Abstract
Background
Cachexia, a devastating syndrome in cancer patients, critically determines survival and life quality. It is characterized by impaired homeostasis of multiple organs including the liver, involves tissue wasting, and is conventionally diagnosed and classified by weight loss (WL). However, recent studies pointed at the problem that WL is not sufficient for precise classification of cancer patients according to disease severity (i.e. prognosis). Tissue inhibitor of metalloproteinases‐1 (TIMP‐1) is an easily accessible cachexia‐associated biomarker in the blood, known to alter liver homeostasis. Here, we investigated the value of combining blood levels of TIMP‐1 with parameters of liver functionality towards establishment of a cachexia‐associated clinical score, which predicts survival of cancer patients, reflects the clinical manifestation of cachexia, and is easily accessible in the clinic.
Methods
The TIMP‐1/liver cachexia (TLC) score, expressed as numerical value ranging from 0 to 1, was calculated by categorizing the blood levels of TIMP‐1 and parameters of liver functionality (C‐reactive protein, ferritin, gamma‐glutamyl transferase, albumin, and total protein) for each patient as below/above a certain risk threshold. The TLC score was tested in a cohort of colorectal cancer (CRC) patients (n = 82, 35.4% women, 64.6% men, median age: 70 years) and validated in a cohort of pancreatic cancer (PC) patients (n = 84, 54.8% women, 45.2% men, median age: 69 years).
Results
In CRC patients, the TLC score positively correlated with presence of cachexia‐related symptoms (WL, impaired liver function), predicted survival [P < 0.001, hazard ratio (HR): 96.91 (9.85–953.90)], and allowed classification of three prognostically distinct patient subpopulations [low (LO)‐risk, intermediate (IM)‐risk, and high (HI)‐risk groups; LO vs. IM: P = 0.003, LO vs. HI: P < 0.001, IM vs. HI: P = 0.029]. The prognostic power of the cachexia‐associated TLC score [P < 0.001, HR: 7.37 (2.80–19.49)] and its application to define risk groups (LO vs. IM: P = 0.032, LO vs. HI: P < 0.001, IM vs. HI: P = 0.014) was confirmed in a cohort of PC patients. The prognostic power of the TLC score was independent of presence of liver metastases in CRC or PC patients and was superior to clinically established staging classifications.
Conclusions
The TLC score, a result of straightforward determination of blood parameters, is an objective cachexia‐associated clinical tool for precise survival prediction of gastrointestinal cancer patients.
Keywords: Cachexia, Prognostic score, Gastrointestinal cancer, TIMP‐1
Introduction
Gastrointestinal (GI) cancers account for more than 3 million deaths worldwide per year. 1 In particular, colorectal cancer (CRC) is the GI cancer accounting for the highest number of absolute cancer deaths, 1 , 2 while pancreatic cancer (PC) is the most lethal GI cancer type. 1 , 2 Cachexia, which occurs exceptionally frequent in CRC and PC, 3 is a devastating multiorgan syndrome critically impacting on life quality and survival of cancer patients. 4 , 5 One common cachexia‐associated symptom is weight loss (WL), resulting from wasting of skeletal muscle mass as well as loss of fat tissue. 3 However, it is recently discussed that the complex syndrome of cancer cachexia can also occur without obvious loss of total body mass, 6 a phenomenon termed ‘hidden cachexia’. 7 Therefore, it is increasingly debated 8 whether WL of cancer patients, which is the conventional and still most widely applied clinical cachexia parameter, 9 is sufficient to classify all pathophysiological manifestations of cachexia, which are known to vary in severity. 3 Thus, novel cachexia‐associated biomarkers are needed to classify patients not only based on overt manifestation of cachexia, but also based on the severity of disease (i.e. the prognosis of these patients), because such differentiation would be a prerequisite for adequate therapeutic intervention strategies. 8 Because cachectic patients often exhibit signs of systemic inflammation, attempts to identify particular pro‐inflammatory cytokines predicting cachexia have been undertaken, but so far failed at large. 10 In addition to inflammatory parameters, anaemia and impaired liver function are recognized as symptoms of cancer cachexia. 3 More specifically, cachexia‐associated changes in liver functionality are discussed to promote increased cancer mortality. 4 , 11 In particular, activation of the hepatic acute phase response as indicator of ongoing inflammation has been observed in cachectic patients with GI cancer. 12 One factor that was shown to be linked to such cachexia‐related changes in cancer patients is tissue inhibitor of metalloproteinases‐1 (TIMP‐1). 13 TIMP‐1 has pro‐inflammatory functions 14 , 15 , 16 , 17 and is known to impair liver homeostasis, 14 , 16 , 18 and elevated TIMP‐1 levels directly correlate with poor prognosis in virtually all cancer entities. 19
Because cancer cachexia is a frequent and heterogeneous cancer‐associated syndrome critically determining disease severity in patients, a precise classification of cancer patients not only based on the mere presence of cachexia itself, but also based on disease prognosis, is needed in the clinic. Such classification would allow better monitoring as well as therapy of at‐risk cachectic cancer patients. Towards this end, this study aimed to establish a cachexia‐associated clinical tool, which (i) precisely predicts the severity of cancer diseases (i.e. the prognosis of cancer patients), (ii) is associated with the clinical manifestation of cachexia, and (iii) is easily accessible upon a single venipuncture.
In the present study, we established the novel TIMP‐1/liver cachexia (TLC) score by linking blood levels of TIMP‐1 to cachexia‐associated liver parameters (factors of acute phase response, damage, and metabolism), allowing classification of cancer patients according to both varying manifestations of cachexia and patient survival.
Material and methods
Patients
This study was approved by the Ethics Committee of the Medical Faculty of the Technical University of Munich (Germany; #1946/07, #409/16S), and written informed consent was obtained from all participants before surgery or before blood sampling. The analysis was conducted on a pseudonymized data set. The study population comprised patients with CRC and PC, who underwent oncological treatment (staging or resection) between 2009 and 2019 in the Department of Surgery, Klinikum Rechts der Isar, Technical University of Munich, and who agreed to participate in the study. Diagnosis of CRC and PC patients was verified by definitive histological examination of retrieved biopsies or, in PC patients without surgery, by cytology or clinical/radiological information to the best of our knowledge. For CRC patients, liver metastases were detected during the staging process using computed tomography (CT), or in case of non‐clear findings in CT by additional MRI, and liver metastases of CRC patients were pathologically confirmed. Eleven out of 82 CRC patients received liver resection because of liver metastases. For PC patients, liver metastases were detected by staging CT or MRI, or during surgical exploration. We defined treatment‐naïve patients as patients without chemotherapy or radiation prior to inclusion in the study. We used the eighth edition of the Union for International Cancer Control (UICC) tumour–node–metastasis (TNM) classification and staging system for CRC and PC. 20 A minor part (n = 36) of the here‐described PC patient cohort (n = 84) was included in a previous study 13 ; however, survival data of all PC patients were updated in 2020. If not stated otherwise, all available patients were included for the respective analyses throughout this study.
Clinical parameters assessment
Blood samples for analysis of blood parameters were taken at the time point of patients' inclusion in the clinical study. This occurred at the day of admission to the hospital or upon a visit of surgical outpatient clinic. Weight was measured at the time of inclusion in the clinical study. Weight histories over the 6 months preceding inclusion in the clinical study were reported, and WL was calculated from the time period 6 months prior to inclusion in the clinical study. WL‐defined cachexia was defined as loss of at least 5% of the original body weight. 9 Analysis of body composition was performed at the time point of inclusion in the clinical study and 6 months afterwards. Staging CT scans were used for the measurement of body composition parameters [visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), intramuscular adipose tissue (IMAT), and skeletal muscle area (SMA)] by employing the Slice‐O‐Matic Software V4.3 as described elsewhere. 21 , 22 Total adipose tissue (TAT) was calculated by summing up the surface area of VAT, SAT, and IMAT. Area values were normalized to stature (height × height, m2) and reported as indices (VATI, SATI, IMATI, TATI, and SMAI). Relative delta values (relative delta VATI, relative delta SATI, relative delta IMATI, relative delta TATI, and relative delta SMAI) were used for correlation analysis. Anaemia was defined as blood haemoglobin <11.0 g/dL according to the recommendations of the World Health Organization (WHO) for moderate and severe anaemia (WHO reference number: WHO/NMH/NHD/MNM/11.1). Normal clinical ranges (NCRs) of blood parameters were set as recommended by the WHO (NCRCRP < 0.3 mg/dL, WHO/NMH/NHD/EPG/14.7 and NCRferritin,female 15–150 ng/mL, NCRferritin,male 15–200 ng/mL, WHO/NMH/NHD/MNM/11.2) or the UK National Health Service (NCRGGT,female < 40 U/L, NCRGGT,male < 60 U/L, NCRalbumin 3.5–5.0 g/dL, NCRtotalprotein 6.0–8.0 g/dL), 23 respectively.
