Abstract
Virtually all adults with Down syndrome (DS) show neuropathological changes of Alzheimer disease (AD) by age 40 years. This association is due partially to overexpression of amyloid precursor protein, encoded by APP, owing to the location of this gene on chromosome 21. Amyloid-β (Aβ) accumulates in the brain across the lifespan of people with DS, which provides a unique opportunity to understand the temporal progression of AD and the epigenetic factors that contribute to the age of dementia onset. This age-dependency in the development of AD in DS can inform research into the presentation of AD in the general population, in whom a longitudinal perspective of the disease is not often available. Comparison of the risk profiles, biomarker profiles and genetic profiles of adults with DS with those of individuals with AD in the general population can help to determine common and distinct pathways as well as mechanisms underlying increased risk of dementia. This Review evaluates of similarities and differences between the pathological cascades and genetics underpinning DS and AD with the aim of providing a platform for common exploration of these disorders.
Introduction
In 1948 — 42 years after Alois Alzheimer gave his famous lecture about the condition that would bear his name — G. A. Jervis described three patients with Down syndrome (DS) who seemed to have a dementia course and neuropathological findings similar to those that Alzheimer previously described in a patient without DS1. Jervis confirmed the existence of many senile plaques in individuals with DS, in addition to neurofibrillary tangles (NFT) and extensive neuronal loss2,3. This remarkable association between DS and AD was supported by many subsequent publications. In 1988, Mann observed that patients with DS over the age of 40 years had high numbers of senile plaques and NFT, much the same as in AD within the general population 4(FIG. 1). Studies also showed a similarity in lesion distribution between individuals with DS and euploid individuals with AD. Today, we understand that the neuropathological changes of AD in DS are characterized by initial formation of senile plaques within the hippocampus and amygdala. NFTs develop in neurons that project to these areas.
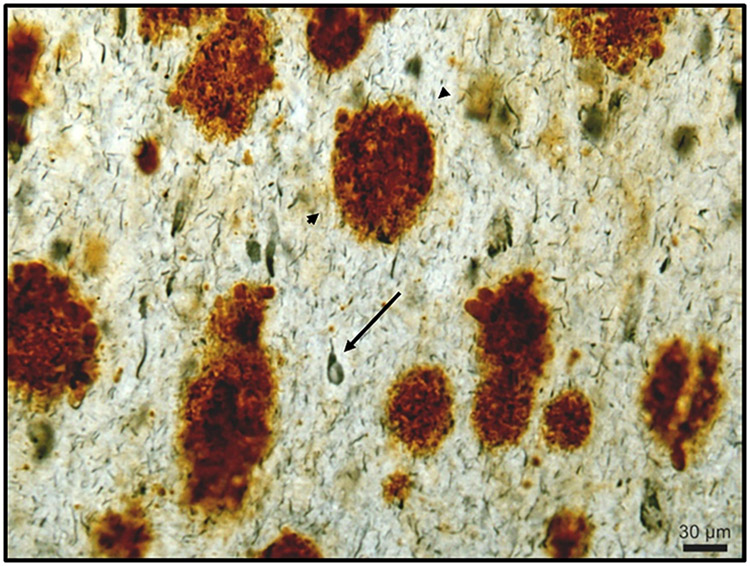
Figure 1 ∣. Amyloid plaques in Down syndrome.
Representative example of Aβ plaques (Aβ1-42; brown staining; arrowheads) and neurofibrillary tangles (PHF-1 antibody; blue staining; arrow) in the frontal cortex of a 46 year old person with DS and endstage AD.
The cloning of APP, the gene encoding amyloid precursor protein (APP), and characterization of its location on chromosome 21 were key factors in the development of the ‘amyloid cascade’ hypothesis, which defines much of the current research in DS and AD 5-7. Interestingly, the original description of amyloid-β (Aβ) was in DS 8 and was one of the observations that led to the formulation of the Aβ hypothesis. APP overexpression and the exponential accumulation of Aβ in the brain is a primary driver of dementia in individuals with DS9. Soluble forms of oligomeric Aβ are thought by some researchers to be the primary noxious species in senile plaques and injure neurons through apoptosis, cytoskeletal disruption and synaptic loss10,11. However, APP is not the only factor driving the pathological features of AD.
The fields of DS and AD research have many points of synergy including genetics, pathogenesis, clinical manifestations and opportunity for translational research. The study of DS affords an opportunity to understand the timing and sequence of pathological changes associated with AD, partially due to the lifelong accumulation of Aβ in individuals with DS. The objective of this Review is to highlight observations in DS that might be informative to researchers of AD in the general population. We address areas such as the relationship of oestrogen receptor genes to the cumulative incidence of dementia; the bidirectional role of apolipoprotein E (APOE) in AD pathogenesis; the common mechanistic features underlying disease in individuals with APP duplication (dup-APP) and individuals with AD in DS; candidate gene analysis and epigenetic observations; lessons from rare patients with partial trisomy 21; amyloid imaging and vulnerability for dementia; the role of chronic amyloid angiopathy in DS; biomarkers such as exosomes and inflammatory markers; and the role of seizures and other comorbidities in DS and AD. Ultimately, we seek to update information on the common pathways and common goals between DS and AD research, particularly in regard to the age-dependency of AD neuropathology, progression and early signs.
Epidemiology of AD in DS
Prevalence
Before 1954, people with DS generally did not survive beyond the age of 30 years, owing to high neonatal mortality and a failure to treat intercurrent disease in childhood. Over the past 30 years, increased attention to treatable medical disorders in DS has doubled life expectancy12. A child with DS born today can expect to live into their 60s and often beyond.
Within the lengthened lifespan of individuals with DS, the prevalence of AD has shown a striking age-dependency. A 2016 study reported the prevalence of progressive cognitive impairment to be 55% in individuals with DS aged 40–49 years and up to 77% in individuals age 60–69 years13. Other studies have shown that the risk of dementia is approximately 23% at 50 years, 45% at age 55 years, and 88% or more at 65 years 14-17. Several factors might confound the calculation of dementia prevalence in DS. One is whether an elderly person with DS lives in an institution or in a community residence: diagnosis of dementia within an institution can be challenging owing to a lack of expertise and the presence of severe intellectual disability in this setting 18. Another confounding factor is the process of physiological ageing, wherein cognitive decline can occur due to factors other than AD 19,20 .
The question arises as to whether protective factors might forestall or prevent dementia in some people with DS. Understanding the variables that affect dementia onset in people with DS affords an opportunity to recognize which factors might be protective or aggravating in the onset of cognitive decline, not only in DS but also in AD in the general population.
Apolipoprotein E
Evidence suggests that the APOE*ε4 allele increases the risk of AD and overall mortality in people with DS, albeit to a lesser extent than in AD in the general population. 21-24. 25 The physiological mechanisms by which the genetic risk of AD is increased by APOE*ε4 are not fully elucidated but a bidirectional link seems to exist between lipid metabolism, APOE status and the processing of amyloid26,27. APOE can be proteolytically cleaved to form a truncated amino terminal fragment, which becomes associated with NFTs and might be a driver in AD pathogenesis 28. In DS, APOE that has undergone proteolytic cleavage and fragmentation is also closely associated with the NFTs, similar to observations in AD in the general population29,30. Further study of the dynamics of APOE function in DS and AD in the general population is likely to inform our understanding of pathogenesis in both conditions.
Menopause and oestrogen
Research over the past 20 years has indicated that the high vulnerability of women in the general population to AD is linked to the loss of ovarian hormones during and after menopause31. The oestrogen receptor β is involved in the development of AD in the general population and might also be a potential target for therapeutic interventions32. Oestrogen use is associated with decreased cognitive decline in APOE*ε4 negative women but not in APOE*ε4 positive women 32. However, some studies are contradictory; for instance, a large study of post-menopausal women found that hormonal replacement did not result in cognitive improvement 33. Additional models are needed to facilitate the study of the effect of oestrogen on neurodegeneration 34. DS might serve as one of these models. Women with DS typically experience menopause between 44–46 years of age compared with an average of 51 years of age in the general population 35. Cognitive function in postmenopausal women with DS is worse than in men with DS at comparable ages 36, possibly owing to reduced bioavailability of oestrogen in these women. Polymorphisms in genes associated with the oestrogen receptor and oestrogen biosynthesis can affect endogenous oestrogen levels and seem to influence age of dementia onset as well as the cumulative incidence of dementia. The short time period between menopause and cognitive decline in women with DS suggests that this population might be well suited for future clinical trials of oestrogen-replacement therapies for AD. This interval between menopause and cognitive decline is longer in euploid patients with AD 37 and therefore longer clinical trials would be required in the general population than in people with DS.
Genetic epidemiology
Most individuals with DS (90–95%) have trisomy of chromosome 21 with translocation occurring in 2–4% and mosaicism in 2–4%38. The percentage of mosaic cells in individuals with DS seems to be tissue specific and changes with age. Mosaicism for chromosome 21 can present with developmental delay and early-onset dementia but without the physical features of DS39. Mosaic aneuploidy of chromosome 21 in brain and peripheral cells has been argued to underlie neurodegeneration in multiple forms of AD 40. Post-mitotic somatic mutations that result in mosaicism can emerge that present a risk factor for AD both in individuals with DS and in the general population, which suggests a common area between these entities that could be investigated in future research 41.
Some individuals develop AD as a result of duplication of a small region of chromosome 21 that includes APP (dup-APP). Pathogenic mechanisms in these individuals might parallel those in individuals who have DS with AD 42. An additional copy of APP is present in both dup-APP and DS with AD, in contrast to conditions in which other genes such as PSEN1 or PSEN2 are mutated wherein the processing of APP is altered independently of gene copy number. Dup-APP shares some common traits with DS including early age of dementia onset (mean age 52 years for dup-APP), AD neuropathology 43,44 and an increased prevalence of cerebral amyloid angiopathy (CAA)45. Phenotypic features of DS do not seem to occur in people with dup-APP 46. Although almost all people with DS have AD neuropathology, the variability in the prevalence of dementia is more marked in DS than in dup-APP, whereas CAA is less prevalent in DS than in dup-APP. These phenotypic differences between DS and dup-APP provide a platform for further understanding the roles that genes on chromosome 21 other than APP might have in AD pathogenesis.
Genome-wide evaluation of AD in DS
Genetic mechanisms in AD and DS have been reviewed extensively elsewhere42. To date, only one genome-wide association study (GWAS) has been reported in DS, which aimed to determine which genes influence age of dementia onset in DS47. Despite the small sample size of this study (n = 67), single nucleotide polymorphisms (SNPs) in PICALM were found to be associated with early onset of dementia in individuals with DS, consistent with previous observations that PICALM is associated with increased risk of AD in the general population. Candidate gene analysis is one approach that can help to explain the relationship between AD and DS — not only for chromosome 21 but also for other chromosomes. In a cohort of 320 individuals with DS (aged 30–78 years), SNPs were adjusted for age, sex, race, level of intellectual disability and APOE status 48. Multiple SNPs in APP, CST3 and MARK4 were identified that were associated with AD in DS, potentially highlighting pathways involved with Aβ, lipid metabolism and tau. An examination of measures of Aβ from this study showed an association between Aβ42 levels, Aβ40 levels or Aβ42:40 ratio and variants in genes linked to the processing of APP (such as CALHM1 and IDE), vesicular trafficking and oxidative stress49. These studies further highlight some fundamental biological similarities between dementia in DS and AD in the general population, and suggest that APP processing genes might contribute to both conditions.
The field of epigenetics evaluates the factors (environmental or otherwise) that influence gene expression. Post-GWASs show how SNPs affect levels of methylation in nearby and distant chromosomal loci50 and thereby affect phenotypic expression. Gene expression in DS can vary in relation to baseline levels of intellectual function51. Epigenetic mechanisms that remodel chromatin transcription include DNA methylation, post-translational histone modifications, nucleosomal positioning, histone variant incorporation and the action of small and long noncoding RNAs 52. Individuals with DS have an epigenetic signature (consisting of a characteristic pattern of DNA methylation in white blood cells) that includes modifications to genes on chromosome 21 and that also shows an ageing phenotype, suggesting a link between developmental defects and disease phenotype 53. A quantitative molecular marker has demonstrated that the ‘epigenetic clock’ (that is, estimation of molecular age based on DNA methylation) confers accelerated ageing in blood and brain tissue in people with DS 54. In addition, a brain tissue study found that a type of histone modification (acetylation of H4K16) occurs in the brains of individuals with AD in the general population, but not in control groups of young or old individuals with normal ageing parameters 55. Epigenetic changes in AD and DS present a potential target for future therapeutic interventions, as they occur before the formation of mature senile plaques and NFTs and are mediated by enzymes that can be inhibited or enhanced 56.
Neuropathogenesis
People with DS have differences in brain structure development that might also exacerbate vulnerability to AD compared with euploid individuals. Studies show that the neural phenotype of DS begins in utero 57. Developmental neuropathological aberrancies include disruptions in neurogenesis, synaptogenesis and myelination 58. As early as 21 weeks of gestation, cell numbers are reduced in the hippocampus and surrounding cortical regions in people with DS compared with euploid individuals59. In DS, fetal neurons overexpress APP, which causes a disruption in axonal guidance within the brain 60. However, APP might also have a trophic role linked to neuronal survival, outgrowth and repair in early brain development 61. The consequences of APP gain-in-function in early brain development in DS is unclear and requires additional research.
