Abstract
Introduction
Displacement of refugees from highly endemic areas of leishmaniasis to adjacent countries is associated with the spread of Leishmania. Syria is a country with a known high endemicity for cutaneous leishmaniasis and the presence of Syrian refugees in Lebanon has contributed to the re-emergence of the disease. The aim of this article is to evaluate the burden of cutaneous leishmaniasis in Lebanon in view of the presence of a large number of Syrian refugees.
Methods
Data regarding all cases of leishmaniasis were collected from reports by the Lebanese Ministry of Public Health-Epidemiology Surveillance (LMPH-ESU), and the World Health Organization (WHO) between 2005 and 2018. All cases were reviewed in terms of area of residence, age and gender, clinical presentation, treatment, and outcome. An extensive literature review was conducted using “PubMed”, “Medline”, and “Google Scholar”.
Results
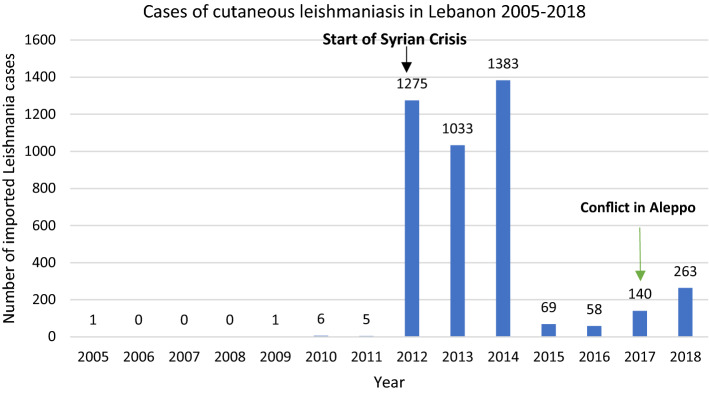
The annual number of leishmaniasis cases recorded in Lebanon between 2005 and 2011 ranged between 0 and 6 cases. In 2012, this number increased to 1275 cases and dropped to 263 in 2018, where all those infected were Syrian refugees from Aleppo, with zero cases of local transmission. Seventy-two percent of cases were seen in patients aged < 20 years. The predominant species of Leishmania was L. tropica followed by L. major.
Conclusion
Lebanon was affected by leishmaniasis following the Syrian crisis, and the influx of refugees to the country. Accurate disease monitoring and strategic training of healthcare personnel based within refugee camps are essential for proper containment. Preventative measures remain the best way to avoid both local and adjacent spread of leishmaniasis.
Keywords: Cutaneous leishmaniasis, Leishmania, Lebanon, Syria, Outbreak
Introduction
Leishmaniasis are vector-borne zoonotic diseases caused by protozoan parasites of the genus Leishmania transmitted by the female sand fly [1]. Wars are the most significant risk factor for the occurrence of leishmaniasis due to the resultant exposure of individuals to the parasite from population displacement and clustering [2]. Poor nutrition, low socioeconomic status, and environmental changes are additional contributors to the spread of this disease [1]. Approximately, 20 different species of the Leishmania protozoa can cause the disease and are distributed worldwide affecting millions of people across Asia, Africa, South America, and the Mediterranean Basin. The Mediterranean region accounts for 57% of the global incidence that reaches about 2 million cases annually [3]. Cases of cutaneous leishmaniasis (CL) reached 82,275 cases in 2018. Afghanistan and Iraq represent the remaining Middle Eastern countries with a heavy burden of leishmaniasis, with 38,407 and 11,426 CL cases, respectively, in 2018 [4, 5. Syria has the highest prevalence of CL in the Middle East and North Africa (MENA) region and the world [6], mainly in the city of Aleppo, which explains the origin of the name “Aleppo boil” [2]. After the start of the Syrian civil war in 2011, more than 6.5 million Syrians were displaced facilitating the spread of leishmaniasis to neighboring countries including Turkey, Iraq, and Lebanon [7]. Lebanon has the largest number of refugees per capita in the world: 1 out of every 4 residents. An estimated number of 1.5 million Syrian refugees were present in Lebanon [947,063 registered with United Nations High Commissioner for Refugees (UNHCR) as of January 2019 and many more present but unregistered] [8]. In this paper, we evaluate the burden of CL in Lebanon after the influx of Syrian refugees post Syrian crisis between 2005 and 2018.
Methods
This is a retrospective descriptive analysis of all cases of leishmaniasis reported to the LMPH-ESU, and the World Health Organization (WHO) between 2005 and 2018. All cases were reviewed in terms of district, clinical presentation, treatment, and outcome.
