear DNA ratio is lower in cachectic cancer mice compared with non‐cachectic cancer mice, 77 , 148 , 187 suggesting a decrease in mitochondrial content. This reduction was associated with body mass loss. 77 , 187 A lower mitochondrial content has also been reported in skeletal muscle of cachectic cancer rats compared with control rats. 112 As observed in human patients, mitochondria also present an altered morphology 187 with a swollen appearance, 112 , 170 , 180 , 188 , 189 a smaller size, 187 and the presence of electron‐lucent areas, 112 , 180 , 189 which all together indicate an alteration in the mitochondrial network and function. Biochemical analyses corroborate ultrastructural information. A reduction in the metabolic flux throughout the Krebs cycle, 188 which has also been confirmed by metabolomic approaches, 94 , 190 , 191 and a decrease in the activities of complexes I, 135 , 192 II, 80 , 99 , 135 , 170 , 192 , 193 III, 193 IV, 72 , 85 , 192 , 193 , 194 and V 72 , 87 have thus been consistently described. Accordingly, mitochondrial respiration rate is also reduced in isolated mitochondria from skeletal muscle of cachectic cancer mice compared with control mice. 192 , 195 , 196 Therefore, animal studies clearly indicate that the entire mitochondrial oxidative pathway is profoundly impaired by cancer cachexia.
Figure 1.
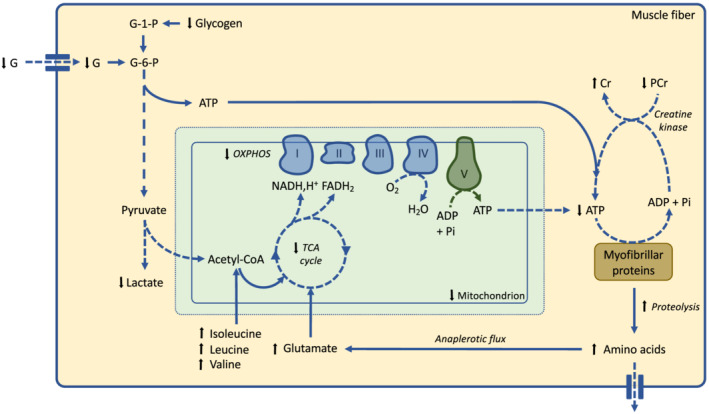
Effects of cancer cachexia on skeletal muscle energetic metabolism. This schematic representation is only based on animal studies. Reduced glycogen and glucose contents in skeletal muscle contribute to alter glycolysis flux. A decrease in mitochondrial content and mitochondrial oxidative phosphorylation, together with a decreased in ATP synthesis from phosphocreatine system, leads to a reduced ATP content in skeletal muscle. Skeletal muscle proteins are degraded and subsequently processed into amino acids. Individual amino acids can be either exported outside the muscle fibre or transported into the mitochondria (anaplerotic flux). Fatty acid metabolism is not represented because there is currently no study available. Black arrows indicate variations in metabolite concentration. Dashed arrow indicates experimentally demonstrated reduction in the corresponding metabolic pathway. Cr, creatine; G, glucose; G‐1‐P, glucose‐1‐phosphate; G‐6‐P, glucose‐6‐phosphate; OXPHOS, oxidative phosphorylation; PCr, phosphocreatine; TCA, tricarboxylic acid cycle.
The oxidative activity of a muscle fibre is also tightly associated with its capillary density, 197 which allows oxygen supply and substrate delivery to the muscle fibre. Although data are scarce, cancer cachexia does not seem to impact capillary density of cachectic cancer patients. 57 In animals, a study reported an increased number of blood vessels in skeletal muscle of cachectic cancer mice. 179 This would not be true angiogenesis but would rather reflect a relative increase in capillary density because of the reduction in muscle fibre cross‐sectional area.
The decrease in oxidative activity is also associated with a decrease in skeletal muscle ATP content, 94 , 170 , 198 a decrease in creatine phosphate concentration, 94 a decrease in glucose concentration, 92 , 199 a decrease in glycogen store, 92 a decrease in glycolysis, 92 , 190 , 199 and a reduction in lactate concentration, 199 which together may contribute to reducing skeletal muscle to synthesize ATP during contraction. Importantly, the decrease in skeletal muscle capacity to generate ATP 113 , 188 may also alter its capacity to perform other essential ATP‐dependent cellular works involved in skeletal muscle homeostasis such as the maintenance of mineral balance.
Amino acid metabolism
The intense catabolism of skeletal muscle proteins during cancer cachexia 71 , 89 , 90 , 105 , 107 , 109 , 110 , 147 , 148 , 200 , 201 , 202 , 203 , 204 , 205 , 206 , 207 , 208 , 209 also raises the question of the metabolic fate of amino acids. Cancer cachexia is associated with an alteration in skeletal muscle amino acid profile, 151 , 199 as well as an altered concentration of circulating amino acids. 83 , 210 In cachectic skeletal muscle, increased provision of glutamate 199 may increase the anaplerotic flux to the Krebs cycle (α‐cetoglutarate production). Similarly, branched chain amino acids may be converted to acetyl‐CoA and then enter the tricarboxylic acid cycle. 199 Furthermore, a recent in vivo metabolomic study revealed that mitochondrial dysfunction in cachectic skeletal muscle tissue also seemed to influence amino acid metabolism. 151 Considering the whole decrease in mitochondrial activity that occurs during cancer cachexia, major adjustments of metabolic fluxes may occur, so that the muscle fibre may adapt skeletal muscle proteolysis.
Amino acids can be also released into the blood compartment, interconverted into gluconeogenic amino acids by the liver, and recycled into glucose through hepatic gluconeogenesis. 211 This may thus rewire amino acid metabolism to promote glucose supply for tumour growth. 47 , 212 Under stress conditions, hepatic protein synthesis also shifts from constitutive proteins (albumin, transferrin) to inflammatory acute‐phase proteins, such as C‐reactive protein, fibrinogen, or serum amyloid A1. How the acute‐phase response is linked to the development of skeletal muscle atrophy during cancer cachexia is not clear, but it has been hypothesized that skeletal muscle can provide the amino acids required for the synthesis of hepatic positive acute‐phase proteins. 210 , 213 Bonetto et al. 214 also showed that cancer cachexia in mice was associated with an increase in liver fibrinogen protein content but more surprisingly also by a strong elevation of fibrinogen and serum amyloid A1 protein content in skeletal muscle. Sustained synthesis of acute‐phase proteins in liver and skeletal muscle may thus represent a spillway for amino acids derived from skeletal muscle proteolysis. 210 , 213 A better knowledge of the contribution of skeletal muscle amino acid metabolism to whole‐body metabolism during cancer cachexia is needed.
Mononucleated cell niche of skeletal muscle fibre during cancer cachexia
The micro‐environment of skeletal muscle fibres contains different mononucleated cell types that are important for skeletal muscle repair and that contribute to skeletal muscle diversity. Upon muscle injury, quiescent resident satellite cells get activated, proliferate, and then fuse with pre‐existing damaged muscle fibres to rebuild new functional fibres. 215 Activation of satellite cells, proliferation of myoblasts, and their differentiation into myotubes require the presence of pro‐inflammatory macrophages (M1) and their transition into anti‐inflammatory macrophages (M2), with M1 and M2 macrophages stimulating, respectively, the early and late phases of regeneration. 215 Fibro‐adipogenic progenitors, fibroblasts, and endothelial cells are also important to achieve proper skeletal muscle regeneration. 215
Pioneering works from Jewesbury and Topley at the beginning of the 20th century 216 and later by Marin and Denny‐Brown 158 already indicated that skeletal muscle fibres from cachectic cancer patients had more nuclei in the vicinity of the sarcolemma even though the intrafibre or extrafibre localization of nuclei was not determined in these studies. Much more recently, cancer cachexia in pancreatic cancer patients was associated with a type of muscle damage characterized by the activation of both satellite cells and non‐satellite muscle progenitor cells. 217 The extent of the activation correlated with body mass loss. 217 The presence of inflammatory cells, 146 macrophages, and fibro‐adipogenic progenitors 218 was also reported in skeletal muscle of cachectic cancer patients. In animal models of cancer cachexia, skeletal muscle also contains a higher number of activated satellite cells and non‐satellite muscle progenitor cells, 217 undifferentiated cells, 73 , 160 and inflammatory cells. 78 , 118 , 147 , 148 , 149 , 219 In contrast, it has been reported that the cachectic muscle of tumour‐bearing mice was enriched in haematopoietic stem cells, but not in inflammatory cells. 167 Together, these data from human and animal studies suggest that an alteration in the mononuclear cell profile of skeletal muscle fibre micro‐environment, and particularly the persistence of inflammatory cells, may contribute to skeletal muscle mass loss during cancer cachexia.
Indeed, a greater fragility of skeletal muscle to micro‐injuries and injuries in the cachectic muscle may lead to episodes of degeneration/regeneration, thus allowing the activation of satellite cells. The existence of ongoing episodes of regeneration in cachectic skeletal muscle is supported by the observation that skeletal muscle of cachectic cancer patients 218 , 220 , 221 and cachectic cancer mice or rats 78 , 118 , 147 , 148 , 149 displays a higher number of muscle fibres with centralized nuclei, indicating the presence of regenerating muscle fibres. Interestingly, internally located nuclei were predominantly found in type 2 muscle fibres, 220 which is consistent with the notion that these fibres would be more prone to cachexia. Importantly, satellite cells of cachectic cancer mice are able to commit to the myogenic program, but not to completely differentiate, as indicated by the persistent expression of Pax7. 217 Together with the deficiency of cancer cachectic skeletal muscle to regenerate after freeze clamping‐induced 70 , 217 or cardiotoxin‐induced 103 muscle injury, these data collectively indicate that an accumulation of unresolved/incomplete episodes of skeletal muscle repair could contribute to the loss of skeletal muscle mass and function during cancer cachexia. Finally, this response could also indicate the existence of a vain compensatory mechanism to limit the extent of skeletal muscle mass loss during cancer cachexia.
Other histological features of skeletal muscle during cancer cachexia
Endomysial space
Skeletal muscle from cachectic cancer patients displays an increased area occupied by collagen that positively correlates with weight loss and poor survival. 218 This increase in fibrosis is in agreement with the increase in endomysial space observed in skeletal muscle of cachectic cancer patients, 178 , 218 but not in non‐cachectic cancer patients. 218 This is also consistent with decreased mRNA level for matrix metalloproteinase 3, an enzyme involved in extracellular matrix protein breakdown. 222 A similar increase in collagen deposition was also reported in skeletal muscle of cachectic cancer mice, 160 , 223 together with an increased area of non‐contractile tissue that may reflect disrupted extracellular matrix remodelling. 84 The progressive development of fibrosis may be the long‐term consequence of an increase in the expansion and differentiation of fibro‐adipogenic progenitor in the cachectic muscle. 218 Surprisingly, a down‐regulation of extracellular matrix gene expression has been also reported in skeletal muscle of cancer cachectic patients 91 and animal models of cancer cachexia 91 , 166 , 172 , 214 , 223 , 224 , 225 , 226 , 227 , 228 with Col1a1, Col3a1, Col5a1, and Col5a2, the most frequently down‐regulated genes. Anyhow, it remains clear that extracellular matrix is markedly disorganized in cachectic skeletal muscle, which may likely alter the mechanical properties of skeletal muscle and may also contribute to render muscle fibres more susceptible to injuries.
