Abstract
Inefficient delivery is a major obstacle to the development of peptide-based drugs targeting the intracellular compartment. We recently showed that selectively inhibiting integrin outside-in signaling using a peptide (mP6) derived from the Gα13-binding ExE motif within the integrin β3 cytoplasmic domain had anti-thrombotic effects. Here, we engineered lipid-stabilized high loading peptide nanoparticles (HLPN), in which a redesigned ExE peptide (M3mP6) constituted up to 70% of the total nanoparticle molarity, allowing efficient in vivo delivery. We observed that M3mP6 HLPNs inhibited occlusive thrombosis more potently than a clopidogrel/aspirin combination without adverse effects on hemostasis in rodents. Furthermore, M3mP6 HLPN synergized with P2Y12 receptor inhibitors or the clopidogrel/aspirin combination in preventing thrombosis, without exacerbating hemorrhage. M3mP6 HLPN also inhibited intravascular coagulation more potently than the P2Y12 inhibitor cangrelor. Post-ischemia injection of M3mP6 HLPN protected the heart from myocardial ischemia-reperfusion injury in a mouse model. This study demonstrates an efficient in vivo peptide delivery strategy for a therapeutic that not only efficaciously prevented thrombosis with minimal bleeding risk but also protected from myocardial ischemia/reperfusion injury in mice.
One Sentence Summary:
In vivo high-loading peptide nanoparticles administration prevented thrombosis and myocardial injury without causing bleeding in mice.
Introduction
The inability to efficiently deliver peptides in vivo into cells is a major obstacle in developing peptide-based drugs targeting the intracellular compartments. Thus, despite the low toxicity and high specificity of natural peptides in general, successes in developing cell-penetrating drugs targeting intracellular compartments are scarce, (1, 2). An efficient method for delivering peptides into cells in vivo would be an important advancement in peptide-based drug development.
Thrombotic cardiovascular disease causes more deaths than any other disease in the world (3). Blood platelets physiologically mediate hemostatic thrombus formation to prevent bleeding but are also critical in the development of occlusive thrombosis (4, 5). Anti-platelet therapy is therefore pivotal in the treatment of thrombotic diseases, and in preventing thrombosis in patients undergoing invasive vascular procedures (6, 7). The important role of platelets in hemostasis and thrombosis requires the adhesion receptor integrin αIIbβ3 (also named glycoprotein (GP) IIb-IIIa) (8). Currently available anti-platelet drugs suppress thrombus formation either by inhibiting the activation of integrin αIIbβ3 (such as the cyclooxygenase (COX) inhibitor aspirin or inhibitors of the adenosine diphosphate (ADP) P2Y12 receptor, clopidogrel, ticagrelor and cangrelor), or directly block the ligand binding function of αIIbβ3 (integrin antagonists abciximab, eptifibatide and tirofiban) (9, 10). The P2Y12 inhibitors such as clopidogrel either with or without aspirin are the current standard of care (9, 10). However, these drugs have the serious adverse effect of excessive bleeding (11–14) because of the importance of integrin-mediated primary platelet adhesion and aggregation in hemostasis. Hemorrhage is strongly associated with poor outcomes and increased mortality (11, 15–17), thus the need for a new generation of anti-platelet drugs that minimally affect hemostasis (18). Recently, inhibitors of thrombin receptors, the protease-activated receptor (PAR) 1 inhibitor vorapaxar (19) and PAR4 inhibitor BMS-986120 (20), have been shown to partially reduce hemorrhage as compared to P2Y12 inhibitors in animal studies. However, clinical trials revealed clear adverse hemorrhagic effects associated with vorapaxar (19). Although the mechanism for reduced hemorrhage with these inhibitors in animal models remains unclear, the hemorrhagic effects of both PAR1 and PAR4 inhibitors are consistent with the knowledge that these thrombin receptors are important for activation of the ligand binding function of integrin αIIbβ3.
Ligand binding to integrin αIIbβ3 not only mediates platelet adhesion but also transmits signals leading to greatly expanded thrombus size, important for vascular occlusion (12, 21). We recently discovered a Gα13-dependent mechanism of integrin outside-in signaling and proposed the concept of selectively targeting this pathway without affecting the ligand binding function of integrin αIIbβ3 (21, 22). Indeed, a synthetic peptide (mP6; Myr-FEEERA), derived from the Gα13 binding ExE motif of integrin β3’s cytoplasmic domain, inhibited thrombosis without affecting hemostasis (21). However, in vivo delivery of this peptide with liposomes or lipid micelles was inefficient and impractical for therapeutic use. To resolve this problem and address the challenge of intracellular peptide delivery in vivo, we engineered lipid-stabilized, high-loading peptide nanoparticles (HLPN) that incorporate high concentrations of the modified ExE peptide, M3mP6 and demonstrated superior characteristics of M3mP6 in inhibiting occlusive thrombosis without causing excessive bleeding. M3mP6 HLPN also synergistically enhanced the anti-thrombotic effects of current standard anti-platelet treatments while minimizing the adverse effect of hemorrhage. Furthermore, post-ischemia injection of M3mP6 HLPN protected heart from ischemia/reperfusion injury in mice.
Results
High-loading ExE peptide nanoparticles as a new anti-platelet drug
The Gα13-binding ExE motif of the β3 cytoplasmic domain (FEEERA) selectively mediates outside-in signaling and occlusive thrombosis (21). Although a synthetic peptide based on this sequence can disrupt Gα13 - β3 interaction and outside-in signaling, it is a challenge to efficiently deliver such a peptide in vivo into cells for therapeutic use. Although liposomes or lipid micelles can be used(2), they only incorporate low concentrations of peptide (21), making it difficult to achieve efficacious doses for clinical use, except for a few extremely high affinity drugs. We found only 1 mM mP6 (4% of total molar content) could be achieved in the lipid micellar suspension (21). Although this concentration had effects in mice when injecting the maximum possible volume (21), is not practical for clinical use. For this purpose, we developed lipid-stabilized, high-loading peptide nanoparticles (HLPN) for intracellular delivery of peptides in vivo. The main component of HLPN is an amphiphilic peptide, which is capable of self-assembling to form micellar nanoparticles. The peptide micellar nanoparticles are stabilized by a low percentage of phosphatidylcholine and protected by 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine (DSPE)-attached polyethylene glycol (PEG) forming the hydrophilic outer layer (Fig. 1A). We re-engineered mP6 to enhance its incorporation into HLPN. The new peptide, M3mP6 (Myr-FEEERL) contains the critical ExE motif, and its N-terminal phenylalanine is myristoylated. The C-terminal alanine was changed to leucine, whose long hydrophobic side chain facilitates packing higher concentrations of the peptide together with lipids into nanoparticles (Fig. 1B). This peptide retained the function of mP6 to inhibit Gα13-β3 interaction as indicated by co-immunoprecipitation (Fig. 1C and D). The M3mP6 HLPN achieved high M3mP6 peptide loading, reaching 70% of the total nanoparticle (mol/mol) and a high peptide concentration of >10 mM in an injectable suspension (>10 times more concentrated than the original lipid micelle formulation of mP6 (21)). As analyzed by dynamic light scattering (DLS), the vast majority (99.8-100%) of these lipid-stabilized, PEG-coated M3mP6 HLPN had an average size ranging from 6 to 25 nm (in different preparations) with occasional appearance of very small populations (0-0.2%) with larger diameters (~50-500 nm) (Fig. 1E). The lyophilized powder of M3mP6 was readily soluble in physiological saline for I.V. injection, and was stable for >18 months with a similar DLS profile and pharmacological effect when stored at −20°C, and for at least 2 weeks at room temperature (22°C) (Fig. S1). In a pilot maximal tolerated dose (MTD) test in mice, we did not observe signs of toxicity after one bolus injection of up to 60 mg peptide/kg (~60 μmol/kg) (Table S1A). Further pilot studies in rats revealed the MTD exceeded 100 mg peptide bolus (80 x converted efficacy dose) (Table S1B), and rats exhibited no observable toxic reaction to M3mP6 HLPN after a 5-day continuous infusion at 150 mg/kg/day and 300 mg/kg/day (Table S1C, S1D). M3mP6 HLPN dose-dependently inhibited human platelet granule secretion and secretion-dependent secondary platelet aggregation induced by low dose thrombin in vitro (Fig. 2 A, B and C), but had no effect on platelet aggregation induced by a high dose of thrombin (Fig. 2 C), although platelet granule secretion was still partially inhibited by M3mP6 even at higher thrombin concentrations (Fig. 2D). M3mP6 HLPN also partially inhibited collagen- (Fig. 2E) and U46619 (thromboxane A2 analog)-induced platelet aggregation (Fig. 2 F), but did not affect ADP-induced platelet aggregation (Fig. 2G). M3mP6 HLPN (Fig. 2H, 2I) or DMSO-dissolved M3mP6 (Fig. S2) did not affect JonA (Fig. 2H, Fig. S2A) or fibrinogen (Fig. 2I, Fig. S2B, S2C) binding to platelets induced by protease-activated receptor 4 agonist peptide (PAR4AP) and, under identical conditions as the fibrinogen binding assay, M3mP6 HLPN had no effect on high dose PAR4AP-induced platelet aggregation, but partially inhibited low dose PAR4AP-induced platelet aggregation (Fig. S2). These data suggest that M3mP6 does not directly affect inside-out signaling nor the ligand binding function of αIIbβ3, but inhibits secondary platelet responses to integrin outside-in signaling. Fluorescence microscopy of fluorescently labeled M3mP6 demonstrated that M3mP6 HLPN entered platelets after incubation (Fig. S3). Using flow cytometry, we found that entry of fluorescent M3mP6 HLPN into platelets was markedly greater than that of fluorescent M3mP6 in DMSO (Fig. 3A). Consistently, we found only one fourth the concentration of M3mP6 in HLPN was required to achieve an inhibitory effect on platelet aggregation comparable to that of M3mP6 dissolved in DMSO (Fig. 3B), underscoring the improved intracellular delivery. The DMSO-dissolved M3mP6 peptide had no in vivo effect on arterial thrombosis (Fig. 3C). In contrast, M3mP6 HLPN dose-dependently inhibited occlusive thrombosis using a robust FeCl3-induced carotid artery thrombosis model in mice (Fig. 3C, 3D; See Fig. S4 for comparison with negative control peptides), suggesting the protective effect of the HLPN on peptide viability in vivo. Near maximal anti-thrombotic effect of M3mP6 HLPN was observed at doses above 5 μmol peptide/kg when administered by retro-orbital injection (Fig. 3D), and 2.5 μmol/kg with tail vein injection (Fig. S5). The HLPN containing 50% (mass/mass) M3mP6 peptide had a similar anti-thrombotic effect to HLPN containing 36% M3mP6 when identical amounts of peptide (5 μmol/kg) were injected, confirming that peptide concentrations but not changes in formulation determined the anti-thrombotic effects (Fig. S6). Pharmacokinetic studies indicated that blood and plasma concentrations of M3mP6 reached a maximum within 10 minutes at 59.63 μg/ml in plasma (Fig. 3E) and 33.36 μg/ml in whole blood (Fig. 3F) following intravenous injection of 5 mg/kg M3mP6 (5 μmol/kg) with t1/2-λz =2.46 hours (Table S2). Consistently, the anti-thrombotic effect of M3mP6 was demonstrated within 5 minutes after injection, and lasted until about 45 minutes after injection (Fig. 3G). A rat 5-day infusion study showed a t1/2-λz (half-life after cessation of infusion) of 3.1 (female) and 3.9 (male) (See Table S2 for PK characteristics). Thus, M3mP6 HLPN is a fast-acting and reversible anti-platelet drug likely suitable for i.v. injection; if needed, its therapeutic effect might be prolonged by continuous infusion.