Laboratory examinations
Blood samples were analysed at the Institute of Clinical Chemistry and Pathobiochemistry, Klinikum rechts der Isar, Munich, according to standard operating procedures. Blood was collected in a 2.7 mL EDTA tube and one 7.5 mL serum tube (S Monovette, Sarstedt, Nümbrecht, Germany) and mixed immediately by gently inverting the tube after collection. Haemoglobin levels were determined by the sodium lauryl sulfate haemoglobin detection method. A photometric biuret‐based test was used for measurement of total serum protein, and the bromcresol green reaction test was used to determine serum albumin. Serum concentrations of C‐reactive protein (CRP) were measured with an immunoturbidimetric assay. Serum ferritin was assessed by electrochemiluminescence immunoassay (Roche Diagnostics, Mannheim, Germany). Serum gamma‐glutamyl transferase (GGT) was determined using the enzymatic colorimetric assay GGT‐2 (Roche Diagnostics, Mannheim, Germany). A turbidimetric method and a Behring Coagulation System analyser (BCS, Siemens Healthcare Diagnostics, Erlangen, Germany) were used to measure thromboplastin time (quick). A kinetic photometric test, according to the International Federation of Clinical Chemistry and Laboratory Medicine, was used for the measurement of alkaline phosphatase. Glutamate–oxaloacetate transaminase and glutamate–pyruvate transaminase were determined by UV test with pyridoxal phosphate activation (Roche Diagnostics, Mannheim, Germany). A photometric diazonium‐based test was used to measure serum bilirubin. The measurements of total protein, bilirubin, albumin, CRP (e 602 module), GGT, glutamate–oxaloacetate transaminase, glutamate–pyruvate transaminase, alkaline phosphatase, and ferritin were performed on a Cobas 8000 platform (Roche Diagnostics, Mannheim, Germany). Jaundice was defined by serum total bilirubin level >3 mg/dL at the time of blood sampling.
ELISA
Blood samples were collected, and plasma was obtained within 30 min by centrifugation of whole blood for 15 min at 1000 g. Plasma samples were immediately snap‐frozen in liquid nitrogen and stored at −80°C. TIMP‐1 levels in plasma were determined using the DuoSet ELISA kit (R&D Systems) according to the manufacturer's instructions.
Statistical analyses
Statistical analysis was performed using the statistical software SPSS version 24.0 (Chicago, IL, USA). Artwork was created using GraphPad Prism version 9.0.0 (San Diego, CA, USA) and Affinity Designer version 1.8.4 (Nottingham, UK). Normal distribution was tested by Shapiro–Wilk tests. Correlations between quantitative variables were tested by Spearman correlations due to absence of normal distribution. Spearman's correlation analysis of several blood parameters at once was complemented by a multiple comparison test employing the Benjamini–Hochberg procedure. 24 For this, the P value of each correlation was compared with its corresponding Benjamini–Hochberg critical value ( , with false discovery rate = 0.20), and the largest P value that has P < critical value as well as all of the P values smaller than it were considered as significant. 25 Groups were compared using Student's t‐test for independent samples in the case of normal distribution, or non‐parametric Mann–Whitney test for independent variables, in the absence of normal distribution. χ2 test (Fisher's exact test) was employed to test differences in the proportion of patients with ≥5% WL, or anaemia, between the low (LO)‐risk, intermediate (IM)‐risk, as well as high (HI)‐risk groups. To derive optimal cut‐off values for blood parameters, maximally selected log–rank statistics was performed by using the R function maxstat.test 26 and employing the R Software version 2.13.0 (R Foundation for Statistical Computing, Vienna, Austria).
Survival analysis
Time‐dependent survival probabilities were estimated with the Kaplan–Meier method. The log–rank test (Mantel–Cox test) was used to compare statistically significant differences between independent subgroups. Cox regression analysis was employed to calculate the significance of a predictive relation between continuous or categorical variables and survival as well as the corresponding hazard ratio (HR) including the corresponding 95.0% confidence interval. Overall survival was censored at the date of the last visit or last phone contact for patients whose deaths could not be confirmed.
Results
Patient cohort
This study employed routinely accessible clinical data and blood samples from CRC as well as PC patients (Supporting Information, Figure S1), which were analysed with respect to cachexia‐associated parameters and patient survival. The CRC cohort, which was employed as test cohort to establish the prognostic score, comprised 82 patients (Supporting Information, Table S1A) with a median age of 70 years, a median relative WL (−3.2%) at time point of diagnosis, as well as a median loss of SATI (−3.9%), VATI (−10.3%), and TATI (−7.9%) 6 months after inclusion in the study (Supporting Information, Table S1B). The PC cohort served as validation cohort in order to validate the usability of the prognostic score in patients diagnosed with a different cancer type. This second cohort comprised 84 PC patients (Supporting Information, Table S1A) with a median age of 69 years, median relative WL (−6.8%) at time point of diagnosis, as well as a median loss SATI (−33.6%), VATI (−37.4%), and TATI (−36.6%) 6 months after inclusion in the study (Supporting Information, Table S1B).
Systemic tissue inhibitor of metalloproteinases‐1 levels differentiate subpopulations of weight loss‐defined cachectic colorectal cancer patients
First, we characterized the CRC patient cohort with respect to conventional WL‐defined cachexia (≥5% WL within 6 months 9 ). Relative WL significantly predicted survival, as CRC patients with high WL (WLHI) exhibited a 3.5‐fold increased risk to die as compared with patients with low or without WL (WLLO) (Figure 1A). Next, we aimed to investigate whether the cachexia‐related blood parameter TIMP‐1 was associated with WL, the conventional cachexia parameter, in CRC patients. In fact, we found a direct correlation between plasma TIMP‐1 levels and WL of CRC patients (Figure 1B), which is in agreement with previous findings in PC patients 13 and indicates that TIMP‐1 is a cachexia‐associated marker in CRC.
Figure 1.
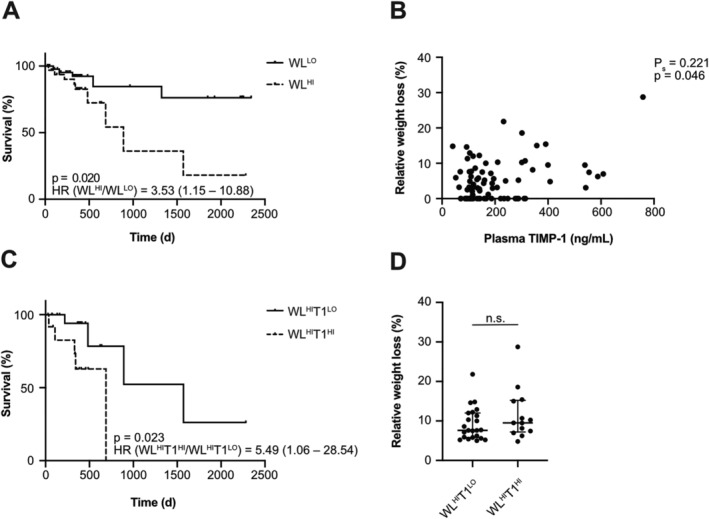
Plasma tissue inhibitor of metalloproteinases‐1 (TIMP‐1) levels differentiate subpopulations of weight loss (WL)‐defined cachectic colorectal cancer (CRC) patients. Survival of CRC patients (n = 80) separated by relative WL (A). Correlation of plasma TIMP‐1 levels with relative WL of CRC patients (n = 82) (B). Survival of CRC patients with increased WL (WLHI, n = 34) separated by plasma TIMP‐1 levels (C, cut‐off: 288.37 ng/mL TIMP‐1). Hazard ratios (HRs) are shown including the corresponding 95.0% confidence interval (A, C). Relative WL of WLHI CRC patients separated by plasma TIMP‐1 levels is shown as individual dots and medians with interquartile range (D).