Fat depot
Computerized tomography analyses of the third lumbar vertebra level show an increase in fat infiltration in skeletal muscle of cachectic cancer patients 12 , 65 , 229 that was not correlated with muscle mass loss. 230 An increase in the size and the number of lipid droplets 231 and an increase in the lipid content 218 have also been reported in skeletal muscle of cachectic cancer patients. This increase in lipid content in skeletal muscle of cachectic cancer patients is due to both an intramyocellular and an extramyocellular accumulation of triglycerides. 232 The expansion and differentiation of fibro‐adipogenic progenitors, which occur in skeletal muscle of cachectic cancer patients, 218 may explain the accumulation of extramyocellular lipids. It has also been proposed that impaired lipid oxidation due to altered mitochondrial function may contribute to the accumulation of intramyocellular lipid droplets in skeletal muscle fibres of cachectic cancer patients. 230 It may be noted that one study reported a decreased lipid content in the slow‐twitch muscle fibres of cachectic patients with late‐stage non‐small‐cell lung cancer. 233 In animals, lipid accumulation in the skeletal muscle fibres of cachectic cancer mice has been also reported. 188 Consequently, an increase in fat depot appears to be a common histological feature of cachectic skeletal muscle in both patients and animal models, but its consequence on skeletal muscle function has to be further explored.
Altered myofibrillar structure
Sarcolemmal alterations, 146 , 157 , 218 which heighten with body mass loss, 217 an increase in the number of damaged 218 and shrunken 178 fibres, a loss of normal cross‐striation pattern, 158 , 178 , 181 , 218 disrupted triads, 186 and a dilated sarcoplasmic reticulum, 181 have been observed in skeletal muscle of cachectic cancer patients. Similarly, a disorganization of the sarcolemma 76 , 179 and basement membrane, 97 , 179 , 224 an alteration of sarcomere structure, 97 , 180 , 188 , 189 disrupted triads, 170 and a dilated sarcoplasmic reticulum 112 have been also reported in skeletal muscle of animal models of cancer cachexia. These structural alterations may impact membrane excitability, calcium transient frequency, and cross‐bridge kinetics, which are major determinants of myofibre contractile performance (see succeeding text).
Figure 2 sums up the most important cellular and subcellular phenotypic changes in skeletal muscle during cancer cachexia.
Figure 2.
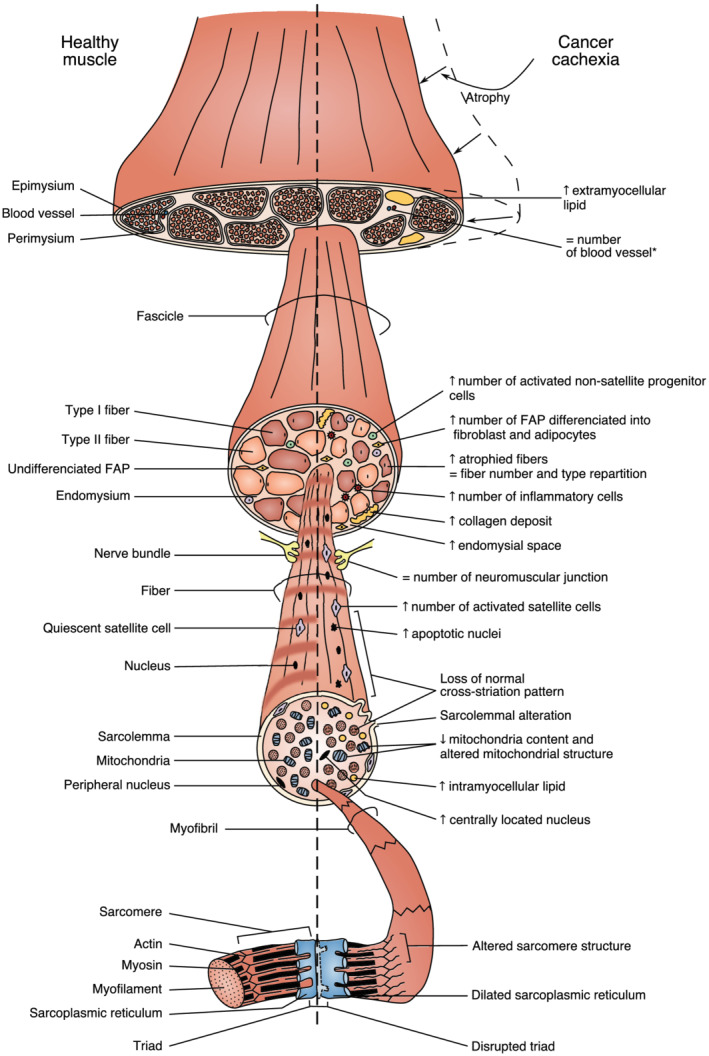
Schematic overview of the main cellular and subcellular phenotypic changes that occur in cachectic cancer skeletal muscle. This schematic representation is based on the analysis of both human and animal studies. Arrows indicate either an increase or a decrease for the corresponding parameters during cancer cachexia. =, the parameter is unchanged with cancer cachexia. * denotes a phenotypic change that has only been reported in animal models of cancer cachexia. FAP, fibro‐adipogenic precursor.
Skeletal muscle function during cancer cachexia
The main function of skeletal muscle is to generate force to maintain posture and produce movement. Any alteration in skeletal muscle mass, metabolism, structure, and organization may profoundly alter its capacity to generate force. The data reported earlier in human patients and in animal models of cancer cachexia highlighting marked alterations in skeletal muscle mass and metabolism indicate that the function of cachectic skeletal muscle must be profoundly modified.
Decrease in skeletal muscle force
Cachectic cancer patients have a lower handgrip force (−7% to −31%) than cancer patients without cachexia. 7 , 10 , 12 , 25 , 32 , 53 Absolute isometric muscle force of knee extensors 55 , 57 , 61 , 146 and knee flexors 55 has also been consistently reported to be lowered in cachectic cancer patients compared with healthy subjects, as well as compared with non‐cachectic cancer patients. 55 The isokinetic force of knee extensors and knee flexors is also reduced in cachectic cancer patients when compared with healthy subjects 54 , 57 or non‐cachectic cancer patients. 54 Accordingly, the speed of contraction is also lower in men, but interestingly not in women, cachectic cancer patients, when compared with gender‐paired healthy control subjects. 61
In murine models of cancer cachexia, a decrease in grip force (−9% to −40%) has also been observed in cachectic cancer mice or rats when compared with control animals. 69 , 73 , 74 , 79 , 80 , 92 , 94 , 95 , 101 , 108 , 150 , 152 , 161 , 162 , 166 , 170 , 177 , 192 , 196 , 207 , 234 , 235 , 236 , 237 , 238 , 239 , 240 , 241 We can note that this decrease was not observed when cachectic cancer mice were compared with mild‐cachectic mice, 79 indicating that moderate cachexia is already associated with functional alterations of skeletal muscle. Maximal contraction force of the extensor digitorum longus, 67 , 81 , 91 , 97 , 100 , 242 soleus, 67 , 97 , 100 , 135 tibialis anterior, 79 , 99 , 102 , 153 and diaphragm 96 muscles are also decreased in cachectic cancer mice compared with control mice, as well as in cachectic cancer mice compared with non‐cachectic cancer mice. 102 Interestingly, skeletal muscles of cachectic cancer mice with a majority of fast fibres, such as extensor digitorum longus, are more prone to a decrease in force, 67 further strengthening the notion that type 2 fibres are more impacted by cachexia. Although some authors did not find any difference, 79 , 242 the speed of muscle contraction and relaxation in response to a single twitch stimulation is also reduced in the tibialis anterior 102 and extensor digitorum longus 81 , 100 muscles of cachectic cancer mice when compared with control mice. Similar results have been obtained when the comparison was done with non‐cachectic cancer mice. 102 nterestingly, the extent of the decrease in speed contraction correlated with body mass loss. 102
Taken together, all these data emphasize that skeletal muscle force could be an important criterion to diagnose cachexia. In this context, it is essential to know the kinetic of skeletal muscle force decrease, because if the loss in muscle force occurs before the loss in muscle mass during cancer cachexia, as it is observed during the geriatric muscle mass loss, 243 the measurement of muscle force would be a precocious and easily measurable predicting factor of cancer cachexia in human cancer patients. However, there is limited information on this topic. An 8 week follow‐up study reported that isometric quadriceps and hamstring muscle force, as well as handgrip force, were stable in cachectic cancer patients, but skeletal muscle mass was not determined in this study. 244 More studies are necessary to provide comparative analyses of the time‐course changes in the loss of skeletal muscle mass and function in human cancer patients. Finally, an increase in muscle fatigue may also contribute to decrease muscle force in cancer cachectic patients. Some studies reported an increase in muscle fatigue that was attributed to an increased central fatigue, 245 , 246 , 247 but no information about the cachectic status of the cancer patients was reported. Therefore, studies aimed at exploring muscle fatigue would be very helpful to further understand the aetiology of the decrease in skeletal muscle force in cancer cachectic patients. In animal models of cancer cachexia, muscle fatigue has been consistently reported to increase. 79 , 80 , 97 , 100 , 102 , 150 , 221 , 242 Importantly, muscle fatigue also correlated with body mass loss. 80
Potential factors involved in muscle force decrease
Beyond the description remains the question of the mechanisms involved in the loss of skeletal muscle force production in the cachectic muscle. A decrease in muscle mass can obviously contribute to explain the decrease in muscle force in human cancer patients. 57 However, when muscle force is normalized to muscle cross‐sectional area or body mass, difference in muscle force between cachectic cancer patients and control subjects still persists. 61 Similarly, in mice models of cancer cachexia, if a decrease in muscle force can be attributed to a loss of body mass 74 or muscle mass 242 or a decrease in skeletal muscle cross‐sectional area, 79 , 91 , 97 , 99 , 135 the loss in muscle force persists even after normalization by body mass, 79 muscle mass, 81 or muscle fibre cross‐sectional area 96 , 100 , 102 or both. 67 Collectively, these data indicate that muscle mass loss does not account entirely for the decrease in force and support the conclusion that cancer cachexia is also associated with intrinsic contractile dysfunctions (i.e. diminished function per unit muscle size).
Factors required for co‐ordinated muscle contractile function involve neuromuscular junction integrity, excitation–contraction coupling, calcium handling, sarcomere structure, and energetic metabolism. 142 Previous studies indicate that the decrease in muscle force would not involve an alteration in neuromuscular junction as cancer cachexia seems to affect neither muscular nor intramuscular nerve bundles in patients, 158 , 181 nor neuromuscular junction integrity, 156 or the number of neuromuscular junctions in the muscle of cachectic cancer mice. 167 However, this concept has recently been questioned at least in C26 tumour‐bearing mice. 155 Anyhow, an in‐depth analysis of skeletal muscle junction and neuromuscular coupling is necessary for cancer patients and animal models of cancer cachexia. Loss in muscle force can also result from impairment in calcium handling. Unexpectedly, isolated muscle fibres from cachectic cancer patients have an increased calcium sensitivity, 143 which could be explained by a shift from slow to fast myosin isoform expression, as type 2 muscle fibres are more calcium sensitive. 248 In contrast, muscle fibre calcium sensitivity is reduced in cachectic cancer mice compared with control mice. 96 Moreover, the calcium‐activated force and cross‐bridge kinetics are reduced in cachectic cancer mice compared with control mice, 96 strongly suggesting that the loss of skeletal muscle force can be due to an alteration of calcium handling. A recent study by Judge et al. also described an increase in calcium deposition in skeletal muscle of cachectic pancreatic cancer patients. 218 Calcium overload within skeletal muscle fibre may exert deleterious effects leading to muscle damage via the activation of calcium‐activated proteases (calpains) and the disruption of sarcolemma integrity. 249 Furthermore, calcium overload can be also sensed by mitochondria, which may further contribute to alter mitochondrial metabolism and worsen cellular damages. 249 Together, this may profoundly alter the capacity of the fibre to generate force. Therefore, and although several evidences suggest that calcium handling may be involved in the loss of muscle force, more investigations are required to clearly establish the role of altered calcium handling.
