Abstract
There has been increasing interest in incorporating β-lactam precision dosing into routine clinical care, but robust population pharmacokinetic models in critically ill children are needed for these purposes. The objective of this study was to demonstrate the feasibility of an opportunistic sampling approach that utilizes scavenged residual blood for future pharmacokinetic studies of cefepime, meropenem and piperacillin. We aimed to show that opportunistic samples would cover the full concentration vs. time profiles and to evaluate stability of the antibiotics in whole blood and plasma to optimize future use of the opportunistic sampling approach.
A prospective observational study was conducted in a single-center pediatric intensive care unit, where patients administered at least one dose of cefepime, meropenem, or piperacillin/tazobactam and had residual blood scavenged from samples obtained for routine clinical care were enrolled. A total of 138 samples from 22 patients were collected in a two-week period. For all three antibiotics, the samples collected covered the entire dosing intervals and were not clustered around specific time points. There was high variability in the free concentrations, as well as the percentage of drug bound to protein. There was less than 15% degradation for meropenem or piperacillin when stored in whole blood or plasma at 4°C after six days. Cefepime degraded by more than 15% after three days.
The opportunistic sampling approach is a powerful and feasible method to obtain sufficient samples to study the variability of drug concentrations and protein binding for future pharmacokinetic studies in the pediatric critical care population.
Keywords: beta-lactams, antibiotics, pharmacokinetics, pediatrics, critical care
Introduction
Critical illness leads to substantial physiologic changes, affecting the pharmacokinetics (PK) of drugs. Sepsis, a life-threatening condition caused by dysregulation of a host’s response to infection, has profound effects on antibiotic PK. Sepsis-induced increase in vascular permeability and fluid resuscitation can increase the volume of distribution (Vd) of hydrophilic drugs, such as β-lactam antibiotics, the most common empiric therapy used in children with severe infectious processes,1 and result in lower drug concentrations.2 If hepatic or renal dysfunction occurs, antibiotic clearance decreases, increasing antibiotic exposure and risk of adverse effects.1 Despite the profound effects that critical illness has on antimicrobial PK, dosing regimens used in sepsis are often based on PK studies in non-critically ill patients,3 and clinicians frequently do not account for organ injury or comorbid conditions when selecting antibiotic dosing regimens.
PK variability of β-lactams and the association of target attainment with clinical outcomes has been widely reported in critically ill adults,3,4 but limited data exist in critically ill children. Since β-lactam antibiotic efficacy depends on the time free drug concentration is above the minimum inhibitory concentration (MIC) of bacteria, it is crucial to ensure appropriate antibiotic doses are given to achieve adequate concentrations in relation to the MIC (termed target attainment). As a result, there has been greater interest in incorporating β-lactam therapeutic drug monitoring (TDM) or model-informed precision dosing into routine clinical care, but robust population PK models in critically ill children are needed for these purposes.
PK studies in children are difficult to perform due to the additional blood draws required for adequate characterization of drug PK. Traditional PK studies may require multiple blood draws of large volumes for each dosing interval to cover the full concentration vs. time profile5,6 (Supplementary Figure 1). Sparse sampling, which reduces frequency of blood draws based on previous PK studies can still have high rates of parental refusal or study withdrawal in our experience.7 Opportunistic sampling takes advantage of residual blood from samples ordered for routine clinical care and is an underutilized approach5,6 (Supplementary Figure 1). Critically ill patients in the pediatric intensive care unit (PICU) often have blood drawn frequently for routine clinical care so are ideally suited for the scavenged opportunistic approach.
Using scavenged blood from opportunistic sampling requires an understanding of the effect of blood or plasma storage on the degradation of β-lactam antibiotics over time. For long term storage, up to one year, solutions or plasma containing β-lactam antibiotics should be stored at −70° to −80 °C to limit degradation and for accurate concentration measurement.8–10 Recent studies have sought to evaluate β-lactam antibiotic stability in human whole blood and plasma at various temperatures. For chromatographic assays, the Food and Drug Administration (FDA) defines stability as being ±15% of the original concentration.11 Multiple studies have showed that various β-lactam antibiotics, including piperacillin, cefepime and meropenem, are stable at room temperature for only a few hours in whole blood or plasma, and some studies have shown β-lactam stability for a few days at 4 °C.9,10,12–14 However, there is no agreement on the best practice for short-term storage of beta-lactam antibiotics to ensure stability.
Our research program uses residual blood from clinical samples (scavenged opportunistic sampling) to evaluate antibiotic concentrations in patients. Depending on the clinical test ordered, blood may be stored as whole blood in tubes containing ethylenediaminetetraacetic acid (EDTA) or in tubes with lithium heparin (Li-Hep) with gel after centrifugation to separate plasma from blood cells. We sought to demonstrate the feasibility of an opportunistic sampling approach to obtain sufficient samples in critically ill children to cover the full antibiotic concentration vs. time profile of three β-lactam antibiotics, cefepime, meropenem and piperacillin, and to determine the stability of these antibiotics in scavenged whole blood and plasma stored at 4°C to optimize future use of the opportunistic sampling approach. We evaluated the variability in antibiotic concentrations and protein binding and investigated the stability of the antibiotics when stored in whole blood in EDTA-containing tubes compared to when stored in plasma in Li-Hep gel tubes.
Materials and methods
Study Design, Ethics
A prospective observational pilot study was conducted in the pediatric intensive care unit (PICU) at Cincinnati Children’s Hospital Medical Center (CCHMC). The study was approved by the CCHMC Institutional Review Board; a waiver of consent was granted.