Fig. 1. M3mP6 HLPN.
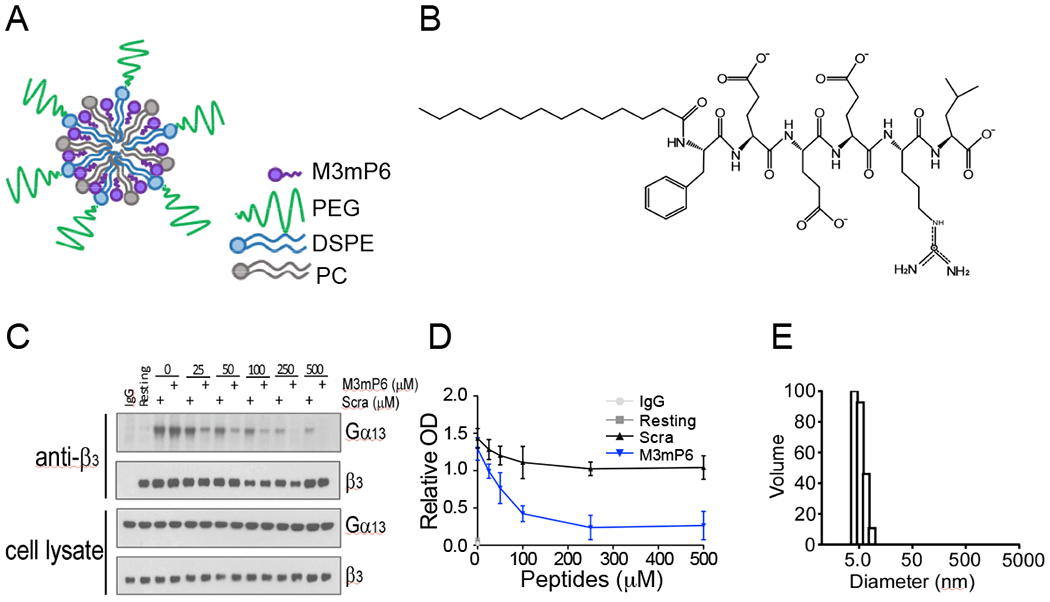
(A) A schematic of lipid-stabilized, high-loading peptide nanoparticles (HLPN). (B) Structure of M3mP6 peptide. (C) Dose-dependent inhibitory effects of M3mP6 on the coimmunoprecipitation of integrin β3 and Gα13 in α-thrombin (0.025 U/mL) stimulated human platelets compared to scrambled peptide. (D) Quantification of coimmunoprecipitation blots in panel C (n=3). OD, optical density. (E) The size distribution of a single preparation of M3mP6 HLPN (6.6 ±0.8 nm) as analyzed by dynamic light scattering (43).
Fig. 2. Function analysis of M3mP6 HLPN.
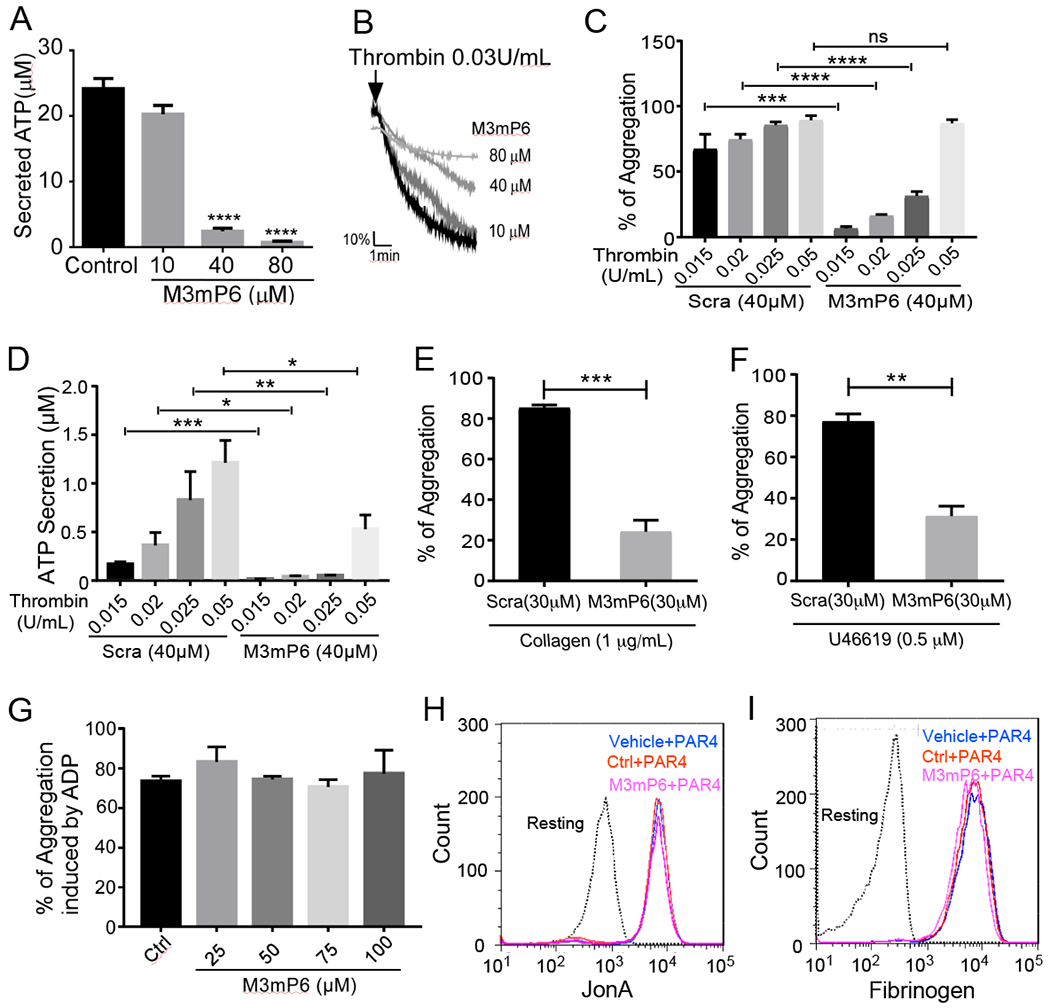
(A) Dose-dependent effects of M3mP6 HLPN on thrombin-induced human platelet secretion in vitro. Data from 3-4 experiments. (B) Dose-dependent effects of M3mP6 HLPN on thrombin (0.03 U/mL)-induced human platelet aggregation. (C) Effects of M3mP6 HLPN on thrombin-induced human platelet aggregation induced by low- and high-dose thrombin (n=3, Scra: scrambled control peptide). (D) Effects of M3mP6 HLPN on low-dose and higher-dose thrombin-induced secretion in human platelets (n=3). (E) Effects of M3mP6 HLPN on collagen (1μg/mL)-induced mouse platelet aggregation compared to scrambled peptide control (n=3). (F) Effects of M3mP6 HLPN on U46619 (0.5μM)-induced mouse platelet aggregation compared to scrambled peptide HLPN. (G) Effects of M3mP6 HLPN on ADP (5μM)-induced human platelet aggregation (PRP). (H) Effects of M3mP6 HLPN on 100 μM PAR4AP-induced binding of PE-conjugated antibody against activated αIIbβ3, JON/A, to mouse platelets. (I) Effects of M3mP6 HLPN on 100 μM agonist (PAR4AP)-induced binding of Oregon Green-labeled fibrinogen to mouse platelets. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, ns, not significant, Unpaired t-test in (A), (C), (D), (E) and (F); data presented as means ± SEM.
Fig. 3. Pharmacokinetics of M3mP6 HLPN.