Further insights into the mechanisms involved in the loss of skeletal muscle force have been also provided by ex vivo analysis of the contractile properties of skeletal muscle fibres 178 or muscle fibre bundles 143 from cachectic cancer patients. The absolute 143 and specific 178 maximal force of isolated fibres has thus been shown to decrease in cachectic cancer patients. Furthermore, specific maximal force correlates with myosin‐to‐actin ratio, 178 indicating that the loss of contractile machinery is a factor contributing to decreased muscle force. However, one may remind that measuring the actin‐to‐myosin ratio is strongly dependent on the extraction conditions and may thus lead to misinterpretations. 250 Another study showed that single fibre isometric tension was reduced in type 2A fibres from non‐cachectic and cachectic cancer patients, which was explained by a reduction in the number of strongly bound cross‐bridges. 251 Myosin–actin cross‐bridge kinetics were also reduced in type 1 fibres from non‐cachectic and cachectic cancer patients. 251 Therefore, a reduction in myofilament protein function may be a potential molecular mechanism contributing to muscle weakness in human cancer patients. Finally, a decrease in the capacity of skeletal muscle to sustain ATP production by mitochondrial oxidative phosphorylation during contraction (see earlier discussion) could also contribute to decrease muscle force and increase muscle fatigue.
Summary and future perspectives
The purpose of this review was to specifically focus on the structure and function of cachectic skeletal muscle in human cancer patients and animal models of cancer cachexia. The extent of skeletal muscle mass loss has largely been described, and the consequences of cachexia on skeletal muscle function are now getting more and more documented. The loss of muscle mass is of course an important factor to consider when studying cancer cachexia, but qualitative factors such as changes in skeletal muscle metabolism, muscle fibre micro‐environment, fibrosis, neuromuscular junction, and sarcomere integrity, as well as calcium handling, are certainly involved in the impaired muscle function and need to be explored in details (Figure 3). A comparative analysis of the time‐course changes of these qualitative factors and skeletal muscle mass during cancer cachexia is also necessary.
Figure 3.
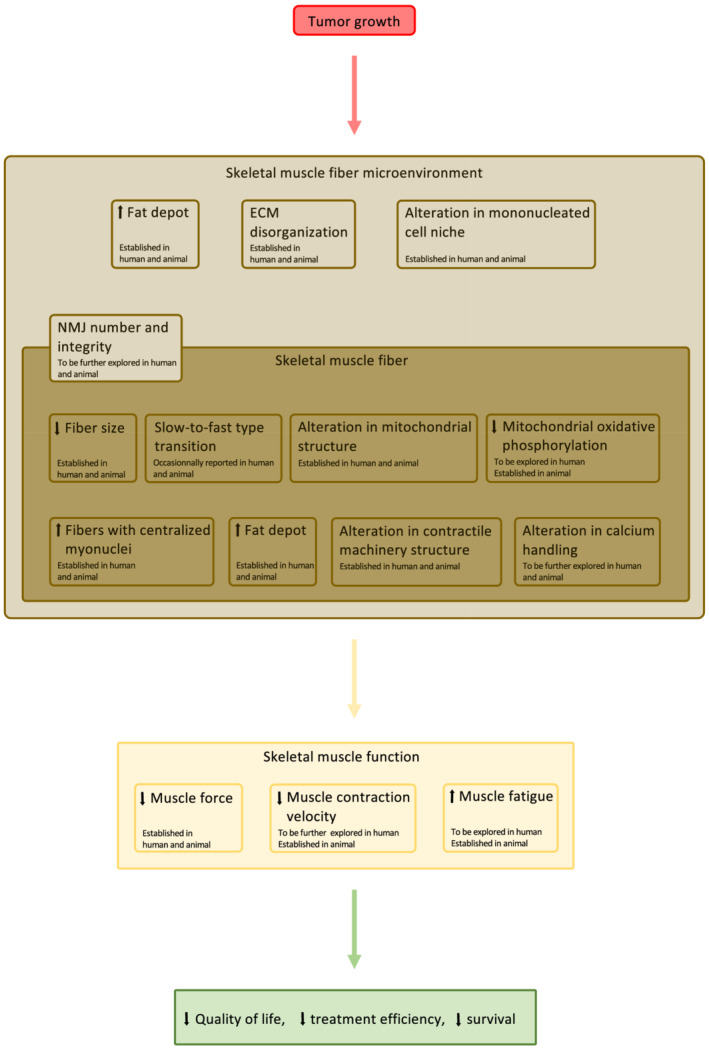
Functional relationship between skeletal muscle fibre phenotypic changes, skeletal muscle function, and patient's quality of life during cancer cachexia. ECM, extracellular matrix; NMJ, neuromuscular junction.
Our review also underlines important methodological aspects that may explain contrasted results between studies. For instance, the choice of the control group is quite heterogeneous between clinical studies (due to obvious constraints related to the recruitment of the subjects). Healthy control subjects, non‐cachectic cancer patients, and cachectic non‐cancer patients have thus been used. A careful analysis of the reference group is therefore necessary. Longitudinal analyses of the kinetic of the loss in skeletal muscle mass and function in cancer patients should be also preferred. This implicates that the detection of cachexia must be carefully and regularly performed with the use of solid image‐based analyses 4 , 37 and standardized functional tests. This also applies to animal models of cancer cachexia where time point analyses are essential to delineate the time‐course changes in the loss of skeletal muscle mass and function occurring during cancer cachexia. Non‐invasive measurement of skeletal muscle force, together with the use of image‐based analysis of skeletal muscle mass, such as computerized tomography or magnetic resonance imaging, should be therefore encouraged whenever it is possible. The analysis of a gender effect in the development of cancer cachexia has also to be explored in both human and animal studies. Differences in skeletal muscle physiology between species 142 , 252 , 253 must be also kept in mind when analysing and translating animal data to human patients, as animal models of cancer cachexia remain very different from human cancer cachexia. 239 , 254 The physiological status of the animal species is also very important to consider. Many studies, especially those using tumour‐bearing models of cancer cachexia, use young growing animals (2 to 3 months old). The strong stimulation of protein synthesis that supports skeletal muscle growth during the post‐natal period may mask/modify numerous cellular events. Non‐growing adult animals should be thus preferentially used when using tumour‐bearing animal models of cancer cachexia. Finally, our analysis emphasizes that measuring skeletal muscle force on a large epidemiologic scale by standardized functional tests is clinically fundamental to have a simple and robust mean to early diagnose cachexia in cancer patients. This could lead to proposing specific physical activity programs that may slow down the progression of cachexia and improve patient's quality of life.
Funding
A.M. was financially supported by the Ministère de l'Enseignement Supérieur de la Recherche et de l'Innovation. This work was supported by the Fondation ARC pour la Recherche sur le Cancer (PJA 2018 1207841).
Conflict of interest
A.M. and D.F. declare that they have no conflict of interest.
Acknowledgements
The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. 255
Martin A., and Freyssenet D. (2021) Phenotypic features of cancer cachexia‐related loss of skeletal muscle mass and function: lessons from human and animal studies, Journal of Cachexia, Sarcopenia and Muscle, 12, 252–273, 10.1002/jcsm.12678
References
- 1. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 2. Anker MS, Holcomb R, Muscaritoli M, von Haehling S, Haverkamp W, Jatoi A, et al. Orphan disease status of cancer cachexia in the USA and in the European Union: a systematic review. J Cachexia Sarcopenia Muscle 2019;10:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lieffers JR, Mourtzakis M, Hall KD, McCargar LJ, Prado CM, Baracos VE. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: contributions of organ and tumor mass to whole‐body energy demands. Am J Clin Nutr 2009;89:1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan BHL, Birdsell LA, Martin L, Baracos VE, Fearon KCH. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res 2009;15:6973–6979. [DOI] [PubMed] [Google Scholar]
- 5. Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol 2012;10:90–99. [DOI] [PubMed] [Google Scholar]
- 6. Takayama K, Atagi S, Imamura F, Tanaka H, Minato K, Harada T, et al. Quality of life and survival survey of cancer cachexia in advanced non‐small cell lung cancer patients—Japan nutrition and QOL survey in patients with advanced non‐small cell lung cancer study. Support Care Cancer 2016;24:3473–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fearon KC, Voss AC, Hustead DS. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr 2006;83:1345–1350. [DOI] [PubMed] [Google Scholar]
- 8. Blum D, Stene GB, Solheim TS, Fayers P, Hjermstad MJ, Baracos VE, et al. Validation of the Consensus‐Definition for Cancer Cachexia and evaluation of a classification model—a study based on data from an international multicentre project (EPCRC‐CSA). Ann Oncol 2014;25:1635–1642. [DOI] [PubMed] [Google Scholar]
- 9. Wallengren O, Lundholm K, Bosaeus I. Diagnostic criteria of cancer cachexia: relation to quality of life, exercise capacity and survival in unselected palliative care patients. Support Care Cancer 2013;21:1569–1577. [DOI] [PubMed] [Google Scholar]
- 10. Vanhoutte G, van de Wiel M, Wouters K, Sels M, Bartolomeeussen L, De Keersmaecker S, et al. Cachexia in cancer: what is in the definition? BMJ Open Gastroenterol 2016;3:e000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andreyev HJN, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer 1998;34:503–509. [DOI] [PubMed] [Google Scholar]
- 12. Loumaye A, de Barsy M, Nachit M, Lause P, Frateur L, van Maanen A, et al. Role of activin A and myostatin in human cancer cachexia. J Clin Endocrinol Metab 2015;100:2030–2038. [DOI] [PubMed] [Google Scholar]
- 13. Mason MC, Garcia JM, Sansgiry S, Walder A, Berger DH, Anaya DA. Preoperative cancer cachexia and short‐term outcomes following surgery. J Surg Res 2016;205:398–406. [DOI] [PubMed] [Google Scholar]
- 14. Ross PJ, Ashley S, Norton A, Priest K, Waters JS, Eisen T, et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer 2004;90:1905–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. da Rocha IMG, Marcadenti A, de Medeiros GOC, Bezerra RA, de Rego JFM, Gonzalez MC, et al. Is cachexia associated with chemotherapy toxicities in gastrointestinal cancer patients? A prospective study. J Cachexia Sarcopenia Muscle 2019;10:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schwarz S, Prokopchuk O, Esefeld K, Gröschel S, Bachmann J, Lorenzen S, et al. The clinical picture of cachexia: a mosaic of different parameters (experience of 503 patients). BMC Cancer 2017;17:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fouladiun M, Korner U, Gunnebo L, Sixt‐Ammilon P, Bosaeus I, Lundholm K. Daily physical‐rest activities in relation to nutritional state, metabolism, and quality of life in cancer patients with progressive cachexia. Clin Cancer Res 2007;13:6379–6385. [DOI] [PubMed] [Google Scholar]
- 18. Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior tochemotherapy in cancer patients. Am J Med 1980;69:491–497. [DOI] [PubMed] [Google Scholar]
- 19. Martin L. Diagnostic criteria for cancer cachexia: data versus dogma. Curr Opin Clin Nutr Metab Care 2016;19:188–198. [DOI] [PubMed] [Google Scholar]
- 20. Martin L, Birdsell L, MacDonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 21. Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, et al. Diagnostic criteria for the classification of cancer‐associated weight loss. J Clin Oncol 2015;33:90–99. [DOI] [PubMed] [Google Scholar]
- 22. Bachmann J, Heiligensetzer M, Krakowski‐Roosen H, Büchler MW, Friess H, Martignoni ME. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J Gastrointest Surg 2008;12:1193–1201. [DOI] [PubMed] [Google Scholar]
- 23. Vagnildhaug OM, Blum D, Wilcock A, Fayers P, Strasser F, Baracos VE, et al. The applicability of a weight loss grading system in cancer cachexia: a longitudinal analysis. J Cachexia Sarcopenia Muscle 2017;8:789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Naumann P, Eberlein J, Farnia B, Liermann J, Hackert T, Debus J, et al. Cachectic body composition and inflammatory markers portend a poor prognosis in patients with locally advanced pancreatic cancer treated with chemoradiation. Cancer 2019;11:1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen X, Zeng Y, Huang Y, Xu J, Meng W, Wang X, et al. Preoperative Cachexia predicts poor outcomes in young rather than elderly gastric cancer patients: a prospective study. Cancer Manag Res 2019;11:8101–8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Warren S. The immediate causes of death in cancer. Am J Med Sci 1932;148:610–615. [Google Scholar]
