Abstract
Background:
Immunotherapy for wart employs ability of immune system to recognize certain viral, bacterial, and fungal antigens in previously sensitized individual inducing Type IV delayed-type hypersensitivity reaction (up-regulated Th1 cytokines IL-1, TNF-α, IFN-γ; down-regulated Th2 cytokines IL-10), not only to injected antigen but also against wart virus.
Aims:
To evaluate and compare the pattern of production of Th1 cytokines (IL-1, TNF-α, IFN-γ) and Th2 cytokines (IL-10) in patients receiving immunotherapy with purified-protein-derivative (PPD), Mycobacterium w (Mw), or mumps-measles-rubella (MMR) vaccine.
Methods:
The cohort study conducted on patients receiving immunotherapy with PPD, Mw, or MMR which was injected intradermally at baseline, repeated every 2 weeks for 6 doses?. Five-millilit?e?r blood was collected for evaluation of cytokines at baseline and 12 weeks of treatment. Blood was centrifuged to separate serum, stored at -80°C. Cytokines were measured by ELISA using a standard kit.
Results:
Nine participants in PPD group, 11 in Mw group, and 12 in MMR group completed the study. IL-1 was raised from baseline in all study arms and was significant in PPD group (P = 0.008). There was a predicted increase in IFN-γ in Mw and MMR groups but not in the PPD group. In the PPD group, IFN-γ was found to be down regulated. IL-10, a Th 2 cytokine was down regulated in all the groups at the study end from baseline, significantly so in the PPD group (P = 0.027) and MMR group (P = 0.001). TNF-α, being a Th1 cytokine was down regulated in all groups instead of an increase. In PPD group, IL-10 was significantly low at study end in patients who had complete resolution of warts.
Limitations:
Longer follow-up could not be done due to logistic issues.
Conclusion:
IL-1, TNF-α upregulation and IL-10 downregulation confirm that cytokine milieu plays an important role in wart immunotherapy. TNF-α has no contributory role. IL-10 can be used as a biomarker of complete response in PPD therapy.
KEY WORDS: Cytokines, IFN-γ, IL-1, IL-10, immunotherapy, MMR, Mw vaccine, PPD, TNF-α, viral warts
Introduction
Viral wart is one of the most commonly known dermatological diseases, caused by human papillomavirus (HPV) which is notorious for being contagious, recurrent, and recalcitrant. Treatment of warts becomes a challenge when they are numerous or present over inaccessible areas. Primary treatment modalities for verrucae include destructive therapies such as topical chemical cautery, cryotherapy, electro-cautery, and excision but none of them gives a guarantee of cure and recurrence is common, apart from producing scarring.[1,2,3] In addition, the destructive modalities are designed to remove the visibly infected lesions; however, nonvisible infected tissues are not targeted by these approaches resulting in recurrences. To overcome the challenges associated with the use of destructive therapies, many immunotherapeutic modalities have been investigated.
Immunotherapy for wart employs the ability of the immune system to recognize certain viral, bacterial, and fungal antigens in previously sensitized individual that “probably” induce a type IV delayed-type hypersensitivity reaction (Th1 cytokines IL-1, IL-12, TNF-α, IFN-γ up-regulated; Th2 cytokines IL-4, IL-10 down-regulated), not only to the injected antigen but also against the wart virus, which increases the ability of the immune system to recognize and clear HPV.[2,3,4] The immunotherapy being researched into are bivalent and quadrivalent HPV vaccines, topical contact sensitizers like dinitrochlorobenzene (DNCB), squaric acid dibutyl ester (SADBE?), and diphencyprone (DPCP), immunomodifier like imiquimod, antigen?s like Bacillus Calmette-Guerin (BCG), purified protein derivative (PPD), candida, trichophyton, mumps, mumps measles rubella vaccine (MMR), Mycobacterium w vaccine, and interferons.[3,5,6]
The mechanism of action of immunotherapy in warts is yet to be elucidated with a confirmation. The studies conducted using immunotherapy were restricted to evaluating the clinical profile of the participants. There is a dearth of research estimating the cytokines after using immune-enhancing therapies. Thus, we conducted our study with the objective to evaluate and compare the pattern of production of Th1 cytokines (IL-1, TNF-α, IFN-γ) and Th2 cytokines (IL-10) in patients receiving PPD or Mycobacterium w (Mw) or mumps measles rubella (MMR) vaccine. We also assessed the contributory role of cytokines in complete resolution of warts.