Next, we determined whether plasma TIMP‐1 levels can be used to identify different subgroups within WL‐defined cachectic patients with respect to survival, which would point at TIMP‐1‐associated differences in severity of cachexia. For this, an optimized cut‐off value to dichotomize the CRC patient cohort based on plasma TIMP‐1 levels was calculated by maximally selected log–rank statistics revealing a cut‐off value of 288.37 ng/mL TIMP‐1 (Supporting Information, Figure S2A) to separate T1LO from T1HI patients. Survival analysis resulted in a significant separation of WL‐defined CRC patients based on plasma TIMP‐1 levels into two distinct subpopulations, where patients with high WL and elevated TIMP‐1 blood levels (WLHIT1HI) showed the worst prognosis, while patients with high WL and low TIMP‐1 blood levels (WLHIT1LO) showed significantly longer survival (Figure 1C). Importantly, WLHIT1HI patients did not show significant differences in relative WL as compared with WLHIT1LO patients (Figure 1D), confirming that the TIMP‐1‐associated difference in survival of cachectic patients was independent of differences in WL itself. Notably, separation of WLLO patients based on plasma TIMP‐1 levels did not reveal prognostically distinct subgroups (Supporting Information, Figure S3). Altogether, these findings suggest that blood levels of TIMP‐1 are suitable to distinguish prognostically different subpopulations of WL‐defined cachectic patients, which, importantly, cannot be identified by WL alone.
Parameters of liver functionality correlate with blood tissue inhibitor of metalloproteinases‐1 levels and predict survival in colorectal cancer patients
The finding that TIMP‐1 levels determined two prognostically distinct subpopulations (WLHIT1LO vs. WLHIT1HI) within the WL‐defined group of cachectic patients raised the question of whether TIMP‐1's impact on liver homeostasis is also reflected in CRC patients. In a first unbiased approach, we tested easily accessible clinical blood parameters and additional cachexia‐associated body composition parameters for correlation with blood TIMP‐1 levels within the whole CRC patient cohort by employing Spearman's correlation adjusted with the Benjamini–Hochberg procedure for multiple comparison tests (Supporting Information, Table S2). Blood levels of TIMP‐1 positively correlated with blood levels of CRP (Figure 2A), ferritin (Figure 2B), and GGT (Figure 2C), markers related to liver inflammation. Furthermore, TIMP‐1 negatively correlated with known markers of liver functionality, namely, albumin (Figure 2D) and total protein (Figure 2E).
Figure 2.
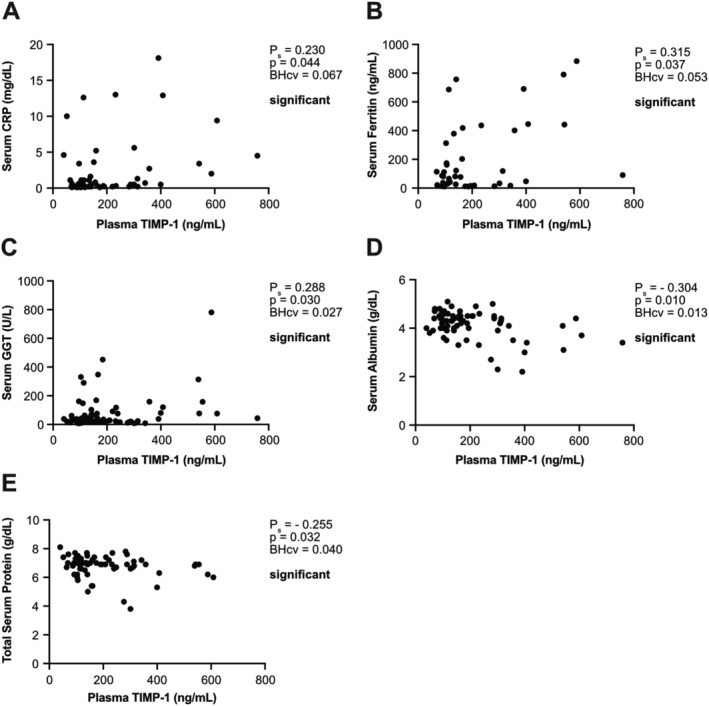
Plasma tissue inhibitor of metalloproteinases‐1 (TIMP‐1) levels correlate with parameters of liver functionality in colorectal cancer (CRC) patients. Correlation of plasma TIMP‐1 levels with serum C‐reactive protein (CRP) (A, n = 57), ferritin (B, n = 45), gamma‐glutamyl transferase (GGT) (C, n = 77), albumin (D, n = 71), or total protein (E, n = 71) of CRC patients. Due to absence of normal distribution, Spearman correlation was employed. Positive (A–C)/negative (D, E) Spearman correlation coefficients Ps indicate a positive/negative correlation. Benjamini–Hochberg critical values (BHcv) were derived from Supporting Information, Table S2.
Next, we assessed whether these TIMP‐1‐associated parameters of liver functionality are of prognostic relevance in the entire CRC cohort. Maximally selected log–rank statistics revealed 0.3 mg/dL CRP (Supporting Information, Figure S2B), 203 ng/mL ferritin (Supporting Information, Figure S2C), 120 U/L GGT (Supporting Information, Figure S2D), 3.9 g/dL albumin (Supporting Information, Figure S2E), as well as 5.8 g/dL total protein (Supporting Information, Figure S2F), respectively, as individual cut‐off values for optimal separation of the CRC patient cohort. Indeed, high blood levels of liver‐related inflammation parameters, such as CRP (Figure 3A), ferritin (Figure 3B), and GGT (Figure 3C), significantly correlated with poor survival of CRC patients. Low blood levels of parameters of liver functionality, such as albumin (Figure 3D) or total protein (Figure 3E), significantly correlated with decreased survival. Taken together, TIMP‐1‐associated liver parameters are of prognostic relevance in CRC patients.
Figure 3.
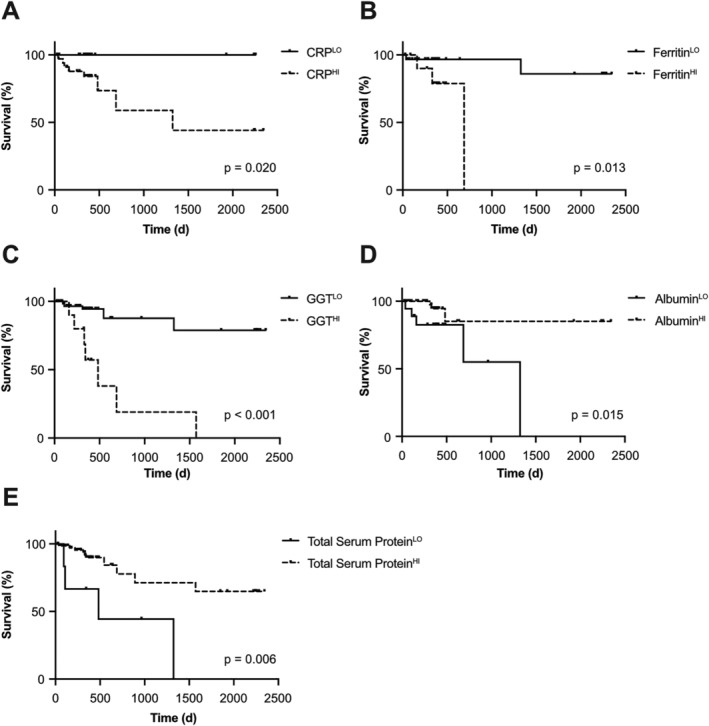
Parameters of liver functionality predict survival of colorectal cancer (CRC) patients. Survival of CRC patients separated by serum levels of CRP [A, n = 56, cut‐off: 0.3 mg/dL C‐reactive protein (CRP)], ferritin (B, n = 43, cut‐off: 203 ng/dL ferritin), gamma‐glutamyl transferase (GGT) (C, n = 75, cut‐off: 120 U/L GGT), albumin (D, n = 70, cut‐off: 3.9 g/dL albumin), or total protein (E, n = 80, cut‐off: 5.8 g/dL total protein).