Sample Collection for Feasibility Study of Opportunistic Scavenging Approach
For two weeks in October 2018, all patients administered at least one dose of cefepime, meropenem and piperacillin/tazobactam were screened for residual blood samples. Clinical samples drawn after administration of a β-lactam dose were requested from the clinical laboratory, which stores residual blood at 4°C within hours of clinical test completion for up to seven days (Supplementary Figure 2A). Samples obtained within 30 minutes of dose administration or more than 30 hours after the most recent dose were excluded. Typical dosing regimens prescribed in our PICU are as follows: cefepime (50 mg/kg/dose q8h-12h (max 2000 mg/dose)), meropenem (20–40 mg/kg/dose q8h (max 2000 mg/dose)), and piperacillin/tazobactam (100 mg/kg/dose q6h (max 3375 mg/dose or 4500 mg/dose)). Samples with sufficient volume (approximately ≥100 μL plasma in lithium-heparin tubes or ≥200 μL whole blood in tubes containing EDTA) were centrifuged (2060 × g, 10–20°C, 10 min), and supernatant was removed and stored at −80°C until concentrations were measured.
Sample Collection for Stability Assays
For each β-lactam antibiotic, we enrolled two patients who were each administered at least one dose of the antibiotic and had blood drawn into both an EDTA tube and a Li-Hep with gel tube. Typically, samples collected in EDTA-containing tubes were obtained for complete blood cell count (CBC), while those collected in Li-Hep with gel tubes were ordered for metabolic panels to measure electrolytes or liver function tests. All tubes with Li-Hep with gel underwent centrifugation using standard clinical laboratory procedures to separate cells from plasma, whereas EDTA-containing tubes did not.
After the clinical laboratory tested for clinical purposes (i.e. CBC, metabolic panels), the residual whole blood from EDTA-containing tubes and plasma from Li-Hep with gel tubes were stored at 4°C (Supplementary Figure 2B). Within 16 hours of the blood being drawn, aliquots from the paired residual whole blood and plasma samples were centrifuged (2060 × g, 10–20°C, 10 min) separately. Supernatants from the aliquots were removed and stored at −80°C until β-lactam concentrations were measured. The remaining residual blood and plasma were continuously stored in the EDTA-containing tubes or Li-Hep with gel tubes, respectively, at 4°C. Centrifugation of an aliquot from each of the paired residual whole blood and plasma samples was performed daily for seven total consecutive days.
β-lactam Concentration Measurement for Feasibility Study and Stability Assays
Supernatants from centrifuged samples were stored at −80°C until β-lactam concentrations could be measured. Samples were frozen for up to 120 days prior to measurement, at which time they were thawed. For the feasibility study, total and free cefepime, meropenem and piperacillin concentrations were measured. For the stability assays, only the total concentrations were measured.
Sample preparation for free β-lactam concentrations in plasma was based on the user guide of Centrifee® Ultrafiltration Devices (Merck Millipore). For each patient sample, plasma was placed into an Amicon Ultra-0.5 mL 30,000 molecular weight cut-off centrifugal filter device (Millipore) and centrifuged for 20 min at 1200 × g After centrifugation, a 100 μL aliquot of filtrate was distributed to an autosampler vial. To this vial, 100 μL internal standard was added. After mixing, a 10 μL aliquot of the sample was injected into the high-performance liquid chromatography with ultraviolet detection (HPLC-UV) system.
Sample preparation for total β-lactam concentrations in plasma was achieved using protein precipitation and ultracentrifugation procedures. To a 100 μL aliquot of plasma sample, 100 μL of internal standard and 300 μL of methanol were added. After vortex-mixing, the sample was centrifuged (10,000 × g, 4°C, 10 min.). After centrifugation, the liquid phase was distributed to an autosampler vial. A 10 μL aliquot of the sample was injected into the HPLC-UV system.
β-Lactam antibiotics were quantified using an automated Hitachi Chromaster™ system (Tarrytown, NY) equipped with Model 5110 quaternary pump, Model 5210 autosampler, Model 5310 column oven, and Model 5410 UV detector. The EZChrom Elite® software was used for monitoring output signal and processing result. The analytical column was a 250-mm × 4.6-mm Validated C18 column (PerkinElmer) with 5-μm spherical particles connected to a Security Guard (Phenomenex) equipped with C18 cartridge (4-mm × 3-mm). MAGNA nylon filter (0.2-μm, 47-mm diameter) was from GE Water & Process Technologies for the filtration of mobile phase.
Measurement of free and total cefepime concentrations were performed using a linear gradient HPLC procedure. Column temperature was maintained at 40°C. The mobile phase was a 50 mM potassium phosphate buffer (pH 4.6) and the flow rate at 1 mL/min. Linear gradient started from 0% acetonitrile at time 0 and increased to 20% acetonitrile in 5 min. UV detection of cefepime and etofylline (used as internal standard) were performed at 260 nm and 270 nm, respectively. The lower limit of quantitation of cefepime was 0.5 μg/mL. The upper limit of quantitation was 200 μg/mL.
Determination of free and total meropenem concentrations were performed using an isocratic HPLC with UV detection. The mobile phase was 8% acetonitrile in 50 mM potassium phosphate buffer (pH 4.6). The column temperature was at 45°C, and the flow rate was at 1 mL/min. UV detection of meropenem and theophylline (used as internal standard) were performed at 300 nm and 270 nm, respectively. The assay range was linear from 0.5 to 200 μg/mL.
Free and total piperacillin measurements were performed using a linear gradient HPLC with UV detection. The mobile phase was 3% acetonitrile in 50 mM potassium phosphate buffer (pH 4.6). Column temperature was set at 30°C and the flow rate set at 1 mL/min. Linear gradient was carried out from 3% acetonitrile at time 0 and increased to 48% acetonitrile in 10 min. UV detection of piperacillin and ferulic acid (used as internal standard) were performed at 210 nm and 320 nm, respectively. The lower limit of quantitation of piperacillin was 0.5 μg/mL. The upper limit of quantitation was 400 μg/mL.