A reduction in the number of neuronal cells during brain development in DS compared with typical brain development might underlie findings in neuroimaging studies in DS, which indicate that the brain has a smaller frontal cortex, occipital cortex and cerebellum compared to typically developing brains at a commensurate age 58,62 (FIG. 2). These anatomic discrepancies become more pronounced with age 62-64 . Cortical regions and the hippocampus show increasing levels of atrophy with age 62. White matter integrity in the frontal cortex is reduced in individuals with DS compared with controls, and this abnormality is further compromised with age 65.
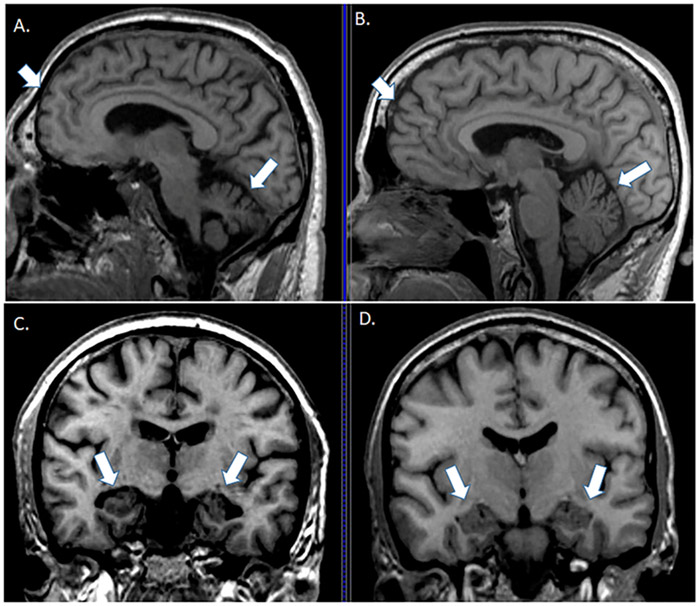
Figure 2 ∣. Brain structural changes in Down syndrome.
Structural differences shown in coronal and sagittal sections from an MRI between a 46 year old person with DS without dementia (parts A and C ) and a person without DS ( parts b and d) highlighting structural differences in frontal cortex (arrowhead in parts a and b), cerebellum (arrow in parts a and b) and hippocampus (arrow in parts c and d).
Role of APP in AD pathogenesis in DS
APP is overexpressed in DS with full trisomy 21 66. Age-dependency, temporal events and the postnatal modifications of Aβ have been discussed in depth elsewhere67-71. To summarize, autopsy studies indicate that Aβ deposition can occur in very young people with DS 69,72,73 but seems to be observed systematically after 30 years of age — still decades earlier than that observed in AD within the general population. Aβ initially appears in diffuse deposits that progress to compact neuritic plaques with increasing age. After age 40 years in individuals with DS, the accumulation of brain amyloid is not linear but exponential, which suggests that disease development enters a phase of acceleration at this age74,75. People with DS show a similar early age of onset of Aβ pathology compared to familial forms of AD such as dup-APP or autosomal dominant early onset AD 42,76. Aβ also accumulates around the cerebral vasculature in DS as AD develops, as occurs in sporadic AD.
Case studies of people with DS who have partial trisomy 21 have provided exciting insights into the major role that APP overexpression has in AD pathogenesis. One study described a woman who had partial trisomy 21 but was disomic for APP, who was mildly impaired intellectually and lived to age 78 years without dementia, plaques or NFTs 77. Another study described a 72 year old man with partial trisomy 21, also disomic for APP, who had mild intellectual impairment, no neuropsychological evidence of dementia over 7 years of testing, dramatically low plasma levels of Aβ, no significant amyloid uptake on 2 consecutive PET scans and no evidence of AD at autopsy78. These rare cases of partial trisomy 21 point to the importance of APP as a key driver of dementia in DS.
Our understanding of how age and cognition are associated with Aβ neuropathology has been accelerated by the development of ligands that bind to Aβ in vivo and can be visualized with PET 79-82. Pittsburgh Compound B (PiB) 83, the first of these Aβ ligands, has now been used in a large number of clinical studies in patients with AD and can detect Aβ accumulation early in the disease 80. PiB binding in DS shows a similar cortical distribution to that observed in AD in the general population, with the exception of the striatum (FIG. 3). Interestingly, PiB binding suggests that the striatum is the earliest site of Aβ neuropathology in DS, with PiB signal typically observed after 35 years of age 84-86. These findings are similar to reports of amyloid deposition in patients with presenilin-1 mutations, which suggests that enhanced Aβ production leads to a common phenotype in both conditions 87-89. Striatal PiB binding might reflect diffuse Aβ accumulation as fibrillar plaques are rarely observed in these brain areas even in elderly individuals with DS90. PiB binding in DS increases with age 84-86,91,92, and is increased in people with cognitive decline 85,91. PiB signal in individuals with DS suggests that Aβ neuropathology progressively spreads to additional cortical regions with age85,93 and correlates to hypometabolism, as detected by fludeoxyglucose (FDG)-PET 94. After appearing in the striatum, reports of PiB signal suggest that amyloid progressively involves different cortical areas, first affecting the rostral prefrontal and cingulo-parietal cortices, then caudal frontal, rostral temporal, primary sensorimotor and occipital cortices and finally parahippocampal cortex, thalamus and amygdala, in a similar pattern to that observed in AD in the general population83,85. Similar results have been reported in PET imaging with different Aβ ligands, such as florbetapir 95-97 and 18F-FDDNP 98. However, neither florbetapir nor 18F-FDDNP show early striatal binding as reported with PiB, which suggests that either the ligands have different affinity for different types of Aβ or that PiB binding might indicate additional neuropathologies. Thus, amyloid imaging studies suggest that DS includes features of Aβ accumulation that are consistent with both sporadic AD in the general population (in which cortical Aβ accumulation is observed) and familial AD (in which striatal Aβ accumulation is observed). In the future, beneficial interventions that target Aβ in DS might inform clinical trials that target Aβ both in sporadic and familial AD. Thus, future collaboration between DS and AD research might focus on the temporal dynamics of striatal and cortical amyloid accumulation in DS and links to clinical changes to provide insights into when to target Aβ accumulation associated with overproduction and reduced clearance typical of sporadic and familial AD.
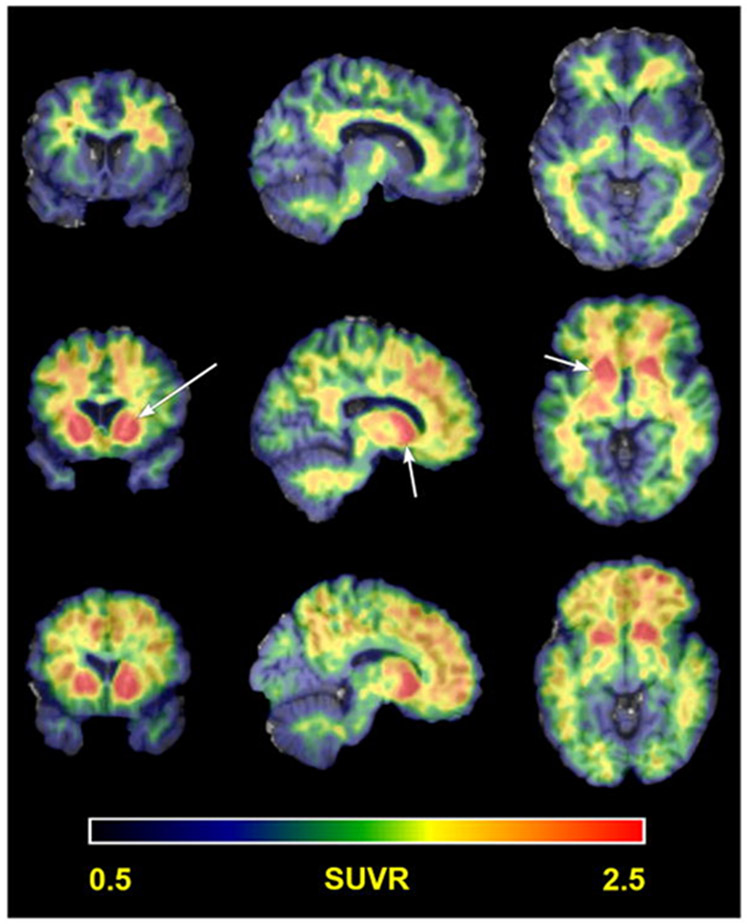
Figure 3 ∣. Amyloid PET in Down syndrome.
Representative examples of Pittsburgh Compound B (PiB) PET neuroimaging for amyloid in people with DS. The pattern of PiB binding highlights striatal PiB uptake (arrows). Three patterns of PiB update are highlighted in this figure from Lao and colleagues (2016)86 showing nonspecific white matter binding in the top row, striatal only PiB uptake in the middle row and striatal with cortical PiB update on the bottom row. SUVR, standard uptake value ratio.
Tau in DS
Tau phosphorylation and aggregation are evident in the brains of individuals with DS and can be found as NFTs and neuropil threads or dystrophic neurites around Aβ plaques. Early tau pathological changes are observed in the outer molecular layer of hippocampus in middle-aged (30–40 years of age) adults with DS 99, with subsequent findings of NFTs in the hippocampal CA1 region and subiculum, and neuronal loss in the entorhinal cortex 100-103. In general, although NFTs seem to follow a similar distribution pattern in DS and in AD — starting in the entorhinal cortex and spreading to hippocampus and then to the neocortex — a higher density of NFTs are observed in DS brain than in AD brain 100. However, large and systematic studies are needed to determine whether the pattern of regions affected with disease progression of NFT accumulation parallels that observed in AD in the general population. Several genes on chromosome 21 might also contribute to acceleration of the early onset of NFT pathology in DS104, including DYRK1A and RCAN1. DYRK1A is a dual specificity kinase that phosphorylates tau protein, facilitating GSK3β phosphorylation. In addition, DYRK1A phosphorylates alternate splicing factors, leading to an increased ratio of 3-repeat to 4-repeat tau that is associated with neurodegeneration105-110. Unsurprisingly, the number NFTs that are positive for DYRK1A and/or 3-repeat tau is increased in brains from middle-aged and elderly adults with DS compared to brains from individuals with AD in the general population110. RCAN1 (Calcipressin-1) can act as a facilitator or inhibitor of calcineurin, a serine–threonine protein phosphatase that activates NFAT (Nuclear Factor of Activated T-cells) leading to transcriptional activation 111,112. RCAN1 can also activate GSK-3, an enzyme that phosphorylates tau 113 and might also lead to enhanced neurofibrillary tangle formation in DS.
Our understanding of the role of tau in AD pathogenesis in people with DS is likely to be enhanced by biomarker studies that measure CSF and/or plasma tau levels and neuroimaging studies that use tau ligands to detect tau in the brain. The few studies available show that levels of total tau and phosphorylated tau, reflecting neurofibrillary tangle formation in the brain, are increased in CSF from individuals with DS compared to controls 104,114. Plasma levels of tau increase with age in DS 115 and were found to be associated with cognitive dysfunction in one study 116 but not another 115. Studies that measure levels of tau from neuronally derived exosomes isolated from plasma show higher levels of tau in DS than in controls 117. In the first study of DS using the tau PET ligand 18F-AV-1451, tau binding was found to occur in people with DS who were amyloid positive (as detected by PiB or florbetapir) but not in those who were amyloid negative, and high tau binding was associated with poor cognition 118. Overall, additional information about NFTs and tau in DS is clearly needed to establish when changes in tau pathology occur in relation to age in people with DS, whether tau pathology correlates with changes in cognition (or possibly precedes these changes) and with conversion to overt dementia. As NFT pathology increases with age in individuals with DS, future research could facilitate understanding of the mechanistic links between Aβ, tau and potential facilitators of tau expression and phosphorylation such as DYRK1A and RCAN1.
Neuroinflammation
Neuroinflammation is a crucial contributor to many neurodegenerative disorders and includes both pro-inflammatory and anti-inflammatory pathways. Yet the role of neuroinflammation in the pathogenesis of AD is relatively unexplored in DS 119,120. The primary mediators of the neuroinflammatory response in the brain are microglial cells 121. Microglial cells show both morphological and pathological changes in people with DS who are over the age of 40 years, including decreased numbers of microglial cells with a resting state morphology and increased numbers of dystrophic microglial cells 122.
GWASs show that genes involved in inflammation such as TREM2 and CD33 are risk factors for AD 123,124. In early studies of neuroinflammation in DS, increased S100β and IL-1β expression was detected in astrocytes 125. S100B, the gene encoding S100β, is on the 21st chromosome. Complement C1q activation, which reflects the activation of the complement pathway that mediates immune functions, seems to be highly prevalent in individuals with DS after 29 years of age 73,126. The brains of people with DS seem to have a unique neuroinflammatory phenotype that differs from that of AD in the general population and is consistent with immune activation due to invasion of serum proteins in the brain 127. The extravasation of serum proteins into brain might be due to the presence of microbleeds in the brains of people with DS, as has been visualized by neuroimaging or at autopsy (described further in the next section). These findings might be relevant for future immunotherapies targeting Aβ or tau, given that immunological approaches might lead to adverse effects in the presence of already heightened inflammation in the brains of people with DS.