Improved accuracy of colorectal cancer survival prediction by combining tissue inhibitor of metalloproteinases‐1 with liver parameters
In a next step, we combined the prognostic power of TIMP‐1 blood levels together with TIMP‐1‐associated liver parameters in order to define an integrative prognostic score for CRC patients. For this, we used the rounded cut‐off values from the survival analyses so far (Supporting Information, Figure S2) as the ‘risk threshold’ (RTh) for each clinical parameter (TIMP‐1, CRP, ferritin, GGT, albumin, and total protein). For each patient of our CRC cohort, blood parameter values were categorized as being above or below the respective RTh. To account for situations where not all blood parameters were available from each patient, the number of parameters above (for TIMP‐1, CRP, ferritin, and GGT) and below (for albumin and total protein) the RThs was normalized to the total number of measured parameters from each patient (Table 1). This provided an integrative score with a numerical value ranging from 0 to 1 for each patient. Of note, this integrative score was applied to the entire CRC patient cohort, irrespective of WL. An initial Cox regression analysis revealed a very significant and powerful predictive relation between the numerical values of this integrative score and CRC patient survival [P < 0.001, HR: 96.91 (9.85–953.90) with 95% confidence interval]. Next, we evaluated whether CRC patients can be classified into prognostically distinct risk categories based on this integrative score. Towards this end, we performed an approximating statistical approach, in which patients were separated into different risk categories with distinct boundaries, which were statistically tested for the strongest significant separation. Strongest separation of different risk groups of CRC patients was achieved by classifying them into three groups, namely, the ‘LO‐risk group’ (score ≤0.25), the ‘IM‐risk group’ (score between 0.25 and 0.60), as well as the ‘HI‐risk group’ (score ≥0.60) (Table 1). In fact, the HI‐risk group showed a significantly increased risk to die as compared with the LO‐risk group and the IM‐risk group (Figure 4A). Taken together, survival was distinct between all three groups that were separated according to the integrative prognostic score (Figure 4A).
Table 1.
Classification of cancer patients based on the TLC score
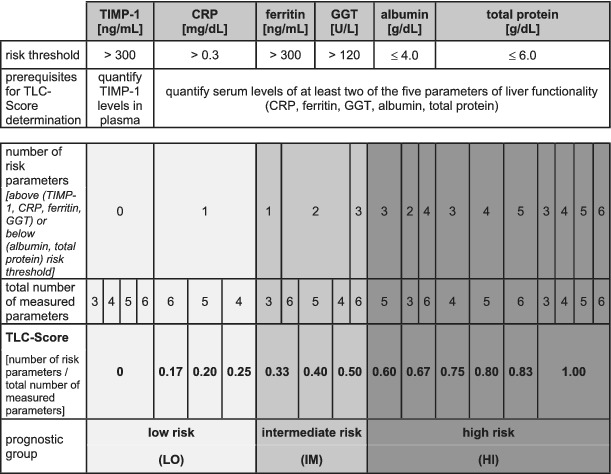
|
Classification into three groups according to the risk to die by (i) measuring the patient's blood levels of TIMP‐1 as well as of at least two additional liver parameters (CRP, ferritin, GGT, albumin, and total protein), (ii) categorizing the patient's blood parameters as below/above the respective RTh (rounded cut‐off values from previous analyses, Supporting Information, Figure S2A), and (iii) normalizing the number of risk parameters to the total number of measured parameters.
CRP, C‐reactive protein; GGT, gamma‐glutamyl transferase; TIMP‐1, tissue inhibitor of metalloproteinases‐1; TLC, TIMP‐1/liver cachexia.
Figure 4.
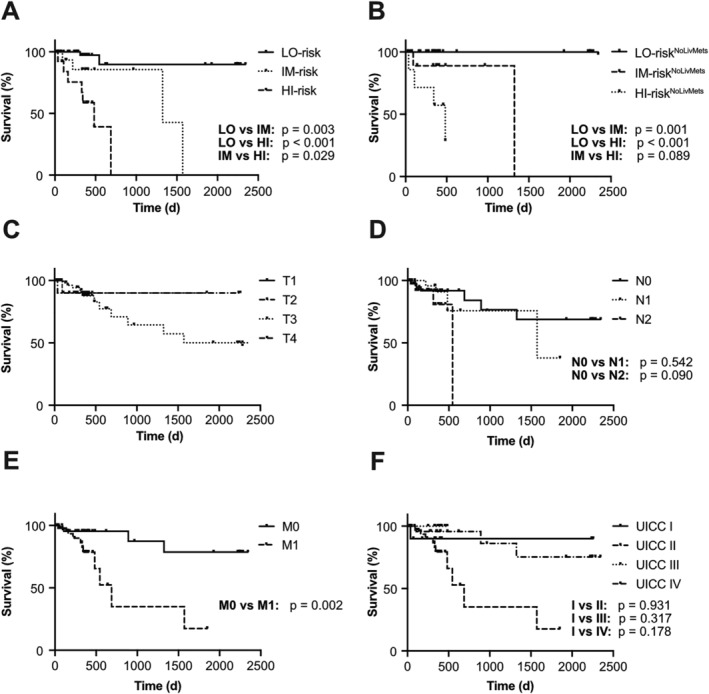
Combination of tissue inhibitor of metalloproteinases‐1 (TIMP‐1) with liver parameters predicts survival of colorectal cancer (CRC) patients. Survival of CRC patients separated by the integrative prognostic score into low‐risk (LO, n = 51), intermediate‐risk (IM, n = 15), or high‐risk (HI, n = 13) groups (A). Survival of CRC patients without liver metastases separated by the integrative prognostic score into low‐risk (LONoLivMets, n = 38), intermediate‐risk (IMNoLivMets, n = 9), or high‐risk (HINoLivMets, n = 7) groups (B). Survival of CRC patients separated by tumour (T, n = 80) status (C), lymph node (N, n = 79) status (D), metastasis (M, n = 80) status (E), or Union for International Cancer Control (UICC) classification (n = 79, F). No P value could be calculated to compare the respective tumour status groups, as there was a too low number of patients with T1 (n = 1).
Importantly, separation of three distinct subpopulations with different prognosis based on the integrative score was also effective upon exclusion of all CRC patients with liver metastases (Figure 4B), indicating that the prognostic power of this integrative score did not depend on the presence of liver metastases. Risk category‐matched survival analyses revealed that patients with liver metastases showed shorter survival as compared with patients without liver metastases only within the LO‐risk group (Supporting Information, Figure S4A), while there were no differences in survival between patients with vs. without liver metastases within the IM‐risk or HI‐risk groups (Supporting Information, Figure S4B and S4C). The prognostic power of separating LO‐risk vs. IM‐risk vs. HI‐risk groups based on the integrative prognostic score (Figure 4A) was even superior to the clinically established T, N, and M classification individually, as well as combined as UICC classification, because neither individual tumour (T) (Figure 4C), lymph node (N) (Figure 4D), and metastasis status (M) (Figure 4E) nor the TNM‐combined UICC status (Figure 4F) reliably separated more than two prognostically distinct subpopulations. These data show that the integrative score is useful for accurate prediction of CRC patient survival.