Data Collection
Data on documented Leishmania infection in Lebanon were collected from the publicly available LMPH-ESU database, which anonymously records these cases based on laboratory, epidemiologic, and clinical criteria. Physicians and health institutions are obliged to report cases to LMPH by filling the Leishmania case investigation form. Data were also collected from the WHO database. An extensive literature review was conducted using “PubMed”, “Medline”, and “Google Scholar”.
Data Analysis
Collected data from reported cases were plotted on a Microsoft Excel spread sheet and analyzed using GraphPad Prism 6. The data were then plotted into tables and graphs.
Results
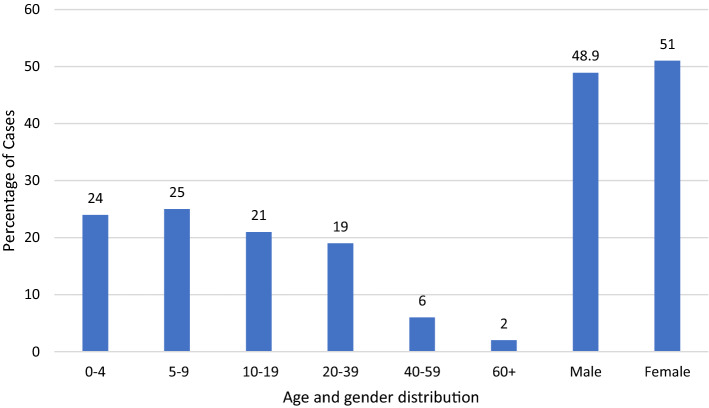
The annual number of leishmaniasis cases recorded in Lebanon between 2005 and 2011 ranged from 0 to 6 cases [9], and in 2012, this number increased to 1275 cases [10]. In 2016, the number of cases dropped to 58 to increase again to 140 cases in 2017 and 263 cases in 2018 [11] (Fig. 1). The Beqaa district comprised the highest percentage (65.2%) of leishmaniasis cases in Lebanon between 2014 and 2018. This number drops in other districts with the South district accounting for 12.8% of the cases and the North district for 9.6% of the cases. The other districts’ combined total was 12.4% of all cases [2]. In 2014, 65.3% of leishmaniasis cases occurred among Syrian refugees while during 2015 and 2017 this number ranged between 81.1 and 89.6%. Children, adolescents, and young adults (< 40 years) represented 85.4% of leishmaniasis cases in Lebanon, while only 10.8% of cases were documented among those older than 40 years. 46.5% of the cases belonged to males, whereas 53.5% of cases were among females. In 2017, 140 cases of Leishmania were reported in Lebanon by the LMPH-ESU. The highest number of cases occurred in the Beqaa district where 126 (90%) cases were reported, while all other districts combined had 14 (10%) cases [2]. Forty-eight CL patients were seen at the Al Bashaer Medical Center in North Lebanon between January and June 2017, originating from CL endemic and non-endemic areas of North Syria, with L. tropica found to be the predominant species followed by L. major [12]. Nodular lesions were the predominant clinical presentation (67%), with the majority of patients presenting with newly active lesions (90%). Treatment was achieved with antimonials, with 46 and 42% of patients requiring one or two courses, respectively. A 2018 study by the WHO on the endemicity of CL showed Lebanon to have the second highest imported cases at 263, followed by Iran at 158 cases. In contrast, there was no reported local transmission by the WHO in Lebanon versus more than 5000 cases in Syria [13].
Fig. 1.