Plasma markers of inflammation might serve as biomarkers for the development of dementia in DS, although peripheral drivers of inflammation (for example, periodontal disease 128) might also contribute to changes in the levels of these markers. Increased serum levels of inflammatory cytokines — including IL-1β, IL-6, TNF-α and IFN-γ — have been detected in individuals with DS 129. In addition, levels of TNF-α, IL-6 and IL-10 were found to be increased in plasma in people with DS compared with euploid individuals, and the levels of these cytokines are further increased in individuals who have DS with dementia 130. The unique inflammatory phenotype observed in the DS brain might provide novel insights into the role of different cytokines or chemokines in AD progression, which might present therapeutic targets and provide novel insights into AD in the general population. However, this area would benefit from additional research to clarify the similarities and important differences in DS-AD compared with AD in individuals without DS.
Cerebrovascular Neuropathology
Cerebrovascular pathology, which occurs in up to 45% of the general population, is now recognized to lower the age of dementia onset and to increase the rate of disease progression131,132. Cerebrovascular disease has been hypothesised to act as a ‘second hit’ necessary for inducing dementia, particularly in combination with a ‘first hit’ of a substantial Aβ burden in the brain 133.
CAA is defined as the deposition of amyloid in the walls of medium-sized and small-sized leptomeningeal and cortical arteries, arterioles and, less frequently, capillaries and veins. CAA can lead to microhaemorrhages and macrohaemorrhages 134 and is observed in elderly individuals with DS (>55 years of age) 135-137. In a 2017 study, we showed a strikingly higher frequency and severity of CAA in DS at autopsy than in AD without DS, and that CAA accumulation increased with age in DS138 (FIG. 4). However, whether the CAA in DS brain leads to more frequent haemorrhage in DS compared with sporadic AD is unknown 135,136. This topic presents an obvious area for future research.
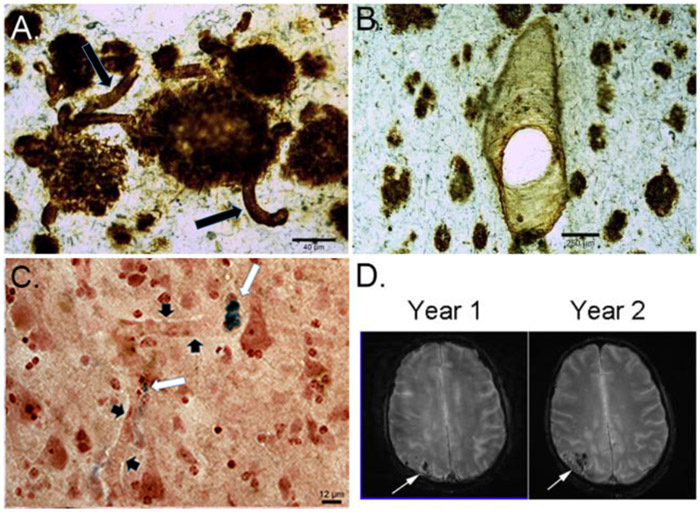
Figure 4 ∣. Cerebrovascular pathology in Down syndrome.
a ∣ Aβ1-42 immunolabeling in a 67 year old man with DS defines plaques clearly but also shows substantial accumulation on blood vessel walls (arrows). b ∣ Aβ1-40 labeling clearly defines vascular pathology but plaque labeling is less than that observed with Aβ1-42. c ∣ panel shows the possible consequences of CAA in a 58 year old man with DS — microhemorrhages, illustrated with a Prussian blue stain as deposits (white arrows) that are blue adjacent to blood vessels (black arrowheads). d ∣ cerebrovascular pathology identified by T2* MR imaging in a 60 year old man. Progressive worsening of bleeds can be seen, particularly in the occipital cortex (arrows). Modified with permission from Wilcock et al., 2016 237.
People with DS are protected from atherosclerosis and hypertension. In a study of 70 adults with DS aged 40–66 years, atheroma was completely absent, in contrast to similarly aged adults without DS139. Furthermore, young adults with DS aged 13–42 years seem to have low blood pressure compared with age matched controls 139 and a study of 86 people with DS from 18–56 years found no systematic increase in blood pressure (i.e. hypertension) with increasing age 140. By studying the brains of people with DS, who exhibit a high incidence and severity of CAA , we can determine the role of CAA in driving cerebrovascular dysfunction and cognitive decline in AD within the general population in a cohort uncomplicated by hypertension and atherosclerosis.
Neuroimaging adds to our understanding of the contribution of cerebrovascular disease to dementia in DS and AD in the general population. For example, characterization of microbleeds by MRI (using T2* or susceptibility-weighted imaging) in individuals with DS 7,62 has shown frequent CAA that increases with dementia76. FDG-PET shows that glucose hypometabolism correlates with the loss of cerebral blood flow in the temporal lobes that is observed with increasing age and dementia in DS95,97,141. Interestingly, the reduction in FDG-PET signal in DS involves the same areas that show a compensatory increase in FDG values in individuals with DS just prior to dementia , which suggests that compensatory responses might occur prior to the loss of function associated with dementia 142. FDG-PET seems to be a valid biomarker for prediction of conversion to dementia in individuals with AD in the general population143, and offers a common platform for studying dementia in DS and in AD in the general population. The study of AD in DS might lead to the identification of unique protective factors for cerebrovascular pathology that could be leveraged in clinical trials for AD in the general population. Furthermore, the study of autopsy tissue from individuals with DS — a population not complicated by atherosclerosis and hypertension — provides exciting opportunities to understand the mechanisms and consequences of CAA.
Other contributors to AD pathogenesis
Here we briefly summarize other potential pathways of AD pathogenesis in DS, which have been reviewed in depth elsewhere. Oxidative damage is consistently observed in DS, beginning at a young age and becoming exacerbated with ageing and AD progression 75,144-147. Other relevant pathways include the autophagy and endosomal systems 148, white matter degeneration 104, neurotransmitter losses 149, neuron loss 150, dysregulation of synaptic protein expression151, consequences of increased gene expression (such as DYRK1A and SYNJ1)152, telomere shortening 153 and losses in neurotrophic factors 104,154-157. All of these pathways and mechanisms that are compromised in DS and that contribute to AD pathogenesis are viable targets for treatment and are also compromised in AD in the general population.
Whether individuals with DS age prematurely presents an intriguing question. Ample evidence certainly shows that markers of oxidative stress are increased in AD as well as DS 158,159 and some of these measures are associated with cognitive decline of the AD type 146. Molecular mechanisms responsible for mitochondrial damage and energy deficits have been found to occur in DS 160. An epigenetic signature is present in DS that is similar to that of premature aging syndromes such as Werner syndrome 53. However, although chronic medical conditions are similar between elderly adults in the general population and young individuals with DS 161, atherosclerotic complications are rare in DS 162, which suggests that this common complication of ageing is lessened in trisomy 21. Further study of commonalities and differences in biological ageing between DS and AD in the general population promises to be illuminating with regard to mitochondria as a potential target for dementia prevention or therapy 163. Overall, however, whether DS represents premature aging or possibly accelerated aging is still debated.
Clinical evaluation
Dementia
Variability of baseline cognitive function in DS makes the diagnosis of dementia challenging. Because of the pathological changes of AD and the age-related prevalence of cognitive decline, the time of transition to dementia can seem arbitrary 13. Indeed, expert clinical judgement of an individual patient with DS might be a more accurate way to diagnose dementia than with International Classification of Disease 10th revision (ICD-10) or Diagnostic and Statistical Manual of Mental disorders 4th edition text revision (DSM-IV-TR) criteria 164. The diagnosis of dementia in DS uses the consensus approach to diagnosis typically used for sporadic AD as a guideline 165, and is dependent on the clinical examination, neuropsychological tests and biomarkers. Single tests usually fall short in assessing the breadth of different cognitive function present in individuals with DS and, as a consequence, a battery of neuropsychological measures is required 166. An ideal battery for testing cognition in DS should measure a wide range of skills, including both strengths and weaknesses. The testing protocol must meet standards of reliability, statistical evaluation, sensitivity, reproducibility and applicability across a wide range of ages. Brain areas that develop later in children with DS than in euploid individuals, such as the hippocampus and prefrontal cortices, seem to be those most susceptible to neurodegeneration in dementia and thus might show early cognitive changes associated with the onset of dementia 167. Difficulties in executive function that reflect frontal lobe dysfunction occur in the preclinical stages of dementia in DS 168,169. This area has also been an important focus in a comprehensive battery test for AD in the general population170,171. The temporal changes in executive function seem to be a common area for comparative studies in DS and AD in the general population.
Mild cognitive impairment (MCI) can be defined as a decline in cognition that reflects an intermediate state between typical brain ageing and dementia. A number of test batteries have been developed to define MCI in adults with DS 13,164,168,171-173. Although no consensus has been reached for a ‘gold standard’ to diagnose MCI in DS, longitudinal observations, monitoring of behavioural changes and domain-specific tests of memory and executive functioning will be at the core of the battery. The domains identified in neuropsychological testing of MCI in the general population are relevant to DS, including general intellectual function, attention, processing speed, language, visuospatial skills, learning, memory and executive function174. Neuropsychiatric symptoms occur in up to 97% of individuals with AD in the general population, with psychosis, agitation, apathy, depression and sleep disturbances representing the most common symptoms 175. These symptoms overlap almost completely with those seen in DS and dementia 176. Individuals with DS and individuals with AD in the general population show similar cognitive profiles on the Severe Impairment Battery test, suggesting that parallel cognitive changes take place in these two groups177. A caveat to diagnosis of psychosis in DS is the prevalence of ‘self-talk’ — private speech in which the individual seems to be talking to imaginary persons. Private speech is seen at an early age and proceeds into adult life, and is often adaptive and not reflective of a systematic disturbance in thinking 178. Although 4–12% of patients with MCI in the general population progress to dementia within any given year 179, up to 53% can improve or revert to normal status 180. Individual variation in gene expression and epigenetic factors might account for similar variability in MCI in DS 181. .
Gait
Disturbances of gait are a common feature of dementia progression in DS and in AD in the general population 182,183. This commonality suggests that shared pathological mechanisms might exist between these groups. In the general population, individuals with AD show a slowing of gait and increased stride variability 184,185. Children with DS show inefficient gait strategies for obstacle crossing 186, and this problem becomes more marked in the early stages of dementia. Taken together, the gait problems in DS and AD can be viewed as a motor dyspraxia, defined in this setting as an inability to use the legs in the absence of other neurological or vascular causes. Gait dyspraxia seems to be associated with brain areas that are important for sensorimotor integration, including the hippocampus and white matter tracts of the frontal and parietal lobes 187.
Seizures
Individuals with DS have a bimodal occurrence of seizures 188. In individuals younger than 1 year of age, infantile spasms are seen along with generalized seizures. Following this first peak of seizure incidence, a second peak begins after the third decade of life and can herald the onset of dementia. In a prospective study of individuals with DS and dementia, as many as 84% developed seizures 136. The presentation of seizures in DS is associated with increased psychiatric co-morbidities 189. Brain tissue studies in individuals with DS and data from a mouse model of DS suggest an imbalance in the excitatory and inhibitory functions associated with the GABAergic system 190. In turn, this change might alter the ratio between excitation and inhibition of neurons and thus propagate epileptic impulses 190. As dementia progresses, it is not unusual for patients with DS to develop myoclonic epilepsy with myoclonic jerks time-locked to EEG abnormalities, particularly upon awakening 191. Interestingly, mutations in CSTB (encoding cystatin B), which is located on chromosome 21, produce senile myoclonic epilepsy as part of the Unverricht–Lundborg syndrome 192. Seizures seem to augment cognitive decline in adults with DS and dementia 193.
Non-motor complex seizures are the predominant presentation of epileptic activity in AD in the general population194. Neuronal excitation associated with the epileptic impulses propagates tau pathology 195 as do perturbations in GABAergic, glutamatergic and cholinergic networks. Long term EEG monitoring in patients with MCI or AD in the general population has shown a high prevalence of subclinical epileptiform discharges 196 particularly around the frontal and temporal lobes and in sleep. Seizure prevalence is increased in individuals with dup-APP or mutations in presenilin, with myoclonic manifestations observed in the latter population197. As in DS, epileptiform activity results in a deleterious cognitive outcome in individuals with dup-APP or mutations in presenilin, 198probably owing to defective remodelling of neuronal circuitry in the hippocampus. 197,199 In a mouse model of AD, elevated levels of APP and Aβ caused neuronal hyperexcitability and made these animals more susceptible to becoming epileptic 194,200. In mouse models for DS, trisomy for APP disrupts retrograde transport of nerve growth factor and the morphology of basal forebrain cholinergic neurons 201. Whether these changes in the DS mouse cause similar electrophysiological changes to those observed in the AD transgenic mouse is a potential subject for future research.