Cachexia is reflected by the prognostic score combining tissue inhibitor of metalloproteinases‐1 and liver parameters
Next, we evaluated whether, in addition to patient survival, clinical manifestations of cachexia are reflected by this integrative score. In the analyses above, we have shown that TIMP‐1 as well as the parameters of liver functionality (albumin, total protein, CRP, ferritin, and GGT) are of prognostic relevance in the entire CRC patient cohort. In the IM‐risk and the HI‐risk group, blood albumin and total protein levels were significantly decreased, while blood CRP and ferritin were significantly increased, as compared with the LO‐risk group (Figure 5A). Blood levels of albumin, total protein, CRP, or ferritin were not different between the IM‐risk and the HI‐risk group (Figure 5A). Blood GGT and TIMP‐1 levels were the only parameters of the integrative prognostic score that were significantly increased in the HI‐risk group as compared with the IM‐risk groups (Figure 5B). It is important to note that a combination of parameters of liver functionality without TIMP‐1, or GGT, neither showed a significant predictive relation between the numerical values of these modified scores and CRC patient survival [score without TIMP‐1: P = 0.835, HR: 1.26 (0.15–10.66); score without GGT: P = 0.937, HR: 0.92 (0.12–7.36), with 95% confidence interval], nor were these modified scores sufficient to separate three distinct prognostic groups (Figure 5C and 5D). These findings highlight the necessity of both parameters as key prognostic factors in this setting.
Figure 5.
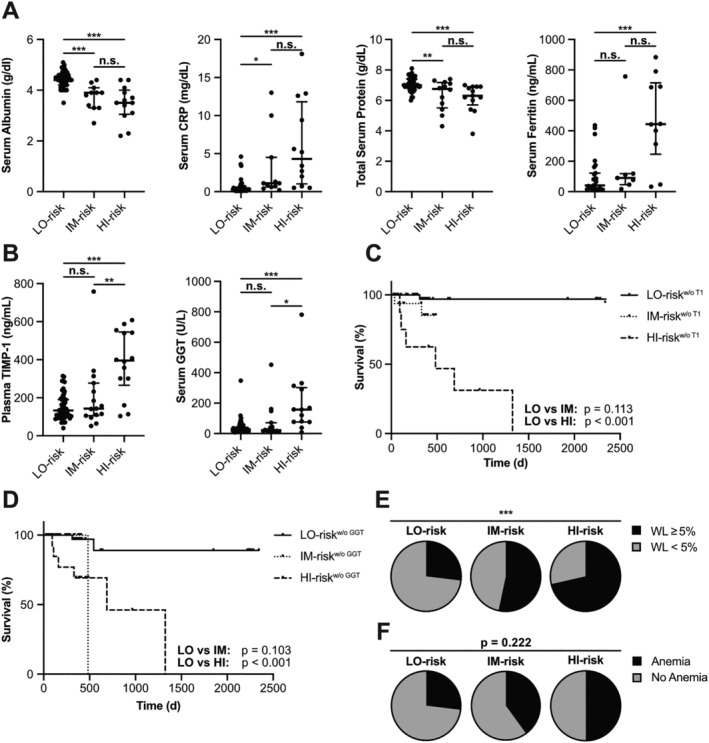
Cachexia is reflected by the tissue inhibitor of metalloproteinases‐1 (TIMP‐1)/liver cachexia (TLC) score combining TIMP‐1 with liver parameters. Serum levels of albumin (A, far left), C‐reactive protein (CRP) (A, center left), total protein (A, center right), ferritin (A, far right), and gamma‐glutamyl transferase (GGT) (B, right) as well as plasma levels of TIMP‐1 (B, left) of colorectal cancer (CRC) patients grouped according to the TLC score are shown as individual dots and medians with interquartile range. Survival of CRC patients separated by the modified TLC score without considering TIMP‐1 (C, n = 71) or GGT (D, n = 71). Proportion of CRC patients with low (<5%) vs. high (≥5%) weight loss (WL) (E), or with absence vs. presence of anaemia (F), grouped according to the TLC score.
In addition to plasma TIMP‐1 levels and parameters of liver functionality, we next assessed whether the three distinct patient subpopulations also differed in additional clinical manifestations of cachexia, namely, the conventional cachexia parameter WL as well as anaemia. In fact, the proportion of patients with WL ≥ 5% significantly increased from ~26% in the LO‐risk group to ~53% in the IM‐risk group and to ~71% in the HI‐risk group (Figure 5E). Of note, a rather high proportion (29%) of patients identified as HI‐risk group did not exhibit WL ≥ 5% (Figure 5E). A similar increasing trend in the proportion of patients with anaemia was observed in the LO‐risk vs. IM‐risk vs. HI‐risk groups (Figure 5F). In addition, the numerical value of the integrative score directly positively correlated with relative WL (Supporting Information, Figure S5A) as well as negatively correlated with blood haemoglobin levels (Supporting Information, Figure S5B), the indicator for anaemia. Taken together, these findings indicate that various syndromes of cachexia such as impaired liver function, WL, and anaemia are reflected by the combination of plasma TIMP‐1 levels and parameters of liver functionality, which led us to designate the integrative prognostic score as TLC score. In fact, applying the TLC score only to CRC patients with WL‐defined cachexia, that is, ≥5% WL, showed a very significant and powerful relation with WLHI patient survival [Cox regression: P = 0.011, HR: 120.74 (3.02–4832.18), with 95% confidence interval] as well as revealed three prognostically distinct subpopulations (Supporting Information, Figure S5C). Of note, separation of patients without WL‐defined cachexia by the TLC score was less powerful [Cox regression: P = 0.043, HR: 46.67 (1.14–1913.09), Supporting Information, Figure S5D]. These data further substantiate the TLC score as cachexia‐associated, powerful clinical tool to classify especially cachectic CRC patients based on the severity (i.e. the prognosis) of cancer disease.
The tissue inhibitor of metalloproteinases‐1/liver cachexia score predicts survival of pancreatic cancer patients
As a proof of concept and to illustrate the applicability of the TLC score also in other cancer entities, we next tested its prognostic value in a validation cohort of PC patients. Of note, patients with pre‐existing jaundice were excluded (Supporting Information, Figure S1) because obstructive jaundice can contribute to liver parenchymal damage and impaired liver function tests. 27 Applying the RTh levels determined above (Table 1) to the PC patient cohort, we found that increased blood levels of TIMP‐1 (Figure 6A) and the parameters of liver inflammation (CRP, Figure 6B) and liver damage (GGT, Figure 6C), as well as decreased levels of the liver function parameters albumin (Figure 6D) predicted shorter survival also for PC patients. Only blood ferritin (Figure 6E) as well as total protein levels (Figure 6F), which were available from only 30 or 62 of 84 PC patients, respectively, showed no association with prognosis of PC patients.
Figure 6.