Within-day and between-day assay precision and accuracy of the quality control for all three drugs were satisfactory (CV <15%). Assay data examined for analytical methods met all criteria recommended by FDA for bioanalytical method validation including accuracy, precision, selectivity, sensitivity, reproducibility, and stability,15 as previously described for β-lactam antibiotics in the literature16 and performed for other medications by our laboratory.17,18
Data Analysis for Feasibility Study and Stability Assays
For the feasibility study, the percentage of drug bound was calculated by dividing free concentrations by total concentrations. Free concentrations were plotted as concentration versus time after the most recent antibiotic dose. For stability assays, percent degradation of total drug was calculated by using the aliquots centrifuged on day 0 (day of clinical sample collection) as the reference samples for concentrations.
Clinical Data for Feasibility Study
Clinical data were collected during the study duration, including age, weight, sex and presence of comorbid conditions. Sepsis was determined if patients met pediatric-specific consensus criteria for sepsis19 and received at least seven days of antibiotics. Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tool.20
Results
Feasibility Study
During the two-week pilot study, 23 patients were prescribed at least one of the three β-lactams of interest, and 22 unique patients had residual blood samples obtained (demographics shown in Table 1). One patient did not have clinical samples obtained or enough residual blood. One patient was readmitted to the PICU and administered a different β-lactam on PICU readmission, and therefore was considered as two separate encounters. Sepsis occurred in 43% of the enrolled patients, and 91% of patients had comorbid conditions, including pulmonary diseases (e.g. asthma, bronchopulmonary dyplasia), immunosuppressive conditions (e.g. oncologic processes, status-post organ transplant), neurologic disorders (e.g. hydrocephalus, cerebral palsy, epilepsy), endocrinologic dysfunction (e.g. hypothyroidism, panhypopituitarism, diabetes) and chronic liver or kidney disease.
Table 1:
Demographic data of cohort with opportunistic sampling.
| Demographics | All Patients (n=23)a | Cefepime (n=10) | Meropenem (n=7) | Piperacillin/Tazobactam (n=9) |
|---|---|---|---|---|
|
Age, years Mean ± Standard Deviation |
12.7 ± 8.3 | 15.2 ± 9.0 | 15.4 ± 7.4 | 8.0 ± 7.1 |
| Median [range] | [10.6–94.8] | [16.1–69.4] | [10.6–94.8] | [10.6–55.9] |
| Males, n (%) | 11 (48) | 2 (20) | 4 (57) | 7 (78) |
|
Comorbid conditionsb, n (%) |
21 (91) | 9 (90) | 7 (100) | 8 (89) |
| Culture negative, n (%) | 8 (80) | 5 (83) | 2 (67) | 3 (75) |
One patient received an organ transplant, readmitted post-op to the PICU, was administered a different β-lactam on PICU readmission, and was considered as 2 separate encounters. Three patients were initiated on one of the 3 β-lactams of interest and switched to one of the other 2 β-lactams within 7 days during index PICU admission; patients who received 2 of the 3 β-lactam antibiotics sequentially were included each respective antibiotic group.
comorbid condition defined as condition requiring medications or subspecialty care
sepsis: defined as meeting pediatric-specific consensus criteria for sepsis and received at least seven days of antibiotics.
A total of 138 residual blood samples were collected and stored at 4°C (median 1.5 days, range 0–4 days) until processing and storage at −80°C. Of the 138 samples, 38 were stored as whole blood in EDTA-containing tubes, and the rest were stored as plasma in Li-Hep with gel tubes. The samples covered entire dosing intervals and were not clustered around specific time points (Figure 1A–C). These data show a large spread in the antibiotic concentrations, most notably towards the end of the dosing intervals. In the last two hours of the dosing intervals, the ranges in free concentrations for cefepime were 5.4 – 86.7 μg/mL, <0.5 μg/mL to 10 μg/mL for meropenem and 3.8 −159.8 μg/mL for piperacillin (Figure 1A–C).
Figure 1: Free Cefepime (A), Meropenem (B) and Piperacillin (C) concentrations obtained from residual blood from clinical samples.