Methodology
The study was conducted after obtaining Institutional Ethics Committee permission. Adult consenting patients of either gender suffering from multiple viral nongenital warts (>5 warts) and were about to receive PPD, Mw, or MMR vaccine as their treatment protocol in the hospital were included. Pregnant, lactating women, presence of mucosal, ulcerated or inflamed warts, those who were suffering from systemic diseases were excluded. Those patients with aberrations in immunity which could interfere with cytokine production were excluded like HIV infection, organ transplantation, long-term steroid use, etc.
Study medications
The included participants received intradermal injections of the antigens on 6 occasions 2 weeks apart as a part of hospital protocol. The participants received either purified protein derivative (Tuberculin Diluted, Span diagnostics private limited, Gujarat, India), Mumps, measles, rubella vaccine (TRESIVAC® manufactured by Serum Institute of India) or Mycobacterium w vaccine (Immuvac, marketed by Cadila Pharmaceuticals, Licenced by National Institute of Immunology, New Delhi, India).
Immunotherapy protocol
Each of the patients received 0.1 mL of either 0.1 ml of PPD or Mw vaccine or 0.3 ml of MMR vaccine intradermally over the deltoid region at 2 weeks interval till a maximum of 6 injections or complete resolution of wart.
Preparation of blood for ELISA
Five-milliliter venous blood was drawn at baseline and 2 weeks after the completion of the six intradermal sessions. The clotted blood was centrifuged at 2000 rpm for 5 min to separate serum. The serum was stored at -80°C.
ELISA
Sandwich ELISA was performed with the stored serum using standard ELISA kits (RayBiotech, Norcross, Georgia, USA) to measure the amount of IL-10, IL-1, TNF-α, and IFN-γ in the samples. All the four ELISA test kits employ a similar principle and procedure. The method is in-vitro enzyme-linked immunosorbent assays (using sandwich ELISA method) for the quantitative measurement of the parameter (TNF-α, IFN-γ, IL-1, IL-10). The assay employs an antibody specific to the antigen (TNF-α, IFN-γ, IL-1, IL-10) coated on each well of a 96 well plate. The calibrators and samples are pipetted into the wells and the antigen present in the sample is bound to the wells by the immobilized antibody. The wells are then washed and a biotinylated second antibody is added. Excess unbound biotinylated antibody is washed and horseradish peroxidase (HRP)-conjugated streptavidin reagent is added to the wells. Wells are again washed to remove unbound HRP-streptavidin. TMB substrate solution is added to the wells and blue color develops in proportion to the bound antigen (TNF-α, IFN-γ, IL-1, IL-10). Stop solution is added which changes color to yellow; the intensity of color produced is measured at 450 nm. From the absorbance of the calibrators, a calibration curve is generated and value of the samples quantified by comparing with the calibration curve.
Sample size calculation
The calculated sample size was 12 participants in each of the three study arms. The total number of participants was 36. The sample size was calculated to note a difference in mean of 7.66 units with respect to TNF-α values before and after treatment with contact immunotherapy.[7] The standard deviation was taken at 6.25 with 80% power, probability of α error as 0.05, drop-out rate at 10%. Thus, each group had 12 participants.
Statistical analysis
Data was tested for normality using Kolmogorov-Smirnov test. All data groups were nonparametric in nature. Numerical data was expressed as mean ± standard deviation. Kruskal–Wallis ANOVA was used to compare the data of individual cytokines between treatment groups. Wilcoxon's test was used to compare data within same treatment group. Subgroups were done according to the achievement of complete resolution of warts and Mann–Whitney U test compared the cytokine changes between these two subgroups. For estimating clinical changes within groups, Friedman's ANOVA with post hoc Dunn's test was used and Kruskal–Wallis ANOVA with post hoc Dunn's test was done for estimating between groups changes. The statistical software Medcalc® v 9.6.4.0 was used for analysis drawing the graphs.
Results
Twelve participants per group were included in the study. One patient in the Mw group and 3 patients in the PPD group were lost to follow-up. The clinicodemographic parameters of the study population are expressed in Table 1.
Table 1.