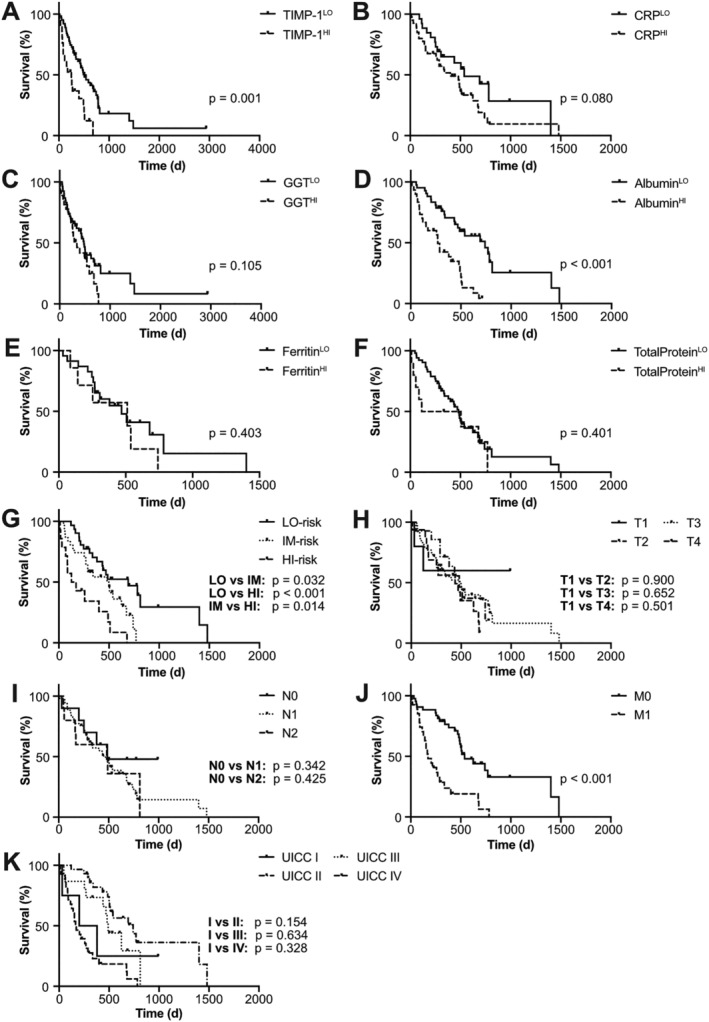
Tissue inhibitor of metalloproteinases‐1 (TIMP‐1)/liver cachexia (TLC) score predicts survival of pancreatic cancer (PC) patients. Survival of PC patients separated by plasma levels of TIMP‐1 (A, n = 84, cut‐off: 300 ng/mL TIMP‐1), or serum levels of C‐reactive protein (CRP) (B, n = 66, cut‐off: 0.3 mg/dL CRP), gamma‐glutamyl transferase (GGT) (C, n = 80, cut‐off: 120 U/L GGT), albumin (D, n = 72, cut‐off: 4.0 g/dL albumin), ferritin (E, n = 30, cut‐off: 300 ng/mL ferritin), or total protein (F, n = 62, cut‐off: 6.0 g/dL total protein). Survival of PC patients separated by the TLC score (G, n = 76), the tumour (T) status (H, n = 74), the lymph node (N) status (I, n = 73), the metastasis (M) status (J, n = 70), or the Union for International Cancer Control (UICC) classification (K, n = 76).
In an analogous approach as applied in the CRC patient cohort (Table 1), we now determined the numerical TLC score value for each PC patient of the entire PC patient cohort, irrespective of WL, by employing the previously calculated RTh (Table 1). Cox regression analysis revealed a very significant and powerful predictive relation between PC patient survival and the numerical TLC score value [P < 0.001, HR: 7.37 (2.80–19.49), with 95% confidence interval]. This finding suggests a general applicability of the TLC score as powerful prognostic tool also in the entire validation cohort of PC patients.
Classification of PC patients into three groups based on the same boundaries as determined previously (Table 1) revealed three PC patient subpopulations with varying prognosis (Supporting Information, Figure S6A). However, the IM‐risk group did not significantly differ in survival as compared with the LO‐risk group (Supporting Information, Figure S6A). In fact, separation of three prognostically very distinct subpopulations could be achieved by fine adjustment of the boundaries for the IM‐risk group from TLC score values 0.33 to 0.50, as previously described for CRC patients (Table 1), to TLC score values 0.25 to 0.60 (Figure 6G), indicating that the boundaries of the TLC score to classify patients into three distinct risk categories can be optimized for each cancer type. In fact, the HI‐risk group showed worst prognosis and an increased risk to die as compared with the LO‐risk group as well as to the IM‐risk group (Figure 6G).
Importantly, separation of three distinct subpopulations with different prognoses based on the integrative score was also effective upon exclusion of all PC patients with liver metastases (Supporting Information, Figure S6B), indicating that the prognostic power of this integrative score did not depend on the presence of liver metastases. The prognostic power of separating LO‐risk vs. IM‐risk vs. HI‐risk groups of PC patients according to the TLC score (Figure 6G) was superior to the clinically established TNM classification, because neither individual tumour (T) (Figure 6H), nor lymph node (N) (Figure 6I), nor metastasis status (M) (Figure 6J) allowed clear separation of more than two prognostically distinct subpopulations. Similarly, the TNM‐combined UICC classification (Figures 4F and 6K) as well as the modified Glasgow Prognostic Score (mGPS) 28 (Supporting Information, Figure S6C and S6D) did not predict patient survival in the CRC and PC cohort. Therefore, the newly established TLC score predicted also the survival of PC patients, indicating a broader clinical applicability.
A standardized tissue inhibitor of metalloproteinases‐1/liver cachexia score as straightforward prognostic tool in gastrointestinal cancer
Finally, we aimed to determine the direct applicability of the TLC score in the clinics. Our results showed that the contribution of TIMP‐1 levels to the prognostic value of the TLC score is essential (Figure 5C), and therefore, plasma TIMP‐1 levels necessarily need to be quantified in the clinics. In addition to TIMP‐1, the TLC score comprised five liver functionality parameters, which are being quantified and compared with the NCR of the respective parameter in a standardized manner in daily clinical routine. In fact, these standardized NCRs are identical (for CRP) or at least in a very similar range (for ferritin, GGT, albumin, and total protein) as compared with the determined optimized RThs for each parameter (NCRCRP < 0.3 mg/dL vs. RThCRP = 0.3 mg/dL; NCRferritin,female 15–150 ng/mL, NCRferritin,male 15–200 ng/mL vs. RThferritin = 300 ng/mL; NCRGGT,female < 40 U/L, NCRGGT,male < 60 U/L vs. RThGGT = 120 U/L; NCRalbumin 3.5–5.0 g/dL vs. RThalbumin = 4.0 g/dL; NCRtotalprotein 6.0–8.0 g/dL vs. RThtotalprotein = 6.0 g/dL). The similarity between the clinically relevant NCRs and the previously determined RThs for each liver functionality parameter led us to test the prognostic value of the NCRs as RThs for each parameter individually. For this, we checked for each patient whether the respective liver functionality parameter was within (‘normal range’) or outside (‘pathological range’) the NCR and analysed the respective prognostic value. For both the CRC and the PC patient cohorts, the NCRGGT (Supporting Information, Figure S7A and S7B) and the NCRCRP (Supporting Information, Figure S7C and S7D) separated prognostically distinct subpopulations, while the NCRferritin (Supporting Information, Figure S7E and S7F) did not separate patients into prognostically distinct groups. For the NCRalbumin and the NCRtotalprotein, there were opposing trends between both cancer types, because the NCRalbumin was of prognostic value only for PC patients (Supporting Information, Figure S7G and S7H) and the NCRtotalprotein was of prognostic value only for CRC patients (Supporting Information, Figure S7I and S7J). We next calculated a standardized TLC score (sTLC score) by employing the NCRs as respective RThs for CRC as well as PC patients. Cox regression analysis revealed a very significant and powerful predictive relation between the numerical values of the sTLC score with CRC [P = 0.001, HR: 55.94 (4.88–641.71), with 95% confidence interval] as well as PC [P = 0.001, HR: 4.75 (1.88–12.01), with 95% confidence interval] patient survival. In fact, the prognostic value of the sTLC score was only slightly less powerful than the TLC score calculated with the previously described RThs (Table 1) [for CRC patients: P < 0.001, HR: 96.91 (9.85–953.90), for PC patients: P < 0.001, HR: 7.37 (2.80–19.49), with 95% confidence interval]. Importantly, separation of three prognostically distinct CRC (Figure 7A) as well as PC (Figure 7B) patient subpopulation with LO, IM, or HI risk was also possible by employing the sTLC score. Taken together, these findings indicate that the clinically easily accessible sTLC score is a straightforward and powerful prognostic tool for evaluation of CRC and PC patients.
Figure 7.