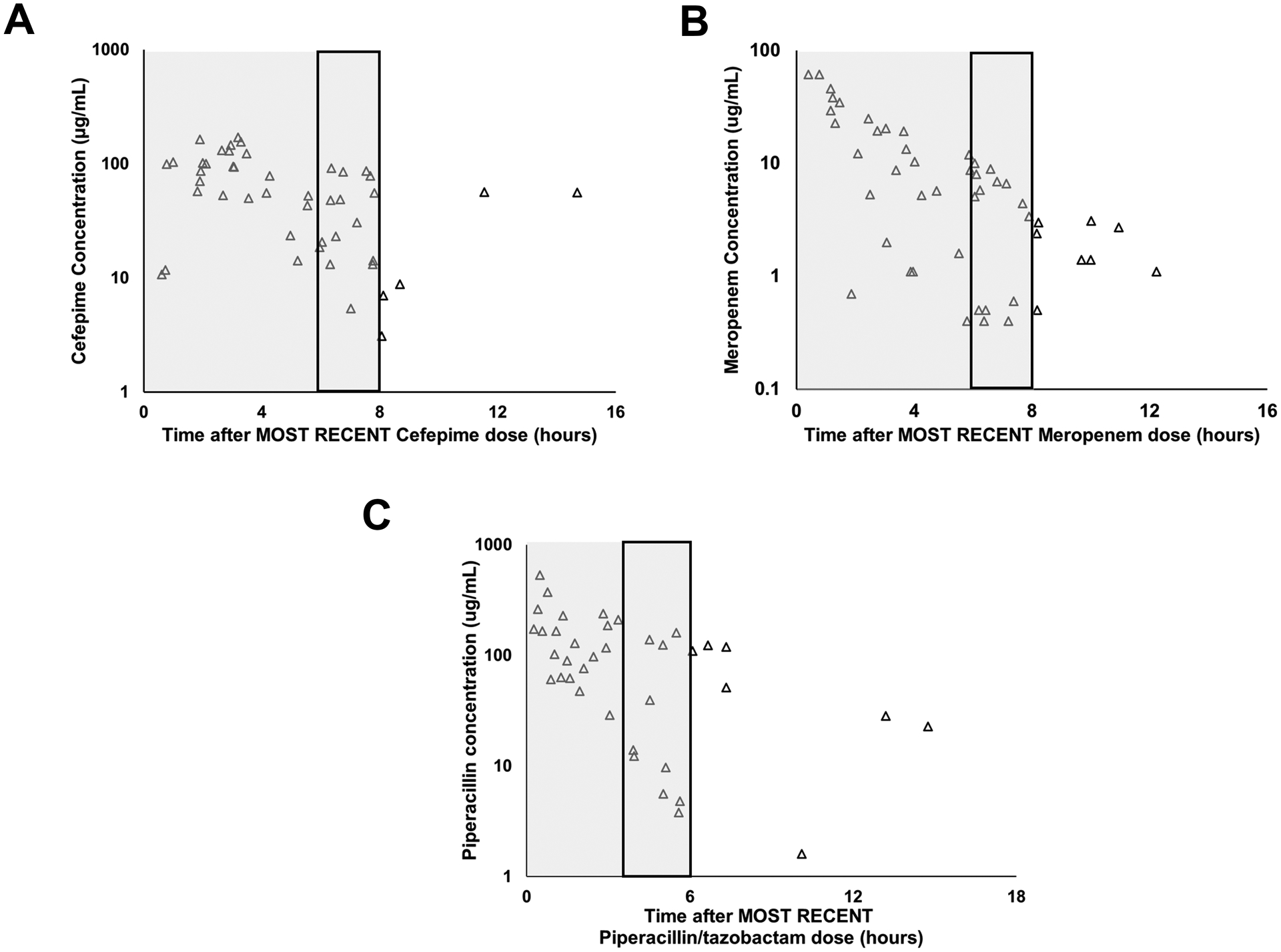
Free drug concentrations were plotted according to time after the most recent dose of β-lactam antibiotic (X-axis). Y- axis represents concentration (μg/mL). Samples obtained from patients who were prescribed cefepime, meropenem and piperacillin/tazobactam showed spread over the entire dosing interval (typically every 6 hours for piperacillin/tazobactam, 8 hours for cefepime and meropenem) and were not clustered around one time point. Shaded areas represent the typical dosing interval. There was high variability in the free concentrations of the antibiotics, particularly towards the end of dosing intervals. Rectangular boxes outlined in black represent the last two hours of the most commonly used dosing interval.
Since critical illness affects albumin concentrations, which can change throughout hospitalization,21and β-lactam efficacy is dependent on free (non-protein bound) concentrations, we investigated the percentage of drug bound to protein. We found that the percentage of bound β-lactam antibiotic had high inter- and intra- patient variability. Across patients, the mean percentage of drug bound to protein for cefepime, meropenem and piperacillin ranged from 0–10%, 0–38% and 13–32%, respectively (Figure 2A–C). Examining individual piperacillin patients, the spread of percent protein binding among samples was large. For example, in patient 14, percent protein binding of piperacillin ranged from 6% to 35% (Figure 2C).
Figure 2: Inter- and Intra- patient variability in protein binding of cefepime (A), meropenem (B) and piperacillin (C).
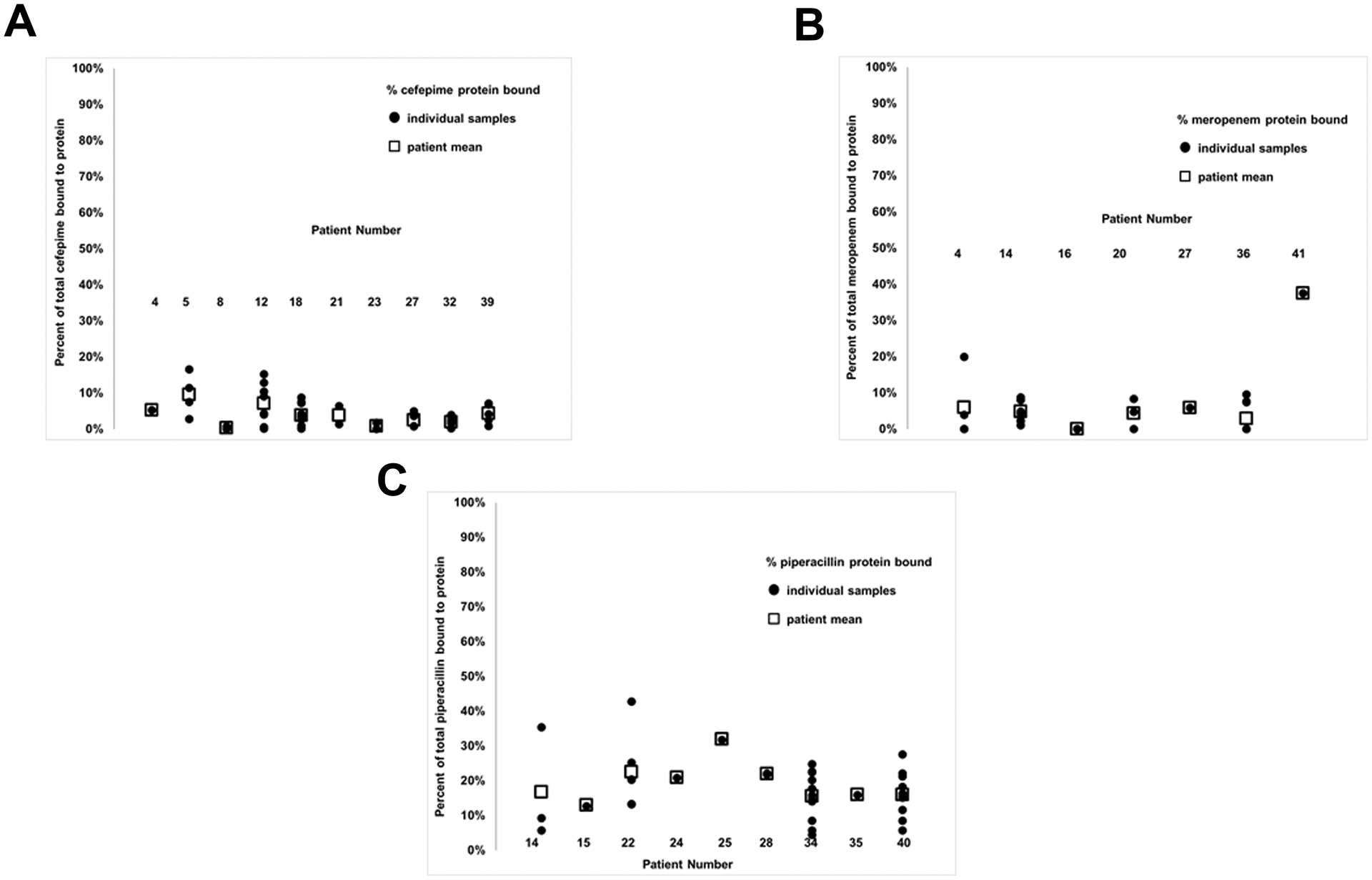
Circles represent the percentage of antibiotic bound for individual samples and squares represent the mean percentage of antibiotic bound for each patient.
Stability Assays
There was up to a 25% difference in cefepime concentrations when comparing whole blood in an EDTA-containing tube and plasma in a Li-Hep with gel tube (Table 2, cefepime, patient 2). The higher concentration was not consistently measured in one type of tube (whole blood in EDTA vs. plasma in Li-Hep with gel) between the two patients. Cefepime degraded at a higher rate than piperacillin and meropenem with more than 15% of drug degradation by day 3 in both plasma samples in Li-Hep with gel tubes. By day 6, approximately 25% of cefepime was degraded in plasma samples in Li-Hep with gel tubes. For the samples containing whole blood in EDTA-containing tubes, the maximum degradation was only 12.9%, and for patient 2, this occurred by day 3.
Table 2: Concentrations and percent change in concentration of cefepime, meropenem and piperacillin over time in tubes containing EDTA and in Li-Hep with gel tubes.
Day 0 concentrations serve as reference concentrations for calculation of percent change. EDTA: ethylenediaminetetraacetic acid; Li-Hep: Lithium-heparin; CBC: complete blood count.
| EDTA tubes (whole blood used for CBC) Concentration (ug/mL) [% degradation] |
Li-Hep with gel Tubes (plasma used for metabolic panels) Concentration (ug/mL) [% degradation] |
|
|---|---|---|
| Cefepime | ||
| Patient 1 (dose 5.4 hours prior to blood draw) | ||
| Day 0 | 56.2 (0.0) | 64.7 (0.0) |
| Day 1 | 52.9 (5.9) | 61.0 (5.7) |
| Day 2 | 51.9 (7.7) | 56.9 (12.1) |
| Day 3 | 56.0 (0.4) | 54.9 (15.1) |
| Day 4 | 56.5 (−0.5) | 51.9 (19.8) |