Sleep disorders
Sleep disorders are common both in DS and in AD in the general population. Certain facial features that are a part of the physical phenotype in DS (such as midfacial hypoplasia and a small oro-pharynx) make obstructive sleep apnoea syndrome (OSAS) more common than in typically developing children 202,203. OSAS has been associated with depression 204 and seems to affect cognition in children with DS due to disruption of verbal learning and executive functioning 205. Insomnia in young adults with DS is linked to a disintegrative disorder characterized by cognitive decline to a dementia-type state and autistic regression, the cause of which is unknown 206. Sleep fragmentation and resulting hypoxaemia across the lifespan in DS has been purported to increase deposition of Aβ in brain, potentially leading to AD207. Sleep might facilitate amyloid clearance from the brain, and disordered sleep might impair amyloid clearance208. Considerable correlations exist between measures of poor sleep, cortical Aβ burden and measures of phosphorylated tau in patients with MCI or AD in the general population 209,210.
Thyroid dysfunction and cognition
Hypothyroidism occurs in more than one-third of children with DS 211 with hyperthyroidism also found in a small proportion of individuals (<3%)212. In addition, the prevalence of autoimmune thyroiditis is increased in DS compared with the general population213. Such alterations in cellular and immunological responses are partly related to increased expression of AIRE (encoding autoimmune regulator) on chromosome 21, which results thyroid dysfunction and other immune manifestations 214. All parameters of B-cell development seem to be altered in DS 215, which can result in increased susceptibility to infections and thyroiditis. Maintenance of a euthyroid status is important for myelination and white matter integrity early in brain development in DS. However, in contrast to DS, AD in the general population is more often associated with hyperthyroidism than hypothyroidism 216. The biological mechanisms underlying the association between thyroid function and cognition in AD is as yet unknown and it is unclear whether AD neuropathology causes thyroid dysfunction or whether thyroid dysfunction can exacerbate AD neuropathology and progression 217.
Healthcare guidelines
Multimorbidity for general health problems seems to be similar for adults with intellectual disabilities with or without DS 218,219. The most prevalent co-morbidities are visual impairment, obesity, epilepsy, constipation and gait disorders related to orthopaedic conditions. Unidentified comorbidities and failure to recognize the behavioural effects of pharmacological medications might confound the diagnosis of dementia in DS. Primary care education and ongoing surveillance of healthcare guideline compliance seems to improve quality of life in adults with DS 220,221, thus simplifying the differential diagnosis of dementia. Current healthcare guidelines for co-occurring medical conditions in adults with DS are in the process of being updates on the basis of data from meta-analyses222.
Clinical trials and translational research
A 2015 literature-based review of randomized clinical trials of anti-dementia medications for adults with DS indicated that insufficient data exist to evaluate the therapeutic effects of acetylcholinesterases, memantine, simvastatin, antioxidants, and L-carnitine in this population223. Although most of the existing studies of anti-dementia therapies in DS were considered to be well conducted, the small number of participants and the outcome methodology employed did not enable results from different trials to be combined. Consequently, larger trials conducted over longer periods of time and that use uniform criteria are needed. In addition, if preventative interventions (as opposed to symptomatic therapy) are to be tested, then biomarkers will be necessary that act as proxy indicators for disease progression 224. This point is also true for AD in the general population in those individuals who have a slow disease onset that allows consideration of preventative therapies 225.
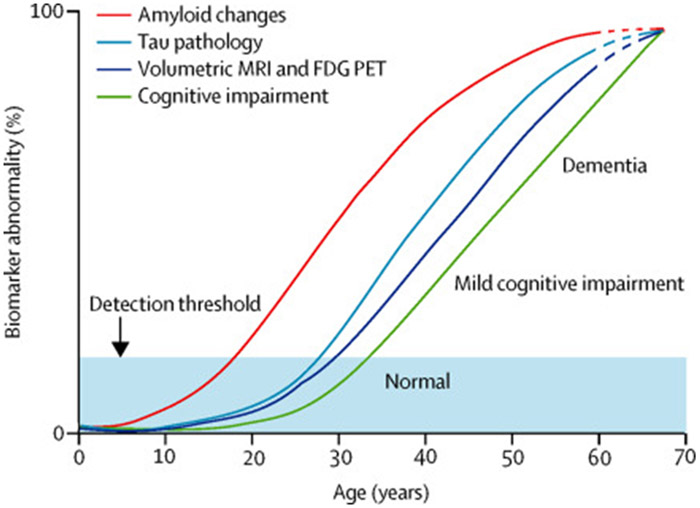
DS might serve as a model for early intervention in AD in the general population. Indeed, an advantage of designing and testing prevention approaches for AD in DS is that virtually all people with DS have full blown AD pathology by 40 years of age. Ballard and colleagues assembled findings of changes in Aβ, tau, volumetric MRI, FDG-PET and cognitive impairment over the lifecourse into a model of biomarker changes in relation to age in AD (FIG. 5). Small sample sizes and short trials are feasible in individuals with DS and dementia compared with AD in the general population. Such trials could be an important first step for the design of prevention studies in AD in the general population. Indeed, it might be possible to identify the temporal progression of many different AD-associated pathological mechanisms such as Aβ, tau, inflammation, cerebrovascular pathology, and others as a function of age in individuals with DS, as suggested hypothetically in FIG 6. Understanding AD pathogenesis as a function of age in DS could enable identification of early molecular abnormalities that can be targeted for interventions both in DS and in AD in the general population.
Figure 5 ∣. Hypothetical model of biomarker and clinical outcomes.
Ballard and colleagues developed a hypothetical model of different biomarker and clinical outcomes reflecting the progression of AD in DS. Amyloid changes are thought to be detectable after 20 years of age whereas tau pathology might not be detectable until 30 years of age. In vivo neuroimaging suggests that changes in brain volume and glucose metabolism (FDG-PET) can develop after 30 years of age, not long after tau pathology develops. Clinical changes are delayed and may not be detectable until after 35 years of age. Each of these biomarkers is thought to get progressively more abnormal as people with DS age. Ages are estimated on the basis of published studies. Reproduced with permission from Ballard et al., 2016 13.
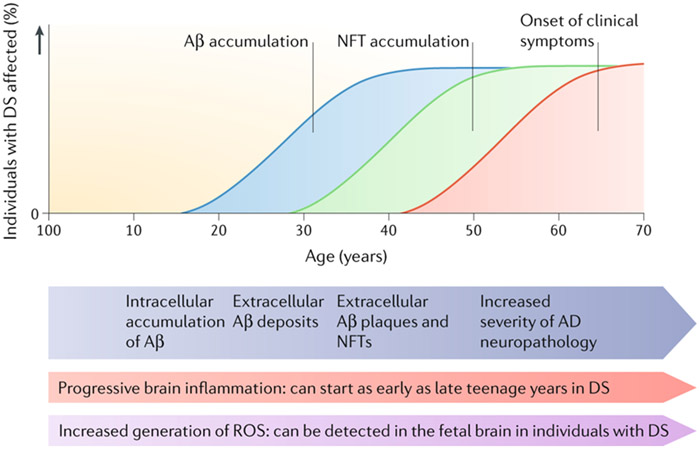
Figure 6. Hypothetical progression of Alzheimer disease neuropathology in Down syndrome.
A proposed timeline from birth to over 60 years of age of Alzheimer disease (AD) pathology in individuals with Down syndrome (DS). Mitochondrial dysfunction and increased generation of reactive oxygen species (ROS) occurs as early as in fetal brain. Brain inflammation can begin as early as in the late teens with the presence of activated microglial cells, which are associated with Aβ plaques later in the disease. By age 40 years, both extracellular Aβ and NFTs are present in sufficient quantities for a neuropathological diagnosis of AD. As individuals with DS age to over 50 years, AD neuropathology increases in severity and clinical signs of dementia become frequent.
However, the pharmacokinetic profile of individuals with DS might differ from that of other groups with AD — for example, as has been observed for donepezil, one of the few compounds systematically tested in DS — which presents a potential confounding factor for clinical trials226. Medication compliance for adults with intellectual disability (including DS) seems to be much better in a residential home setting than in the patient’s own home 227 and this compliance needs to be taken into account during a clinical trial. The heterogeneity of baseline intellectual disability in DS and the difficulty in choosing neuropsychological outcome measures is an ongoing challenge in DS 228. In this regard, a new approach to outcome measures might be appropriate. Goal attainment scaling (GAS) attempts to tailor outcome measures to the unique expectations of individual participants in a clinical trial. The GAS was a successful outcome measure in a trial of galantamine in AD in the general population, 229 with improvement in verbal repetition used as a treatment goal. The GAS seemed to track improvement better than the AD Assessment Scale–Cognitive Subscale 230.
More than 600 genes are overexpressed as a consequence of trisomy 21. As a result, additional off-target effects of any pharmacological intervention will need to be considered. For example, one therapeutic strategy in AD is alteration of beta-secretase 1 (BACE1) activity as a means to reduce Aβ production; however, BACE1 has several targets, which might include proteins encoded by genes on chromosome 21. Furthermore, BACE2, a close homolog of BACE1, is on chromosome 21, which might complicate the interpretation of clinical trial outcomes. None of these challenges are insurmountable but require careful consideration of the unique genetic characteristics of trisomy 21. 152,231.
Preclinical studies are a necessary step to identify therapeutic targets and to ensure safety in future clinical trials in DS 232. However, preclinical studies present a challenge for AD in DS 233. Mouse models of DS are exceptionally good at capturing developmental phenotypes 234,235 and it might also be possible to model early features of AD pathogenesis in DS with mouse models 233. However, mouse models of DS do not develop Aβ plaques or tangles with age, probably owing to the triplication and overexpression of murine APP, which leads to murine Aβ that does not aggregate in the way that human-Aβ can. . In addition, mouse models of AD capture some of the ageing phenotypes of people with DS but these models often have mutations that are typically not observed in DS 236. Thus, depending on the pathways being targeted as a possible treatment for AD in DS, use of multiple mouse models might present the most efficacious approach to determine efficacy and safety of potential new therapeutics.
Conclusions
In this Review, we attempt to identify some areas in which research on dementia in DS can inform similar areas of AD research. DS provides an opportunity to address temporal events and mechanistic pathways that are important to AD across the lifespan. Research areas of interest include the similarities and differences in the molecular drivers of pathogenesis; targets for therapeutic intervention; timing of preventative studies; the evolution of clinically meaningful biomarkers; and the course of lifelong accumulation of amyloid in the brains of individuals with DS and its relationship to dementia onset. Systematic examination of large cohorts will be required in DS, including assessment of clinical, neuroimaging, biomarker and autopsy outcomes. Such a systematic approach will require uniform clinical and neuropathology databases and consensus between sites and across different age groups in terms of the clinical measures used to assess cognition . Interestingly, however, the sample sizes required for these studies (for example, in clinical trials) might be considerably smaller than those needed to achieve the same level of power for AD in the general population due to less variability in AD phenotype for dementia in DS. Several gaps in clinical knowledge exist that once addressed in DS might also lead to novel insights in AD in the general population. These include understanding the similarities and differences in Braak staging in DS compared with AD in euploid individuals, the contribution of CAA to dementia and the mechanisms underlying white matter integrity losses. Future research in these areas is likely to aid the design of clinical trials in both cohorts. We hope that a focus on DS will continue play an important part in the objectives of AD research centres.
Key points.
Virtually all people with Down syndrome (DS) have Alzheimer disease (AD) pathology by 40 years of age; this association facilitates an increased understanding of the temporal progression of AD pathogenesis and provides unique insights for AD in the general population.
Understanding the role of amyloid precursor protein in DS might lead to a greater understanding of its role in both sporadic AD and familial AD in the general population
The study of neuroinflammation in DS might provide unique insights into AD in the general population and highlight key pathways that might be amenable to therapeutic intervention
Investigation of cerebrovascular pathology and its role in dementia might be simplified by the study of DS cohorts and lead to novel hypotheses regarding the causes and consequences of cerebral amyloid angiopathy
Co-morbidities in DS, such as sleep disturbances, seizures and psychiatric conditions, overlap with those conditions seen in AD in the general population
Acknowledgements
Supported by NIA UO1AG 051412 and AG P50 16573 to ITL and NIH R01HD064993 to EH. The authors are grateful to N. Schupf and J. Lee at Columbia University, New York, NY, USA for their helpful comments on this manuscript.