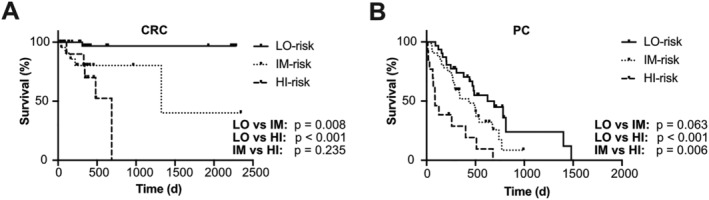
A standardized tissue inhibitor of metalloproteinases‐1 (TIMP‐1)/liver cachexia (sTLC) score predicts survival of colorectal cancer (CRC) and pancreatic cancer (PC) patients. Survival of CRC (A, n = 70) or PC (B, n = 76) patients separated by the sTLC score (RThTIMP‐1: 300 ng/mL, NCRGGT,female < 40 U/L, NCRGGT,male < 60 U/L, NCRCRP < 0.3 mg/dL, NCRferritin,female 15–150 ng/mL, NCRferritin,male 15–200 ng/mL, NCRalbumin 3.5–5.0 g/dL, NCRtotalprotein 6.0–8.0 g/dL).
Discussion
Here, we establish the novel TLC score, which precisely predicts survival in patients with GI cancers. For this, we employed a test cohort of CRC patients as well as a validation cohort of PC patients. In fact, the majority of these CRC and PC patients showed apparent WL as well as loss of adipose tissue, which is in line with previous reports demonstrating that cachexia is frequent in CRC as well as PC. 3 Moreover, we found that CRC patients with WL‐defined cachexia exhibited a 3.5‐fold increased risk to die as compared with non‐cachectic patients, which is consistent with the finding of a previous study employing a larger cohort of CRC patients. 29 Taken together, the here‐employed patient cohorts nicely reflect characteristics from large populations of GI cancer patients, indicating that our findings would also be confirmed in larger populations.
Based on this TLC score, we were able to separate three distinct cancer patient subpopulations (LO‐risk, IM‐risk, and HI‐risk groups) that differ not only in survival but also in clinical parameters of cachexia. As cachexia‐associated symptoms such as WL, anaemia, or impaired liver homeostasis turned out to be most prominent in the HI‐risk group and more frequent in the IM‐risk group as compared with the LO‐risk group, we propose that the TLC score is associated with cachexia varying from minor (LO‐risk) to moderate (IM‐risk) or more severe (HI‐risk) forms. Our finding that these clinical parameters of cachexia were associated with shorter patient survival (LO‐risk vs. IM‐risk vs. HI‐risk group) supports the notion that cachexia significantly contributes to cancer deaths. 30
So far, WL represented the most widely clinically applied parameter to identify cachectic patients. 9 Importantly, application of the cachexia‐associated TLC score enabled us to identify patients with low vs. high risk to die. The majority of LO‐risk patients did not show WL, while a remarkable proportion of 26% showed rather good prognosis despite exhibiting WL. As expected, the majority of HI‐risk patients showed increased WL, but this HI‐risk subpopulation also consisted of about 29% of patients without evidence for WL, despite having an increased risk to die. Although these findings are generally in line with previous reports showing that WL‐defined cachexia significantly contributes to cancer severity, 9 they also point to the fact that WL per se is not necessarily associated with poor prognosis. In fact, severe WL‐independent manifestations of cachexia that were previously summarized as ‘hidden cachexia’ have already been described 6 , 7 and further substantiate the notion that WL alone is not sufficient to precisely evaluate the severity of cachexia for cancer patients. In addition, weight data are mostly self‐reported by the patients and therefore lack robustness as well as objectivity. 31 Moreover, WL is also a changing and quite dynamic parameter, which requires measurements over a period of time and is usually recorded only for a fraction of patients (in our clinic: ~30%). These limitations are also true for WL‐based clinical scores such as the CAchexia SCOre 32 , 33 or the cachexia staging score. 34 The advantage of the TLC score over these scores is that it is independent from self‐reported WL measurements and, in addition, allows cachexia‐associated survival prediction. In fact, the TLC score can be quantified by straightforward, reliable, and simple clinical chemistry from a single venipuncture. In addition, follow‐up determination of the TLC score upon subsequent venipunctures may be straightforward and useful for assessment of cachexia therapy response. Because comparison of the patients' blood levels of CRP, ferritin, GGT, albumin, and total protein to the NCR of the respective parameter is already carried out in clinical chemistry in a standardized manner, only TIMP‐1 plasma levels have to be additionally quantified and compared with the here‐described RTh of 288.37 ng/mL in order to determine the sTLC score in the clinics. The mGPS, which is based on the quantification of blood CRP and albumin levels, 28 exhibits similar advantages. However, in comparison with the TLC score, the mGPS exhibited remarkably less prognostic power for CRC and PC patients. The objective classification of patients with increased signs of cachexia, as demonstrated by direct correlation of the TLC score with WL, and high risk to die may allow early and differential initiation of appropriate measures (therapies, pre‐rehabilitation and rehabilitation interventions, and adjuvant dietary) to combat cachexia in order to improve the quality of life as well as survival of patients. 4
Importantly, the versatility of the TLC score lies in the fact that its prognostic power was not demonstrated in one distinct subpopulation of patients, but in entire, heterogeneous cohorts comprising a range of different tumour stages (43% of CRC patients were UICC I and II, 44% of PC patients were UICC I and II). Because the TLC score relies on liver functionality parameters and CRC as well as PC notoriously metastasize to the liver, 35 it is important to emphasize that the TLC score also predicted survival of liver metastases‐free CRC and PC patients, demonstrating the independence of the TLC score from liver metastases. Another strong and unique feature of our study is that we provide long‐term survival (6.8 years in CRC or 8.2 years in PC) compared with other studies showing 1‐year survival 34 or mortality within 90 days. 28
The broader clinical relevance of the here‐described TLC score is underlined by the finding that the TLC score, which we had developed in a CRC patient cohort, also efficiently predicted survival in a PC patient cohort. These two cancer types represent the most mortal and lethal GI cancers, 1 , 2 respectively, in which cachexia is exceptionally frequent and crucially contributes to cancer‐related mortality. 3 Because blood levels of TIMP‐1, 19 one essential component of the TLC score, as well as of parameters of liver functionality 36 , 37 show prognostic relevance also in several other cancer types, the TLC score might be applicable to many tumour entities beyond the GI cancer entities. In the future, we expect that the TLC score could easily be determined during oncological treatment as a means to monitor the cachexia status, which is important to adjust for each patient the adequate oncological treatment.
Author contributions
O.P., C.D.H., and B.S. contributed in the data acquisition, data processing, and manuscript writing. H.F. and M.E.M. provided patient material and clinical data. O.L.P. worked on data acquisition. U.N. helped in data processing and manuscript editing. P.K. edited the manuscript. A.K. contributed to the conceptualization, supervision, design of the study, and manuscript writing.
Conflict of interest
The authors have no conflict of interest.
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting, or dissemination plans of this research study.
Funding
This work was supported by grants to A.K. from the Deutsche Forschungsgemeinschaft, Bonn, Germany (KR 2047/8‐1), and the Wilhelm‐Sander‐Stiftung, Munich, Germany (2016.124.1 and 2016.124.2). O.P. was supported by a Clinical Leave Stipend from the German Center of Infection Research (DZIF, grant TI07.001).
Supporting information
Figure S1 Supporting Information
Figure S2 Supporting Information
Figure S3 Supporting Information
Figure S4 Supporting Information
Figure S5 Supporting Information
Figure S6 Supporting Information
Figure S7 Supporting Information
Table S1 Supporting Information
Table S2 Supporting Information
Acknowledgements
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 38
Open access funding enabled and organized by Projekt DEAL.