| Day 5 | 53.9 (4.1) | 50.3 (22.3) |
| Day 6 | 50.3 (10.5) | 49.0 (24.3) |
| Patient 2 (dose 9.9 hours prior to blood draw) | ||
| Day 0 | 16.3 (0.0) | 12.3 (0.0) |
| Day 1 | 15.5 (4.9) | 12.6 (−2.4) |
| Day 2 | 14.7 (9.8) | 11.7 (4.9) |
| Day 3 | 14.2 (12.9) | 10.4 (15.4) |
| Day 4 | 16.5 (−1.2) | 9.5 (23.6) |
| Day 5 | 16.3 (0) | 9.3 (24.4) |
| Day 6 | 15.6 (4.3) | 9.8 (20.3) |
| Meropenem | ||
| Patient 1 (dose 6.1 hours prior to blood draw) | ||
| Day 0 | 1.2 (0.0) | 1.7 (0.0) |
| Day 1 | 1.4 (−16.7) | 1.8 (−5.9) |
| Day 2 | 1.4 (−16.7) | 1.9 (−11.8) |
| Day 3 | 1.5 (−25.0) | 1.8 (−5.9) |
| Day 4 | 1.3 (−8.3) | 1.7 (0) |
| Day 5 | 1.3 (−8.3) | 1.8 (−5.9) |
| Day 6 | 1.4 (−16.7) | 1.8 (−5.9) |
| Patient 2 (dose 6.4 hours prior to blood draw) | ||
| Day 0 | 1.0 (0.0) | 1.6 (0.0) |
| Day 1 | 1.1 (−10.0) | 1.7 (−6.2) |
| Day 2 | 1.0 (0.0) | 1.6 (0.0) |
| Day 3 | 1.1 (−10.0) | 1.5 (6.2) |
| Day 4 | 1.0 (0.0) | 1.7 (−6.2) |
| Day 5 | 1.1 (−10.0) | 1.7 (−6.2) |
| Day 6 | 1.0 (0.0) | 1.6 (0.0) |
| Piperacillin | ||
| Patient 1 (dose 3.6 hours prior to blood draw) | ||
| Day 0 | 13.5 (0.0) | 11.6 (0.0) |
| Day 1 | 12.8 (5.2) | 12.7 (−9.5) |
| Day 2 | 12.3 (8.9) | 11.8 (−1.7) |
| Day 3 | 12.1 (10.4) | 11.4 (1.7) |
| Day 4 | 12.3 (8.9) | 11.3 (2.6) |
| Day 5 | 11.8 (12.6) | 10.8 (6.9) |
| Day 6 | 11.6 (14.1) | 10.7 (7.8) |
| Patient 2 (dose 7.7 hours prior to blood draw) | ||
| Day 0 | 10.9 (0.0) | 12.4 (0.0) |
| Day 1 | 10.6 (2.8) | 11.7 (5.6) |
| Day 2 | 10.4 (4.6) | 11.5 (7.3) |
| Day 3 | 10.8 (0.9) | 12.2 (1.6) |
| Day 4 | 9.9 (9.2) | 11.0 (11.3) |
| Day 5 | 10.3 (5.5) | 11.6 (6.5) |
| Day 6 | 10.5 (3.7) | 10.6 (14.5) |
Meropenem concentrations differed by 29% (patient 1) and 38% (patient 2) between whole blood samples in EDTA-containing tubes and plasma samples in Li-Hep with gel tubes on day 0 (Table 2, meropenem). The large difference between samples is likely due to the low meropenem concentrations in all the samples. However, there was minimal degradation in any of the samples, with only up to 6.3% of the meropenem being degraded in one of the plasma samples in a Li-Hep with gel tube.
For piperacillin patient 1, the difference between concentrations in whole blood collected in an EDTA-containing tube and in plasma in a Li-Hep with gel tube on day 0 (day of sample collection) was 14%, with the higher concentration being measured in whole blood (Table 2, piperacillin, patient 1). For patient 2, however, the piperacillin concentration was higher by 12% in plasma collected in a Li-Hep with gel tube. After 6 days of storage at 4°C, there was less than 15% degradation of piperacillin in all four samples and minimal difference in degradation in each of the two types of tubes (EDTA: 14.1%; Li-Heparin with gel: 14.5%).
Discussion
We sought to demonstrate the feasibility of using the scavenged opportunistic sampling approach to obtain adequate sample numbers for future PK studies and to evaluate the stability of drugs of interest over time under relevant storage conditions. We show that an opportunistic sampling method in a single-center PICU covers the concentration vs. time profiles for three β-lactam antibiotics (cefepime, meropenem and piperacillin). Further, we demonstrate the stability of these antibiotics at 4°C over time.
Feasibility Study