- 27. Fearon KCH, Preston T. Body composition in cancer cachexia. Infusionstherapie 1990;17:63–66. [DOI] [PubMed] [Google Scholar]
- 28. Cohn SH, Gartenhaus W, Sawitsky A, Rai K, Zanzi I, Vaswani A, et al. Compartmental body composition of cancer patients by measurement of total body nitrogen, potassium, and water. Metabolism 1981;30:222–229. [DOI] [PubMed] [Google Scholar]
- 29. Rier HN, Jager A, Sleijfer S, Maier AB, Levin M‐D. The prevalence and prognostic value of low muscle mass in cancer patients: a review of the literature. Oncologist 2016;21:1396–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moley JF, Aamodt R, Rumble W, Kaye W, Norton JA. Body cell mass in cancer‐bearing and anorexic patients. J Parenter Enteral Nutr 1987;11:219–222. [DOI] [PubMed] [Google Scholar]
- 31. Weerink LBM, van der Hoorn A, van Leeuwen BL, de Bock GH. Low skeletal muscle mass and postoperative morbidity in surgical oncology: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle 2020;11:636–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang D‐D, Wang S‐L, Zhuang C‐L, Zheng B‐S, Lu J‐X, Chen F‐F, et al. Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Colorectal Dis 2015;17:256–264. [DOI] [PubMed] [Google Scholar]
- 33. Jung H‐W, Kim JW, Kim J‐Y, Kim S‐W, Yang HK, Lee JW, et al. Effect of muscle mass on toxicity and survival in patients with colon cancer undergoing adjuvant chemotherapy. Support Care Cancer 2015;23:687–694. [DOI] [PubMed] [Google Scholar]
- 34. Mir O, Coriat R, Blanchet B, Durand J‐P, Boudou‐Rouquette P, Michels J, et al. Sarcopenia predicts early dose‐limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS One 2012;7:e37563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tan BHL, Brammer K, Randhawa N, Welch NT, Parsons SL, James EJ, et al. Sarcopenia is associated with toxicity in patients undergoing neo‐adjuvant chemotherapy for oesophago‐gastric cancer. Eur J Surg Oncol 2015;41:333–338. [DOI] [PubMed] [Google Scholar]
- 36. Wendrich AW, Swartz JE, Bril SI, Wegner I, de Graeff A, Smid EJ, et al. Low skeletal muscle mass is a predictive factor for chemotherapy dose‐limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol 2017;71:26–33. [DOI] [PubMed] [Google Scholar]
- 37. Prado CM, Sawyer MB, Ghosh S, Lieffers JR, Esfandiari N, Antoun S, et al. Central tenet of cancer cachexia therapy: do patients with advanced cancer have exploitable anabolic potential? Am J Clin Nutr 2013;98:1012–1019. [DOI] [PubMed] [Google Scholar]
- 38. Choi MH, Oh SN, Lee IK, Oh ST, Won DD. Sarcopenia is negatively associated with long‐term outcomes in locally advanced rectal cancer. J Cachexia Sarcopenia Muscle 2018;9:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Basile D, Parnofiello A, Vitale MG, Cortiula F, Gerratana L, Fanotto V, et al. The IMPACT study: early loss of skeletal muscle mass in advanced pancreatic cancer patients. J Cachexia Sarcopenia Muscle 2019;10:368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. da Cunha LP, Silveira MN, Mendes MCS, Costa FO, Macedo LT, de Siqueira NS, et al. Sarcopenia as an independent prognostic factor in patients with metastatic colorectal cancer: a retrospective evaluation. Clin Nutr ESPEN 2019;32:107–112. [DOI] [PubMed] [Google Scholar]
- 41. Ebner N, Anker SD, von Haehling S. Recent developments in the field of cachexia, sarcopenia, and muscle wasting: highlights from the 12th Cachexia Conference. J Cachexia Sarcopenia Muscle 2020;11:274–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Argilés JM, López‐Soriano FJ, Busquets S. Mechanisms and treatment of cancer cachexia. Nutr Metab Cardiovasc Dis 2013;23:S19–S24. [DOI] [PubMed] [Google Scholar]
- 43. Argilés JM, Busquets S, Stemmler B, López‐Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 2014;14:754–762. [DOI] [PubMed] [Google Scholar]
- 44. Argilés JM, Stemmler B, López‐Soriano FJ, Busquets S. Inter‐tissue communication in cancer cachexia. Nat Rev Endocrinol 2018;15:9–20. [DOI] [PubMed] [Google Scholar]
- 45. Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer‐associated cachexia. Nat Rev Dis Primers 2018;4:17105. [DOI] [PubMed] [Google Scholar]
- 46. Johns N, Stephens NA, Fearon KCH. Muscle wasting in cancer. Int J Biochem Cell Biol 2013;45:2215–2229. [DOI] [PubMed] [Google Scholar]
- 47. Rohm M, Zeigerer A, Machado J, Herzig S. Energy metabolism in cachexia. EMBO Rep 2019;20:e47258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schmidt SF, Rohm M, Herzig S, Berriel Diaz M. Cancer cachexia: more than skeletal muscle wasting. Trends Cancer 2018;4:849–860. [DOI] [PubMed] [Google Scholar]
- 49. Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 2009;89:381–410. [DOI] [PubMed] [Google Scholar]
- 50. Roeland EJ, Ma JD, Nelson SH, Seibert T, Heavey S, Revta C, et al. Weight loss versus muscle loss: re‐evaluating inclusion criteria for future cancer cachexia interventional trials. Support Care Cancer 2017;25:365–369. [DOI] [PubMed] [Google Scholar]
- 51. Blauwhoff‐Buskermolen S, Langius JAE, Becker A, Verheul HMW, de van der Schueren MAE. The influence of different muscle mass measurements on the diagnosis of cancer cachexia: muscle measurements in the diagnosis of cachexia. J Cachexia Sarcopenia Muscle 2017;8:615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Heymsfield SB, Gonzalez MC, Lu J, Jia G, Zheng J. Skeletal muscle mass and quality: evolution of modern measurement concepts in the context of sarcopenia. Proc Nutr Soc 2015;74:355–366. [DOI] [PubMed] [Google Scholar]
- 53. Lerner L, Hayes TG, Tao N, Krieger B, Feng B, Wu Z, et al. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. J Cachexia Sarcopenia Muscle 2015;6:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Op den Kamp C, Gosker HR, Lagarde S, Tan DY, Snepvangers FJ, Dingemans A‐MC, et al. Preserved muscle oxidative metabolic phenotype in newly diagnosed non‐small cell lung cancer cachexia. Proc Nutr Soc 2015;6:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Op den Kamp CM, Langen RC, Snepvangers FJ, de Theije CC, Schellekens JM, Laugs F, et al. Nuclear transcription factor B activation and protein turnover adaptations in skeletal muscle of patients with progressive stages of lung cancer cachexia. Am J Clin Nutr 2013;98:738–748. [DOI] [PubMed] [Google Scholar]
- 56. Tardif N, Klaude M, Lundell L, Thorell A, Rooyackers O. Autophagic‐lysosomal pathway is the main proteolytic system modified in the skeletal muscle of esophageal cancer patients. Am J Clin Nutr 2013;98:1485–1492. [DOI] [PubMed] [Google Scholar]
- 57. Weber M‐A, Krakowski‐Roosen H, Schröder L, Kinscherf R, Krix M, Kopp‐Schneider A, et al. Morphology, metabolism, microcirculation, and strength of skeletal muscles in cancer‐related cachexia. Acta Oncol 2009;48:116–124. [DOI] [PubMed] [Google Scholar]
- 58. Dejong CHC, Busquets S, Moses AGW, Schrauwen P, Ross JA, Argiles JM, et al. Systemic inflammation correlates with increased expression of skeletal muscle ubiquitin but not uncoupling proteins in cancer cachexia. Oncol Rep 2005;14:257–263. [PubMed] [Google Scholar]
- 59. Aversa Z, Pin F, Lucia S, Penna F, Verzaro R, Fazi M, et al. Autophagy is induced in the skeletal muscle of cachectic cancer patients. Sci Rep 2016;6:30340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Falconer JS, Fearon KC, Plester CE, Ross JA, Carter DC. Cytokines, the acute‐phase response, and resting energy expenditure in cachectic patients with pancreatic cancer. Ann Surg 1994;219:325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stephens NA, Gray C, MacDonald AJ, Tan BH, Gallagher IJ, Skipworth RJE, et al. Sexual dimorphism modulates the impact of cancer cachexia on lower limb muscle mass and function. Clin Nutr 2012;31:499–505. [DOI] [PubMed] [Google Scholar]
- 62. Weber M‐A, Kinscherf R, Krakowski‐Roosen H, Aulmann M, Renk H, Künkele A, et al. Myoglobin plasma level related to muscle mass and fiber composition: a clinical marker of muscle wasting? J Mol Med 2007;85:887–896. [DOI] [PubMed] [Google Scholar]
- 63. Gomez‐Perez SL, Haus JM, Sheean P, Patel B, Mar W, Chaudhry V, et al. Measuring abdominal circumference and skeletal muscle from a single cross‐sectional computed tomography image: a step‐by‐step guide for clinicians using National Institutes of Health ImageJ. J Parenter Enteral Nutr 2016;40:308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 65. Narasimhan A, Greiner R, Bathe OF , Baracos V, Damaraju S. Differentially expressed alternatively spliced genes in skeletal muscle from cancer patients with cachexia: alternatively spliced genes in cancer cachexia. J Cachexia Sarcopenia Muscle 2018;9:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen JL, Walton KL, Qian H, Colgan TD, Hagg A, Watt MJ, et al. Differential effects of IL6 and activin A in the development of cancer‐associated cachexia. Cancer Res 2016;76:5372–5382. [DOI] [PubMed] [Google Scholar]
- 67. Choi E, Carruthers K, Zhang L, Thomas N, Battaglino RA, Morse LR, et al. Concurrent muscle and bone deterioration in a murine model of cancer cachexia. Physiol Rep 2013;1:e00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shadfar S, Couch ME, McKinney KA, Weinstein LJ, Yin X, Rodríguez JE, et al. Oral resveratrol therapy inhibits cancer‐induced skeletal muscle and cardiac atrophy in vivo. Nutr Cancer 2011;63:749–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, et al. Reversal of cancer cachexia and muscle wasting by ActRIIb antagonism leads to prolonged survival. Cell 2010;142:531–543. [DOI] [PubMed] [Google Scholar]
- 70. Coletti D, Aulino P, Pigna E, Barteri F, Moresi V, Annibali D, et al. Spontaneous physical activity downregulates Pax7 in cancer cachexia. Stem Cells Int 2016;2016:6729268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. White JP, Baynes JW, Welle SL, Kostek MC, Matesic LE, Sato S, et al. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apc Min/+ mouse. PLoS One 2011;6:e24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Padrão AI, Oliveira P, Vitorino R, Colaço B, Pires MJ, Márquez M, et al. Bladder cancer‐induced skeletal muscle wasting: disclosing the role of mitochondria plasticity. Int J Biochem Cell Biol 2013;45:1399–1409. [DOI] [PubMed] [Google Scholar]