Clinicodemographic characteristics of study participants
| Clinicodemographic parameters | PPD group (n=9) | Mw group (n=11) | MMR group (n=12) | P (between groups) |
|---|---|---|---|---|
| Age | ||||
| Mean±SD | 25.11±11.53 | 29±13.08 | 26.67±6.87 | 0.286 |
| Median (IQR) | 20 (20, 24.75) | 23 (19, 37) | 28 (20, 31) | |
| Gender | ||||
| Male: Female | 3:6 | 3:8 | 8:4 | 0.124 |
| Occupation | ||||
| Student | 4 | 4 | 2 | 0.098 |
| Skilled worker | 2 | 3 | 3 | |
| Unskilled worker | 0 | 0 | 5 | |
| Homemaker | 3 | 4 | 2 | |
| Residence | ||||
| Urban: Rural | 8:1 | 7:4 | 7:5 | 0.295 |
| Education | ||||
| Literate: Illiterate | 9:0 | 11:0 | 10:2 | 0.169 |
| Income | ||||
| APL: BPL | 9:0 | 10:1 | 8:4 | 0.087 |
| Duration (in months) | ||||
| Mean±SD | 29.56±14.76 | 30.36±25.15 | 22.83±20.21 | 0.622 |
| Median (IQR) | 26 (21, 36) | 24 (12, 36) | 15 (8, 30) | |
| Aggravation factor | ||||
| Absent | 7 | 10 | 6 | 0.087 |
| Threading | 1 | 1 | 0 | |
| Soaking in water | 0 | 0 | 4 | |
| Walking | 1 | 0 | 2 | |
| Köebnarization | ||||
| Present: Absent | 1:8 | 6:5 | 6:6 | 0.102 |
| Type of wart | ||||
| Verruca vulgaris | 3 | 5 | 6 | 0.672 |
| Verruca plana | 1 | 2 | 0 | |
| Periungual | 1 | 1 | 0 | |
| Palmoplantar | 4 | 3 | 5 | |
| Filliform | 0 | 0 | 1 | |
| Previous treatment | ||||
| Absent | 0 | 4 | 6 | |
| Chemical cautery | 3 | 2 | 1 | 0.219 |
| Radiofrequency ablation | 0 | 1 | 1 | |
| Excision | 0 | 0 | 1 | |
| Alternative medicine | 6 | 4 | 3 |
IL-1 was raised from baseline in all study arms, and the rise was significant in PPD group (P = 0.008). Immunotherapy thus boosted IL-1 action [Table 2]. TNF-α, being a Th1 cytokine was down regulated in all groups instead of an increase, significantly so in the Mw and MMR group [Table 3]. There was a predicted increase in IFN-γ in Mw and MMR groups but not in the PPD group. In the PPD group, IFN-γ was found to be down regulated. In MMR group, there was near significant (P = 0.052) rise in IFN-γ value [Table 4]. IL-10, a Th 2 cytokine was down regulated in all the groups at the study end from baseline, significantly so in the PPD group (P = 0.027) and MMR group (P = 0.001) [Table 5].
Table 2.
Changes in IL-1 between treatment arms
| IL-1 | PPD group (n=9) | Mw group (n=11) | MMR group (n=12) | P (between groups) |
|---|---|---|---|---|
| Baseline | 3.22±1.56 | 8.09±9.08 | 5.58±2.23 | 0.017 |
| 3 (2, 4.25) | 6 (4.25, 7.5) | 5.58 (4, 7) | ||
| End of treatment | 6.78±4.21 | 9±8.81 | 25.75±59.41 | 0.830 |
| 5 (4.75, 7.5) | 7 (3.5, 8) | 5 (2.5, 19.5) | ||
| P (within groups) | 0.008 | 0.465 | 0.432 |
• P-value for between groups by Kruskal–Wallis ANOVA. • P-value for within groups by Wilcoxon test
Table 3.
Changes in TNF between treatment arms
| TNF | PPD group (n=9) | Mw group (n=11) | MMR group (n=12) | P (between groups) |
|---|---|---|---|---|
| Baseline | 247.22±359.50 | 250.45±582.40 | 103.50±64.01 | 0.165 |
| 83 (60, 287.5) | 58 (52.25, 84.25) | 73.5 (65.5, 133.5) | ||
| End of treatment | 129.22±179.19 | 51.82±4.94 | 55.75±10.70 | 0.281 |
| 60 (51, 101.75) | 52 (49.5, 55) | 54.5 (51, 65) | ||
| P (within groups) | 0.203 | 0.042 | 0.0005 |
P-value for between groups by Kruskal–Wallis ANOVA. P-value for within groups by Wilcoxon test
Table 4.