References cited:
- 1.Jervis GA Early senile dementia in mongoloid idiocy. Am J Psychiatry 105, 102–106, doi: 10.1176/ajp.105.2.102 (1948). [DOI] [PubMed] [Google Scholar]
- 2.Struwe F Histopathologische Untersuchungen uber Entstehung und Wesen der senilen Plaques. Z. ges. Neurol. Psychiat 122, 291–307 (1929). [Google Scholar]
- 3.Bertrand I & Koffas D Cas d'idioti mongolienne adulte avec nombreuses plaques senile et concretions calcaires pallidales. Rev. Neurol 78, 338 (1946). [Google Scholar]
- 4.Mann DMA The pathological association between Down syndrome and Alzheimer disease. Mech. Ageing and Develop 43, 99–136 (1988). [DOI] [PubMed] [Google Scholar]
- 5.Hardy J The discovery of Alzheimer-causing mutations in the APP gene and the formulation of the "amyloid cascade hypothesis". FEBS J 284, 1040–1044, doi: 10.1111/febs.14004 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Selkoe DJ & Hardy J The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO molecular medicine 8, 595–608, doi: 10.15252/emmm.201606210 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Head E, Helman AM, Powell D & Schmitt FA Down syndrome, beta-amyloid and neuroimaging. Free Radic Biol Med 114, 102–109, doi: 10.1016/j.freeradbiomed.2017.09.013 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glenner GG, and Wong CW Alzheimer's disease and Down's syndrome sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun 120, 885–890 (1984). [DOI] [PubMed] [Google Scholar]
- 9.Holler CJ et al. BACE2 expression increases in human neurodegenerative disease. Am J Pathol 180, 337–350, doi:S0002-9440(11)00927-8 [pii] 10.1016/j.ajpath.2011.09.034 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiss AB, Arain HA, Stecker MM, Siegart NM & Kasselman LJ Amyloid toxicity in Alzheimer's disease. Rev Neurosci, doi: 10.1515/revneuro-2017-0063 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Di Domenico F et al. Impairment of proteostasis network in Down syndrome prior to the development of Alzheimer's disease neuropathology: redox proteomics analysis of human brain. Biochim Biophys Acta 1832, 1249–1259, doi: 10.1016/j.bbadis.2013.04.013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartley D et al. Down syndrome and Alzheimer's disease: Common pathways, common goals. Alzheimers Dement 11, 700–709, doi: 10.1016/j.jalz.2014.10.007 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballard C, Mobley W, Hardy J, Williams G & Corbett A Dementia in Down's syndrome. Lancet Neurol 15, 622–636, doi: 10.1016/S1474-4422(16)00063-6 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Prasher VP & Filer A Behavioural disturbance in people with Down's syndrome and dementia. J Intellect Disabil Res. 39, 432–436 (1995). [DOI] [PubMed] [Google Scholar]
- 15.Holland AJ, Hon J, Huppert FA, Stevens F Incidence and course of dementia in people with Down's syndrome: findings from a population-based study. J Intellect Disabil Res 44, 138–146 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Zigman WB, Schupf N, Sersen E & Silverman W Prevalence of dementia in adults with and without Down syndrome. Am J Ment Retard 100, 403–412 (1996). [PubMed] [Google Scholar]
- 17.McCarron M et al. A prospective 20-year longitudinal follow-up of dementia in persons with Down syndrome. J Intellect Disabil Res 61, 843–852, doi: 10.1111/jir.12390 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Jarrett S The meaning of 'community' in the lives of people with intellectual disabilities: an historical perspective. Int J Dev Disabil 61, 107–112, doi: 10.1179/2047386914Z.00000000094 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devenny DA et al. Normal ageing in adults with Down's syndrome: a longitudinal study. J Intellect Disabil Res 40 ( Pt 3), 208–221 (1996). [PubMed] [Google Scholar]
- 20.Margallo-Lana ML et al. Cognitive decline in Down syndrome. Arch Neurol 60, 1024; author reply 1024, doi: 10.1001/archneur.60.7.1024-a (2003). [DOI] [PubMed] [Google Scholar]
- 21.Rohn TT, McCarty KL, Love JE & Head E Is Apolipoprotein E4 an Important Risk Factor for Dementia in Persons with Down Syndrome? Journal of Parkinson's disease and Alzheimer's disease 1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zigman WB, Jenkins EC, Tycko B, Schupf N & Silverman W Mortality is associated with apolipoprotein E epsilon4 in nondemented adults with Down syndrome. Neurosci Lett 390, 93–97, doi: 10.1016/j.neulet.2005.08.002 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Prasher VP et al. Significant effect of APOE epsilon 4 genotype on the risk of dementia in Alzheimer's disease and mortality in persons with Down syndrome. Int J Geriatr Psychiatry 23, 1134–1140, doi: 10.1002/gps.2039 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schupf N et al. Onset of dementia is associated with apolipoprotein E epsilon4 in Down's syndrome. Ann Neurol 40, 799–801, doi: 10.1002/ana.410400518 (1996). [DOI] [PubMed] [Google Scholar]
- 25.Eisenstein M Genetics: finding risk factors. Nature 475, S20–22, doi: 10.1038/475S20a (2011). [DOI] [PubMed] [Google Scholar]
- 26.Huynh TV, Davis AA, Ulrich JD & Holtzman DM Apolipoprotein E and Alzheimer's disease: the influence of apolipoprotein E on amyloid-beta and other amyloidogenic proteins. J Lipid Res 58, 824–836, doi: 10.1194/jlr.R075481 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grimm MOW, Michaelson DM & Hartmann T Omega-3 fatty acids, lipids, and apoE lipidation in Alzheimer's disease: a rationale for multi-nutrient dementia prevention. J Lipid Res 58, 2083–2101, doi: 10.1194/jlr.R076331 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rohn TT Proteolytic cleavage of apolipoprotein E4 as the keystone for the heightened risk associated with Alzheimer's disease. Int J Mol Sci 14, 14908–14922, doi: 10.3390/ijms140714908 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Day RJ, McCarty KL, Ockerse KE, Head E & Rohn TT Proteolytic Cleavage of Apolipoprotein E in the Down Syndrome Brain. Aging and disease 7, 267–277, doi: 10.14336/AD.2015.1020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rohn TT, Catlin LW, Coonse KG & Habig JW Identification of an amino-terminal fragment of apolipoprotein E4 that localizes to neurofibrillary tangles of the Alzheimer's disease brain. Brain Res 1475, 106–115, doi: 10.1016/j.brainres.2012.08.003 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Zhao L, Woody SK & Chhibber A Estrogen receptor beta in Alzheimer's disease: From mechanisms to therapeutics. Ageing Res Rev 24, 178–190, doi: 10.1016/j.arr.2015.08.001 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaffe K, Haan M, Byers A, Tangen C & Kuller L Estrogen use, APOE, and cognitive decline: evidence of gene-environment interaction. Neurology 54, 1949–1954 (2000). [DOI] [PubMed] [Google Scholar]
- 33.Gleason CE et al. Effects of Hormone Therapy on Cognition and Mood in Recently Postmenopausal Women: Findings from the Randomized, Controlled KEEPS-Cognitive and Affective Study. PLoS medicine 12, e1001833; discussion e1001833, doi: 10.1371/journal.pmed.1001833 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henderson VW Alzheimer's disease: review of hormone therapy trials and implications for treatment and prevention after menopause. The Journal of steroid biochemistry and molecular biology 142, 99–106, doi: 10.1016/j.jsbmb.2013.05.010 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schupf N et al. Onset of dementia is associated with age at menopause in women with Down's syndrome. Ann Neurol 54, 433–438, doi: 10.1002/ana.10677 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Patel BN, Seltzer GB, Wu HS & Schupf N Effect of menopause on cognitive performance in women with Down syndrome. Neuroreport 12, 2659–2662 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Mosconi L et al. Perimenopause and emergence of an Alzheimer's bioenergetic phenotype in brain and periphery. PLoS One 12, e0185926, doi: 10.1371/journal.pone.0185926 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papavassiliou P, Charalsawadi C, Rafferty K & Jackson-Cook C Mosaicism for trisomy 21: a review. Am J Med Genet A 167A, 26–39, doi: 10.1002/ajmg.a.36861 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Ringman JM, Rao PN, Lu PH & Cederbaum S Mosaicism for trisomy 21 in a patient with young-onset dementia: a case report and brief literature review. Arch Neurol 65, 412–415 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Potter H, Granic A & Caneus J Role of Trisomy 21 Mosaicism in Sporadic and Familial Alzheimer's Disease. Curr Alzheimer Res 13, 7–17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leija-Salazar M, Piette CL & Proukakis C Somatic mutations in neurodegeneration. Neuropathol Appl Neurobiol, doi: 10.1111/nan.12465 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Wiseman FK et al. A genetic cause of Alzheimer disease: mechanistic insights from Down syndrome. Nat Rev Neurosci 16, 564–574, doi: 10.1038/nrn3983 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rovelet-Lecrux A et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet 38, 24–26 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Cabrejo L et al. Phenotype associated with APP duplication in five families. Brain 129, 2966–2976, doi: 10.1093/brain/awl237 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Wallon D et al. The French series of autosomal dominant early onset Alzheimer's disease cases: mutation spectrum and cerebrospinal fluid biomarkers. J Alzheimers Dis 30, 847–856, doi: 10.3233/JAD-2012-120172 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Hooli BV et al. Role of common and rare APP DNA sequence variants in Alzheimer disease. Neurology 78, 1250–1257, doi: 10.1212/WNL.0b013e3182515972 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones EL et al. Evidence that PICALM affects age at onset of Alzheimer's dementia in Down syndrome. Neurobiol Aging 34, 2441 e2441–2445, doi: 10.1016/j.neurobiolaging.2013.03.018 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JH et al. Candidate gene analysis for Alzheimer's disease in adults with Down syndrome. Neurobiol Aging 56, 150–158, doi: 10.1016/j.neurobiolaging.2017.04.018 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schupf N et al. Candidate genes for Alzheimer's disease are associated with individual differences in plasma levels of beta amyloid peptides in adults with Down syndrome. Neurobiol Aging 36, 2907 e2901–2910, doi: 10.1016/j.neurobiolaging.2015.06.020 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Do C, Xing Z, Yu YE & Tycko B Trans-acting epigenetic effects of chromosomal aneuploidies: lessons from Down syndrome and mouse models. Epigenomics 9, 189–207, doi: 10.2217/epi-2016-0138 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Megarbane A et al. The intellectual disability of trisomy 21: differences in gene expression in a case series of patients with lower and higher IQ. European journal of human genetics : EJHG 21, 1253–1259, doi: 10.1038/ejhg.2013.24 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weber D et al. Mechanisms of epigenetic and cell-type specific regulation of Hey target genes in ES cells and cardiomyocytes. J Mol Cell Cardiol 79, 79–88, doi: 10.1016/j.yjmcc.2014.11.004 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Bacalini MG et al. Identification of a DNA methylation signature in blood cells from persons with Down Syndrome. Aging 7, 82–96, doi: 10.18632/aging.100715 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horvath S et al. Accelerated epigenetic aging in Down syndrome. Aging Cell 14, 491–495, doi: 10.1111/acel.12325 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Narayan PJ, Lill C, Faull R, Curtis MA & Dragunow M Increased acetyl and total histone levels in post-mortem Alzheimer's disease brain. Neurobiol Dis 74, 281–294, doi: 10.1016/j.nbd.2014.11.023 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Fyfe I Alzheimer disease: Epigenetics links ageing with Alzheimer disease. Nat Rev Neurol, doi: 10.1038/nrneurol.2018.36 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Edgin JO, Clark CA, Massand E & Karmiloff-Smith A Building an adaptive brain across development: targets for neurorehabilitation must begin in infancy. Frontiers in behavioral neuroscience 9, 232, doi: 10.3389/fnbeh.2015.00232 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidt-Sidor B, Wisniewski KE, Shepard TH & Sersen EA Brain growth in Down syndrome subjects 15 to 22 weeks of gestational age and birth to 60 months. Clin Neuropathol 9, 181–190 (1990). [PubMed] [Google Scholar]
- 59.Guidi S et al. Neurogenesis impairment and increased cell death reduce total neuron number in the hippocampal region of fetuses with Down syndrome. Brain Pathol 18, 180–197, doi: 10.1111/j.1750-3639.2007.00113.x (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sosa LJ et al. Dosage of amyloid precursor protein affects axonal contact guidance in Down syndrome. FASEB J 28, 195–205, doi: 10.1096/fj.13-232686 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dawkins E & Small DH Insights into the physiological function of the beta-amyloid precursor protein: beyond Alzheimer's disease. J Neurochem 129, 756–769, doi: 10.1111/jnc.12675 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neale N, Padilla C, Fonseca LM, Holland T & Zaman S Neuroimaging and other modalities to assess Alzheimer's disease in Down syndrome. NeuroImage. Clinical 17, 263–271, doi: 10.1016/j.nicl.2017.10.022 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teipel SJ et al. Age-related cortical grey matter reductions in non-demented Down's syndrome adults determined by MRI with voxel-based morphometry. Brain 127, 811–824 (2004). [DOI] [PubMed] [Google Scholar]