- 73. Penna F, Costamagna D, Fanzani A, Bonelli G, Baccino FM, Costelli P. Muscle wasting and impaired myogenesis in tumor bearing mice are prevented by ERK inhibition. PLoS One 2010;5:e13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Puppa MJ, Murphy EA, Fayad R, Hand GA, Carson JA. Cachectic skeletal muscle response to a novel bout of low‐frequency stimulation. J Appl Physiol 2014;116:1078–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rivadeneira DE, Naama HA, McCarter MD, Fujita J, Evoy D, Mackrell P, et al. Glucocorticoid blockade does not abrogate tumor‐induced cachexia. Nutr Cancer 1999;35:202–206. [DOI] [PubMed] [Google Scholar]
- 76. Talbert EE, Metzger GA, He WA, Guttridge DC. Modeling human cancer cachexia in colon 26 tumor‐bearing adult mice. J Cachexia Sarcopenia Muscle 2014;5:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. White JP, Baltgalvis KA, Puppa MJ, Sato S, Baynes JW, Carson JA. Muscle oxidative capacity during IL‐6‐dependent cancer cachexia. Am J Physiol Regul Integr Comp Physiol 2010;300:R201–R211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mehl KA, Davis JM, Berger FG, Carson JA. Myofiber degeneration/regeneration is induced in the cachectic Apc Min/+ mouse. J Appl Physiol 2005;99:2379–2387. [DOI] [PubMed] [Google Scholar]
- 79. Murphy KT, Chee A, Trieu J, Naim T, Lynch GS. Importance of functional and metabolic impairments in the characterization of the C‐26 murine model of cancer cachexia. Dis Model Mech 2012;5:533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Murphy KT, Struk A, Malcontenti‐Wilson C, Christophi C, Lynch GS. Physiological characterization of a mouse model of cachexia in colorectal liver metastases. Am J Physiol Regul Integr Comp Physiol 2013;304:R854–R864. [DOI] [PubMed] [Google Scholar]
- 81. van Norren K, Kegler D, Argilés JM, Luiking Y, Gorselink M, Laviano A, et al. Dietary supplementation with a specific combination of high protein, leucine, and fish oil improves muscle function and daily activity in tumour‐bearing cachectic mice. Br J Cancer 2009;100:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Diffee GM, Kalfas K, Al‐Majid S, McCarthy DO. Altered expression of skeletal muscle myosin isoforms in cancer cachexia. Am J Physiol Cell Physiol 2002;283:C1376–C1382. [DOI] [PubMed] [Google Scholar]
- 83. Gallot YS, Durieux A‐C, Castells J, Desgeorges MM, Vernus B, Plantureux L, et al. Myostatin gene inactivation prevents skeletal muscle wasting in cancer. Cancer Res 2014;74:7344–7356. [DOI] [PubMed] [Google Scholar]
- 84. Hardee JP, Mangum JE, Gao S, Sato S, Hetzler KL, Puppa MJ, et al. Eccentric contraction‐induced myofiber growth in tumor‐bearing mice. J Appl Physiol 2015;120:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Julienne CM, Dumas J‐F, Goupille C, Pinault M, Berri C, Collin A, et al. Cancer cachexia is associated with a decrease in skeletal muscle mitochondrial oxidative capacities without alteration of ATP production efficiency. J Cachexia Sarcopenia Muscle 2012;3:265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lima M, Sato S, Enos RT, Baynes JW, Carson JA. Development of an UPLC mass spectrometry method for measurement of myofibrillar protein synthesis: application to analysis of murine muscles during cancer cachexia. J Appl Physiol 2013;114:824–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Antunes D, Padrão AI, Maciel E, Santinha D, Oliveira P, Vitorino R, et al. Molecular insights into mitochondrial dysfunction in cancer‐related muscle wasting. Biochim Biophys Acta 2014;1841:896–905. [DOI] [PubMed] [Google Scholar]
- 88. Costelli P, Muscaritoli M, Bonetto A, Penna F, Reffo P, Bossola M, et al. Muscle myostatin signalling is enhanced in experimental cancer cachexia. Eur J Clin Invest 2008;38:531–538. [DOI] [PubMed] [Google Scholar]
- 89. Baracos VE, DeVivo C, Hoyle DH, Goldberg AL. Activation of the ATP‐ubiquitin‐proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. Am J Physiol Endocrinol Metab 1995;268:E996–E1006. [DOI] [PubMed] [Google Scholar]
- 90. Pin F, Minero VG, Penna F, Muscaritoli M, De Tullio R, Baccino FM, et al. Interference with Ca2+‐dependent proteolysis does not alter the course of muscle wasting in experimental cancer cachexia. Front Physiol 2017;8:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Talbert EE, Cuitiño MC, Ladner KJ, Rajasekerea PV, Siebert M, Shakya R, et al. Modeling human cancer‐induced cachexia. Cell Rep 2019;28:1612–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tseng YC, Kulp SK, Lai IL, Hsu EC, He WA, Frankhouser DE, et al. Preclinical investigation of the novel histone deacetylase inhibitor AR‐42 in the treatment of cancer‐induced cachexia. J Natl Cancer Inst 2015;107:djv274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Costelli P, Muscaritoli M, Bossola M, Penna F, Reffo P, Bonetto A, et al. IGF‐1 is downregulated in experimental cancer cachexia. Am J Physiol Regul Integr Comp Physiol 2006;291:R674–R683. [DOI] [PubMed] [Google Scholar]
- 94. Pin F, Barreto R, Couch ME, Bonetto A, O'Connell TM. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J Cachexia Sarcopenia Muscle 2019;10:140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bonetto A, Penna F, Minero VG, Reffo P, Bonelli G, Baccino FM, et al. Deacetylase inhibitors modulate the myostatin/follistatin axis without improving cachexia in tumor‐bearing mice. Curr Cancer Drug Targets 2009;9:608–616. [DOI] [PubMed] [Google Scholar]
- 96. Roberts BM, Ahn B, Smuder AJ, Al‐Rajhi M, Gill LC, Beharry AW, et al. Diaphragm and ventilatory dysfunction during cancer cachexia. FASEB J 2013;27:2600–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Aulino P, Berardi E, Cardillo VM, Rizzuto E, Perniconi B, Ramina C, et al. Molecular, cellular and physiological characterization of the cancer cachexia‐inducing C26 colon carcinoma in mouse. BMC Cancer 2010;10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Segatto M, Fittipaldi R, Pin F, Sartori R, Dae Ko K, Zare H, et al. Epigenetic targeting of bromodomain protein BRD4 counteracts cancer cachexia and prolongs survival. Nat Commun 2017;8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Murphy KT, Chee A, Gleeson BG, Naim T, Swiderski K, Koopman R, et al. Antibody‐directed myostatin inhibition enhances muscle mass and function in tumor‐bearing mice. Am J Physiol Regul Integr Comp Physiol 2011;301:R716–R726. [DOI] [PubMed] [Google Scholar]
- 100. Roberts BM, Frye GS, Ahn B, Ferreira LF, Judge AR. Cancer cachexia decreases specific force and accelerates fatigue in limb muscle. Biochem Biophys Res Commun 2013;435:488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhang G, Liu Z, Ding H, Zhou Y, Doan HA, Sin KWT, et al. Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat Commun 2017;8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. VanderVeen BN, Hardee JP, Fix DK, Carson JA. Skeletal muscle function during the progression of cancer cachexia in the male Apc Min/+ mouse. J Appl Physiol 2017;124:684–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Inaba S, Hinohara A, Tachibana M, Tsujikawa K, Fukada S. Muscle regeneration is disrupted by cancer cachexia without loss of muscle stem cell potential. PLoS One 2018;13:e0205467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Baltgalvis KA, Berger FG, Peña MMO, Mark Davis J, White JP, Carson JA. Activity level, apoptosis, and development of cachexia in Apc Min/+ mice. J Appl Physiol 2010;109:1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Smith KL, Tisdale MJ. Increased protein degradation and decreased protein synthesis in skeletal muscle during cancer cachexia. Br J Cancer 1993;67:680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Goncalves MD, Hwang S‐K, Pauli C, Murphy CJ, Cheng Z, Hopkins BD, et al. Fenofibrate prevents skeletal muscle loss in mice with lung cancer. Proc Natl Acad Sci U S A 2018;115:E743–E752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Khal J, Wyke SM, Russell ST, Hine AV, Tisdale MJ. Expression of the ubiquitin‐proteasome pathway and muscle loss in experimental cancer cachexia. Br J Cancer 2005;93:774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Penna F, Busquets S, Pin F, Toledo M, Baccino FM, López‐Soriano FJ, et al. Combined approach to counteract experimental cancer cachexia: eicosapentaenoic acid and training exercise. J Cachexia Sarcopenia Muscle 2011;2:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Llovera M, Garcia‐Martinez C, López‐Soriano J, Agell N, López‐Soriano FJ, Garcia I, et al. Protein turnover in skeletal muscle of tumour‐bearing transgenic mice overexpressing the soluble TNF receptor‐1. Cancer Lett 1998;130:19–27. [DOI] [PubMed] [Google Scholar]
- 110. Llovera M, Garcia‐Martinez C, López‐Soriano J, Carbó N, Agell N, López‐Soriano FJ, et al. Role of TNF receptor 1 in protein turnover during cancer cachexia using gene knockout mice. Mol Cell Endocrinol 1998;142:183–189. [DOI] [PubMed] [Google Scholar]
- 111. Marin‐Corral J, Fontes CC, Pascual‐Guardia S, Sanchez F, Olivan M, Argilés JM, et al. Redox balance and carbonylated proteins in limb and heart muscles of cachectic rats. Antioxid Redox Signal 2009;12:365–380. [DOI] [PubMed] [Google Scholar]
- 112. Fontes‐Oliveira CC, Busquets S, Toledo M, Penna F, Aylwin MP, Sirisi S, et al. Mitochondrial and sarcoplasmic reticulum abnormalities in cancer cachexia: altered energetic efficiency? Biochim Biophys Acta 2013;1830:2770–2778. [DOI] [PubMed] [Google Scholar]
- 113. Constantinou C, Constantinou C, de Oliveira F, Cristine C, de Oliveira F, Cristine C, et al. Nuclear magnetic resonance in conjunction with functional genomics suggests mitochondrial dysfunction in a murine model of cancer cachexia. Int J Mol Med 2011;27:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Penna F, Bonetto A, Muscaritoli M, Costamagna D, Minero VG, Bonelli G, et al. Muscle atrophy in experimental cancer cachexia: is the IGF‐1 signaling pathway involved? Int J Cancer 2010;127:1706–1717. [DOI] [PubMed] [Google Scholar]
- 115. Figueras M, Busquets S, Carbó N, Barreiro E, Almendro V, Argilés JM, et al. Interleukin‐15 is able to suppress the increased DNA fragmentation associated with muscle wasting in tumour‐bearing rats. FEBS Lett 2004;569:201–206. [DOI] [PubMed] [Google Scholar]
- 116. Busquets S, Figueras MT, Fuster G, Almendro V, Moore‐Carrasco R, Ametller E, et al. Anticachectic effects of formoterol: a drug for potential treatment of muscle wasting. Cancer Res 2004;64:6725–6731. [DOI] [PubMed] [Google Scholar]
- 117. Bonetto A, Rupert JE, Barreto R, Zimmers TA. The colon‐26 carcinoma tumor‐bearing mouse as a model for the study of cancer cachexia. J Vis Exp 2016;117:e54893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Salazar‐Degracia A, Busquets S, Argilés JM, Bargalló‐Gispert N, López‐Soriano FJ, Barreiro E. Effects of the beta2 agonist formoterol on atrophy signaling, autophagy, and muscle phenotype in respiratory and limb muscles of rats with cancer‐induced cachexia. Biochimie 2018;149:79–91. [DOI] [PubMed] [Google Scholar]