Number of imported cutaneous leishmaniasis cases between 2005 and 2018 in Lebanon
Discussion
The Center for Disease Prevention and Control (CDC) defines several factors that could contribute to the emergence of an infectious disease [14]. Most notably are the changes in human demographics and behavior, and deficiencies in public health infrastructure (Table 1). Human population movement caused by migration or war is one of the main factors contributing to the spread of Leishmania in Lebanon [2]. Following the initial success in containing the leishmaniasis outbreak that started in 2012 as a result of the Syrian war and mass exodus of refugees to Lebanon, the country witnessed another increase in cases, albeit with lower intensity in 2017 and 2018, as a result of the eruption of conflict in the Aleppo region in Syria [2] (Fig. 1). The large-scale offensive by the Islamic State in Northern Aleppo led to a massive displacement of refugees [15]. The predominance of L. tropica in cases diagnosed in Lebanon relates to the endemicity of the species in Aleppo and surrounding regions [12]. In actuality, L. tropica is common in the Eastern Mediterranean, Middle East, North India, Afghanistan, and Northeast and South Africa, while L. major extends from West Africa to Central Asia [16]. Nevertheless, cases of L. major in Lebanon originated from Raqqa, the then capital of the Islamic State terrorist group. The appearance of L. major in Raqqa likely resulted from a mass migration from neighboring cities combined with ecological disruption of Phlebotomus papatasi (L. major vector) habitats [12, 17]. Progressive increase in the number of CL cases was already evident in Syria between 2007 and 2010 with a surge observed after the onset of conflict [18] (Table 2). Approximately, 18% of Syrian refugees live in informal tent settlements in Lebanon, while the rest in areas without any regional control plan [12]. The northern district, in particular, is one of the most deprived regions with a high rate of poverty and a high number of migrant refugees [12]. A 2018 report by the United Nations Office for the Coordination of Humanitarian Affairs (OCHA) found that half of the population of Lebanon’s northern districts are refugees living below the poverty line with the most vulnerable localities distributed along the border with Syria and east and north of Tripoli [19]. The poorest of Lebanon’s governates, Akkar shares a 100 km border with Syria and has three official crossing points. Due to this proximity to the border, the high number of refugees who migrated during the conflict localized in majority to Akkar governate [19]. Moreover, only three cases of potential local CL transmission were reported among migrant refugees in Lebanon, but it is unknown if these cases were asymptomatic carriers [12]. However, as cases of CL are likely underreported in Lebanon and no empirical assessments of CL underreporting are available from the LMPH-ESU, the true number of locally transmitted cases may be higher than reported by the LMPH and the literature.
Table 1.
Factors in infectious disease emergence and its applicability to the outbreak of leishmaniasis in Lebanon
| Factor | Applicability to Lebanon |
|---|---|
| Ecological changes (including those due to economic development and land use) | Poor sanitary infrastructure in refugee camps promotes the survival of the vector P. sergenti |
| Human demographics, behavior | Population migration from Syria during the war/civil conflict |
| International travel commerce | N/A |
| Technology and industry | N/A |
| Microbial adaptation and change | N/A |
| Breakdown in public health measures | The Syrian refugee camps have poor sanitary infrastructure, and no programs are in place for prevention and control of the disease and its vector |
Adapted from https://wwwnc.cdc.gov/eid/article/1/1/95-0102-t2
Table 2.
New cases of cutaneous leishmaniasis diagnosed at collaborating health centers, northern Syria
| Province | New cases observed during study period | Health centers | Mobile clinics | % program coverage of area | New cases estimated (Standardized cases for 1 year per province) | New cases reported in 2008 (1,17) | Incidence ratio |
|---|---|---|---|---|---|---|---|
| Aleppo | 12,296 | 43 | 21 | 70 | 13,174 | 18,603 | 0.7 |
| Idlib | 21,451 | 40 | 18 | 80 | 20,110 | 3,883 | 5.2 |
| Hama | 10,103 | 7 | 12 | 50 | 15,155 | 2,219 | 6.8 |
| Al-Raqqa | 5,546 | 7 | 5 | 65 | 11,376 | 290 | 39.2 |
| Al-Hasakah | 439 | 8 | 0 | 45 | 4,683 | 290 | 16.1 |
| Total | 49,835 | 105 | 56 | 64,498 | 25,285 | 2.6 |
Adapted from a 2018 study by Rehman et al. [18]
The lack of migratory control of Syrian refugees contributed to the second factor that favors outbreak, the breakdown of public health measures and deficiencies in public health infrastructure [14]. The influx into the poorer northern districts of Lebanon overextended the already scarce resources in these areas. Lack of proper sanitary infrastructure prevented access to clean water and hygienic services while also promoting the accumulation of waste, creating a breeding ground for the vector Phlebotomus sergenti (carrier of L.tropica) [8]. Consequently, an outbreak of CL becomes more likely with the combination of a suitable environment, the presence of sandfly vectors, and refugee migrants presenting with either symptomatic or asymptomatic CL (carriers) [12]. Following the coronavirus (COVID-19) pandemic, the humanitarian situation is Syria worsened with the current socioeconomic situation in the country deemed as one of the most challenging humanitarian conditions experienced in the past ten years of crisis by the OCHA [20]. This situation could create a vicious cycle whereby refugees returning to their country will be at high risk of contracting Leishmania due to the worsening health and sanitary resources and will be prompted to remigrate to Lebanon for more stable living conditions thereby creating a never-ending reservoir for the disease that could spread in Lebanon.