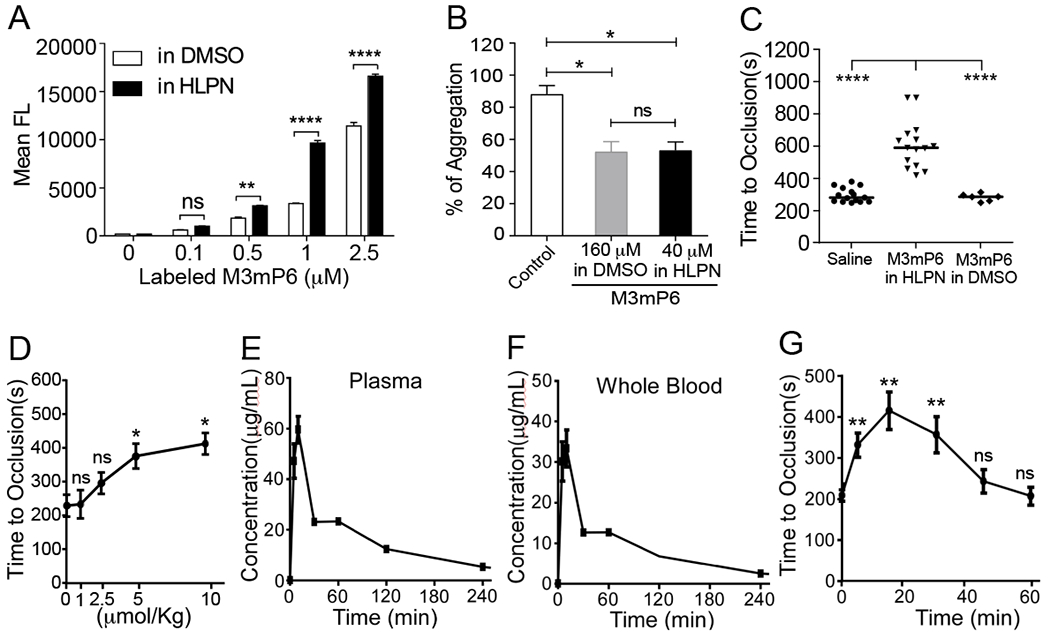
(A) Flow cytometry comparison of the uptake of fluorescence-labeled M3mP6 dissolved in DMSO with that in HLPN into mouse platelets (n=3). (B) Comparable effect of 40 μM M3mP6 in HLPN with 160 μM DMSO-solubilized M3mP6 on human platelet aggregation (n=3). (C) Effects of M3mP6 HLPN (10 μmol/kg) on FeCl3-induced carotid artery thrombosis (n=15), as compared with 10 μmol/kg DMSO-solubilized M3mP6 (n=6) and with saline control (n=15). (D) Dose response of M3mP6 HLPN in inhibiting FeCl3-induced carotid artery occlusive thrombosis following retro-orbital injection 15 minutes before procedure (n=3). (E) Pharmacokinetic study on plasma concentrations of M3mP6 HLPN following retro-orbital injection (5 μmol/kg). (F) Pharmacokinetic study on whole blood levels concentrations of M3mP6 HLPN following retro-orbital injection (5 μmol/kg). (G) Kinetics of anti-thrombotic effect of 5 μmol/kg M3mP6 HLPN (retro-orbital injection) on FeCl3-induced carotid artery occlusive thrombosis (n=3~5). **P<0.01, ****P<0.0001, n.s., not significant, one-way ANOVA with multiple comparisons in (A) and (B); Mann-Whitney test in (C); unpaired t-test in (D) and (G); data presented as means ± SEM.
Comparison of anti-thrombotic efficacy between M3mP6 HLPN and aspirin.
Next, we used a robust FeCl3-induced mouse carotid artery thrombosis model to compare M3mP6 HLPN with aspirin in inhibiting occlusive thrombosis. We used mouse models because the β3 cytoplasmic domain is identical between humans and mice. Following injury induction, control C57BL/6 mice formed stable occlusive thrombosis with a median time of 199 seconds, which was significantly delayed in mice injected with a one-time bolus of M3mP6 HLPN (5 or 10 μmol/kg, 15 min before procedure; retro-orbital, P<0.0001) (Fig. 4A). Occlusion time in aspirin-treated mice (4.3 mg/kg, 2 hours prior to the procedure, oral) was similar to controls (Fig. 4A). To exclude the possibility of insufficient aspirin dosing, we also tested the effect of a very high dose of aspirin (36 mg/kg, i.p., 1 hour prior to experiment, sufficient to severely prolong bleeding (see below)). This dose of aspirin still had no obvious effect on occlusive thrombus formation (Fig. 4B), which contrasted with the marked anti-thrombotic effect of M3mP6 HLPN (5 μmol/kg). Combined use of 5 μmol/kg M3mP6 HLPN and 36 mg/kg aspirin was similar to M3mP6 HLPN alone in inhibiting thrombosis (Fig. 4B). The results suggest that M3mP6 HLPN might be superior to aspirin in inhibiting FeCl3-induced arterial thrombosis.
Fig. 4. Comparison of the effect of M3mP6 HLPN with current anti-platelet drugs on 7.5% FeCl3-induced mouse carotid artery thrombosis, and their synergism.
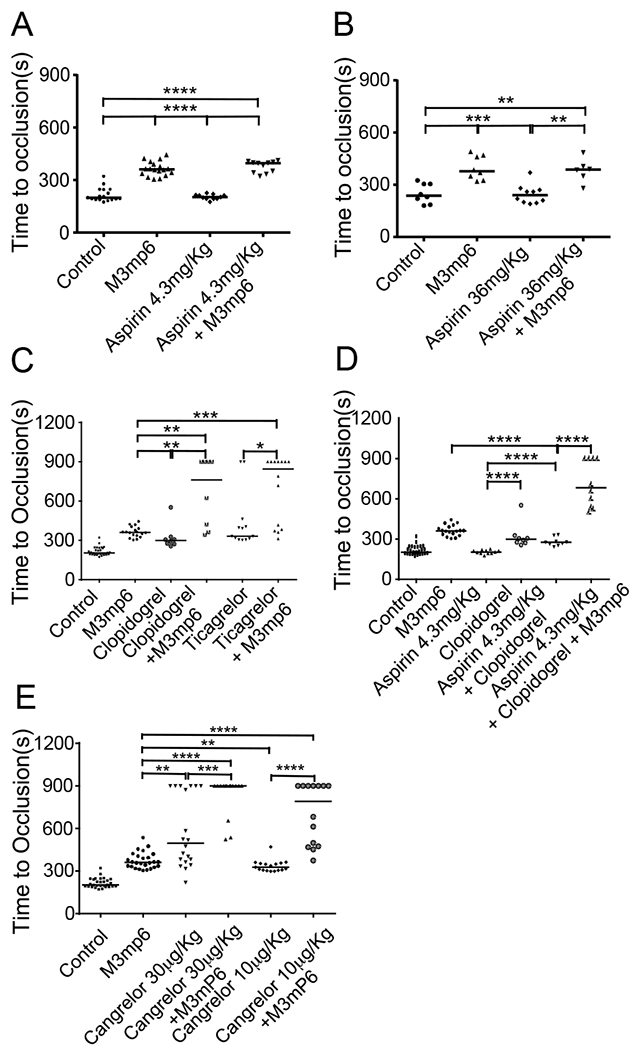
(A) Comparison of saline contro (n=17), M3mP6 HLPN (10 μmol/kg, 15 minutes before procedure, n=18), aspirin (4.3 mg/kg, oral 2 hours before procedure, n=11), and M3mP6 HLPN plus aspirin (n=11) treatments. (B) Comparison of saline control (n=8), M3mP6 HLPN (5 μmol/kg, 15 minutes before procedure, n=8), high dose aspirin (36 mg/kg, i.p. 1 hour before procedure, n=10), and M3mP6 HLPN plus high dose aspirin (n=6) treatments. (C) Comparison of saline control (n=30), M3mP6 HLPN (10 μmol/kg, n=18), clopidogrel (4 mg/kg, 2 hours before procedure, n=8), M3mP6 HLPN plus clopidogrel (n=8), ticagrelor (3 mg/kg, 2 hours before procedure, n=13), and M3mP6 HLPN plus ticagrelor (n=14) treatments. (D). Comparison of the saline control (n=27), M3mP6 HLPN (n=18), aspirin (n=11), clopidogrel (n=8), clopidogrel plus aspirin (n=9), and M3mP6 HLPN plus clopidogrel plus aspirin (n=10) treatments. (E) Comparison of the saline control (n=29), M3mP6 HLPN (10 μmol/kg, n=26), a high dose cangrelor (30 μg/kg, n=20), the high dose cangrelor (30 μg/kg) plus M3mP6 HLPN (n=15), a low dose cangrelor (10 μg/kg, n=17), and the low dose cangrelor (10 μg/kg) plus M3mP6 HLPN (n=14) treatments. Certain identical experimental data are used for different comparisons in different panels. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, Mann-Whitney test.
Comparison of anti-thrombotic efficacy of M3mP6 HLPN and oral P2Y12 inhibitors
We further compared the anti-thrombotic effect of M3mP6 HLPN with P2Y12 antagonist clopidogrel, which is a current standard-of-care anti-platelet drug more potent than aspirin. One-time oral administration of a loading dose of clopidogrel (4 mg/kg, 2 hours prior to procedure) delayed occlusive thrombus formation following FeCl3-induced injury but was less effective than M3mP6 HLPN (Fig. 4C). We also compared the anti-thrombotic effect of M3mP6 HLPN with the more potent oral P2Y12 antagonist, ticagrelor, which does not require hepatic conversion. One-time injection of M3mP6 HLPN had similar anti-thrombotic effects to a one-time ingestion of high dose ticagrelor (3 mg/kg, 2 hours, oral) (Fig. 4C). We found that the median occlusion time in mice treated with M3mP6 HLPN together with clopidogrel (762”) or ticagrelor (845”) was longer than that of mice treated with either of the drugs alone (Fig. 4C), and was longer than the total median occlusion time calculated by adding that of M3mP6 HLPN group with that of either clopidogrel (660”) or ticagrelor (692”) groups (Fig. 4C). These data suggest that M3mP6 HLPN might have synergistic effects with clopidogrel and ticagrelor in inhibiting thrombosis.
Comparison of anti-thrombotic efficacy of M3mP6 HLPN and clopidogrel plus aspirin
Clopidogrel in combination with aspirin is the recommended anti-platelet treatment for patients with myocardial infarction. Thus, we investigated the effects of M3mP6 when combined with clopidogrel plus aspirin. One-time oral administration of a loading dose of clopidogrel combined with aspirin had a similar antithrombotic effect to clopidogrel alone, and was significantly less effective in inhibiting occlusive thrombosis than one-time M3mP6 HLPN injection alone (Fig. 4D, P<0.0001). M3mP6 HLPN in combination with aspirin and clopidogrel had a significantly enhanced anti-thrombotic effect compared to clopidogrel combined with aspirin (Fig. 4D, P<0.0001), but was similar to the anti-thrombotic effect of M3mP6 HLPN combined with clopidogrel alone (Fig. 4D). These data suggest that M3mP6 HLPN might be superior to the combination of aspirin and clopidogrel in preventing arterial thrombosis and that M3mP6 HLPN might exert a synergistic anti-thrombotic effect when used in combination with the clopidogrel and aspirin.