- 119. Augusto V, Padovani CR, Campos GR. Skeletal muscle fiber types in C57BL6J mice. Braz J Morphol Sci 2004;21:89–94. [Google Scholar]
- 120. Martignoni ME, Dimitriu C, Bachmann J, Krakowski‐Rosen H, Ketterer K, Kinscherf R, et al. Liver macrophages contribute to pancreatic cancer‐related cachexia. Oncol Rep 2009;21:363–369. [PubMed] [Google Scholar]
- 121. Shiono M, Huang K, Downey RJ, Consul N, Villanueva N, Beck K, et al. An analysis of the relationship between metastases and cachexia in lung cancer patients. Cancer Med 2016;5:2641–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tomasin R, Martin ACBM, Cominetti MR. Metastasis and cachexia: alongside in clinics, but not so in animal models. J Cachexia Sarcopenia Muscle 2019;10:1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Huot JR, Novinger LJ, Pin F, Narasimhan A, Zimmers TA, O'Connell TM, et al. Formation of colorectal liver metastases induces musculoskeletal and metabolic abnormalities consistent with exacerbated cachexia. JCI Insight 2020;5:e136687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Huot JR, Novinger LJ, Pin F, Bonetto A. HCT116 colorectal liver metastases exacerbate muscle wasting in a mouse model for the study of colorectal cancer cachexia. Dis Model Mech 2020;13:dmm043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Bozzetti F. Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol 2017;28:2107–2118. [DOI] [PubMed] [Google Scholar]
- 126. Ryan AM, Prado CM, Sullivan ES, Power DG, Daly LE. Effects of weight loss and sarcopenia on response to chemotherapy, quality of life, and survival. Nutrition 2019;67, 110539–68. [DOI] [PubMed] [Google Scholar]
- 127. Barreto R, Waning DL, Gao H, Liu Y, Zimmers TA, Bonetto A. Chemotherapy‐related cachexia is associated with mitochondrial depletion and the activation of ERK1/2 and p38 MAPKs. Oncotarget 2016;7:43442–43460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Barreto R, Kitase Y, Matsumoto T, Pin F, Colston KC, Couch KE, et al. ACVR2B/Fc counteracts chemotherapy‐induced loss of muscle and bone mass. Sci Rep 2017;7:14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Barreto R, Mandili G, Witzmann FA, Novelli F, Zimmers TA, Bonetto A. Cancer and chemotherapy contribute to muscle loss by activating common signaling pathways. Front Physiol 2016;7:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hain BA, Xu H, Wilcox JR, Mutua D, Waning DL. Chemotherapy‐induced loss of bone and muscle mass in a mouse model of breast cancer bone metastases and cachexia. JCSM Rapid Commun 2019;2:1–12. [PMC free article] [PubMed] [Google Scholar]
- 131. Hain BA, Jude B, Xu H, Smuin DM, Fox EJ, Elfar JC, et al. Zoledronic acid improves muscle function in healthy mice treated with chemotherapy. J Bone Miner Res 2020;35:368–381. [DOI] [PubMed] [Google Scholar]
- 132. Damrauer JS, Stadler ME, Acharyya S, Baldwin AS, Couch ME, Guttridge DC. Chemotherapy‐induced muscle wasting: association with NF‐κB and cancer cachexia. Eur J Transl Myol 2018;28:7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chen J, Splenser A, Guillory B, Luo J, Mendiratta M, Belinova B, et al. Ghrelin prevents tumour‐ and cisplatin‐induced muscle wasting: characterization of multiple mechanisms involved. J Cachexia Sarcopenia Muscle 2015;6:132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Essex AL, Pin F, Huot JR, Bonewald LF, Plotkin LI, Bonetto A. Bisphosphonate treatment ameliorates chemotherapy‐induced bone and muscle abnormalities in young mice. Front Endocrinol 2019;10:809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Gilliam LAA, Lark DS, Reese LR, Torres MJ, Ryan TE, Lin C‐T, et al. Targeted overexpression of mitochondrial catalase protects against cancer chemotherapy‐induced skeletal muscle dysfunction. Am J Physiol Endocrinol Metab 2016;311:E293–E301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Junior EA de L, Yamashita AS, Pimentel GD, Sousa LGOD, Santos RVT, Gonçalves CL, et al. Doxorubicin caused severe hyperglycaemia and insulin resistance, mediated by inhibition in AMPk signalling in skeletal muscle. J Cachexia Sarcopenia Muscle 2016;7:615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hulmi JJ, Nissinen TA, Räsänen M, Degerman J, Lautaoja JH, Hemanthakumar KA, et al. Prevention of chemotherapy‐induced cachexia by ACVR2B ligand blocking has different effects on heart and skeletal muscle: prevention of chemotherapy‐induced cachexia. J Cachexia Sarcopenia Muscle 2018;9:417–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Braun TP, Szumowski M, Levasseur PR, Grossberg AJ, Zhu X, Agarwal A, et al. Muscle atrophy in response to cytotoxic chemotherapy is dependent on intact glucocorticoid signaling in skeletal muscle. PLoS One 2014;9:e106489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Chen M‐C, Hsu W‐L, Hwang P‐A, Chen Y‐L, Chou T‐C. Combined administration of fucoidan ameliorates tumor and chemotherapy‐induced skeletal muscle atrophy in bladder cancer‐bearing mice. Oncotarget 2016;7:51608–51618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hatakeyama S, Summermatter S, Jourdain M, Melly S, Minetti GC, Lach‐Trifilieff E. ActRII blockade protects mice from cancer cachexia and prolongs survival in the presence of anti‐cancer treatments. Skelet Muscle 2016;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Liu D, Qiao X, Ge Z, Shang Y, Li Y, Wang W, et al. IMB0901 inhibits muscle atrophy induced by cancer cachexia through MSTN signaling pathway. Skelet Muscle 2019;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev 2011;91:1447–1531. [DOI] [PubMed] [Google Scholar]
- 143. Taskin S, Stumpf VI, Bachmann J, Weber C, Martignoni ME, Friedrich O. Motor protein function in skeletal abdominal muscle of cachectic cancer patients. J Cell Mol Med 2014;18:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Johns N, Hatakeyama S, Stephens NA, Degen M, Degen S, Frieauff W, et al. Clinical classification of cancer cachexia: phenotypic correlates in human skeletal muscle. PLoS One 2014;9:e83618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Schmitt TL, Martignoni ME, Bachmann J, Fechtner K, Friess H, Kinscherf R, et al. Activity of the Akt‐dependent anabolic and catabolic pathways in muscle and liver samples in cancer‐related cachexia. J Mol Med 2007;85:647–654. [DOI] [PubMed] [Google Scholar]
- 146. Puig‐Vilanova E, Rodriguez DA, Lloreta J, Ausin P, Pascual‐Guardia S, Broquetas J, et al. Oxidative stress, redox signaling pathways, and autophagy in cachectic muscles of male patients with advanced COPD and lung cancer. Free Radic Biol Med 2015;79:91–108. [DOI] [PubMed] [Google Scholar]
- 147. Chacon‐Cabrera A, Fermoselle C, Urtreger AJ, Mateu‐Jimenez M, Diament MJ, de Kier Joffé EDB, et al. Pharmacological strategies in lung cancer‐induced cachexia: effects on muscle proteolysis, autophagy, structure, and weakness. J Cell Physiol 2014;229:1660–1672. [DOI] [PubMed] [Google Scholar]
- 148. Chacon‐Cabrera A, Mateu‐Jimenez M, Langohr K, Fermoselle C, García‐Arumí E, Andreu AL, et al. Role of PARP activity in lung cancer‐induced cachexia: effects on muscle oxidative stress, proteolysis, anabolic markers,and phenotype. J Cell Physiol 2017;232:3744–3761. [DOI] [PubMed] [Google Scholar]
- 149. Salazar‐Degracia A, Blanco D, Vilà‐Ubach M, de Biurrun G, de Solórzano CO, Montuenga LM, et al. Phenotypic and metabolic features of mouse diaphragm and gastrocnemius muscles in chronic lung carcinogenesis: influence of underlying emphysema. J Transl Med 2016;14:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ham DJ, Murphy KT, Chee A, Lynch GS, Koopman R. Glycine administration attenuates skeletal muscle wasting in a mouse model of cancer cachexia. Clin Nutr 2014;33:448–458. [DOI] [PubMed] [Google Scholar]
- 151. Kunzke T, Buck A, Prade VM, Feuchtinger A, Prokopchuk O, Martignoni ME, et al. Derangements of amino acids in cachectic skeletal muscle are caused by mitochondrial dysfunction. J Cachexia Sarcopenia Muscle 2020;11:226–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Bohnert KR, Gallot YS, Sato S, Xiong G, Hindi SM, Kumar A. Inhibition of ER stress and unfolding protein response pathways causes skeletal muscle wasting during cancer cachexia. FASEB J 2016;30:3053–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Winbanks CE, Murphy KT, Bernardo BC, Qian H, Liu Y, Sepulveda PV, et al. Smad7 gene delivery prevents muscle wasting associated with cancer cachexia in mice. Sci Transl Med 2016;8:348ra98. [DOI] [PubMed] [Google Scholar]
- 154. Salmons S, Sréter FA. Significance of impulse activity in the transformation of skeletal muscle type. Nature 1976;263:30–34. [DOI] [PubMed] [Google Scholar]
- 155. Daou N, Hassani M, Matos E, De Castro GS, Galvao Figueredo Costa R, Seelaender M, et al. Displaced myonuclei in cancer cachexia suggest altered innervation. Int J Mol Sci 2020;21:1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Boehm I, Miller J, Wishart TM, Wigmore SJ, Skipworth RJE, Jones RA, et al. Neuromuscular junctions are stable in patients with cancer cachexia. J Clin Invest 2020;130:1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Zhang Y, Wang J, Wang X, Gao T, Tian H, Zhou D, et al. The autophagic‐lysosomal and ubiquitin proteasome systems are simultaneously activated in the skeletal muscle of gastric cancer patients with cachexia. Am J Clin Nutr 2020;111:570–579. [DOI] [PubMed] [Google Scholar]
- 158. Marin O, Denny‐Brown D. Changes in skeletal muscle associated with cachexia. Am J Pathol 1962;41:23–39. [PMC free article] [PubMed] [Google Scholar]
- 159. Hall DT, Griss T, Ma JF, Sanchez BJ, Sadek J, Tremblay AMK, et al. The AMPK agonist 5‐aminoimidazole‐4‐carboxamide ribonucleotide (AICAR), but not metformin, prevents inflammation‐associated cachectic muscle wasting. EMBO Mol Med 2018;10:e8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Mu X, Agarwal R, March D, Rothenberg A, Voigt C, Tebbets J, et al. Notch signaling mediates skeletal muscle atrophy in cancer cachexia caused by osteosarcoma. Sarcoma 2016;2016:3758162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Shakri AR, Zhong TJ, Ma W, Coker C, Kim S, Calluori S, et al. Upregulation of ZIP14 and altered zinc homeostasis in muscles in pancreatic cancer cachexia. Cancer 2020;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Zhuang P, Zhang J, Wang Y, Zhang M, Song L, Lu Z, et al. Reversal of muscle atrophy by Zhimu and Huangbai herb pair via activation of IGF‐1/Akt and autophagy signal in cancer cachexia. Support Care Cancer 2016;24:1189–1198. [DOI] [PubMed] [Google Scholar]