The lack of familiarity of many Lebanese physicians with the manifestations of the disease, the absence of well-trained laboratory personnel required to detect the parasite, and the limited access to treatment are other contributing factors [2]. Moreover, leishmaniasis seems to be a disease of younger people with 72% of the cases seen in patients aged < 20 years, and males and females sharing the disease almost equally (Fig. 2). One study hypothesized that human odor, CO2, and other elements in the breath could attract vectors into the house and thereby contribute to the higher proportion of young children affected [21]. Additionally, children and young adults are more likely to engage in outdoor activities, thereby increasing risk of exposure to sandflies. However, CL is a scarring disease with permanent effects that can lead to depression, anxiety, and an overall decrease in the quality of life of infected individuals [22]. Proper psychological services are, therefore, another necessity that needs to be provided to tackle the mental and socioeconomic burden imposed by CL.
Fig. 2.
Distribution of the cumulative cases of cutaneous leishmaniasis from 2005 to 2018 in Lebanon based on age and gender
Concerning environmental and geographical factors, a meta-analysis depicting the seasonality of P.papatasi at the global scale was published in 2020 [23]. The vector was found to have the highest activity between − 4o and 58° longitudinally and between 27° and 35° latitudinally, with August and September at a latitude of 33° showing the highest density of the vector at a global scale [23]. By comparison, Lebanon’s coordinates are 33° 52′ 24.81" latitude and 35° 51′ 49.50" longitude, which corresponds to the optimal coordinates where P.papatasi was found to have the highest activity [24]. Additionally, the vector has been found to favor dry seasons, a climate found in Lebanon during the summer months (June–August) [23, 25]. However, globally, habitat preference also varied by climate as indoor activity was recorded in colder periods (April–July), while outdoor activity was mainly seen during warmer periods (August–September) [23]. Another study was conducted in Israel, where different Phlebotomus sandflies were trapped over two years, and found that the P.sergenti population increase began in April, peaked in August–September (warm period), and significantly decreased in December (coldest period) [26]. As Syrian refugees reside in poorly made camps and tents, they are essentially without proper protection as either their shelters are without adequate insulation and roofs, or the winter storms have dismantled their shelters exposing them to the environment [27]. Consequently, the refugees are at high risk for sandfly exposure during both winter and summer months.
Previously, the LMPH assigned treatment centers distributed along all districts in the country to achieve accessibility and made reporting of all Leishmania cases to the ESU mandatory. In each center, a dermatologist or an infectious diseases specialist was responsible for the diagnosis and treatment of all the leishmaniasis cases. Suspected cases of leishmaniasis were recorded in a specific form to track patient history, presentation, diagnosis, and management. Punch biopsies were also performed and sent to the pathology department in a tertiary care center to confirm the diagnosis. Following laboratory confirmation, free of charge treatment was provided by the LMPH, resulting in a major drop of leishmaniasis cases from 1033 in 2013 to 69 in 2015 [2, 28]. However, in 2017, the conflict in Syria was exacerbated especially in the Aleppo region, leading to a surge in immigration and a re-increase in CL cases reaching 263 in 2018 [29]. Aside from a medical management standpoint, Lebanon has still not implemented appropriate prevention and control measures, a task that is seemingly more difficult in the wake of the COVID-19 pandemic and the 2019 economic recession [30]. First, poor sanitary infrastructure in refugee camps promotes the survival of the Phlebotomus vector as poor housing and domestic sanitary conditions (e.g., lack of waste management) may increase sandfly breeding sites. Sleeping outdoors or on the ground may also increase risk [31]. Therefore, a plan must be put in place to provide proper shelter to refugees with access to adequate sanitary services. Second, malnutrition, as is common with poverty, can aggravate the severity of leishmaniasis, and providing refugees with a stable food supply would facilitate disease management [32]. The primary ways to prevent leishmaniasis would therefore include screening of refugees for early diagnosis and effective prompt treatment, vector control, most notably through adequate personal protection (personal protection, insecticides, nets), effective disease surveillance, and education of the community and healthcare personnel about the disease [31].
Limitations
The primary limitation of our study is the poor data availability regarding leishmaniasis in the country. The LMPH stopped recording its data in 2018, and the data already available are likely an underestimate of the true number of cases. Additionally, most cases reported by the LMPH lack details about the species of Leishmania present within each region of the country. The only studies to identify species were conducted in 2012 and 2017 with the last entomological data concerning sandflies in Lebanon being related to a phlebotomine sandfly collection from 1995 [33].