Changes in IFN-γ between treatment arms
| IFN | PPD group (n=9) | Mw group (n=11) | MMR group (n=12) | P (between groups) |
|---|---|---|---|---|
| Baseline | 21.06±36.32 | 30.92±41.11 | 24.29±22.27 | 0.690 |
| 0.9 (0.6, 25.25) | 21 (0.6, 28.75) | 18 (12, 33.5) | ||
| End of treatment | 19.78±29.53 | 50.62±61.92 | 54.15±84.80 | 0.466 |
| 3 (0.48, 37.75) | 33 (21.25, 59.5) | 84.80 (0.5, 52) | ||
| P (within groups) | 0.820 | 0.413 | 0.052 |
P-value for between groups by Kruskal–Wallis ANOVA. P-value for within groups by Wilcoxon test
Table 5.
Changes in IL-10 between treatment arms
| IL-10 | PPD group (n=9) | Mw group (n=11) | MMR group (n=12) | P (between groups) |
|---|---|---|---|---|
| Baseline | 35.42±37.58 | 56.07±47.65 | 53.42±30.33 | 0.222 |
| 19 (7.9, 63.5) | 37 (26.25, 66.5) | 47.5 (28, 78) | ||
| End of treatment | 19.27±25.25 | 36.51±32.61 | 32.05±32.56 | 0.396 |
| 5 (0.88, 29.75) | 21 (8.25, 68.25) | 21.05 (2.5, 63.5) | ||
| P (within groups) | 0.027 | 0.123 | 0.001 |
P-value for between groups by Kruskal–Wallis ANOVA. P-value for within groups by Wilcoxon test
The size of warts at baseline for the three study arms was comparable (P = 0.495, Kruskal–Wallis ANOVA). The size of warts progressively and significantly decreased in all the groups from the 2nd follow-up onwards till study end (P < 0.001, Friedman's ANOVA followed by post hoc Dunn's test). However, between group comparison showed no significant change in 1st follow-up (P = 0.461), 2nd follow-up (P = 0.979), 3rd follow-up (P = 0.582), 4th follow-up (P = 0.554), 5th follow-up (P = 0.715), and 6th follow-up (P = 0.213) (Kruskal–Wallis ANOVA). The number of warts was statistically similar at baseline (P = 0.229). The number of warts steadily decreased and the reduction was statistically significant (P < 0.001) from the 2nd follow-u?p onwards in the Mw and MMR group till study end and from the 3rd follow-up onwards for PPD group. Between groups, comparison showed comparable results at all follow-up visits (1st follow-up: P = 0.227, 2nd follow-up: P = 0.289, 3rd follow-up: P = 0.374, 4th follow-up: P = 0.665, 5th follow-up: 0.559, 6th follow-up: P = 0.316) [Figures 1 and 2].
Figure 1.
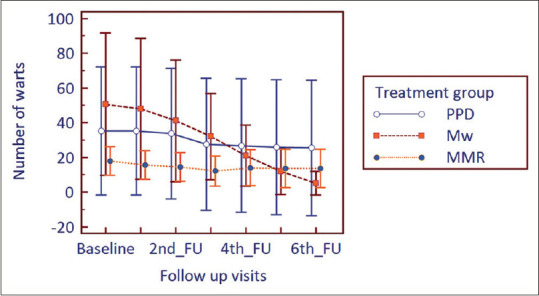
Changes in the number of warts from baseline to study end in treatment arms (FU = follow-up)
Figure 2.
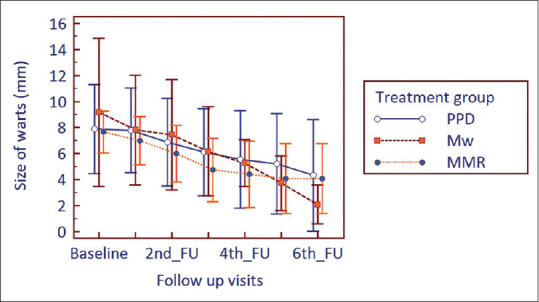
Changes in the size of warts from baseline to study end in treatment groups (FU = follow-up)
In viral warts, complete resolution, i.e., number of warts at study end 0, is usually the clinician's target. Complete resolution was achieved in 4 participants (44.44%) in the PPD group, 5 patients each in the Mw group (45.45%) and MMR group (41.66%). In the PPD group, IL-10 was significantly low at the study end in the patients who had complete resolution of warts than the partial responders [Tables 6–8].