- 64.Teipel SJ & Hampel H Neuroanatomy of Down syndrome in vivo: a model of preclinical Alzheimer's disease. Behav Genet 36, 405–415, doi: 10.1007/s10519-006-9047-x (2006). [DOI] [PubMed] [Google Scholar]
- 65.Powell D et al. Frontal white matter integrity in adults with Down syndrome with and without dementia. Neurobiol Aging, doi: 10.1016/j.neurobiolaging.2014.01.137 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rumble B et al. Amyloid A4 and its precursor in Down's syndrome and Alzheimer's disease. New Engl. J. Med 320, 1446–1462 (1989). [DOI] [PubMed] [Google Scholar]
- 67.Head E & Lott IT Down syndrome and beta-amyloid deposition. Curr Opin Neurol 17, 95–100 (2004). [DOI] [PubMed] [Google Scholar]
- 68.Head E, Powell D, Gold BT & Schmitt FA Alzheimer's Disease in Down Syndrome. European journal of neurodegenerative disease 1, 353–364 (2012). [PMC free article] [PubMed] [Google Scholar]
- 69.Lemere CA et al. Sequence of deposition of heterogeneous amyloid beta-peptides and APOE in Down Syndrome: Implications for initial events in amyloid plaque formation. Neurobiology of Disease 3, 16–32 (1996). [DOI] [PubMed] [Google Scholar]
- 70.Fonseca MI, Head E, Velazquez P, Cotman CW & Tenner AJ The presence of isoaspartic acid in beta-amyloid plaques indicates plaque age. Exp Neurol 157, 277–288, doi:S0014-4886(99)97058-9 [pii] 10.1006/exnr.1999.7058 (1999). [DOI] [PubMed] [Google Scholar]
- 71.Azizeh BY et al. Molecular dating of senile plaques in the brains of individuals with Down syndrome and in aged dogs. Exp Neurol 163, 111–122, doi: 10.1006/exnr.2000.7359 S0014-4886(00)97359-X [pii] (2000). [DOI] [PubMed] [Google Scholar]
- 72.Leverenz JB & Raskind MA Early amyloid deposition in the medial temporal lobe of young Down syndrome patients: a regional quantitative analysis. Experimental neurology 150, 296–304, doi: 10.1006/exnr.1997.6777 (1998). [DOI] [PubMed] [Google Scholar]
- 73.Stoltzner SE, Grenfell TJ, Mori C, Wisniewski KE, Wisniewski TM, Selkoe DJ, and Lemere CA Temporal accrual of complement proteins in amyloid plaques in Down's syndrome with Alzheimer's disease. American Journal of Pathology 156, 489–499 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nistor M et al. Alpha- and beta-secretase activity as a function of age and beta-amyloid in Down syndrome and normal brain. Neurobiol Aging 28, 1493–1506 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cenini G et al. Association between frontal cortex oxidative damage and beta-amyloid as a function of age in Down syndrome. Biochim Biophys Acta 1822, 130–138, doi: 10.1016/j.bbadis.2011.10.001 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carmona-Iragui M et al. Cerebral amyloid angiopathy in Down syndrome and sporadic and autosomal-dominant Alzheimer's disease. Alzheimers Dement 13, 1251–1260, doi: 10.1016/j.jalz.2017.03.007 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prasher VP et al. Molecular mapping of Alzheimer-type dementia in Down's syndrome. Ann Neurol 43, 380–383 (1998).9506555 [Google Scholar]
- 78.Doran E et al. Down Syndrome, Partial Trisomy 21, and Absence of Alzheimer's Disease: The Role of APP. J Alzheimers Dis 56, 459–470, doi: 10.3233/JAD-160836 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sperling R, Mormino E & Johnson K The evolution of preclinical Alzheimer's disease: implications for prevention trials. Neuron 84, 608–622, doi: 10.1016/j.neuron.2014.10.038 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cohen AD & Klunk WE Early detection of Alzheimer's disease using PiB and FDG PET. Neurobiol Dis 72 Pt A, 117–122, doi: 10.1016/j.nbd.2014.05.001 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cohen Kadosh K, Johnson MH, Dick F, Cohen Kadosh R & Blakemore SJ Effects of age, task performance, and structural brain development on face processing. Cereb Cortex 23, 1630–1642, doi: 10.1093/cercor/bhs150 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mintun MA et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 67, 446–452, doi: 10.1212/01.wnl.0000228230.26044.a4 (2006). [DOI] [PubMed] [Google Scholar]
- 83.Klunk WE et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol 55, 306–319, doi: 10.1002/ana.20009 (2004). [DOI] [PubMed] [Google Scholar]
- 84.Handen BL et al. Imaging brain amyloid in nondemented young adults with Down syndrome using Pittsburgh compound B. Alzheimers Dement 8, 496–501, doi: 10.1016/j.jalz.2011.09.229 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Annus T et al. The pattern of amyloid accumulation in the brains of adults with Down syndrome. Alzheimers Dement 12, 538–545, doi: 10.1016/j.jalz.2015.07.490 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lao PJ et al. The effects of normal aging on amyloid-beta deposition in nondemented adults with Down syndrome as imaged by carbon 11-labeled Pittsburgh compound B. Alzheimers Dement 12, 380–390, doi: 10.1016/j.jalz.2015.05.013 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Klunk WE et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci 27, 6174–6184, doi: 10.1523/JNEUROSCI.0730-07.2007 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Villemagne VL et al. High striatal amyloid beta-peptide deposition across different autosomal Alzheimer disease mutation types. Arch Neurol 66, 1537–1544, doi: 10.1001/archneurol.2009.285 (2009). [DOI] [PubMed] [Google Scholar]
- 89.Koivunen J et al. PET amyloid ligand [11C]PIB uptake shows predominantly striatal increase in variant Alzheimer's disease. Brain 131, 1845–1853, doi: 10.1093/brain/awn107 (2008). [DOI] [PubMed] [Google Scholar]
- 90.Mann DM & Iwatsubo T Diffuse plaques in the cerebellum and corpus striatum in Down's syndrome contain amyloid beta protein (A beta) only in the form of A beta 42(43). Neurodegeneration 5, 115–120 (1996). [DOI] [PubMed] [Google Scholar]
- 91.Hartley SL et al. Cognitive functioning in relation to brain amyloid-beta in healthy adults with Down syndrome. Brain 137, 2556–2563, doi: 10.1093/brain/awu173 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Landt J et al. Using positron emission tomography and Carbon 11-labeled Pittsburgh Compound B to image Brain Fibrillar beta-amyloid in adults with down syndrome: safety, acceptability, and feasibility. Arch Neurol 68, 890–896, doi: 10.1001/archneurol.2011.36 (2011). [DOI] [PubMed] [Google Scholar]
- 93.Hartley SL et al. Cognitive decline and brain amyloid-beta accumulation across 3 years in adults with Down syndrome. Neurobiol Aging 58, 68–76, doi: 10.1016/j.neurobiolaging.2017.05.019 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lao PJ et al. Alzheimer-Like Pattern of Hypometabolism Emerges with Elevated Amyloid-beta Burden in Down Syndrome. J Alzheimers Dis 61, 631–644, doi: 10.3233/JAD-170720 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sabbagh MN et al. Florbetapir PET, FDG PET, and MRI in Down syndrome individuals with and without Alzheimer's dementia. Alzheimers Dement 11, 994–1004, doi: 10.1016/j.jalz.2015.01.006 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sabbagh MN et al. Positron emission tomography and neuropathologic estimates of fibrillar amyloid-beta in a patient with Down syndrome and Alzheimer disease. Arch Neurol 68, 1461–1466, doi: 10.1001/archneurol.2011.535 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rafii MS et al. The down syndrome biomarker initiative (DSBI) pilot: proof of concept for deep phenotyping of Alzheimer's disease biomarkers in down syndrome. Frontiers in behavioral neuroscience 9, 239, doi: 10.3389/fnbeh.2015.00239 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nelson LD et al. Positron emission tomography of brain beta-amyloid and tau levels in adults with Down syndrome. Arch Neurol 68, 768–774, doi: 10.1001/archneurol.2011.104 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Head E et al. Parallel Compensatory and Pathological events Associated with Tau Pathology in Middle Aged Individuals with Down Syndrome. J. Neuropath, Exp. Neurol 62, 917–926 (2003). [DOI] [PubMed] [Google Scholar]
- 100.Hof PR et al. Age-related distribution of neuropathologic changes in the cerebral cortex of patients with Down's syndrome. Quantitative regional analysis and comparison with Alzheimer's disease. Arch Neurol 52, 379–391 (1995). [DOI] [PubMed] [Google Scholar]
- 101.Hyman BT, West HL, Rebeck GW, Lai F, and Mann DM Neuropathological changes in Down's syndrome hippocampal formation. Effect of age and apolipoprotein E genotype. Arch Neurol-Chicago 52, 373–378 (1995). [DOI] [PubMed] [Google Scholar]
- 102.Mann DM & Esiri MM The pattern of acquisition of plaques and tangles in the brains of patients under 50 years of age with Down's syndrome. J Neurol Sci 89, 169–179 (1989). [DOI] [PubMed] [Google Scholar]
- 103.Mann DM, Royston MC & Ravindra CR Some morphometric observations on the brains of patients with Down's syndrome: their relationship to age and dementia. J Neurol Sci 99, 153–164 (1990). [DOI] [PubMed] [Google Scholar]
- 104.Head E, Lott IT, Wilcock DM & Lemere CA Aging in Down Syndrome and the Development of Alzheimer's Disease Neuropathology. Curr Alzheimer Res 13, 18–29 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dowjat WK et al. Trisomy-driven overexpression of DYRK1A kinase in the brain of subjects with Down syndrome. Neurosci Lett 413, 77–81, doi:S0304-3940(06)01230-4 [pii] 10.1016/j.neulet.2006.11.026 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kimura R et al. The DYRK1A gene, encoded in chromosome 21 Down syndrome critical region, bridges between beta-amyloid production and tau phosphorylation in Alzheimer disease. Hum Mol Genet 16, 15–23, doi: 10.1093/hmg/ddl437 (2007). [DOI] [PubMed] [Google Scholar]
- 107.Liu F et al. Overexpression of Dyrk1A contributes to neurofibrillary degeneration in Down syndrome. FASEB J 22, 3224–3233, doi:fj.07-104539 [pii] 10.1096/fj.07-104539 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ryoo SR et al. DYRK1A-mediated hyperphosphorylation of Tau. A functional link between Down syndrome and Alzheimer disease. J Biol Chem 282, 34850–34857, doi: 10.1074/jbc.M707358200 (2007). [DOI] [PubMed] [Google Scholar]
- 109.Wegiel J et al. The role of overexpressed DYRK1A protein in the early onset of neurofibrillary degeneration in Down syndrome. Acta Neuropathol 116, 391–407, doi: 10.1007/s00401-008-0419-6 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wegiel J et al. Link between DYRK1A overexpression and several-fold enhancement of neurofibrillary degeneration with 3-repeat tau protein in Down syndrome. J Neuropathol Exp Neurol 70, 36–50, doi: 10.1097/NEN.0b013e318202bfa1 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hogan PG, Chen L, Nardone J & Rao A Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev 17, 2205–2232, doi: 10.1101/gad.1102703 17/18/2205 [pii] (2003). [DOI] [PubMed] [Google Scholar]
- 112.Klee CB, Ren H & Wang X Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem 273, 13367–13370 (1998). [DOI] [PubMed] [Google Scholar]
- 113.Ermak G, Harris CD, Battocchio D & Davies KJ RCAN1 (DSCR1 or Adapt78) stimulates expression of GSK-3beta. Febs J 273, 2100–2109 (2006). [DOI] [PubMed] [Google Scholar]
- 114.Dekker AD, Fortea J, Blesa R & De Deyn PP Cerebrospinal fluid biomarkers for Alzheimer's disease in Down syndrome. Alzheimer's & dementia 8, 1–10, doi: 10.1016/j.dadm.2017.02.006 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kasai T et al. Increased levels of plasma total tau in adult Down syndrome. PLoS One 12, e0188802, doi: 10.1371/journal.pone.0188802 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee NC et al. Blood Beta-Amyloid and Tau in Down Syndrome: A Comparison with Alzheimer's Disease. Frontiers in aging neuroscience 8, 316, doi: 10.3389/fnagi.2016.00316 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hamlett ED et al. Neuronal exosomes reveal Alzheimer's disease biomarkers in Down syndrome. Alzheimers Dement, doi: 10.1016/j.jalz.2016.08.012 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rafii MS et al. PET Imaging of Tau Pathology and Relationship to Amyloid, Longitudinal MRI, and Cognitive Change in Down Syndrome: Results from the Down Syndrome Biomarker Initiative (DSBI). J Alzheimers Dis 60, 439–450, doi: 10.3233/JAD-170390 (2017). [DOI] [PubMed] [Google Scholar]
- 119.Wilcock DM Neuroinflammation in the aging down syndrome brain; lessons from Alzheimer's disease. Curr Gerontol Geriatr Res 2012, 170276, doi: 10.1155/2012/170276 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wilcock DM & Griffin WS Down's syndrome, neuroinflammation, and Alzheimer neuropathogenesis. J Neuroinflammation 10, 84, doi: 10.1186/1742-2094-10-84 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Akiyama H et al. Inflammation and Alzheimer's disease. Neurobiol Aging 21, 383–421 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xue QS & Streit WJ Microglial pathology in Down syndrome. Acta Neuropathol 122, 455–466, doi: 10.1007/s00401-011-0864-5 (2011). [DOI] [PubMed] [Google Scholar]