Conclusion
The Syrian crisis and the consequent mass exodus of refugees have resulted in a significant increase in the number of leishmaniasis cases in Lebanon, as compared to the pre-Syrian war era. Lebanese physicians should familiarize themselves with the diagnosis and management of CL. The differential diagnosis of any chronic skin lesion, mainly on exposed areas, should include CL. Efforts to contain the disease should target both the refugee and host communities; however, given the current economic crisis the country is facing and the COVID-19 pandemic, proper measures will be hard to implement.
Author Contributions
The authors of this paper would like to acknowledge WA and NB as Joint First Authors. WA contributed to the study design, implementation, literature review, data collection, analysis, and interpretation, writing of the manuscript, and final revision. NB contributed to data collection, literature review, and writing of the manuscript. MK contributed to data analysis and writing of the manuscript. UM contributed to the study design and implementation and writing of the manuscript. NG and AB contributed to study implementation and writing of the manuscript. ARB contributed to study design, implementation, writing of the manuscript, and approval of final version.
Declarations
Conflict of Interest
The authors wish to declare that they do not have any conflict of interests.
Footnotes
The authors of this paper would like to acknowledge Walid Alam and Nazih A. Bizri as joint first authors.
Contributor Information
Nazih A. Bizri, Email: Nazih.ar.bizri@gmail.com
Walid Alam, Email: alamwalid94@gmail.com.
Michel Khoury, Email: melkhoury@northwell.edu.
Umayya Musharrafieh, Email: um00@aub.edu.lb.
Nada Ghosn, Email: esumohlb@gmail.com.
Atika Berri, Email: aberrymd@hotmail.com.
Abdul Rahman Bizri, Email: ab00@aub.edu.lb.
References
- 1.González U. Cochrane reviews on neglected diseases: the case of cutaneous leishmaniasis. Cochrane Database Syst Rev. 2013;3:ED000055. doi: 10.1002/14651858.ED000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alawieh A, Musharrafieh U, Jaber A, Berry A, Ghosn N, Bizri AR. Revisiting leishmaniasis in the time of war: the Syrian conflict and the Lebanese outbreak. Int J Infect Dis. 2014;29:115–119. doi: 10.1016/j.ijid.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 3.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 4.Gayer M, Legros D, Formenty P, Connolly MA. Conflict and emerging infectious diseases. Emerg Infect Dis. 2007;13(11):1625–1631. doi: 10.3201/eid1311.061093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Global Health Observatory (GHO) data. Neglected tropical diseases. Leishmaniasis.https://apps.who.int/neglected_diseases/ntddata/leishmaniasis/leishmaniasis.html. Accessed Sept 2020
- 6.McDowell MA, Rafati S, Ramalho-Ortigao M, Ben SA. Leishmaniasis: Middle East and North Africa research and development priorities. PLoS Negl Trop Dis. 2011;5(7):e1219. doi: 10.1371/journal.pntd.0001219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharara SL, Kanj SS. War and infectious diseases: challenges of the Syrian civil war. PLoS Pathog. 2014;10(10):e1004438. doi: 10.1371/journal.ppat.1004438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghanem N (2016) Local governance under pressure. Research in social stability in T5 area, North Lebanon. Arezzo, Italy: Oxfam Italia. https://data2.unhcr.org/en/documents/details/43854. Accessed Sept 2020
- 9.MOPH. Notifiable Communicable Diseases. Leishmaniasis. https://www.moph.gov.lb/en/DynamicPages/index/8#/en/view/196/general-surveillance-data-past-years. Accessed Sept 2020
- 10.Saroufim M, Charafeddine K, Issa G, et al. Ongoing epidemic of cutaneous leishmaniasis among Syrian refugees, Lebanon. Emerg Infect Dis. 2014;20(10):1712–1715. doi: 10.3201/eid2010.140288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO (2018) Status of endemicity of cutaneous leishmaniasis worldwide. https://www.who.int/leishmaniasis/burden/GHO_CL_2018.pdf?ua=1. Accessed Sept 2020