Comparison between M3mP6 HLPN and the intravenous P2Y12 inhibitor cangrelor
Recently, an intravenous direct P2Y12 inhibitor, cangrelor was approved by the FDA for acute anti-thrombosis treatment, which exhibits a more potent anti-thrombotic effect than oral P2Y12 inhibitors, but also causes more severe bleeding(23, 24). We compared the effects of a loading dose of cangrelor with M3mP6 HLPN in preventing occlusive carotid artery thrombosis induced by higher concentrations of FeCl3. Under this condition, a one-time injection of a loading dose of cangrelor (30 μg/kg, retro-orbital) caused a variable inhibition of occlusive thrombosis in different individual mice or experiments. In the majority of mice tested, the effect on vessel occlusion was similar to that of M3mP6 HLPN (Fig. 4E). However, a small population of mice treated with cangrelor showed much longer occlusion time (Fig. 4E). Thus, the overall effect of cangrelor was moderately better than M3mP6 HLPN (Fig. 4E). M3mP6 HLPN combined with cangrelor yielded a longer median occlusion time than M3mP6 HLPN or cangrelor alone (Fig. 4E), which was longer than that calculated by adding the individual increases caused by M3mP6 HLPN and cangrelor alone (>900” vs 857” at 30 μg/kg cangrelor, 791” vs 688” at 10 μg/kg cangrelor), suggesting a likely synergism between M3mP6 HLPN and cangrelor in anti-thrombotic efficacy.
Comparison between M3mP6 HLPN and current anti-platelet drugs in tail bleeding time analysis.
A mouse tail bleeding time test was used to evaluate the effect of M3mP6 HLPN on hemostasis. M3mP6 HLPN, when injected at the same doses that potently inhibited occlusive thrombosis (Fig 4A), showed no effects on mouse tail bleeding time (Fig. 5A). In contrast, the high dose aspirin, which had a minimal anti-thrombotic effect in the FeCl3-induced thrombosis model (Fig. 4B), increased tail bleeding time (Fig. 5B). Also, clopidogrel (Fig 5C), clopidogrel/aspirin combination (Fig. 5D), ticagrelor (Fig 5C) and cangrelor (Fig 5E) increased tail bleeding time in mice. Furthermore, the bleeding time in mice treated with M3mP6 HLPN combined with aspirin, clopidogrel, ticagrelor or cangrelor was similar to mice treated with each of these drugs alone (Fig. 5A–5E), again highlighting the benefit of M3mP6 HLPN in minimizing bleeding risk. Taken together, our data indicate that M3mP6 HLPN might be effective in in potently inhibiting thrombosis without causing excessive bleeding.
Fig. 5. Comparison of effects of M3mP6 HLPN with current anti-platelet drugs on mouse tail bleeding time.
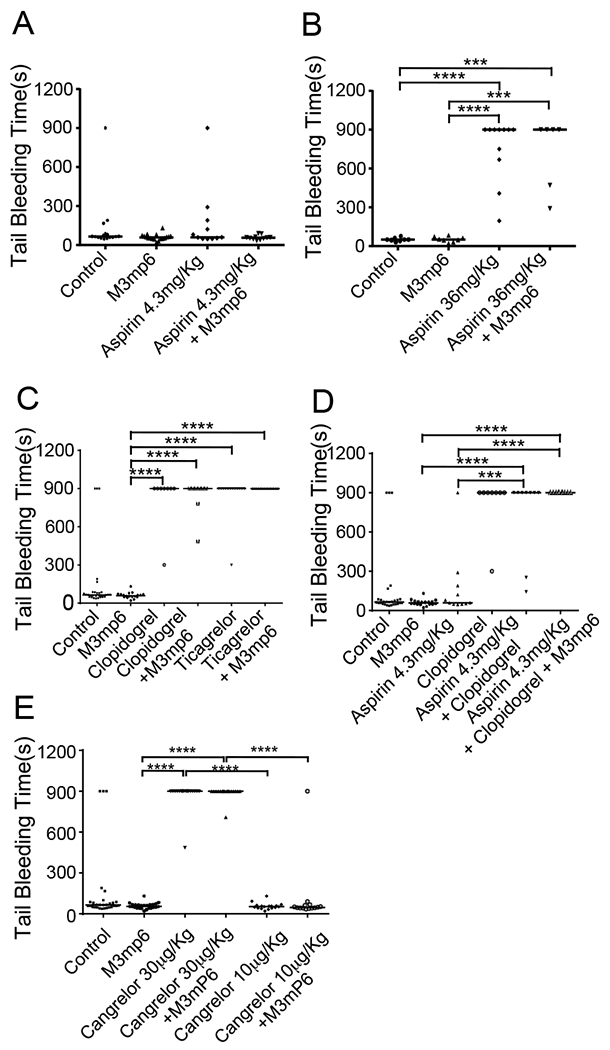
(A) Comparison of saline control (n=16), M3mP6 HLPN (10 μmol/kg, 15 minutes before procedure, n=18), aspirin (4.3 mg/kg, oral 2 hours before procedure, n=11), and M3mP6 HLPN plus aspirin (n=11) treatments. (B) Comparison of saline control (n=8), M3mP6 HLPN (5 μmol/kg, 15 minutes before procedure, n=8), high dose aspirin (36 mg/kg, i.p. 1 hour before procedure, n=11), and M3mP6 HLPN plus high dose aspirin (n=6) treatments. (C) Comparison of saline control (n=30), M3mP6 HLPN (10 μmol/kg, n=18), clopidogrel (4 mg/kg, 2 hours before procedure, n=8), M3mP6 HLPN plus clopidogrel (n=8), ticagrelor (3 mg/kg, 2 hours before procedure, n=13), and M3mP6 HLPN plus ticagrelor (n=14) treatments. (D) Comparison of saline control (n=27), M3mP6 HLPN (n=18), aspirin (n=11), clopidogrel (n=8), clopidogrel plus aspirin (n=9), and M3mP6 HLPN plus clopidogrel plus aspirin (n=10) treatments. (E) Comparison of saline control (n=29), M3mP6 HLPN (10 μmol/kg, n=25), a high dose cangrelor (30 μg/kg, n=17), high dose cangrelor (30 μg/kg) plus M3mP6 HLPN (n=17), a low dose cangrelor (10 μg/kg, n=17), and the low dose cangrelor (10 μg/kg) plus M3mP6 HLPN (n=14) treatments. Certain identical data are used for different comparisons in different panels. ***P<0.001, ****P<0.0001, Mann-Whitney test.
Comparison of M3mP6 HLPN with cangrelor in an artery perforation model of surgical bleeding
Clinically, the risk of hemorrhage during anti-platelet therapy is often associated with traumatic intervention, particularly intravascular intervention when perforation of a vascular wall is a necessity. To more closely mimic hemorrhage during intravascular/surgical procedures, we designed a carotid artery perforation model of surgical hemorrhage (Fig. 6A). In this model, the common carotid artery was surgically exposed, and perforated with a needle. The site of perforation was immediately covered with a hemostatic pad (CERTI-GAUZE) to stop bleeding replicating the procedure performed during vascular intervention. Blood absorbed on the pad was eluted and quantified to indicate amounts of hemorrhage. In normal controls, bleeding at the perforation site was quickly stopped by the hemostatic pad with the extravasation of a minimal amount of blood, but the loading dose of cangrelor caused excessive bleeding despite the use of the hemostatic pad (Fig. 6B). In contrast, M3mP6 HLPN-treated mice were not different from control mice (Fig. 6B), further demonstrating M3mP6 HLPN to be a safe anti-platelet drug that does not cause excessive bleeding under a condition mimicking an invasive vascular intervention. We used this model to verify that M3mP6 HLPN does not exacerbate the adverse bleeding effect of a P2Y12 antagonist. Indeed, there was no difference in hemorrhage between groups administered cangrelor alone or cangrelor plus M3mP6 HLPN, either at high or low dose cangrelor (Fig. 6B). These data demonstrated that M3mP6 HLPN exerted a potent anti-platelet effect with minimal bleeding risk. Moreover, M3mP6 HLPN combined with cangrelor did not increase bleeding risk over that of cangrelor treatment alone.
Fig. 6. Effects of M3mP6 in a mouse artery perforation model of surgical bleeding and dog buccal mucosal bleeding time (BMBT) test.
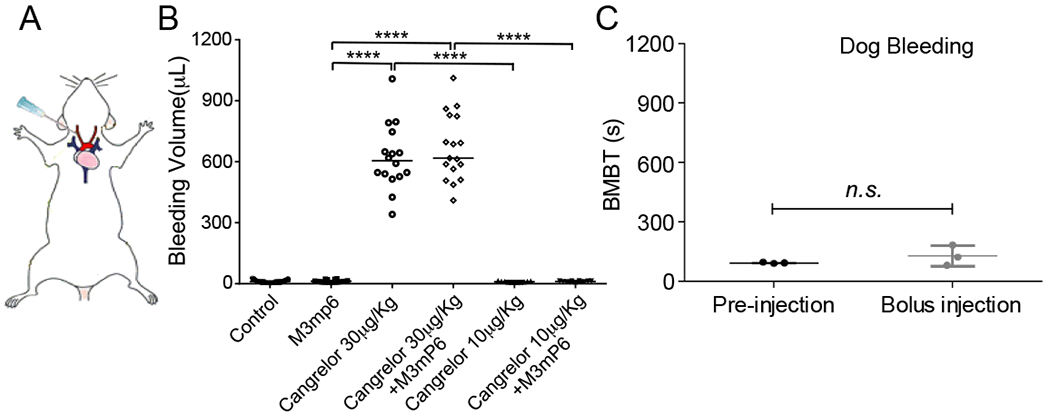
(A) Illustration of a mouse surgical bleeding model. (B) Comparison of M3mP6 HLPN (10 μmol/kg, n=20) with high dose cangrelor (30 μg/kg, n=16), high dose cangrelor plus M3mP6 HLPN (n=17), low dose cangrelor (10 μg/kg, n=11)), low dose cangrelor plus M3mP6 HLPN (n=12) and normal control (n=27) in the artery perforation model of surgical bleeding. (C) BMBT test of the effect of M3mP6 HLPN on hemostasis in dogs (n=3) ****P<0.0001, n.s.: not significant; Mann-Whitney test in (B), unpaired t-test in (C); data presented as means ± SEM.