Opportunistic sampling balances clinical care with the needs of research sampling for PK studies. A necessary component for population PK modeling is covering the full concentration-time profile. The samples we collected using an opportunistic sampling approach from patients admitted to a single-center PICU fulfilled this requirement. Using scavenged residual blood samples added no additional risk to the patient or disrupted nursing workflow. A limitation of using scavenged samples is the potential for low residual blood volumes, resulting in insufficient concentration measurement, but this occurred less than 10% of the time in our study.
We show high variability in the concentrations and protein binding of β-lactam antibiotics in critically ill children. β-lactam efficacy depends on the percentage of the dosing interval during which free antibiotic concentrations are above the MIC of the targeted bacteria. Therefore, high variability in free concentrations can affect clinical outcomes. There are likely multiple factors contributing to the large variability in free concentrations found in our study, including pathophysiologic changes from critical illness and variability in protein binding, as well as patient factors. Since albumin is the primary protein that binds drugs, hypoalbuminemia, commonly seen in critical illness,21 may lead to an increase in the unbound fraction of the drug.22 However, hypoalbuminemia increases Vd, lowering free drug concentrations. Furthermore, since the unbound drug fraction is the portion cleared by the kidneys, an increase in free drug concentrations may lead to increase in drug clearance, and thus result in overall low drug exposures.22 Albumin concentrations may change frequently based on clinical status, leading to intra-patient variability in antibiotic protein binding. Thus, it is important to evaluate total and free concentrations to ensure efficacy while limiting adverse events.
While the percentages of meropenem bound to protein for most patients were comparable to the 2% reported in the package insert for meropenem,23 one meropenem sample showed 38% protein binding. This reduction in free meropenem concentrations may lead to failure to attain pharmacodynamic targets to eradicate bacteria. The increase in protein binding may be due to patient and clinical factors, including albumin concentrations, concomitant medications and underlying disease. A recent study in adults also found protein binding of meropenem to be nearly 40%, and demonstrated high variability in protein binding of cefepime, meropenem and piperacillin.24 Another study showed that measured unbound concentrations of piperacillin tended to be higher (i.e. lower protein binding) in critically ill adults than what would have been predicted by using reported percentage of protein binding in literature and total concentrations.25 For meropenem, however, the unbound concentrations were found to be lower (i.e. higher protein binding), but the differences were not significant. Both these studies and our study demonstrate that there is variability in protein binding of drugs in critically ill adult and pediatric patients, and direct measurement of free concentrations should be considered when implementing in clinical practice. Future studies to identify clinical factors that affect protein binding and impact variability are needed to ensure appropriate dosing of antibiotics.