Table 6.
Comparison of cytokines in complete and partial resolution of warts in PPD group
| PPD | Complete resolution of wart number (n=4) | Partial resolution of wart number (n=5) | P |
|---|---|---|---|
| IL-10 | |||
| Baseline | 13.65±10.10 | 52.84±43.53 | 0.286 |
| 14 (5.8, 21.5) | 60 (14.55, 83) | ||
| EOT | 7.43±10.57 | 28.74±30.64 | 0.556 |
| 2.95 (0.85, 14) | 22 (0.925, 55) | ||
| IL-1 | |||
| Baseline | 2.75±2.22 | 3.60±0.89 | 0.219 |
| 2 (1.5, 4) | 3 (3, 4.25) | ||
| EOT | 4.25±0.96 | 8.80±4.82 | 0.027 |
| 4.5 (3.5, 5) | 7 (5.75, 11) | ||
| IFN | |||
| Baseline | 33.85±53.06 | 10.82±15.25 | 0.713 |
| 11.45 (0.7, 67) | 0.9 (0.6, 21.5) | ||
| EOT | 29.55±36.37 | 11.96±24.08 | 0.556 |
| 19 (3.1, 56) | 0.9 (0.46, 16) |
P-value between groups determined by Mann–Whitney U test
Table 8.
Comparison of cytokines in complete and partial resolution of warts in MMR group
| MMR | Complete resolution of wart number (n=5) | Partial resolution of wart number (n=7) | P |
|---|---|---|---|
| IL-10 | |||
| Baseline | 56.20±29.04 | 51.43±33.36 | 0.745 |
| 56 (33.75, 81.00) | 44 (24.5, 71.25) | ||
| EOT | 42.46±25.40 | 24.61±36.85 | 0.639 |
| 46 (28.33, 61.25) | 4.5 (2.45, 59.03) | ||
| IL-1 | |||
| Baseline | 6.00±1.22 | 5.29±2.81 | 0.624 |
| 6 (5.5, 7) | 6 (3.25, 7.5) | ||
| EOT | 45.0±93.38 | 12.0±11.37 | 0.624 |
| 4 (1.75, 57.5) | 6 (3, 20.25) | ||
| IFN | |||
| Baseline | 14.7±14.20 | 31.14±25.36 | 0.372 |
| 17 (0.83, 24.25) | 19 (12.25, 45.75) | ||
| EOT | 31.38±28.24 | 70.41±109.15 | 0.935 |
| 52 (0.48, 52) | 41 (9.13, 52.25) |
•P-value between groups determined by Mann–Whitney U test
Table 7.
Comparison of cytokines in complete and partial resolution of warts in Mw group
| Mw | Complete resolution of wart number (n=5) | Partial resolution of wart number (n=6) | P |
|---|---|---|---|
| IL-10 | |||
| Baseline | 76.40±63.82 | 39.13±22.93 | 0.523 |
| 37 (26.75, 143) | 39.5 (25, 56) | ||
| EOT | 57.00±35.13 | 19.43±19.24 | 0.100 |
| 76 (19.5, 82.75) | 13 (5, 40) | ||
| IL-1 | |||
| Baseline | 5.60±1.82 | 10.17±12.29 | 0.855 |
| 6 (4.5, 6.5) | 5.5 (4, 8) | ||
| EOT | 10.80±11.97 | 7.50±5.89 | 0.927 |
| 7 (4.5, 13.25) | 7.5 (3, 8) | ||
| IFN | |||
| Baseline | 50.60±56.44 | 14.52±11.05 | 0.411 |
| 30 (0.55, 110.5) | 19.5 (0.6, 22) | ||
| EOT | 82.20±81.32 | 24.30±22.00 | 0.144 |
| 55 (30, 115.5) | 22 (0.6, 40) |
•P-value between groups determined by Mann–Whitney U test
Discussion
Of the Th1 cytokines, there was a predicted rise in the IL-1 values from baseline in all the groups after treatment. Immunotherapy, thus, boosted IL-1 action and also IL-1 helped to clear virus as it has direct virucidal action.[8] IL-1 having a direct virucidal effect contributes to virus clearing and augments the immunotherapeutic action, thus plays a key role among other cytokines. In the PPD group, this rise was significant which in-turn confirms the immunotherapeutic action of PPD in viral wart.