No effect of M3mP6 HLPN in a dog Buccal Mucosal Bleeding Time (BMBT) test
To determine whether M3mP6 may affect hemostasis in large animals, we used BMBT, a routine bleeding time test, in 3 dogs before and during M3mP6 HLPN infusion. No differences in bleeding time were observed between pre- and post-administration of M3mP6 HLPN, both of which fell in the normal bleeding time range (<4 minutes in dogs) (Fig. 6C). These data showed that M3mP6 HLPN did not cause excessive bleeding not only in rodents but also in dogs.
Synergistic anti-thrombotic effect without excessive bleeding using a combination of M3mP6 HLPN with low dose cangrelor
Our results showed that a loading dose of cangrelor had potent anti-thrombotic efficacy but also dramatically increased hemorrhage, which contrasted with M3mP6 HLPN’s selective inhibition of thrombosis without causing bleeding (Fig. 4E, 5E, 6B). Our data also demonstrated that M3mP6 HLPN combined with this high dose of cangrelor enhanced anti-thrombotic efficacy compared to high dose cangrelor alone (Fig. 4E), but appeared to have an adverse effect on bleeding similar to that of cangrelor alone (Fig. 5E, 6B). Thus, we hypothesized that the combination of M3mP6 HLPN with low dose cangrelor may synergistically enhance anti-thrombotic efficacy but with reduced adverse effects on bleeding. Indeed, M3mP6 HLPN in combination with low dose cangrelor (10 μg/kg) had an anti-thrombotic effect that was greater than even the maximum clinical dose of cangrelor (Fig. 4E), however tail bleeding time or bleeding in a carotid artery perforation model of surgical hemorrhage was not increased over control or either of the drugs individually (Fig. 5E, 6B). Thus, M3mP6 HLPN used together with low-dose cangrelor might be an optimal choice for acute anti-thrombotic treatment due to its powerful anti-thrombotic therapeutic effect and lack of increased bleeding risk.
Acute post-ischemic anti-thrombotic effects of M3mP6 HLPN in comparison with cangrelor.
An advantage for intravenous anti-thrombotics is fast action, which makes them suitable for emergency treatment of acute thrombosis. We thus compared the acute anti-thrombotic effect of cangrelor with M3mP6 HLPN under experimental conditions mimicking a thrombotic emergency, where the drugs were retro-orbitally injected after the initiation of carotid arterial thrombosis. There was no difference between M3mP6 (10 μmol/kg) and a loading dose of cangrelor (30 μg/kg) in anti-thrombotic effect under these conditions (Fig. 7A). However, cangrelor but not M3mP6 HLPN showed a prolonged tail bleeding time (Fig. 7B), indicating the superior bleeding safety of M3mP6 HLPN.
Fig. 7. Effects of post-injury injection of M3mP6 and cangrelor on thrombosis and hemostasis, and effects of M3mP6 HLPN on laser-induced cremaster arteriolar platelet thrombus formation and clotting.
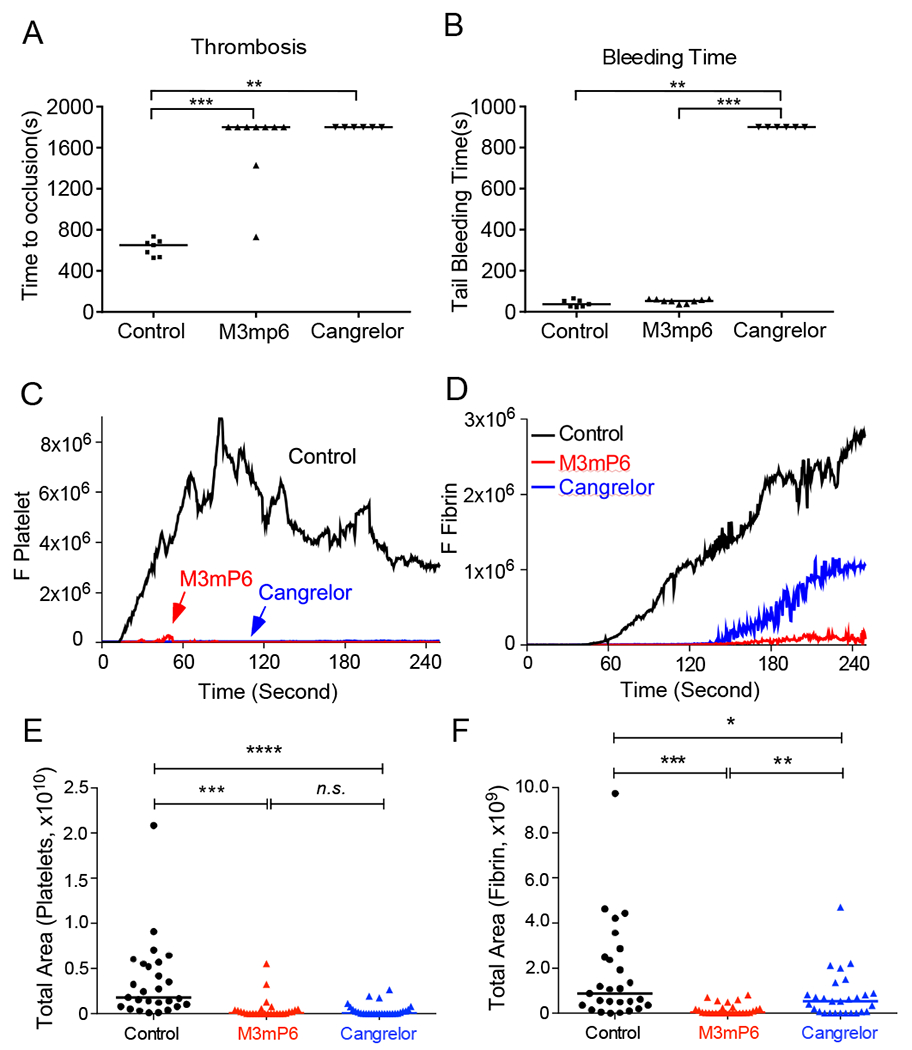
(A) Effects of M3mP6 HLPN (10 μmol/kg) and cangrelor (30 μg/kg) on occlusive thrombosis when injected 5 minutes after FeCl3 (3.75%)-induced carotid artery injury. (B) Tail bleeding time of mice as described in (A). A and B: n=7 for normal control, n=9 for M3mP6 HLPN, n=6 for cangrelor. (C) Comparison of the median integrated platelet fluorescence intensity (DyLight 649-labeled anti-GPIbβ) at the sites of laser-induced injury in cremaster arterioles of control (black), M3mP6 HLPN (10 μmol/kg)-treated (red) and cangrelor (30 μg/kg)-treated (blue) mice (27 injury sites each for control, M3mP6- and cangrelor-treated groups). (D) Comparison of the median integrated fibrin fluorescence intensity (Alexa Fluor 488-labeled anti-fibrin) at the sites of the laser-induced injury in cremaster arterioles of control (black), M3mP6 HLPN (10 μmol/kg)-treated (red) and cangrelor (30 μg/kg)-treated (blue) mice. (E) and (F) Quantification of total fluorescence as detected over time in C for platelet thrombus (E) and in D for fibrin (F) (27 injury sites each for control, M3mP6- and cangrelor-treated groups). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, n.s:. not significant, Mann-Whitney test.
Effects of M3mP6 HLPN and cangrelor on platelet thrombus formation and intravascular coagulation using a laser-induced cremaster arteriolar thrombosis model in mice.
Recent studies demonstrated that integrin outside-in signaling plays an important role not only in platelet thrombus formation but also intravascular coagulation under flow shear(25). Current anti-platelet drugs are ineffective in inhibiting intravascular coagulation(26, 27). Thus, we further tested the effects of M3mP6 HLPN compared with cangrelor in inhibiting both platelet thrombus formation and intravascular coagulation using a laser-induced cremaster arterial thrombosis model. When pre-injected intravenously, M3mP6 HLPN (10 μmol/kg) was similar to a loading dose of cangrelor (30 μg/kg) in potently inhibiting platelet thrombus formation (Fig. 7C, 7E). However, M3mP6 HLPN also almost completely inhibited intravascular fibrin clot formation at the site of vascular injury, whereas cangrelor had only a moderate partial effect (Fig. 7D, 7F). Thus, M3mP6 HLPN was not only effective in inhibiting platelet thrombus formation but also intravascular coagulation in vivo, and this effect might be superior to that achieved by the most potent P2Y12 inhibitor cangrelor.
Treatment of myocardial ischemia-reperfusion (MI/R) injury with M3mP6 HLPN
The prevailing treatment for myocardial infarction/ischemia (MI) is to perform surgical or percutaneous coronary interventions to invasively reperfuse the occluded artery. Reperfusion of ischemic tissues, however, may cause myocardial ischemia/reperfusion (MI/R) injury, where an acute thrombo-inflammatory reaction of the ischemic tissue occurs upon re-exposure to oxygenated blood, resulting in damage to cardiac function and death. To evaluate the potential therapeutic effect of M3mP6 HLPN in treating MI and MI/R injury, a severe MI was induced in mice by ligating the left anterior descending (LAD) branch coronary artery for 45 minutes followed by reperfusion. To mimic the clinical setting in which anti-thrombotic therapies are provided during reperfusion, M3mP6 or control HLPN were injected post-ischemically, 35 minutes after the induction of MI (Fig. 8A). Compared with the control group, the M3mP6 HLPN treatment group showed lower infarct area/risk area ratio (Fig. 8B–C) but similar risk area/total area ratio (Fig. 8D) as indicated by triphenyltetrazolium chloride (TTC)/Evans Blue staining, suggesting reduced infarction under similar ischemia/reperfusion assault. M3mP6 HLPN also prevented damage of cardiac function as indicated by echocardiography performed at 24 hours after the procedure (Fig. 8E and F). Immunohistochemistry indicated that MI/R-induced microvascular thrombosis (Fig. 8G) and neutrophil infiltration (Fig. 8H) in the reperfused cardiac tissue were both reduced by the M3mP6 HLPN treatment. M3mP6 also reduced plasma MPO concentrations (Fig. 8I), which is an indicator of neutrophil activation, in MI/R mice. In addition, M3mP6 greatly reduced mortality rate during the 7-day post-procedure monitoring (Fig. 8J). These data suggest that M3mP6 was effective treatment of MI/R-induced thrombosis/inflammation and cardiac injury in the mouse model.