Prokopchuk O., Hermann C. D., Schoeps B., Nitsche U., Prokopchuk O. L., Knolle P., Friess H., Martignoni M. E., and Krüger A. (2021) ">A novel tissue inhibitor of metalloproteinases‐1/liver/cachexia score predicts prognosis of gastrointestinal cancer patients, Journal of Cachexia, Sarcopenia and Muscle, 12, 378–392, 10.1002/jcsm.12680
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Quaresma M, Coleman MP, Rachet B. 40‐year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971–2011: a population‐based study. The Lancet 2015;385:1206–1218. [DOI] [PubMed] [Google Scholar]
- 3. Stewart GD, Skipworth RJ, Fearon KC. Cancer cachexia and fatigue. Clin Med (Lond) 2006;6:140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmidt SF, Rohm M, Herzig S, Berriel Diaz M. Cancer cachexia: more than skeletal muscle wasting. Trends Cancer 2018;4:849–860. [DOI] [PubMed] [Google Scholar]
- 5. Argiles JM, Stemmler B, Lopez‐Soriano FJ, Busquets S. Inter‐tissue communication in cancer cachexia. Nat Rev Endocrinol 2018;15:9–20. [DOI] [PubMed] [Google Scholar]
- 6. Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 7. Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol 2013;10:90–99. [DOI] [PubMed] [Google Scholar]
- 8. Talbert EE, Lewis HL, Farren MR, Ramsey ML, Chakedis JM, Rajasekera P, et al. Circulating monocyte chemoattractant protein‐1 (MCP‐1) is associated with cachexia in treatment‐naive pancreatic cancer patients. J Cachexia Sarcopenia Muscle 2018;9:358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 10. Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 2012;16:153–166. [DOI] [PubMed] [Google Scholar]
- 11. Jones A, Friedrich K, Rohm M, Schafer M, Algire C, Kulozik P, et al. TSC22D4 is a molecular output of hepatic wasting metabolism. EMBO Mol Med 2013;5:294–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fearon KC, Barber MD, Falconer JS, McMillan DC, Ross JA, Preston T. Pancreatic cancer as a model: inflammatory mediators, acute‐phase response, and cancer cachexia. World J Surg 1999;23:584–588. [DOI] [PubMed] [Google Scholar]
- 13. Prokopchuk O, Grünwald B, Nitsche U, Jager C, Prokopchuk OL, Schubert EC, et al. Elevated systemic levels of the matrix metalloproteinase inhibitor TIMP‐1 correlate with clinical markers of cachexia in patients with chronic pancreatitis and pancreatic cancer. BMC Cancer 2018;18:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seubert B, Grünwald B, Kobuch J, Cui H, Schelter F, Schaten S, et al. Tissue inhibitor of metalloproteinases (TIMP)‐1 creates a premetastatic niche in the liver through SDF‐1/CXCR4‐dependent neutrophil recruitment in mice. Hepatology 2015;61:238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kobuch J, Cui H, Grünwald B, Saftig P, Knolle PA, Krüger A. TIMP‐1 signaling via CD63 triggers granulopoiesis and neutrophilia in mice. Haematologica 2015;100:1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grünwald B, Harant V, Schaten S, Fruhschutz M, Spallek R, Höchst B, et al. Pancreatic pre‐malignant lesions secrete TIMP1, which activates hepatic stellate cells via CD63 signaling to create a pre‐metastatic niche in the liver. Gastroenterology 2016;151:1011–1024. [DOI] [PubMed] [Google Scholar]
- 17. Grünwald B, Schoeps B, Krüger A. Recognizing the molecular multifunctionality and interactome of TIMP‐1. Trends Cell Biol 2019;29:6–19. [DOI] [PubMed] [Google Scholar]
- 18. Kopitz C, Gerg M, Bandapalli OR, Ister D, Pennington CJ, Hauser S, et al. Tissue inhibitor of metalloproteinases‐1 promotes liver metastasis by induction of hepatocyte growth factor signaling. Cancer Res 2007;67:8615–8623. [DOI] [PubMed] [Google Scholar]
- 19. Eckfeld C, Häußler D, Schoeps B, Hermann CD, Krüger A. Functional disparities within the TIMP family in cancer: hints from molecular divergence. Cancer Metastasis Rev 2019;38:469–481. [DOI] [PubMed] [Google Scholar]
- 20. Brierley J, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours, Eighth ed. Oxford: John Wiley & Sons, Inc; 2017. [Google Scholar]
- 21. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 22. Martin L, Hopkins J, Malietzis G, Jenkins JT, Sawyer MB, Brisebois R, et al. Assessment of computed tomography (CT)‐defined muscle and adipose tissue features in relation to short‐term outcomes after elective surgery for colorectal cancer: a multicenter approach. Ann Surg Oncol 2018;25:2669–2680. [DOI] [PubMed] [Google Scholar]
- 23. Fuggle S. Clinical Biochemistry Reference Ranges Handbook. BIJ, Vol. 11; 2018. [Google Scholar]
- 24. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a pratical and powerful approach to multiple testing. J R Statist Soc B 1995;57:289–300. [Google Scholar]
- 25. McDonald JH. Handbook of Biological Statistics, 3rd ed. Baltimore, Maryland: Sparky House Publishing; 2014. [Google Scholar]
- 26. Hothorn T, Zeileis A. Generalized maximally selected statistics. Biometrics 2008;64:1263–1269. [DOI] [PubMed] [Google Scholar]
- 27. Hong JY, E FS, Hiramoto K, Nishikawa M, Inoue M. Mechanism of liver injury during obstructive jaundice: role of nitric oxide, splenic cytokines, and intestinal flora. J Clin Biochem Nutr 2007;40:184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Silva GAD, Wiegert EVM, Calixto‐Lima L, Oliveira LC. Clinical utility of the modified Glasgow Prognostic Score to classify cachexia in patients with advanced cancer in palliative care. Clin Nutr 2020;39:1587–1592. [DOI] [PubMed] [Google Scholar]
- 29. Gannavarapu BS, Lau SKM, Carter K, Cannon NA, Gao A, Ahn C, et al. Prevalence and survival impact of pretreatment cancer‐associated weight loss: a tool for guiding early palliative care. J Oncol Pract 2018;14:e238–e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Argiles JM, Busquets S, Stemmler B, Lopez‐Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 2014;14:754–762. [DOI] [PubMed] [Google Scholar]
- 31. Niedhammer I, Bugel I, Bonenfant S, Goldberg M, Leclerc A. Validity of self‐reported weight and height in the French GAZEL cohort. Int J Obes Relat Metab Disord 2000;24:1111–1118. [DOI] [PubMed] [Google Scholar]
- 32. Argiles JM, Betancourt A, Guardia‐Olmos J, Pero‐Cebollero M, Lopez‐Soriano FJ, Madeddu C, et al. Validation of the CAchexia SCOre (CASCO). Staging cancer patients: the use of miniCASCO as a simplified tool. Front Physiol 2017;8:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Argiles JM, Lopez‐Soriano FJ, Toledo M, Betancourt A, Serpe R, Busquets S. The cachexia score (CASCO): a new tool for staging cachectic cancer patients. J Cachexia Sarcopenia Muscle 2011;2:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou T, Wang B, Liu H, Yang K, Thapa S, Zhang H, et al. Development and validation of a clinically applicable score to classify cachexia stages in advanced cancer patients. J Cachexia Sarcopenia Muscle 2018;9:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Budczies J, von Winterfeld M, Klauschen F, Bockmayr M, Lennerz JK, Denkert C, et al. The landscape of metastatic progression patterns across major human cancers. Oncotarget 2015;6:570–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsai CC, Huang SC, Tai MH, Chien CC, Huang CC, Hsu YC. Hepatoma‐derived growth factor upregulation is correlated with prognostic factors of early‐stage cervical adenocarcinoma. Int J Mol Sci 2014;15:21492–21504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pavo N, Raderer M, Goliasch G, Wurm R, Strunk G, Cho A, et al. Subclinical involvement of the liver is associated with prognosis in treatment naive cancer patients. Oncotarget 2017;8:81250–81260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Supporting Information
Figure S2 Supporting Information
Figure S3 Supporting Information
Figure S4 Supporting Information
Figure S5 Supporting Information
Figure S6 Supporting Information
Figure S7 Supporting Information
Table S1 Supporting Information
Table S2 Supporting Information