Stability Assays
When blood was collected in either a tube containing EDTA or a Li-Hep with gel tube, piperacillin remained stable, having less than 15% degradation over 6 days at 4°C. Although all samples with meropenem had low concentrations (<2 μg/mL), and thus a change in 0.1 μg/mL resulted in a significant percent change, no degradation was noted in samples from either type of tube from the first patient, and the maximum degradation in plasma in a Li-Hep with gel tube for patient 2 was only 6.2%. These findings show stability for longer periods of time at 4°C than previous studies. Defining stability as less than 15% degradation, Mortensen and colleagues showed that piperacillin, tazobactam, meropenem and ceftazidime were stable in whole blood and ethylenediaminetetraacetic acid (EDTA)-anticoagulated plasma at 4°C for 3 days,12 and Bugnon and colleagues observed cefepime stability in plasma for at least 12 hours at 4°C.14 Another study showed decay of <15% of meropenem in plasma in 4 days at 6°C.26 One major difference between our study and the Mortensen and Bugnon studies is that our samples were obtained from actual patients treated with antibiotics and not whole blood and plasma from healthy volunteers spiked with known concentrations of antibiotics.
Unlike piperacillin and meropenem, cefepime had significant degradation after six days. If the goal for stability is less than 15% degradation according to the FDA and the College of American Pathologists guidance, then cefepime clinical samples obtained and processed more than three days after blood draw should not be used. For PK modeling and estimation, a more stringent degradation cut-off, such as <10%, should be considered. In this case, cefepime samples should be processed within 2 days of collection, meropenem samples within 6 days, and piperacillin samples within 5 days.
There were differences in concentrations for all three antibiotics between the two different tubes on day 0. Since the concentrations were not consistently higher in one type of tube versus the other, its significance is unclear. One possible reason for the difference is that hemolysis in whole blood samples may affect concentrations as hemolyzed blood specimens result in larger sample volumes and lead to variation in drug concentration. There was not a difference in maximum degradation over six days for piperacillin and meropenem when comparing whole blood in EDTA-containing samples and plasma in Li-Hep with gel tubes. However, for cefepime, there was a higher rate of degradation in plasma in Li-Hep with gel tubes, with maximum degradation approaching 25% after six days, as compared to 10.5% in whole blood in EDTA-containing tubes. In addition to only using samples stored at 4°C for fewer than three days, it would be recommended that only whole blood collected in tubes containing EDTA be used when measuring cefepime from scavenged samples.
Limitations
This study is not without limitations. For the feasibility study, we did not account for patient factors, such as severity of illness, volume of fluids used for resuscitation or organ injury. These factors from our heterogeneous patient population likely contributed to the high observed variability in our study. The number of samples collected in two weeks was not enough for robust population PK analysis to assess factors contributing to variability. However, our data do provide evidence that similar dosing regimens without regard to patient factors may result in a wide range of concentrations, which may cause adverse effects.
Our sample size for the stability assays is small with having only two patients with paired samples for each drug as our goal was to reproduce some of the existing stability data in our own laboratory. Since concentrations were not measured in real-time (i.e. immediately after clinical sample was drawn) and day 0 samples were not processed for nearly 16 hours after blood draw due to the time it takes for research personnel to obtain residual blood from samples used by the clinical laboratory, it is unknown how much degradation may have occurred in the 16 hours while it was stored. However, given that all 3 drugs remained stable at 4°C for at least two days, the amount of degradation in the first 16 hours is likely minimal. In addition, due to the small amount of residual blood obtained from the samples and the need to divide it among seven aliquots for the stability assays, only total concentration was measured, instead of free concentration. However, assuming that the total amount of protein in the samples remained constant throughout the six days, it is unlikely that the rate of degradation of free drug would be different than total drug.
Conclusions
Until real-time beta-lactam antibiotic assays are readily available to use at the bedside for therapeutic drug monitoring, the opportunistic scavenged sampling approach is a feasible method to study the variability of drug concentrations to inform future drug monitoring. This approach is endorsed by the Pediatric Trials Network and has been successfully used in neonates and for other antibiotics.27 Our data support its use to study β-lactam antibiotics in critically ill children. We report wide variability in the free concentrations and protein binding of β-lactam antibiotics. Our findings show that piperacillin and meropenem remain stable for up to six days in both whole blood in EDTA-containing tubes and plasma in Li-Hep with gel tubes, but scavenged samples containing cefepime should be processed within three days and ideally be measured from whole blood collected in EDTA-containing tubes. The opportunistic scavenged sampling approach will be used for future population pharmacokinetic modeling studies of drugs in critically ill children.
Supplementary Material
Acknowledgements:
We are grateful to Dr. John M. Morrison for critical review of the manuscript. This work was supported by the National Institute of Child Health and Development Cincinnati Pediatric Clinical Pharmacology Postdoctoral Training Program [5T32HD069054-09]; National Center for Advancing Translational Sciences of the National Institutes of Health [Award Number 5UL1TR001425]; the Cincinnati Children’s Hospital Medical Center Arnold W. Strauss Fellow Award; and the Cincinnati Children’s Hospital Medical Center Hospital Medicine Fellow Award.
Conflict of Interest:
J.M.K. reports financial support to the institution (Cincinnati Children’s Hospital Medical Center [CCHMC]) for work on a DSMB of a clinical trial (Eli Lilly) not related in any way to the work in the manuscript. For the remaining authors, none were declared.