Fig. 8. Effects of post-ischemia injection of M3mP6 HLPN on myocardial ischemia/reperfusion (MI/R) injury.
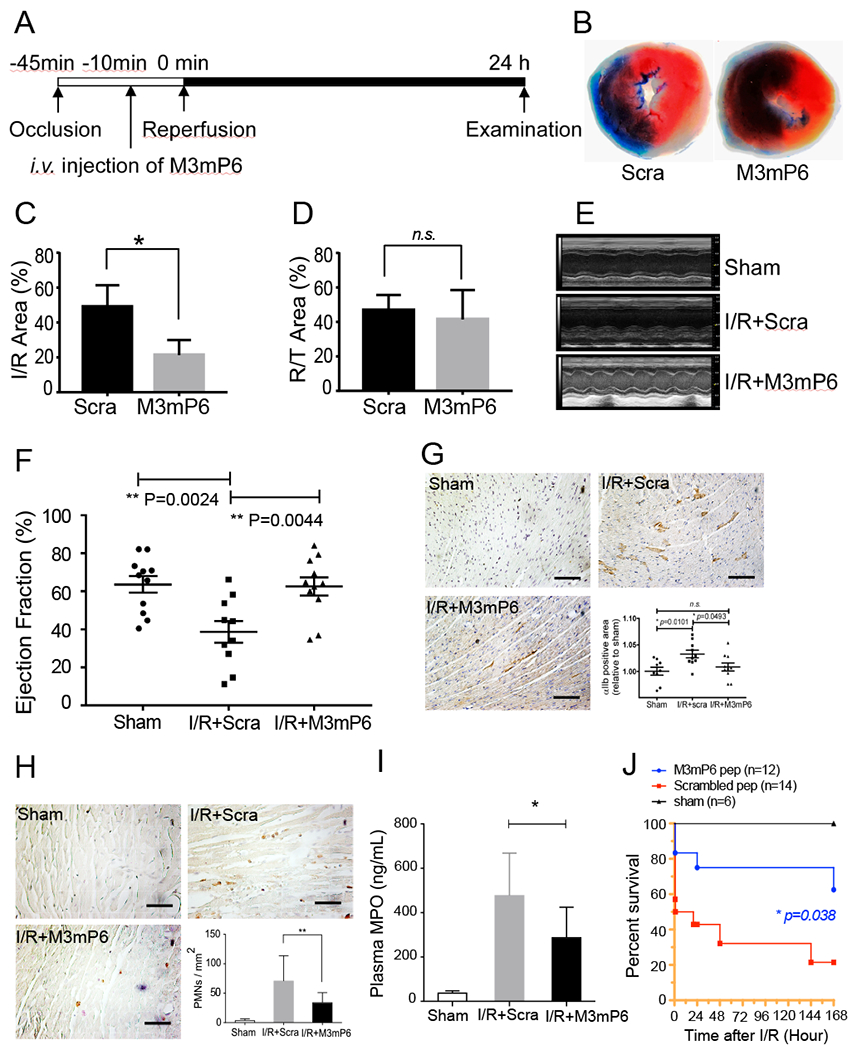
(A) Schematic protocol of MI/R study. (B) Representative images of heart sections from mice treated with M3mP6- or scrambled peptide HLPN (Evans blue/TTC stain). (C) Quantification of the infarct area (white) as percentage of the area at risk (white and red) as shown in A (n=4 for each group, t-test, *P<0.05). (D) Quantification of risk area as percentage of the entire heart section. (n=4 for each group, t-test, ns, not significant (P>0.05) (E) Representative M-mode long-axis echo images for (i) sham control; (ii) MI/R treated with scrambled peptide HLPN; and (iii) MI/R treated with M3mP6 HLPN. (F) Mouse left ventricle ejection fraction (n=11 for sham and M3mP6 groups, n=10 for scrambled peptide group, mean ± SEM, one-way ANOVA). (G) Immunohistochemistry staining of platelets in mouse heart sections 24 h after MI/R using rat anti-integrin αIIb antibody. The representative sections are from (i) sham control; (ii) MI/R treated with scrambled peptide HLPN; and (iii) MI/R treated with M3mP6 HLPN. (iv) αIIb positive staining was quantified using image J and presented as percentage of total tissue area (n=9 for sham, n=10 for both M3mP6 and scrambled peptide groups, mean±SEM, Mann-Whitney test). (H) Immunohistochemistry staining of neutrophils in mouse heart sections 24 h after MI/R using rat anti-Ly6G antibody. The representative sections are from (i) sham control; (ii) MI/R treated with scrambled peptide HLPN; and (iii) MI/R treated with M3mP6 HLPN. (iv) Ly6G-positive cells/mm2 were counted using imageJ software (n=6 for sham, n=22 for scrambled peptide, n=12 for M3mP6, mean±SEM, Mann-Whitney test, **P<0.01) (I) Mouse plasma MPO concentrations 24 hours after MI/R. (sham: n=6, scrambled HLPN treatment: n=11, M3mP6 HLPN treatment: n=9, mean±SEM, t-test, *P<0.05) (J) Kaplan-Meier survival curve of mice 7 days after MI/R surgery treated with M3mP6 HLPN or scrambled peptide HLPN (n=12 for M3mP6 treatment, n=14 for scrambled peptide treatment). Sham surgery caused no death in 6 tested mice.
Discussion
Three important advances are reported in this study: (1) we have developed lipid-stabilized, HLPN for efficient delivery of an ExE motif peptide (M3mP6) in vivo into the intracellular compartment for therapeutic use; (2) we showed that M3mP6 HLPN potently inhibited occlusive thrombosis without observable adverse effect on hemorrhage in mice and dogs; and (3) post-ischemia injection of M3mP6 HLPN inhibited microvascular thrombosis/inflammation and improved cardiac function and survival in a mouse model of MI/R injury. This compound showed not only anti-thrombotic synergism with the current standard of care anti-platelet drugs (P2Y12 inhibitors) without increasing bleeding risk, but also demonstrated a marked therapeutic effect in the treatment of ischemia/reperfusion injury following MI in animal models. Thus, M3mP6 HLPN has the potential to improve current anti-platelet therapy in the treatment of myocardial infarction while concomitantly improving the safety profile.
Despite having advantages of high specificity due to unique amino acid sequences and low risk of toxic nonspecific effects, development of peptide-based drugs targeting intracellular compartments has lagged (1, 2). Among the major reasons is the lack of technology to efficiently deliver sufficient amounts of peptides into cells in vivo. Thus, facilitating intracellular delivery of peptides should accelerate the development and clinical use of peptide-based therapeutic agents. Although numerous tools have been developed to enable peptides to cross cell membranes, none have been completely satisfactory for therapeutic use (1, 2). Among them, lipidization is effective to render peptides membrane permeable. However, myristoylated peptide M3mP6, although membrane permeable and able to inhibit platelet function in vitro, was ineffective in vivo. Thus, membrane permeability alone is not sufficient to render peptides effective in vivo. Liposomes and lipid micelles have been relatively more efficient and often used for drug delivery in vivo(2). However, these approaches usually require large amounts of lipid to encapsulate a limited amount of peptide, thus necessitating unusually high peptide affinity or avidity for its targets. For example, a lipid micellar formulation of the synthetic peptide mP6 contains a maximal 4% (mol/mol) peptide yielding a maximal concentration of mP6 of <1 mM, which is insufficient for clinical use (21). The lipid-stabilized M3mP6 HLPN in the present study contains up to 70% (mol/mol) peptide, achieving a high peptide concentration of >10 mM, suitable for bolus IV injection in humans. The lipid-stabilized HLPN also facilitates peptide drug entry into cells, resulting in much less peptide being required for comparable anti-platelet efficacy compared with the peptide alone. These superior characteristics rendered M3mP6 HLPN a potent anti-thrombotic in vivo. Thus, our data demonstrate that the lipid-stabilized HLPN improves efficiency of intracellular delivery of a peptide-based drug in vivo, enabling effective in vivo use of peptide drugs having relatively moderate affinity for their intracellular targets. This approach may be useful for facilitating development of peptide-based drugs targeting the intracellular compartment for therapeutic use.
Circulating platelets are normally in a resting state and become activated only when exposed to platelet agonists. Platelet agonists elicit platelet activation via various receptor-mediated intracellular signaling pathways (28, 29). These intracellular signals converge as an “inside-out” signal to transform αIIbβ3 from a ‘resting’ state to an ‘activated’ state (8, 30). This enables integrin’s ligand binding function, a requirement for platelet adhesion and aggregation (12). Current anti-platelet drugs either inhibit platelet activation signaling pathways leading to integrin activation or directly inhibit the ligand binding function of the integrin αIIbβ3 (10, 12). Although these drugs have become cornerstones in the treatment of patients with cardiovascular diseases (6, 12), they also inhibit hemostasis, causing excessive bleeding which can be life-threatening (11–14). Bleeding effects of these drugs also limits the use of optimal doses and therefore their anti-thrombotic efficacy. Indeed, bleeding in thrombotic patients receiving a percutaneous coronary intervention is associated with poor prognosis and increased mortality (11, 15–17). Hence the balance of optimal platelet inhibition with minimal bleeding complications is an important current clinical challenge, particularly for patients requiring traumatic procedures or suffering from thrombotic stroke, in which hemorrhage is life-threatening. Recently, various thrombin receptor inhibitors were shown to have reduced adverse effects on hemorrhage in animal models (19, 20). However, this reduction in adverse effect on hemorrhage was measured relative to clopidogrel and was only partial (19, 20). Animals administered thrombin receptor inhibitors still had increased bleeding compared to normal controls (19, 20). In contrast, we observed no difference in bleeding between normal controls and animals administered M3mP6 HLPN. Clinical trials of the PAR1 inhibitor vorapaxar reported excessive bleeding in human patients (19), suggesting that this drug still poses a clinically relevant hemorrhagic risk. The adverse hemorrhagic effects of these drugs are consistent with them inhibiting the activation of the ligand binding function of integrin αIIbβ3 and are thus not fundamentally different from the current anti-platelet drugs (29). The compound described here is based on a different concept of selectively targeting integrin outside-in signaling without directly affecting the ligand binding function of the platelet integrin αIIb,β3. Thus, M3mP6 permits primary platelet adhesion and aggregation to occur but prevents outside-in signaling-mediated secondary thrombus expansion and consequent vascular occlusion (21, 22). As primary platelet aggregation in the collagen-rich tissues outside the vascular lumen is likely to be robust, the secondary outside-in amplification signals are unlikely to be required for hemostasis in most hemorrhagic situations, but are critical during thrombosis intravascularly. Furthermore, outside-in signaling is important for activating platelet procoagulant activity, which is selectively required for intravascular coagulation and thrombus stability in the presence of blood flow shear (25), providing a second mechanism by which selectively blocking outside-in signaling does not affect hemostasis, but inhibits occlusive intravascular thrombi. Thus, our combination of an innovative HLPN delivery system with a peptide-based outside-in signaling inhibitor allowed us to demonstrate the ability of M3mP6 HLPN to inhibit occlusive thrombosis without observable effects on hemostasis using three different bleeding assays in mice and dogs.