- 163. Marino FE, Risbridger G, Gold E. Activin‐C modulates cachexia by repressing the ubiquitin‐proteasome and autophagic degradation pathways. J Cachexia Sarcopenia Muscle 2015;6:365–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Cai D, Frantz JD, Tawa NE, Melendez PA, Oh B‐C, Lidov HGW, et al. IKKβ/NF‐κB activation causes severe muscle wasting in mice. Cell 2004;119:285–298. [DOI] [PubMed] [Google Scholar]
- 165. Reed SA, Sandesara PB, Senf SM, Judge AR. Inhibition of FoxO transcriptional activity prevents muscle fiber atrophy during cachexia and induces hypertrophy. FASEB J 2011;26:987–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Wang G, Biswas AK, Ma W, Kandpal M, Coker C, Grandgenett PM, et al. Metastatic cancers promote cachexia through ZIP14 upregulation in skeletal muscle. Nat Med 2018;24:770–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Berardi E, Aulino P, Murfuni I, Toschi A, Padula F, Scicchitano BM, et al. Skeletal muscle is enriched in hematopoietic stem cells and not inflammatory cells in cachectic mice. Neurol Res 2008;30:160–169. [DOI] [PubMed] [Google Scholar]
- 168. Molinari F, Pin F, Gorini S, Chiandotto S, Pontecorvo L, Penna F, et al. The mitochondrial metabolic reprogramming agent trimetazidine as an ‘exercise mimetic’ in cachectic C26‐bearing mice. J Cachexia Sarcopenia Muscle 2017;8:954–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Sciorati C, Touvier T, Buono R, Pessina P, François S, Perrotta C, et al. Necdin is expressed in cachectic skeletal muscle to protect fibers from tumor‐induced wasting. J Cell Sci 2009;122:1119–1125. [DOI] [PubMed] [Google Scholar]
- 170. Pin F, Busquets S, Toledo M, Camperi A, Lopez‐Soriano FJ, Costelli P, et al. Combination of exercise training and erythropoietin prevents cancer‐induced muscle alterations. Oncotarget 2015;6:43202–43215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Martinelli GB, Olivari D, Re Cecconi AD, Talamini L, Ottoboni L, Lecker SH, et al. Activation of the SDF1/CXCR4 pathway retards muscle atrophy during cancer cachexia. Oncogene 2016;35:6212–6222. [DOI] [PubMed] [Google Scholar]
- 172. Cornwell EW, Mirbod A, Wu C‐L, Kandarian SC, Jackman RW. C26 cancer‐induced muscle wasting is IKKβ‐dependent and NF‐kappaB‐independent. PLoS One 2014;9:e87776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Paul PK, Gupta SK, Bhatnagar S, Panguluri SK, Darnay BG, Choi Y, et al. Targeted ablation of TRAF6 inhibits skeletal muscle wasting in mice. J Cell Biol 2010;191:1395–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Pettersen K, Andersen S, Degen S, Tadini V, Grosjean J, Hatakeyama S, et al. Cancer cachexia associates with a systemic autophagy‐inducing activity mimicked by cancer cell‐derived IL‐6 trans‐signaling. Sci Rep 2017;7:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Schwarzkopf M, Coletti D, Sassoon D, Marazzi G. Muscle cachexia is regulated by a p53–PW1/Peg3‐dependent pathway. Genes Dev 2006;20:3440–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Padilha CS, Borges FH, Costa Mendes da Silva LE, Frajacomo FTT, Jordao AA, Duarte JA, et al. Resistance exercise attenuates skeletal muscle oxidative stress, systemic pro‐inflammatory state, and cachexia in Walker‐256 tumor‐bearing rats. Appl PhysiolNutr Metab 2017;42:916–923. [DOI] [PubMed] [Google Scholar]
- 177. Assi M, Derbré F, Lefeuvre‐Orfila L, Rébillard A. Antioxidant supplementation accelerates cachexia development by promoting tumor growth in C26 tumor‐bearing mice. Free Radic Biol Med 2016;91:204–214. [DOI] [PubMed] [Google Scholar]
- 178. Banduseela V, Ochala J, Lamberg K, Kalimo H, Larsson L. Muscle paralysis and myosin loss in a patient with cancer cachexia. Acta Myol 2007;26:136–144. [PMC free article] [PubMed] [Google Scholar]
- 179. Acharyya S, Butchbach MER, Sahenk Z, Wang H, Saji M, Carathers M, et al. Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell 2005;8:421–432. [DOI] [PubMed] [Google Scholar]
- 180. Shum AMY, Mahendradatta T, Taylor RJ, Painter AB, Moore MM, Tsoli M, et al. Disruption of MEF2C signaling and loss of sarcomeric and mitochondrial integrity in cancer‐induced skeletal muscle wasting. Aging (Albany NY) 2012;4:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Mendell JR, Engel WK. The fine structure of type II muscle fiber atrophy. Neurology 1971;21:358–365. [DOI] [PubMed] [Google Scholar]
- 182. Baltgalvis KA, Berger FG, Peña MMO, Davis JM, White JP, Carson JA. Muscle wasting and interleukin‐6‐induced atrogin‐I expression in the cachectic Apc Min/+ mouse. Pflugers Arch 2009;457:989–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Braun TP, Grossberg AJ, Krasnow SM, Levasseur PR, Szumowski M, Zhu XX, et al. Cancer‐ and endotoxin‐induced cachexia require intact glucocorticoid signaling in skeletal muscle. FASEB J 2013;27:3572–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Bossola M, Mirabella M, Ricci E, Costelli P, Pacelli F, Tortorelli AP, et al. Skeletal muscle apoptosis is not increased in gastric cancer patients with mild–moderate weight loss. Int J Biochem Cell Biol 2006;38:1561–1570. [DOI] [PubMed] [Google Scholar]
- 185. Busquets S, Deans C, Figueras M, Moore‐Carrasco R, López‐Soriano FJ, Fearon KCH, et al. Apoptosis is present in skeletal muscle of cachectic gastro‐intestinal cancer patients. Clin Nutr 2007;26:614–618. [DOI] [PubMed] [Google Scholar]
- 186. de Castro GS, Simoes E, Lima JDCC, Ortiz‐Silva M, Festuccia WT, Tokeshi F, et al. Human cachexia induces changes in mitochondria, autophagy and apoptosis in the skeletal muscle. Cancers (Basel) 2019;11:1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. White JP, Puppa MJ, Sato S, Gao S, Price RL, Baynes JW, et al. IL‐6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the Apc Min/+ mouse. Skelet Muscle 2012;2:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Tzika AA, Tzika AA, Fontes‐Oliveira CC, Fontes‐Oliveira CC, Shestov AA, Shestov AA, et al. Skeletal muscle mitochondrial uncoupling in a murine cancer cachexia model. Int J Oncol 2013;43:886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Shum AMY, Poljak A, Bentley NL, Turner N, Tan TC, Polly P. Proteomic profiling of skeletal and cardiac muscle in cancer cachexia: alterations in sarcomeric and mitochondrial protein expression. Oncotarget 2018;9:22001–22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Der‐Torossian H, Wysong A, Shadfar S, Willis MS, McDunn J, Couch ME. Metabolic derangements in the gastrocnemius and the effect of Compound A therapy in a murine model of cancer cachexia. J Cachexia Sarcopenia Muscle 2013;4:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Lautaoja JH, Lalowski M, Nissinen TA, Hentilä J, Shi Y, Ritvos O, et al. Muscle and serum metabolomes are dysregulated in colon‐26 tumor‐bearing mice despite amelioration of cachexia with activin receptor type 2B ligand blockade. Am J Physiol Endocrinol Metab 2019;316:E852–E865. [DOI] [PubMed] [Google Scholar]
- 192. Fermoselle C, García‐Arumí E, Puig‐Vilanova E, Andreu AL, Urtreger AJ, De EKJ, et al. Mitochondrial dysfunction and therapeutic approaches in respiratory and limb muscles of cancer cachectic mice. Exp Physiol 2013;98:1349–1365. [DOI] [PubMed] [Google Scholar]
- 193. Ushmorov A, Hack V, Dröge W. Differential reconstitution of mitochondrial respiratory chain activity and plasma redox state by cysteine and ornithine in a model of cancer cachexia. Cancer Res 1999;59:3527–3534. [PubMed] [Google Scholar]
- 194. VanderVeen BN, Fix DK, Carson JA. Disrupted skeletal muscle mitochondrial dynamics, mitophagy, and biogenesis during cancer cachexia: a role for inflammation. Oxid Med Cell Longev 2017;2017:3292087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Neyroud D, Nosacka RL, Judge AR, Hepple RT. Colon 26 adenocarcinoma (C26)‐induced cancer cachexia impairs skeletal muscle mitochondrial function and content. J Muscle Res Cell Motil 2019;40:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Penna F, Ballarò R, Martinez‐Cristobal P, Sala D, Sebastian D, Busquets S, et al. Autophagy exacerbates muscle wasting in cancer cachexia and impairs mitochondrial function. J Mol Biol 2019;431:2674–2686. [DOI] [PubMed] [Google Scholar]
- 197. Romanul FCA. Distribution of capillaries in relation to oxidative metabolism of skeletal muscle fibres. Nature 1964;201:307–308. [DOI] [PubMed] [Google Scholar]
- 198. Hochwald SN, Harrison LE, Port JL, Blumberg D, Brennan MF, Burt M. Depletion of high energy phosphate compounds in the tumor‐bearing state and reversal after tumor resection. Surgery 1996;120:534–541. [DOI] [PubMed] [Google Scholar]
- 199. Cui P, Huang C, Guo J, Wang Q, Liu Z, Zhuo H, et al. Metabolic profiling of tumors, sera, and skeletal muscles from an orthotopic murine model of gastric cancer associated‐cachexia. J Proteome Res 2019;18:1880–1892. [DOI] [PubMed] [Google Scholar]
- 200. Beck SA, Smith KL, Tisdale MJ. Anticachectic and antitumor effect of eicosapentaenoic acid and its effect on protein turnover. Cancer Res 1991;51:6089–6093. [PubMed] [Google Scholar]
- 201. Smith HJ, Greenberg NA, Tisdale MJ. Effect of eicosapentaenoic acid, protein and amino acids on protein synthesis and degradation in skeletal muscle of cachectic mice. Br J Cancer 2004;91:408–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Samuels SE, Knowles AL, Tilignac T, Debiton E, Madelmont JC, Attaix D. Higher skeletal muscle protein synthesis and lower breakdown after chemotherapy in cachectic mice. Am J Physiol Regul Integr Comp Physiol 2001;281:R133–R139. [DOI] [PubMed] [Google Scholar]
- 203. Temparis S, Asensi M, Taillandier D, Aurousseau E, Larbaud D, Obled A, et al. Increased ATP‐ubiquitin‐dependent proteolysis in skeletal muscles of tumor‐bearing rats. Cancer Res 1994;54:5568–5573. [PubMed] [Google Scholar]
- 204. Costelli P, García‐Martínez C, Llovera M, Carbó N, López‐Soriano FJ, Agell N, et al. Muscle protein waste in tumor‐bearing rats is effectively antagonized by a beta 2‐adrenergic agonist (clenbuterol). Role of the ATP‐ubiquitin‐dependent proteolytic pathway. J Clin Invest 1995;95:2367–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Tessitore L, Costelli P, Baccino FM. Pharmacological interference with tissue hypercatabolism in tumour‐bearing rats. Biochem J 1994;299:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Tessitore L, Costelli P, Bonetti G, Baccino FM. Cancer cachexia, malnutrition, and tissue protein turnover in experimental animals. Arch Biochem Biophys 1993;306:52–58. [DOI] [PubMed] [Google Scholar]
- 207. Toledo M, Busquets S, Penna F, Zhou X, Marmonti E, Betancourt A, et al. Complete reversal of muscle wasting in experimental cancer cachexia: additive effects of activin type II receptor inhibition and β‐2 agonist. Int J Cancer 2016;138:2021–2029. [DOI] [PubMed] [Google Scholar]