Footnotes
Data Sharing: Please contact the corresponding author if interested in raw data
References
- 1.Droege ME, Van Fleet SL, Mueller EW. Application of Antibiotic Pharmacodynamics and Dosing Principles in Patients With Sepsis. Crit Care Nurse. 2016;36(2):22–32. [DOI] [PubMed] [Google Scholar]
- 2.Sime FB, Udy AA, Roberts JA. Augmented renal clearance in critically ill patients: etiology, definition and implications for beta-lactam dose optimization. Curr Opin Pharmacol. 2015;24:1–6. [DOI] [PubMed] [Google Scholar]
- 3.Sime FB, Roberts MS, Peake SL, Lipman J, Roberts JA. Does Beta-lactam Pharmacokinetic Variability in Critically Ill Patients Justify Therapeutic Drug Monitoring? A Systematic Review. Ann Intensive Care. 2012;2(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miglis C, Rhodes NJ, Kuti JL, Nicolau DP, Van Wart SA, Scheetz MH. Defining the impact of severity of illness on time above the MIC threshold for cefepime in Gram-negative bacteraemia: a ‘Goldilocks’ window. Int J Antimicrob Agents. 2017;50(3):487–490. [DOI] [PubMed] [Google Scholar]
- 5.Laughon MM, Benjamin DK Jr., Capparelli EV, et al. Innovative clinical trial design for pediatric therapeutics. Expert Rev Clin Pharmacol. 2011;4(5):643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girdwood ST, Kaplan J, Vinks AA. Methodologic Progress Note: Opportunistic Sampling for Pharmacology Studies in Hospitalized Children. J Hosp Med. 2020;15(2):E1–E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang Girdwood SC, Mizuno T, Krallman KA, et al. Route of Oseltamivir Administration Affects Metabolite Concentrations in Critically Ill Children. Pediatr Infect Dis J. 2019;38(12):1224–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nickolai DJ, Lammel CJ, Byford BA, et al. Effects of storage temperature and pH on the stability of eleven beta-lactam antibiotics in MIC trays. J Clin Microbiol. 1985;21(3):366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kipper K, Barker CIS, Standing JF, Sharland M, Johnston A. Development of a Novel Multipenicillin Assay and Assessment of the Impact of Analyte Degradation: Lessons for Scavenged Sampling in Antimicrobial Pharmacokinetic Study Design. Antimicrob Agents Chemother. 2018;62(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinder N, Brenner T, Swoboda S, Weigand MA, Hoppe-Tichy T. Therapeutic drug monitoring of beta-lactam antibiotics - Influence of sample stability on the analysis of piperacillin, meropenem, ceftazidime and flucloxacillin by HPLC-UV. J Pharm Biomed Anal. 2017;143:86–93. [DOI] [PubMed] [Google Scholar]
- 11.Food and Drug Administration. Bioanalytical Method Validation: Guidance for Industry. In: U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine, ed. Silver Spring, MD. 2018. [Google Scholar]
- 12.Mortensen JS, Jensen BP, Zhang M, Doogue M. Preanalytical Stability of Piperacillin, Tazobactam, Meropenem, and Ceftazidime in Plasma and Whole Blood Using Liquid Chromatography-Tandem Mass Spectrometry. Ther Drug Monit. 2019;41(4):538–543. [DOI] [PubMed] [Google Scholar]
- 13.Carlier M, De Waele JJ, Verstraete AG, Stove V. Exploration of the pre-analytical stability of beta-lactam antibiotics in plasma and blood--implications for therapeutic drug monitoring and pharmacokinetic studies. Clin Chem Lab Med. 2015;53(9):e227–230. [DOI] [PubMed] [Google Scholar]
- 14.Bugnon D, Giannoni E, Majcherczyk P, Glauser MP, Moreillon P. Pitfalls in cefepime titration from human plasma: plasma- and temperature-related drug degradation in vitro. Antimicrob Agents Chemother. 2002;46(11):3654–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Analytical Procedures and Methods Validation for Drugs and Biologics. 2015. [Google Scholar]
- 16.Denooz R, Charlier C. Simultaneous determination of five beta-lactam antibiotics (cefepim, ceftazidim, cefuroxim, meropenem and piperacillin) in human plasma by high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;864(1–2):161–167. [DOI] [PubMed] [Google Scholar]
- 17.Tang PH. Drug monitoring and toxicology: a simple procedure for the monitoring of felbamate by HPLC-UV detection. J Anal Toxicol. 2008;32(5):373–378. [DOI] [PubMed] [Google Scholar]
- 18.Tang PH. Determination of Posaconazole in Plasma/Serum by High-Performance Liquid Chromatography with Fluorescence Detection. Separations. 2017;4(2). [Google Scholar]
- 19.Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric S. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. [DOI] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth. 2000;85(4):599–610. [DOI] [PubMed] [Google Scholar]
- 22.Ulldemolins M, Roberts JA, Rello J, Paterson DL, Lipman J. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin Pharmacokinet. 2011;50(2):99–110. [DOI] [PubMed] [Google Scholar]
- 23.Dainippon Sumitomo Pharma Co. L. Merrem I.V. (meropenem for injection), FDA label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050706s022lbl.pdf. Published 2006. Accessed.
- 24.Al-Shaer MH, Alghamdi WA, Graham E, Peloquin CA. Meropenem, Cefepime, and Piperacillin Protein Binding in Patient Samples. Ther Drug Monit. 2020;42(1):129–132. [DOI] [PubMed] [Google Scholar]
- 25.Wong G, Briscoe S, Adnan S, et al. Protein binding of beta-lactam antibiotics in critically ill patients: can we successfully predict unbound concentrations? Antimicrob Agents Chemother. 2013;57(12):6165–6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martens-Lobenhoffer J, Monastyrski D, Troger U, Bode-Boger SM. Stability of meropenem in plasma versus dried blood spots (DBS). J Pharm Biomed Anal. 2019;170:279–284. [DOI] [PubMed] [Google Scholar]
- 27.Pediatric Trials Network. Pharmacokinetics of Understudied Drugs Administered to Children per Standard of Care (PTN POPS). Eunice Kennedy Shriver National Institute of Child Health and Human Development. https://pediatrictrials.org/pharmacokinetics-of-understudied-drugs-administered-to-children-per-standard-of-care-ptn-pops/. Accessed July 2, 2020. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