M3mP6 HLPN is not only itself a potent anti-thrombotic but it also exerts a synergistic effect when used in combination with the current standard of care, P2Y12 inhibitors with or without aspirin. This synergistic effect occurred with clopidogrel and with the more potent new direct P2Y12 inhibitors ticagrelor and cangrelor, even at the highest clinical doses. These synergistic effects suggest that the integrin-dependent secondary amplification of thrombus formation requires release of known agonists such as ADP and thromboxane A2 (TXA2) and also activation of additional signaling pathways that are not inhibited by ADP receptor antagonists or inhibitors of the TXA2 synthesis pathway. M3mP6 HLPN did not increase the bleeding risks when combined with aspirin and P2Y12 inhibitors, suggesting that this synergistic effect has the potential to increase anti-thrombotic efficacy to an extent that is not currently possible with anti-platelet therapy without bleeding risk. Consistent with this notion, we showed that the combination of M3mP6 with low-dose cangrelor not only enhanced anti-thrombotic efficacy beyond that achievable with the highest clinical dose of cangrelor but also abolished the bleeding risk associated with high-dose cangrelor in mice. Thus, combined use of M3mP6 HLPN with low doses of a P2Y12 inhibitor represents a potential anti-thrombotic strategy that might have important implications for improving clinical outcomes. Our data further suggest that this compound might be useful for treating acute myocardial ischemia/reperfusion (I/R) injury. Post-ischemia injection of M3mP6 HLPN inhibited both microvascular thrombosis and inflammation in reperfused cardiac tissue, rescued cardiac function and improved survival in a mouse model of myocardial ischemia/reperfusion. It is important to note that the therapeutic effect of M3mP6 HLPN on MI/R injury may not necessarily be due specifically to its anti-platelet effect, but it is also possible that this drug may affect integrin signaling in other cells such as leukocytes. Both thrombosis and leukocyte-mediated inflammation (31) play important roles in MI/R injury.
A major limitation of our study is that our in vivo data are primarily obtained in mice, which show similarities with humans in integrin outside-in signaling and β3 cytoplasmic domain sequence, but also differences in thrombosis and inflammation. Also, M3mP6 HLPN has only been shown to be effective intravenously and has a relatively short half-life in blood, which limits its out-of-hospital use or prolonged use. Thus, M3mP6 in current formulation is not for routine prevention of thrombosis in place of current oral anti-platelet drugs. However, M3mP6 HLPN is fast-acting, has a relatively longer in plasma half-life (~3.5 hours in rats) than cangrelor (3-6 minutes), and thus might be suitable for bolus i.v. injection in emergency in patients suffering from acute myocardial infarction. Future preclinical safety studies and clinical trials should demonstrate whether the compound and the strategy herein is suitable for use in patients.
In summary, we have engineered lipid-stabilized HLPN to efficiently deliver an inhibitor peptide of integrin outside-in signaling in vivo, resulting in a fast acting, reversible and potent anti-thrombotic compound with minimal bleeding risk in mice. Post-ischemic injection of this compound effectively reduces MI/R injury and mortality in mice. Furthermore, we developed an anti-platelet strategy in which M3mP6 HLPN in combination with a low dose of current anti-platelet drugs enhances anti-thrombotic efficacy while minimizing bleeding risk in mice.
Materials and Methods
Study design
This study is aimed at developing high-loading peptide nanoparticles (HLPN) for in vivo delivery of an inhibitor peptide, M3mP6, to its intracellular target for treating thrombosis and MI/R injury. We performed a cohort of experiments using multiple in vitro and in vivo approaches to characterize the properties and anti-platelet/anti-thrombotic/hemorrhagic effects of M3mP6 HLPN, the details of which are described in Supplemental Methods and in previous publications (21, 32–35). We compared the anti-thrombotic effects of M3mP6 HLPN with current anti-platelet drugs using the FeCl3-induced mouse carotid artery thrombosis (34, 36) and laser-induced mouse cremaster arterial thrombosis models (37). We compared the bleeding effects of current anti-platelet drugs with M3mP6 HLPN using mouse tail bleeding time (21, 38), mouse carotid artery perforation bleeding analysis (we developed in this study) and dog Buccal Mucosal Bleeding Time (BMBT) test (39). In all these experiments, mice of either sexes (8-10 weeks of age) were randomly selected into control and different treatment groups. Per animal protection requirements and when scientifically valid, data collected in identical in vivo experiments were used for multiple purposes to avoid unnecessary mouse sacrifice. The thrombosis and bleeding tests were not blinded due to the obviousness of the differences in occlusion times between M3mP6 HLPN and control or aspirin-treated groups, and in bleeding between M3mP6 HLPN and P2Y12 antagonists during procedures. The mouse left anterior descending artery (LAD) ligation model (40, 41) was used to study the effect of M3mP6 HLPN in treating MI/R injury, and this study was blinded by concealing the identities of treatment groups to the operator. The confidence and sample sizes of key experiments were evaluated by Power analysis using GPower 3.1 software (42) (see source files for details). For in vitro experiments, sample size prediction was performed based on our previous experience with n=3~6 (21, 25). To determine the suitability of controls used for our studies, we compared HLPN of scrambled peptide and AAA mutant peptide with physiological saline to show their similarity (Fig. S4).
Statistics
Sample normality and distribution tests were performed using Graphpad PRISM 5.0. For parametric data, statistical significance was analyzed using Student’s t-test (2-group comparison) or ANOVA (multiple group comparison) using Graphpad PRISM 5.0. For nonparametric data, statistical significance was determined using Mann-Whitney test. Power analysis were performed using G-Power 3.1 softwares. Survival analysis was performed using the Kaplan-Meier method. P-value <0.05 was considered as significant.
Supplementary Material
Fig. S1. Stability of M3mP6 HLPN during storage.
Fig. S2. Effect of M3mP6 peptide and HLPN on JonA and fibrinogen binding to platelets and platelet aggregation-induced by PAR4-agonist peptide (PAR4-AP).
Fig. S3. Uptake of fluorescently labeled M3mP6 HLPN into mouse platelets.
Fig. S4. The comparison of the effects of M3mP6 HLPN, scrambled control peptide (Scra) HLPN, AAA mutant peptide (Myr-FAAARL) HLPN and physiological saline solution on 7.5% FeCl3-induced carotid artery thrombosis.
Fig. S5. Dose response analysis of tail vein-injected M3mP6 HLPN in inhibiting occlusive thrombosis using the 7.5% FeCl3-induced carotid artery thrombosis model.
Fig. S6. Comparison of effects of 50% vs 36.8% M3mP6 HLPN on FeCl3-induced thrombosis.
Table S1. Summary of toxicological studies of M3mP6 HLPN.
Table S2: M3mP6 Peptide Pharmacokinetic Parameters
Movie S1. Laser-induced cremaster arteriolar thrombosis after saline injection as indicated by platelet (red) and fibrin (green) stains as described in the method.
Movie S2: Laser-induced cremaster arteriolar thrombosis 15 minutes after bolus injection of M3mP6 HLPN as indicated by platelet (red) and fibrin (green) stains.
Movie S3: Laser-induced cremaster arteriolar thrombosis immediately following injection of cangrelor as indicated by platelet (red) and fibrin (green) stains.
Acknowledgments:
We thank Dr. David Phillips for comments and suggestions, and thank Mr. Xiang Shen for contributing to experiments.
Funding:
This work is partially supported by National Heart, Lung and Blood Institute Vascular Interventions/Innovations and Therapeutic Advances (VITA) contracts Stage-A (HHSN268201400007C) to XD and Stage B (HHSN268201700002C) to MG, grants RO1HL080264, RO1HL062350, and RO1HL125356 to XD, R43 HL142396 to MKD and RAS and R01HL045638, P01HL060678 and P01HL077806 to ABM.).
Footnotes
List of Supplementary Materials
Competing interests: Patents related to this study: US Provisional Application No. 62/932,024 titled “PEPTIDES AND METHODS OF TREATING SEPSIS, ATHEROSCLEROSIS, THROMBOSIS, STROKE, HEART ATTACK AND INFLAMMATION” (University of Illinois). X.D. has ownership interests in DMT, Inc., which licenses the university patents.
Data and materials availability: The data necessary for this work are present in the main text or in the supplementary material.