- 12.El Safadi D, Merhabi S, Rafei R, Mallat H, Hamze M, Acosta-Serrano A. Cutaneous leishmaniasis in north Lebanon: re-emergence of an important neglected tropical disease. Trans R Soc Trop Med Hyg. 2019;113(8):471–476. doi: 10.1093/trstmh/trz030. [DOI] [PubMed] [Google Scholar]
- 13.WHO. Leishmaniasis, Burden. https://www.who.int/leishmaniasis/burden/en/. Accessed Sept 2020
- 14.CDC (1995) Factors in the Emergence of Infectious Diseases. https://wwwnc.cdc.gov/eid/article/1/1/95-0102_article. Accessed 11 Feb 2021
- 15.Relief Web (2016) Closing Borders, Shifting Routes: Summary of Regional Migration Trends Middle East – May, 2016. https://reliefweb.int/report/world/closing-borders-shifting-routes-summary-regional-migration-trends-middle-east-may-2016
- 16.Ghatee MA, Taylor WR, Karamian M. The geographical distribution of cutaneous leishmaniasis causative agents in iran and its neighboring countries, a review. Front Public Health. 2020;8:11. doi: 10.3389/fpubh.2020.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Salem WS, Pigott DM, Subramaniam K, et al. Cutaneous leishmaniasis and conflict in Syria. Emerg Infect Dis. 2016;22(5):931–933. doi: 10.3201/eid2205.160042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rehman K, Walochnik J, Mischlinger J, Alassil B, Allan R, Ramharter M. Leishmaniasis in Northern Syria during Civil War. Emerg Infect Dis. 2018;24(11):1973–1981. doi: 10.3201/eid2411.172146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Relief Web (2018) Lebanon: North & Akkar Governorates Profile. https://reliefweb.int/report/lebanon/lebanon-north-akkar-governorates-profile-october-2018
- 20.Relief Web (2020) UNICEF Whole of Syria Humanitarian Situation Report - End-of-year 2020. https://reliefweb.int/report/syrian-arab-republic/unicef-whole-syria-humanitarian-situation-report-end-year-2020
- 21.Pinto MC, Campbell-Lendrum DH, Lozovei AL, Teodoro U, Davies CR. Phlebotomine sandfly responses to carbon dioxide and human odour in the field [published correction appears in Med Vet Entomol 2001 Sep; 15(3):349] Med Vet Entomol. 2001;15(2):132–139. doi: 10.1046/j.1365-2915.2001.00294.x. [DOI] [PubMed] [Google Scholar]
- 22.Yanik M, Gurel MS, Simsek Z, Kati M. The psychological impact of cutaneous leishmaniasis. Clin Exp Dermatol. 2004;29(5):464–467. doi: 10.1111/j.1365-2230.2004.01605.x. [DOI] [PubMed] [Google Scholar]
- 23.Karmaoui A. Seasonal distribution of phlebotomus papatasi, vector of zoonotic cutaneous leishmaniasis. Acta Parasitol. 2020;65(3):585–598. doi: 10.2478/s11686-020-00201-6. [DOI] [PubMed] [Google Scholar]
- 24.https://latitude.to/map/lb/lebanon. Accessed 7 Apr 2021
- 25.https://www.climatestotravel.com/climate/lebanon. Accessed 7 Apr 2021
- 26.Orshan L, Szekely D, Khalfa Z, Bitton S. Distribution and seasonality of Phlebotomus sand flies in cutaneous leishmaniasis foci, Judean Desert. Israel J Med Entomol. 2010;47(3):319–328. doi: 10.1603/me09096. [DOI] [PubMed] [Google Scholar]
- 27.https://www.hrw.org/news/2021/01/19/lebanon-dire-conditions-syrian-refugees-border-town. Accessed 7 Apr 7 2021
- 28.MOPH. Leishmaniasis investigation form. https://www.moph.gov.lb/userfiles/files/Esu_resources/Esu_forms/formi_lei1.pdf. Accessed 7 Apr 2021
- 29.Human Rights Watch. Syria, Events of 2017. https://www.hrw.org/world-report/2018/country-chapters/syria. Accessed 7 Apr 2021
- 30.Relief Web (2020) Lebanon economic monitor: the deliberate depression. https://reliefweb.int/report/lebanon/lebanon-economic-monitor-deliberate-depression-fall-2020
- 31.WHO. Fact Sheets, Leishmaniasis. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis
- 32.Nweze JA, Nweze EI, Onoja US. Nutrition, malnutrition, and leishmaniasis. Nutrition. 2020;73:110712. doi: 10.1016/j.nut.2019.110712. [DOI] [PubMed] [Google Scholar]
- 33.Haddad N, Léger N, Sadek R. Les phlébotomes du Liban. Inventaire faunistique [Sandflies of Lebanon: faunistic inventory] Parasite. 2003;10(2):99–110. doi: 10.1051/parasite/200310299. [DOI] [PubMed] [Google Scholar]