- 123.Di Bona D et al. Association between the interleukin-1beta polymorphisms and Alzheimer's disease: a systematic review and meta-analysis. Brain Res Rev 59, 155–163, doi:S0165-0173(08)00073-8 [pii] 10.1016/j.brainresrev.2008.07.003 (2008). [DOI] [PubMed] [Google Scholar]
- 124.Karch CM & Goate AM Alzheimer's disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry 77, 43–51, doi: 10.1016/j.biopsych.2014.05.006 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL and Araoz C Brain interleukin I and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer's disease. Proc. Natl. Acad. Sci. USA 86, 7611–7615 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Head E et al. Complement association with neurons and beta-amyloid deposition in the brains of aged individuals with Down Syndrome. Neurobiol Dis 8, 252–265. (2001). [DOI] [PubMed] [Google Scholar]
- 127.Wilcock DM et al. Down syndrome individuals with Alzheimer's disease have a distinct neuroinflammatory phenotype compared to sporadic Alzheimer's disease. Neurobiol Aging 36, 2468–2474, doi: 10.1016/j.neurobiolaging.2015.05.016 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kamer AR et al. Periodontal disease's contribution to Alzheimer's disease progression in Down syndrome. Alzheimer's & dementia 2, 49–57, doi: 10.1016/j.dadm.2016.01.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rodrigues R et al. Alterations of ectonucleotidases and acetylcholinesterase activities in lymphocytes of Down syndrome subjects: relation with inflammatory parameters. Clin Chim Acta 433, 105–110, doi: 10.1016/j.cca.2014.03.002 (2014). [DOI] [PubMed] [Google Scholar]
- 130.Iulita MF et al. An inflammatory and trophic disconnect biomarker profile revealed in Down syndrome plasma: Relation to cognitive decline and longitudinal evaluation. Alzheimers Dement 12, 1132–1148, doi: 10.1016/j.jalz.2016.05.001 (2016). [DOI] [PubMed] [Google Scholar]
- 131.Snyder HM et al. Vascular contributions to cognitive impairment and dementia including Alzheimer's disease. Alzheimers Dement 11, 710–717, doi: 10.1016/j.jalz.2014.10.008 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jellinger KA Pathology and pathogenesis of vascular cognitive impairment-a critical update. Frontiers in aging neuroscience 5, 17, doi: 10.3389/fnagi.2013.00017 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Provenzano FA et al. White matter hyperintensities and cerebral amyloidosis: necessary and sufficient for clinical expression of Alzheimer disease? JAMA neurology 70, 455–461, doi: 10.1001/jamaneurol.2013.1321 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vinters HV Cerebral amyloid angiopathy. A critical review. Stroke 18, 311–324 (1987). [DOI] [PubMed] [Google Scholar]
- 135.Ikeda S et al. Variability of beta-amyloid protein deposited lesions in Down's syndrome brains. Tohoku J Exp Med 174, 189–198 (1994). [DOI] [PubMed] [Google Scholar]
- 136.Lai F, and Williams MD A prospective study of Alzheimer Disease in Down Syndrome. Arch Neurol-Chicago 46, 849–853 (1989). [DOI] [PubMed] [Google Scholar]
- 137.Belza MG & Urich H Cerebral amyloid angiopathy in Down's syndrome. Clin Neuropathol 5, 257–260 (1986). [PubMed] [Google Scholar]
- 138.Head E et al. Cerebrovascular pathology in Down syndrome and Alzheimer disease. Acta neuropathologica communications 5, 93, doi: 10.1186/s40478-017-0499-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Murdoch JC, Rodger JC, Rao SS, Fletcher CD & Dunnigan MG Down's syndrome: an atheroma-free model? Br Med J 2, 226–228 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pucci F et al. Blood pressure levels and body mass index in Brazilian adults with Down syndrome. Sao Paulo Med J 134, 330–334, doi: 10.1590/1516-3180.2016.0057180316 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Matthews DC et al. Dissociation of Down syndrome and Alzheimer's disease effects with imaging. Alzheimers Dement (N Y) 2, 69–81, doi: 10.1016/j.trci.2016.02.004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Haier RJ, Head K, Head E & Lott IT Neuroimaging of individuals with Down's syndrome at-risk for dementia: evidence for possible compensatory events. Neuroimage 39, 1324–1332 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Garibotto V et al. Clinical validity of brain fluorodeoxyglucose positron emission tomography as a biomarker for Alzheimer's disease in the context of a structured 5-phase development framework. Neurobiol Aging 52, 183–195, doi: 10.1016/j.neurobiolaging.2016.03.033 (2017). [DOI] [PubMed] [Google Scholar]
- 144.Barone E, Arena A, Head E, Butterfield DA & Perluigi M Disturbance of redox homeostasis in Down Syndrome: Role of iron dysmetabolism. Free Radic Biol Med 114, 84–93, doi: 10.1016/j.freeradbiomed.2017.07.009 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Di Domenico F et al. Redox proteomics analysis of HNE-modified proteins in Down syndrome brain: clues for understanding the development of Alzheimer disease. Free Radic Biol Med 71C, 270–280, doi: 10.1016/j.freeradbiomed.2014.03.027 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Perluigi M & Butterfield DA Oxidative Stress and Down Syndrome: A Route toward Alzheimer-Like Dementia. Curr Gerontol Geriatr Res 2012, 724904, doi: 10.1155/2012/724904 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Perluigi M et al. Neuropathological role of PI3K/Akt/mTOR axis in Down syndrome brain. Biochim Biophys Acta 1842, 1144–1153, doi: 10.1016/j.bbadis.2014.04.007 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Colacurcio DJ, Pensalfini A, Jiang Y & Nixon RA Dysfunction of autophagy and endosomal-lysosomal pathways: Roles in pathogenesis of Down syndrome and Alzheimer's Disease. Free Radic Biol Med 114, 40–51, doi: 10.1016/j.freeradbiomed.2017.10.001 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Phillips C et al. Noradrenergic System in Down Syndrome and Alzheimer's Disease A Target for Therapy. Curr Alzheimer Res 13, 68–83 (2016). [DOI] [PubMed] [Google Scholar]
- 150.Lockrow JP, Fortress AM & Granholm AC Age-related neurodegeneration and memory loss in down syndrome. Curr Gerontol Geriatr Res 2012, 463909, doi: 10.1155/2012/463909 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Martin SB et al. Synaptophysin and Synaptojanin-1 in Down Syndrome are Differentially Affected by Alzheimer's Disease. J Alzheimers Dis, doi: 10.3233/JAD-140795 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Head E, Lott IT, Patterson D, Doran E & Haier RJ Possible compensatory events in adult Down syndrome brain prior to the development of Alzheimer disease neuropathology: targets for nonpharmacological intervention. J Alzheimers Dis 11, 61–76 (2007). [DOI] [PubMed] [Google Scholar]
- 153.Jenkins EC et al. Longitudinal telomere shortening and early Alzheimer's disease progression in adults with down syndrome. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics 174, 772–778, doi: 10.1002/ajmg.b.32575 (2017). [DOI] [PubMed] [Google Scholar]
- 154.Hartley D et al. Down syndrome and Alzheimer's disease: Common pathways, common goals. Alzheimers Dement, doi: 10.1016/j.jalz.2014.10.007 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen XQ, Sawa M & Mobley WC Dysregulation of neurotrophin signaling in the pathogenesis of Alzheimer disease and of Alzheimer disease in Down syndrome. Free Radic Biol Med 114, 52–61, doi: 10.1016/j.freeradbiomed.2017.10.341 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Iulita MF, Caraci F & Cuello AC A Link Between Nerve Growth Factor Metabolic Deregulation and Amyloid-beta-Driven Inflammation in Down Syndrome. CNS Neurol Disord Drug Targets 15, 434–447 (2016). [DOI] [PubMed] [Google Scholar]
- 157.Iulita MF & Cuello AC The NGF Metabolic Pathway in the CNS and its Dysregulation in Down Syndrome and Alzheimer's Disease. Curr Alzheimer Res 13, 53–67 (2016). [DOI] [PubMed] [Google Scholar]
- 158.Zigman WB Atypical aging in down syndrome. Developmental disabilities research reviews 18, 51–67, doi: 10.1002/ddrr.1128 (2013). [DOI] [PubMed] [Google Scholar]
- 159.Cheignon C et al. Oxidative stress and the amyloid beta peptide in Alzheimer's disease. Redox biology 14, 450–464, doi: 10.1016/j.redox.2017.10.014 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Helguera P et al. Adaptive downregulation of mitochondrial function in down syndrome. Cell metabolism 17, 132–140, doi: 10.1016/j.cmet.2012.12.005 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Carfi A et al. Characteristics of adults with down syndrome: prevalence of age-related conditions. Front Med (Lausanne) 1, 51, doi: 10.3389/fmed.2014.00051 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Englund A, Jonsson B, Zander CS, Gustafsson J & Anneren G Changes in mortality and causes of death in the Swedish Down syndrome population. Am J Med Genet A 161A, 642–649, doi: 10.1002/ajmg.a.35706 (2013). [DOI] [PubMed] [Google Scholar]
- 163.Valenti D et al. Mitochondria as pharmacological targets in Down syndrome. Free Radic Biol Med 114, 69–83, doi: 10.1016/j.freeradbiomed.2017.08.014 (2018). [DOI] [PubMed] [Google Scholar]
- 164.Hithersay R, Hamburg S, Knight B & Strydom A Cognitive decline and dementia in Down syndrome. Curr Opin Psychiatry 30, 102–107, doi: 10.1097/YCO.0000000000000307 (2017). [DOI] [PubMed] [Google Scholar]
- 165.McKhann GM et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7, 263–269, doi: 10.1016/j.jalz.2011.03.005 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Edgin JO et al. Development and validation of the Arizona Cognitive Test Battery for Down syndrome. Journal of neurodevelopmental disorders 2, 149–164, doi: 10.1007/s11689-010-9054-3 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Edgin JO et al. The Arizona Cognitive Test Battery for Down Syndrome: Test-Retest Reliability and Practice Effects. Am J Intellect Dev Disabil 122, 215–234, doi: 10.1352/1944-7558-122.3.215 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lautarescu BA, Holland AJ & Zaman SH The Early Presentation of Dementia in People with Down Syndrome: a Systematic Review of Longitudinal Studies. Neuropsychology review 27, 31–45, doi: 10.1007/s11065-017-9341-9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ball SL, Holland AJ, Treppner P, Watson PC & Huppert FA Executive dysfunction and its association with personality and behaviour changes in the development of Alzheimer's disease in adults with Down syndrome and mild to moderate learning disabilities. Br J Clin Psychol 47, 1–29, doi: 10.1348/014466507X230967 (2008). [DOI] [PubMed] [Google Scholar]
- 170.Weintraub S et al. Version 3 of the Alzheimer Disease Centers' Neuropsychological Test Battery in the Uniform Data Set (UDS). Alzheimer Dis Assoc Disord, doi: 10.1097/WAD.0000000000000223 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Belleville S et al. Neuropsychological Measures that Predict Progression from Mild Cognitive Impairment to Alzheimer's type dementia in Older Adults: a Systematic Review and Meta-Analysis. Neuropsychology review 27, 328–353, doi: 10.1007/s11065-017-9361-5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sinai A, Hassiotis A, Rantell K & Strydom A Assessing Specific Cognitive Deficits Associated with Dementia in Older Adults with Down Syndrome: Use and Validity of the Arizona Cognitive Test Battery (ACTB). PLoS One 11, e0153917, doi: 10.1371/journal.pone.0153917 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Krinsky-McHale SJ & Silverman W Dementia and mild cognitive impairment in adults with intellectual disability: issues of diagnosis. Developmental disabilities research reviews 18, 31–42, doi: 10.1002/ddrr.1126 (2013). [DOI] [PubMed] [Google Scholar]
- 174.Lu PH & Lee GJ The Role of Neuropsychology in the Assessment of the Cognitively Impaired Elderly. Neurol Clin 35, 191–206, doi: 10.1016/j.ncl.2017.01.002 (2017). [DOI] [PubMed] [Google Scholar]
- 175.Lanctot KL et al. Neuropsychiatric signs and symptoms of Alzheimer's disease: New treatment paradigms. Alzheimers Dement (N Y) 3, 440–449, doi: 10.1016/j.trci.2017.07.001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dekker AD et al. Behavioural and psychological symptoms of dementia in Down syndrome: Early indicators of clinical Alzheimer's disease? Cortex 73, 36–61, doi: 10.1016/j.cortex.2015.07.032 (2015). [DOI] [PubMed] [Google Scholar]
- 177.Dick MB, Doran E, Phelan M & Lott IT Cognitive Profiles on the Severe Impairment Battery Are Similar in Alzheimer Disease and Down Syndrome With Dementia. Alzheimer Dis Assoc Disord 30, 251–257, doi: 10.1097/WAD.0000000000000132 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Glenn SM & Cunningham CC Parents' reports of young people with Down syndrome talking out loud to themselves. Ment Retard 38, 498–505, doi: (2000). [DOI] [PubMed] [Google Scholar]