References and Notes:
- 1.Fosgerau K, Hoffmann T, Peptide therapeutics: current status and future directions. Drug Discov Today 20, 122–128 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Tan ML, Choong PF, Dass CR, Recent developments in liposomes, microparticles and nanoparticles for protein and peptide drug delivery. Peptides 31, 184–193 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American C Heart Association Statistics, S. Stroke Statistics, Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 135, e146–e603 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coller BS, Historical perspective and future directions in platelet research. J Thromb Haemost 9 Suppl 1, 374–395 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruggeri ZM, Platelets in atherothrombosis. Nat Med 8, 1227–1234. (2002). [DOI] [PubMed] [Google Scholar]
- 6.Libby P, Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med 368, 2004–2013 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Bledzka K, Smyth SS, Plow EF, Integrin alphaIIbbeta3: from discovery to efficacious therapeutic target. Circ Res 112, 1189–1200 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shattil SJ, Kim C, Ginsberg MH, The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol 11, 288–300 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr., Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Stevenson WG, Yancy CW, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 61, e78–140 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Metharom P, Berndt MC, Baker RI, Andrews RK, Current state and novel approaches of antiplatelet therapy. Arterioscler Thromb Vasc Biol 35, 1327–1338 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Berger PB, Bhatt DL, Fuster V, Steg PG, Fox KA, Shao M, Brennan DM, Hacke W, Montalescot G, Steinhubl SR, Topol EJ, Investigators C, Bleeding complications with dual antiplatelet therapy among patients with stable vascular disease or risk factors for vascular disease: results from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Circulation 121, 2575–2583 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Estevez B, Shen B, Du X, Targeting integrin and integrin signaling in treating thrombosis. Arterioscler Thromb Vasc Biol 35, 24–29 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehilli J, Kastrati A, Schulz S, Frungel S, Nekolla SG, Moshage W, Dotzer F, Huber K, Pache J, Dirschinger J, Seyfarth M, Martinoff S, Schwaiger M, Schomig A, Bavarian I Reperfusion Alternatives Evaluation-3 Study, Abciximab in patients with acute ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention after clopidogrel loading: a randomized double-blind trial. Circulation 119, 1933–1940 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Safley DM, Venkitachalam L, Kennedy KF, Cohen DJ, Impact of Glycoprotein IIb/IIIa Inhibition in Contemporary Percutaneous Coronary Intervention for Acute Coronary Syndromes: Insights From the National Cardiovascular Data Registry. JACC Cardiovasc Interv 8, 1574–1582 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Ndrepepa G, Kastrati A, Bleeding complications in patients undergoing percutaneous coronary interventions: current status and perspective. Coron Artery Dis 25, 247–257 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Mehran R, Pocock S, Nikolsky E, Dangas GD, Clayton T, Claessen BE, Caixeta A, Feit F, Manoukian SV, White H, Bertrand M, Ohman EM, Parise H, Lansky AJ, Lincoff AM, Stone GW, Impact of bleeding on mortality after percutaneous coronary intervention results from a patient-level pooled analysis of the REPLACE-2 (randomized evaluation of PCI linking angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC Cardiovasc Interv 4, 654–664 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Ndrepepa G, Guerra E, Schulz S, Fusaro M, Cassese S, Kastrati A, Weight of the bleeding impact on early and late mortality after percutaneous coronary intervention. J Thromb Thrombolysis 39, 35–42 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Moscucci M, Frequency and costs of ischemic and bleeding complications after percutaneous coronary interventions: rationale for new antithrombotic agents. J Invasive Cardiol 14 Suppl B, 55B–64B (2002). [PubMed] [Google Scholar]
- 19.French SL, Arthur JF, Tran HA, Hamilton JR, Approval of the first protease-activated receptor antagonist: Rationale, development, significance, and considerations of a novel anti-platelet agent. Blood Rev 29, 179–189 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Wong PC, Seiffert D, Bird JE, Watson CA, Bostwick JS, Giancarli M, Allegretto N, Hua J, Harden D, Guay J, Callejo M, Miller MM, Lawrence RM, Banville J, Guy J, Maxwell BD, Priestley ES, Marinier A, Wexler RR, Bouvier M, Gordon DA, Schumacher WA, Yang J, Blockade of protease-activated receptor-4 (PAR4) provides robust antithrombotic activity with low bleeding. Sci Transl Med 9, (2017). [DOI] [PubMed] [Google Scholar]
- 21.Shen B, Zhao X, O’Brien KA, Stojanovic-Terpo A, Delaney MK, Kim K, Cho J, Lam SC, Du X, A directional switch of integrin signalling and a new anti-thrombotic strategy. Nature 503, 131–135 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong H, Shen B, Flevaris P, Chow C, Lam SC, Voyno-Yasenetskaya TA, Kozasa T, Du X, G protein subunit Galpha13 binds to integrin alphaIIbbeta3 and mediates integrin “outside-in” signaling. Science 327, 340–343 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatt DL, Lincoff AM, Gibson CM, Stone GW, McNulty S, Montalescot G, Kleiman NS, Goodman SG, White HD, Mahaffey KW, Pollack CV Jr., Manoukian SV, Widimsky P, Chew DP, Cura F, Manukov I, Tousek F, Jafar MZ, Arneja J, Skerjanec S, Harrington RA, Investigators CP, Intravenous platelet blockade with cangrelor during PCI. N Engl J Med 361, 2330–2341 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Bhatt DL, Stone GW, Mahaffey KW, Gibson CM, Steg PG, Hamm CW, Price MJ, Leonardi S, Gallup D, Bramucci E, Radke PW, Widimsky P, Tousek F, Tauth J, Spriggs D, McLaurin BT, Angiolillo DJ, Genereux P, Liu T, Prats J, Todd M, Skerjanec S, White HD, Harrington RA, Investigators CP, Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med 368, 1303–1313 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Pang A, Cui Y, Chen Y, Cheng N, Delaney MK, Gu M, Stojanovic-Terpo A, Zhu C, Du X, Shear-induced integrin signaling in platelet phosphatidylserine exposure, microvesicle release, and coagulation. Blood 132, 533–543 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jasuja R, Furie B, Furie BC, Endothelium-derived but not platelet-derived protein disulfide isomerase is required for thrombus formation in vivo. Blood 116, 4665–4674 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivanciu L, Krishnaswamy S, Camire RM, New insights into the spatiotemporal localization of prothrombinase in vivo. Blood 124, 1705–1714 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z, Delaney MK, O’Brien KA, Du X, Signaling During Platelet Adhesion and Activation. Arterioscler Thromb Vasc Biol 30, 2341–2349 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Estevez B, Du X, New Concepts and Mechanisms of Platelet Activation Signaling. Physiology (Bethesda) 32, 162–177 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hynes RO, Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687. (2002). [DOI] [PubMed] [Google Scholar]
- 31.Yago T, Petrich BG, Zhang N, Liu Z, Shao B, Ginsberg MH, McEver RP, Blocking neutrophil integrin activation prevents ischemia-reperfusion injury. J Exp Med 212, 1267–1281 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin H, Stojanovic-Terpo A, Xu W, Corken A, Zakharov A, Qian F, Pavlovic S, Krbanjevic A, Lyubimov AV, Wang ZJ, Ware J, Du X, Role for platelet glycoprotein Ib-IX and effects of its inhibition in endotoxemia-induced thrombosis, thrombocytopenia, and mortality. Arterioscler Thromb Vasc Biol 33, 2529–2537 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen H, Banerjee AA, Mlynarska P, Hautman M, Hong S, Kapetanovic IM, Lyubimov AV, Liu Y, Enhanced oral bioavailability of a cancer preventive agent (SR13668) by employing polymeric nanoparticles with high drug loading. J Pharm Sci 101, 3877–3885 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Estevez B, Stojanovic-Terpo A, Delaney MK, O’Brien KA, Berndt MC, Ruan C, Du X, LIM kinase-1 selectively promotes glycoprotein Ib-IX-mediated TXA2 synthesis, platelet activation, and thrombosis. Blood 121, 4586–4594 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen B, Estevez B, Xu Z, Kreutz B, Karginov A, Bai Y, Qian F, Norifumi U, Mosher D, Du X, The interaction of Galpha13 with integrin beta1 mediates cell migration by dynamic regulation of RhoA. Mol Biol Cell 26, 3658–3670 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Brien KA, Stojanovic-Terpo A, Hay N, Du X, An important role for Akt3 in platelet activation and thrombosis. Blood 118, 4215–4223 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Estevez B, Kim K, Delaney MK, Stojanovic-Terpo A, Shen B, Ruan C, Cho J, Ruggeri ZM, Du X, Signaling-mediated cooperativity between glycoprotein Ib-IX and protease-activated receptors in thrombin-induced platelet activation. Blood 127, 626–636 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marjanovic JA, Li Z, Stojanovic A, Du X, Stimulatory roles of nitric-oxide synthase 3 and guanylyl cyclase in platelet activation. J Biol Chem 280, 37430–37438 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Brooks M, Catalfamo J, Buccal mucosa bleeding time is prolonged in canine models of primary hemostatic disorders. Thromb Haemost 70, 777–780 (1993). [PubMed] [Google Scholar]
- 40.Xu Z, Alloush J, Beck E, Weisleder N, A murine model of myocardial ischemia-reperfusion injury through ligation of the left anterior descending artery. J Vis Exp, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu L, Xu C, Huo X, Hao H, Wan Q, Chen H, Zhang X, Breyer RM, Huang Y, Cao X, Liu DP, FitzGerald GA, Wang M, The cyclooxygenase-1/mPGES-1/endothelial prostaglandin EP4 receptor pathway constrains myocardial ischemia-reperfusion injury. Nat Commun 10, 1888 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faul F, Erdfelder E, Buchner A, Lang AG, Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 41, 1149–1160 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Donovan AJ, Kalkowski J, Smith SA, Morrissey JH, Liu Y, Size-controlled synthesis of granular polyphosphate nanoparticles at physiologic salt concentrations for blood clotting. Biomacromolecules 15, 3976–3984 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Stability of M3mP6 HLPN during storage.
Fig. S2. Effect of M3mP6 peptide and HLPN on JonA and fibrinogen binding to platelets and platelet aggregation-induced by PAR4-agonist peptide (PAR4-AP).
Fig. S3. Uptake of fluorescently labeled M3mP6 HLPN into mouse platelets.
Fig. S4. The comparison of the effects of M3mP6 HLPN, scrambled control peptide (Scra) HLPN, AAA mutant peptide (Myr-FAAARL) HLPN and physiological saline solution on 7.5% FeCl3-induced carotid artery thrombosis.
Fig. S5. Dose response analysis of tail vein-injected M3mP6 HLPN in inhibiting occlusive thrombosis using the 7.5% FeCl3-induced carotid artery thrombosis model.
Fig. S6. Comparison of effects of 50% vs 36.8% M3mP6 HLPN on FeCl3-induced thrombosis.
Table S1. Summary of toxicological studies of M3mP6 HLPN.
Table S2: M3mP6 Peptide Pharmacokinetic Parameters
Movie S1. Laser-induced cremaster arteriolar thrombosis after saline injection as indicated by platelet (red) and fibrin (green) stains as described in the method.
Movie S2: Laser-induced cremaster arteriolar thrombosis 15 minutes after bolus injection of M3mP6 HLPN as indicated by platelet (red) and fibrin (green) stains.
Movie S3: Laser-induced cremaster arteriolar thrombosis immediately following injection of cangrelor as indicated by platelet (red) and fibrin (green) stains.