- 208. Lorite MJ, Thompson MG, Drake JL, Carling G, Tisdale MJ. Mechanism of muscle protein degradation induced by a cancer cachectic factor. Br J Cancer 1998;78:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Costelli P, Carbó N, Tessitore L, Bagby GJ, Lopez‐Soriano FJ, Argilés JM, et al. Tumor necrosis factor‐alpha mediates changes in tissue protein turnover in a rat cancer cachexia model. J Clin Invest 1993;92:2783–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Preston T, Slater C, McMillan DC, Falconer JS, Shenkin A, Fearon KC. Fibrinogen synthesis is elevated in fasting cancer patients with an acute phase response. J Nutr 1998;128:1355–1360. [DOI] [PubMed] [Google Scholar]
- 211. Singh J, Grigor MR, Thompson MP. Glucose homeostasis in rats bearing a transplantable sarcoma. Cancer Res 1980;40:1699–1706. [PubMed] [Google Scholar]
- 212. Argilés JM, Stemmler B, López‐Soriano FJ, Busquets S. Nonmuscle tissues contribution to cancer cachexia. Mediators Inflamm 2015;2015:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Stephens NA, Skipworth RJE, Fearon KCH. Cachexia, survival and the acute phase response. Curr Opin Support Palliat Care 2008;2:267–274. [DOI] [PubMed] [Google Scholar]
- 214. Bonetto A, Aydogdu T, Kunzevitzky N, Guttridge DC, Khuri S, Koniaris LG, et al. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLoS One 2011;6:e22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Bentzinger CF, Wang YX, Dumont NA, Rudnicki MA. Cellular dynamics in the muscle satellite cell niche. EMBO Rep 2013;14:1062–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Jewesbury RC, Topley WWC. On certain changes occurring in the voluntary muscles in general diseases. J Pathol 1912;17:432–453. [Google Scholar]
- 217. He WA, Berardi E, Cardillo VM, Acharyya S, Aulino P, Thomas‐Ahner J, et al. NF‐κB–mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J Clin Invest 2013;123:4821–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Judge SM, Nosacka RL, Delitto D, Gerber MH, Cameron ME, Trevino JG, et al. Skeletal muscle fibrosis in pancreatic cancer patients with respect to survival. JNCI Cancer Spectr 2018;2:pky043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Salazar‐Degracia A, Busquets S, Argilés JM, López‐Soriano FJ, Barreiro E. Formoterol attenuates increased oxidative stress and myosin protein loss in respiratory and limb muscles of cancer cachectic rats. PeerJ 2017;5:e4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Zampieri S, Doria A, Adami N, Biral D, Vecchiato M, Savastano S, et al. Subclinical myopathy in patients affected with newly diagnosed colorectal cancer at clinical onset of disease: evidence from skeletal muscle biopsies. Neurol Res 2010;32:20–25. [DOI] [PubMed] [Google Scholar]
- 221. Pigna E, Berardi E, Aulino P, Rizzuto E, Zampieri S, Carraro U, et al. Aerobic exercise and pharmacological treatments counteract cachexia by modulating autophagy in colon cancer. Sci Rep 2016;6:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Gallagher IJ, Stephens NA, MacDonald AJ, Skipworth RJE, Husi H, Greig CA, et al. Suppression of skeletal muscle turnover in cancer cachexia: evidence from the transcriptome in sequential human muscle biopsies. Clin Cancer Res 2012;18:2817–2827. [DOI] [PubMed] [Google Scholar]
- 223. Nosacka RL, Delitto AE, Delitto D, Patel R, Judge SM, Trevino JG, et al. Distinct cachexia profiles in response to human pancreatic tumours in mouse limb and respiratory muscle. J Cachexia Sarcopenia Muscle 2020;11:820–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Fernandez GJ, Ferreira JH, Vechetti IJJ, de Moraes LN, Cury SS, Freire PP, et al. MicroRNA‐mRNA co‐sequencing identifies transcriptional and post‐transcriptional regulatory networks underlying muscle wasting in cancer cachexia. Front Genet 2020;11:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 2004;18:39–51. [DOI] [PubMed] [Google Scholar]
- 226. Shum AMY, Fung DCY, Corley SM, McGill MC, Bentley NL, Tan TC, et al. Cardiac and skeletal muscles show molecularly distinct responses to cancer cachexia. Physiol Genomics 2015;47:588–599. [DOI] [PubMed] [Google Scholar]
- 227. Judge SM, Wu C‐L, Beharry AW, Roberts BM, Ferreira LF, Kandarian SC, et al. Genome‐wide identification of FoxO‐dependent gene networks in skeletal muscle during C26 cancer cachexia. BMC Cancer 2014;14:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Fontes‐Oliveira CC, Busquets S, Fuster G, Ametller E, Figueras M, Olivan M, et al. A differential pattern of gene expression in skeletal muscle of tumor‐bearing rats reveals dysregulation of excitation–contraction coupling together with additional muscle alterations. Muscle Nerve 2014;49:233–248. [DOI] [PubMed] [Google Scholar]
- 229. Gabiatti CTB, Martins MCL, Miyazaki DL, Silva LP, Lascala F, Macedo LT, et al. Myosteatosis in a systemic inflammation‐dependent manner predicts favorable survival outcomes in locally advanced esophageal cancer. Cancer Med 2019;8:6967–6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Stretch C, Aubin J‐M, Mickiewicz B, Leugner D, Al‐manasra T, Tobola E, et al. Sarcopenia and myosteatosis are accompanied by distinct biological profiles in patients with pancreatic and periampullary adenocarcinomas. PLoS One 2018;13:e0196235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Stephens NA, Skipworth RJ, Macdonald AJ, Greig CA, Ross JA, Fearon KC. Intramyocellular lipid droplets increase with progression of cachexia in cancer patients. J Cachexia Sarcopenia Muscle 2011;2:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Bhullar AS, Anoveros‐Barrera A, Dunichand‐Hoedl A, Martins K, Bigam D, Khadaroo RG, et al. Lipid is heterogeneously distributed in muscle and associates with low radiodensity in cancer patients. J Cachexia Sarcopenia Muscle 2020;11:735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Murton AJ, Maddocks M, Stephens FB, Marimuthu K, England R, Wilcock A. Consequences of late‐stage non–small‐cell lung cancer cachexia on muscle metabolic processes. Clin Lung Cancer 2017;18:e1–e11. [DOI] [PubMed] [Google Scholar]
- 234. Bae T, Jang J, Lee H, Song J, Chae S, Park M, et al. Paeonia lactiflora root extract suppresses cancer cachexia by down‐regulating muscular NF‐κB signalling and muscle‐specific E3 ubiquitin ligases in cancer‐bearing mice. J Ethnopharmacol 2020;246:112222. [DOI] [PubMed] [Google Scholar]
- 235. Busquets S, Toledo M, Sirisi S, Orpí M, Serpe R, Coutinho J, et al. Formoterol and cancer muscle wasting in rats: effects on muscle force and total physical activity. Exp Ther Med 2011;2:731–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Busquets S, Toledo M, Orpí M, Massa D, Porta M, Capdevila E, et al. Myostatin blockage using actRIIB antagonism in mice bearing the Lewis lung carcinoma results in the improvement of muscle wasting and physical performance. J Cachexia Sarcopenia Muscle 2012;3:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Levolger S, Wiemer EAC, Vugt JLA, Huisman SA, van Vledder MG, van Damme‐van Engel S, et al. Inhibition of activin‐like kinase 4/5 attenuates cancer cachexia associated muscle wasting. Sci Rep 2019;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Parajuli P, Kumar S, Loumaye A, Singh P, Eragamreddy S, Nguyen TL, et al. Twist1 activation in muscle progenitor cells causes muscle loss akin to cancer cachexia. Dev Cell 2018;45:712–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Penna F, Busquets S, Argilés JM. Experimental cancer cachexia: evolving strategies for getting closer to the human scenario. Semin Cell Dev Biol 2016;54:20–27. [DOI] [PubMed] [Google Scholar]
- 240. Toledo M, Busquets S, Sirisi S, Serpe R, Orpí M, Coutinho J, et al. Cancer cachexia: physical activity and muscle force in tumour‐bearing rats. Oncol Rep 2011;25:189–193. [PubMed] [Google Scholar]
- 241. Kir S, Komaba H, Garcia AP, Economopoulos KP, Liu W, Lanske B, et al. PTH/PTHrP receptor mediates cachexia in models of kidney failure and cancer. Cell Metab 2016;23:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Gorselink M, Vaessen SFC, van der Flier LG, Leenders I, Kegler D, Caldenhoven E, et al. Mass‐dependent decline of skeletal muscle function in cancer cachexia. Muscle Nerve 2006;33:691–693. [DOI] [PubMed] [Google Scholar]
- 243. Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006;61:1059–1064. [DOI] [PubMed] [Google Scholar]
- 244. Gale N, Wasley D, Roberts S, Backx K, Nelson A, van Deursen R, et al. A longitudinal study of muscle strength and function in patients with cancer cachexia. Support Care Cancer 2019;27:131–137. [DOI] [PubMed] [Google Scholar]
- 245. Cai B, Allexandre D, Rajagopalan V, Jiang Z, Siemionow V, Ranganathan VK, et al. Evidence of significant central fatigue in patients with cancer‐related fatigue during repetitive elbow flexions till perceived exhaustion. PLoS One 2014;9:e115370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Yavuzsen T, Davis MP, Ranganathan VK, Walsh D, Siemionow V, Kirkova J, et al. Cancer‐related fatigue: central or peripheral? J Pain Symptom Manage 2009;38:587–596. [DOI] [PubMed] [Google Scholar]
- 247. Kisiel‐Sajewicz K, Siemionow V, Seyidova‐Khoshknabi D, Davis MP, Wyant A, Ranganathan VK, et al. Myoelectrical manifestation of fatigue less prominent in patients with cancer related fatigue. PLoS One 2013;8:e83636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Bottinelli R, Pellegrino MA, Canepari M, Rossi R, Reggiani C. Specific contributions of various muscle fibre types to human muscle performance: an in vitro study. J Electromyogr Kinesiol 1999;9:87–95. [DOI] [PubMed] [Google Scholar]
- 249. Gissel H. The role of Ca2+ in muscle cell damage. Ann N Y Acad Sci 2005;1066:166–180. [DOI] [PubMed] [Google Scholar]
- 250. Cosper PF, Leinwand LA. Myosin heavy chain is not selectively decreased in murine cancer cachexia. Int J Cancer 2012;130:2722–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Toth MJ, Miller MS, Callahan DM, Sweeny AP, Nunez I, Grunberg SM, et al. Molecular mechanisms underlying skeletal muscle weakness in human cancer: reduced myosin‐actin cross‐bridge formation and kinetics. J Appl Physiol 2013;114:858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Jones RA, Harrison C, Eaton SL, Hurtado ML, Graham LC, Alkhammash L, et al. Cellular and molecular anatomy of the human neuromuscular junction. Cell Rep 2017;21:2348–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Mierzejewski B, Archacka K, Grabowska I, Florkowska A, Ciemerych MA, Brzoska E. Human and mouse skeletal muscle stem and progenitor cells in health and disease. Semin Cell Dev Biol 2020;104:93–104. [DOI] [PubMed] [Google Scholar]
- 254. Talbert EE, Cuitino MC, Ladner KJ, Rajasekerea PV, Siebert M, Shakya R, et al. Modeling human cancer‐induced cachexia. Cell Rep 2019;28:1612–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Haehling S, von Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]