- 179.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E Mild cognitive impairment: clinical characterization and outcome. Arch Neurol-Chicago 56, 303–308 (1999). [DOI] [PubMed] [Google Scholar]
- 180.Kryscio RJ, Schmitt FA, Salazar JC, Mendiondo MS & Markesbery WR Risk factors for transitions from normal to mild cognitive impairment and dementia. Neurology 66, 828–832, doi: 10.1212/01.wnl.0000203264.71880.45 (2006). [DOI] [PubMed] [Google Scholar]
- 181.Karmiloff-Smith A et al. The importance of understanding individual differences in Down syndrome. F1000Research 5, doi: 10.12688/f1000research.7506.1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Anderson-Mooney AJ, Schmitt FA, Head E, Lott IT & Heilman KM Gait dyspraxia as a clinical marker of cognitive decline in Down syndrome: A review of theory and proposed mechanisms. Brain and cognition 104, 48–57, doi: 10.1016/j.bandc.2016.02.007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chhetri JK, Chan P, Vellas B & Cesari M Motoric Cognitive Risk Syndrome: Predictor of Dementia and Age-Related Negative Outcomes. Front Med (Lausanne) 4, 166, doi: 10.3389/fmed.2017.00166 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Scherder E et al. Gait in ageing and associated dementias; its relationship with cognition. Neurosci Biobehav Rev 31, 485–497, doi: 10.1016/j.neubiorev.2006.11.007 (2007). [DOI] [PubMed] [Google Scholar]
- 185.Beauchet O et al. Association between high variability of gait speed and mild cognitive impairment: a cross-sectional pilot study. J Am Geriatr Soc 59, 1973–1974, doi: 10.1111/j.1532-5415.2011.03610_9.x (2011). [DOI] [PubMed] [Google Scholar]
- 186.Chen HL, Yu WH & Yeh HC Obstacle crossing in 7-9-year-old children with Down syndrome. Res Dev Disabil 48, 202–210, doi: 10.1016/j.ridd.2015.11.004 (2016). [DOI] [PubMed] [Google Scholar]
- 187.Tian Q et al. The brain map of gait variability in aging, cognitive impairment and dementia-A systematic review. Neurosci Biobehav Rev 74, 149–162, doi: 10.1016/j.neubiorev.2017.01.020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Menendez M Down syndrome, Alzheimer's disease and seizures. Brain Dev 27, 246–252, doi: 10.1016/j.braindev.2004.07.008 (2005). [DOI] [PubMed] [Google Scholar]
- 189.Gholipour T, Mitchell S, Sarkis RA & Chemali Z The clinical and neurobehavioral course of Down syndrome and dementia with or without new-onset epilepsy. Epilepsy & behavior : E&B 68, 11–16, doi: 10.1016/j.yebeh.2016.12.014 (2017). [DOI] [PubMed] [Google Scholar]
- 190.Araujo BH, Torres LB & Guilhoto LM Cerebal overinhibition could be the basis for the high prevalence of epilepsy in persons with Down syndrome. Epilepsy & behavior : E&B 53, 120–125, doi: 10.1016/j.yebeh.2015.10.004 (2015). [DOI] [PubMed] [Google Scholar]
- 191.d'Orsi G, Specchio LM & Apulian Study Group on Senile Myoclonic, E. Progressive myoclonus epilepsy in Down syndrome patients with dementia. J Neurol 261, 1584–1597, doi: 10.1007/s00415-014-7376-x (2014). [DOI] [PubMed] [Google Scholar]
- 192.Lehesjoki AE Molecular background of progressive myoclonus epilepsy. EMBO J 22, 3473–3478, doi: 10.1093/emboj/cdg338 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lott IT et al. Down syndrome and dementia: seizures and cognitive decline. J Alzheimers Dis 29, 177–185, doi: 10.3233/JAD-2012-111613 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Vossel KA, Tartaglia MC, Nygaard HB, Zeman AZ & Miller BL Epileptic activity in Alzheimer's disease: causes and clinical relevance. Lancet Neurol 16, 311–322, doi: 10.1016/S1474-4422(17)30044-3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wu JW et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci 19, 1085–1092, doi: 10.1038/nn.4328 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Vossel KA et al. Incidence and impact of subclinical epileptiform activity in Alzheimer's disease. Ann Neurol 80, 858–870, doi: 10.1002/ana.24794 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shea YF et al. Novel presenilin 1 mutation (p.F386I) in a Chinese family with early-onset Alzheimer's disease. Neurobiol Aging 50, 168 e169–168 e111, doi: 10.1016/j.neurobiolaging.2016.10.015 (2017). [DOI] [PubMed] [Google Scholar]
- 198.Kleen JK, Wu EX, Holmes GL, Scott RC & Lenck-Santini PP Enhanced oscillatory activity in the hippocampal-prefrontal network is related to short-term memory function after early-life seizures. J Neurosci 31, 15397–15406, doi: 10.1523/JNEUROSCI.2196-11.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Noebels J A perfect storm: Converging paths of epilepsy and Alzheimer's dementia intersect in the hippocampal formation. Epilepsia 52 Suppl 1, 39–46, doi: 10.1111/j.1528-1167.2010.02909.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chan J, Jones NC, Bush AI, O'Brien TJ & Kwan P A mouse model of Alzheimer's disease displays increased susceptibility to kindling and seizure-associated death. Epilepsia 56, e73–77, doi: 10.1111/epi.12993 (2015). [DOI] [PubMed] [Google Scholar]
- 201.Salehi A et al. Increased App expression in a mouse model of Down's syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron 51, 29–42, doi: 10.1016/j.neuron.2006.05.022 (2006). [DOI] [PubMed] [Google Scholar]
- 202.Trois MS et al. Obstructive sleep apnea in adults with Down syndrome. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine 5, 317–323 (2009). [PMC free article] [PubMed] [Google Scholar]
- 203.Jayaratne YSN et al. The facial morphology in Down syndrome: A 3D comparison of patients with and without obstructive sleep apnea. Am J Med Genet A 173, 3013–3021, doi: 10.1002/ajmg.a.38399 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Capone GT, Aidikoff JM, Taylor K & Rykiel N Adolescents and young adults with Down syndrome presenting to a medical clinic with depression: co-morbid obstructive sleep apnea. Am J Med Genet A 161A, 2188–2196, doi: 10.1002/ajmg.a.36052 (2013). [DOI] [PubMed] [Google Scholar]
- 205.Breslin J et al. Obstructive sleep apnea syndrome and cognition in Down syndrome. Dev Med Child Neurol 56, 657–664, doi: 10.1111/dmcn.12376 (2014). [DOI] [PubMed] [Google Scholar]
- 206.Worley G et al. Down Syndrome Disintegrative Disorder: New-Onset Autistic Regression, Dementia, and Insomnia in Older Children and Adolescents With Down Syndrome. J Child Neurol 30, 1147–1152, doi: 10.1177/0883073814554654 (2015). [DOI] [PubMed] [Google Scholar]
- 207.Fernandez F & Edgin JO Poor Sleep as a Precursor to Cognitive Decline in Down Syndrome : A Hypothesis. J Alzheimers Dis Parkinsonism 3, 124, doi: 10.4172/2161-0460.1000124 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Xie L et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377, doi: 10.1126/science.1241224 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Polsek D et al. Obstructive sleep apnoea and Alzheimer's disease: In search of shared pathomechanisms. Neurosci Biobehav Rev 86, 142–149, doi: 10.1016/j.neubiorev.2017.12.004 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mander BA, Winer JR, Jagust WJ & Walker MP Sleep: A Novel Mechanistic Pathway, Biomarker, and Treatment Target in the Pathology of Alzheimer's Disease? Trends Neurosci 39, 552–566, doi: 10.1016/j.tins.2016.05.002 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lavigne J et al. Thyroid dysfunction in patients with Down syndrome: Results from a multi-institutional registry study. Am J Med Genet A 173, 1539–1545, doi: 10.1002/ajmg.a.38219 (2017). [DOI] [PubMed] [Google Scholar]
- 212.King K, O'Gorman C & Gallagher S Thyroid dysfunction in children with Down syndrome: a literature review. Ir J Med Sci 183, 1–6, doi: 10.1007/s11845-013-0994-y (2014). [DOI] [PubMed] [Google Scholar]
- 213.Iughetti L et al. Ten-year longitudinal study of thyroid function in children with Down's syndrome. Horm Res Paediatr 82, 113–121, doi: 10.1159/000362450 (2014). [DOI] [PubMed] [Google Scholar]
- 214.Guaraldi F et al. Endocrine Autoimmunity in Down's Syndrome. Front Horm Res 48, 133–146, doi: 10.1159/000452912 (2017). [DOI] [PubMed] [Google Scholar]
- 215.Carsetti R et al. Reduced numbers of switched memory B cells with high terminal differentiation potential in Down syndrome. Eur J Immunol 45, 903–914, doi: 10.1002/eji.201445049 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chaker L et al. Age-dependent association of thyroid function with brain morphology and microstructural organization: evidence from brain imaging. Neurobiol Aging 61, 44–51, doi: 10.1016/j.neurobiolaging.2017.09.014 (2018). [DOI] [PubMed] [Google Scholar]
- 217.Tan ZS & Vasan RS Thyroid function and Alzheimer's disease. J Alzheimers Dis 16, 503–507, doi: 10.3233/JAD-2009-0991 (2009). [DOI] [PubMed] [Google Scholar]
- 218.Kinnear D et al. Prevalence of physical conditions and multimorbidity in a cohort of adults with intellectual disabilities with and without Down syndrome: cross-sectional study. BMJ open 8, e018292, doi: 10.1136/bmjopen-2017-018292 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Maatta T et al. Healthcare and guidelines: a population-based survey of recorded medical problems and health surveillance for people with Down syndrome. J Intellect Dev Disabil 36, 118–126, doi: 10.1080/13668250.2011.570253 (2011). [DOI] [PubMed] [Google Scholar]
- 220.Santoro SL, Martin LJ, Pleatman SI & Hopkin RJ Stakeholder Buy-In and Physician Education Improve Adherence to Guidelines for Down Syndrome. J Pediatr 171, 262–268 e261-262, doi: 10.1016/j.jpeds.2015.12.026 (2016). [DOI] [PubMed] [Google Scholar]
- 221.Wexler ID et al. Optimizing health care for individuals with Down syndrome in Israel. The Israel Medical Association journal : IMAJ 11, 655–659 (2009). [PubMed] [Google Scholar]
- 222.Capone GT et al. Co-occurring medical conditions in adults with Down syndrome: A systematic review toward the development of health care guidelines. Am J Med Genet A 176, 116–133, doi: 10.1002/ajmg.a.38512 (2018). [DOI] [PubMed] [Google Scholar]
- 223.Livingstone N, Hanratty J, McShane R & Macdonald G Pharmacological interventions for cognitive decline in people with Down syndrome. Cochrane Database Syst Rev 10, CD011546, doi: 10.1002/14651858.CD011546.pub2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Castro P, Zaman S & Holland A Alzheimer's disease in people with Down's syndrome: the prospects for and the challenges of developing preventative treatments. J Neurol 264, 804–813, doi: 10.1007/s00415-016-8308-8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Nelson L & Tabet N Slowing the progression of Alzheimer's disease; what works? Ageing Res Rev 23, 193–209, doi: 10.1016/j.arr.2015.07.002 (2015). [DOI] [PubMed] [Google Scholar]
- 226.Hefti E & Blanco JG Pharmacotherapeutic Considerations for Individuals with Down Syndrome. Pharmacotherapy 37, 214–220, doi: 10.1002/phar.1880 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hom CL et al. The relationship between living arrangement and adherence to antiepileptic medications among individuals with developmental disabilities. J Intellect Disabil Res 59, 48–54, doi: 10.1111/jir.12123 (2015). [DOI] [PubMed] [Google Scholar]
- 228.Rafii MS Improving Memory and Cognition in Individuals with Down Syndrome. CNS Drugs 30, 567–573, doi: 10.1007/s40263-016-0353-4 (2016). [DOI] [PubMed] [Google Scholar]
- 229.Rockwood K, Fay S, Jarrett P & Asp E Effect of galantamine on verbal repetition in AD: a secondary analysis of the VISTA trial. Neurology 68, 1116–1121, doi: 10.1212/01.wnl.0000258661.61577.b7 (2007). [DOI] [PubMed] [Google Scholar]
- 230.Rockwood K, Howlett SE, Hoffman D, Schindler R & Mitnitski A Clinical meaningfulness of Alzheimer's Disease Assessment Scale-Cognitive subscale change in relation to goal attainment in patients on cholinesterase inhibitors. Alzheimers Dement 13, 1098–1106, doi: 10.1016/j.jalz.2017.02.005 (2017). [DOI] [PubMed] [Google Scholar]
- 231.Antonarakis SE Down syndrome and the complexity of genome dosage imbalance. Nat Rev Genet 18, 147–163, doi: 10.1038/nrg.2016.154 (2017). [DOI] [PubMed] [Google Scholar]
- 232.Herault Y et al. Rodent models in Down syndrome research: impact and future opportunities. Dis Model Mech 10, 1165–1186, doi: 10.1242/dmm.029728 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Choong XY, Tosh JL, Pulford LJ & Fisher EM Dissecting Alzheimer disease in Down syndrome using mouse models. Frontiers in behavioral neuroscience 9, 268, doi: 10.3389/fnbeh.2015.00268 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gardiner K et al. Down syndrome: from understanding the neurobiology to therapy. J Neurosci 30, 14943–14945, doi: 10.1523/JNEUROSCI.3728-10.2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gardiner KJ Pharmacological approaches to improving cognitive function in Down syndrome: current status and considerations. Drug Des Devel Ther 9, 103–125, doi: 10.2147/DDDT.S51476 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sasaguri H et al. APP mouse models for Alzheimer's disease preclinical studies. EMBO J 36, 2473–2487, doi: 10.15252/embj.201797397 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wilcock DM, Schmitt FA & Head E Cerebrovascular contributions to aging and Alzheimer's disease in Down syndrome. Biochim Biophys Acta 1862, 909–914, doi: 10.1016/j.bbadis.2015.11.007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]