IFN γ was upregulated in Mw vaccine and MMR group but down-regulated in the PPD group. IFN γ, thus, has minimal immunotherapeutic role during treatment with PPD, which can be explained by the fact that rise in IL-1 plays the lead role with PPD immunotherapy and do not require the participation of other Th1 cytokines to exert its immunotherapeutic effect. On the other hand, Mw vaccine and MMR rely on IFN-γ to exert their effect. A similar increase in IFN-γ levels was also f?ound in a study by Mitsuishi et al.[9] In the abovementioned study, cimetidine treatment for viral warts has shown enhancement in IL-2 and IFN-γ expression but not IL-18 expression in lesional skin. Cimetidine activates Th1 cells to produce IL-2 and IFN- γ and that their expression correlates with wart remission.[9]
TNF-α was downregulated instead of showing a rise in all the three treatment arms establishing that it has minimal role in immunotherapy with warts. Our findings regarding TNF-α refute the results obtained by the study on local expression of TNF-α mR?NA in wart tissue by Azim et al., where a significant increase was obtained compared to healthy controls.[10]
The downregulation of Th2 cytokine, IL-10 played an important part in eradicating the di?sease. In a study by Park et al., it was found that contact immunotherapy with squaric acid dibutyl ester caused the percentages of CD3+/IL-4+ and CD3+/IL-10 to increase after treatment in poor responders but not in excellent responders.[7] They concluded that response to contact immunotherapy can vary depending on the types of cytokine changes occurring after treatment. An excessive shift toward a type 2 pattern and increased IL-10 production after contact immunotherapy may be the factors that hinder HPV clearance. Thus, our study supports the study by Park et al. in that decrease in IL-10 is associated with a positiv?e response. Recently Cao et al.[11] suggested that contact immunotherapy might be a promising treatment for HPV infection. They reported high expression of IL-10 and TGF-β by FOXP3 Tregs in large warts. Thus, high expression of IL-10 is associate?d with persistence of warts promoting their increase in size. In our study, a decrease in IL-10 also led to decrease in size and number of wart lesions.
The upregulation of IL-1, INF-γ and the downregulation of IL-10 lead to induction of Type IV hypersensitivity reaction against the wart virus. The immunity induced by immunotherapy was effective clinically, evidenced by the significant reduction of the size and number of the warts in all the three treatment groups.
The study also assessed if cytokines play a role in the complete resolution of warts. In the PPD group, IL-10 was significantly low at the study end in the patients who had complete resolution of warts than the partial responders suggesting that IL-10 played the key role in complete responders. In the other treatment arms, such association was not found with any single cytokine. IL-10 has paramount importance in the persistence and progres?sion of viral diseases. Though it was once considered to be merely a Th-2 cytokine, it is now accepted that IL-10 is not restricted to T helper cells but is secreted by all leucocytes and even epithelial cells and keratinocytes. IL-10 via its receptor IL-10 R1, IL-10 R2 activates JAK/STAT signaling leading to inhibition of proinflammatory mediator, antigen presentation, and phagocytosis. IL-10 also releases IL-1 receptor antagonist, soluble TNFα receptor, and IL-27 and can enhance regulatory T cells, CD 8 + T cells, mast cells, NK cells, B cells. In viral infection, the role of IL-10 becomes conspicuous by its ability to prevent maturation of antigen-presenting cells like macrophage and dendritic cells. The lowering of IL-10 by immunotherapy gives a clue that it is a predominant cytokine that influences the outcome with immunotherapy.[12]
Cytokines can be used as a biomarker to predict the end point of therapy. The number of sessions of intradermal injection of antigen ranges from 1 to 10 weeks in various studies.[3,13] The number of injections can be modified according to the increase in titer of IL-1, IFN-γ and the decrease in titer of IL-10. For PPD immunotherapy, IL-10 can be used as a biomarker of complete response.
We evaluated and compared head to head the changes in cytokines using PPD, MMR, and Mw vaccines. Such estimation and comparison have not been evaluated previously by any such immunotherapy study on warts. In a way, this study has pioneered in the estimation of baseline cytokines and also after treatment cytokines. However, much more data is required using other immunotherapeutic agents to pin point the responsible cytokine in those treatments. The way forward of this study is the estimation of intracellular cytokines in the peripheral blood mononuclear cells.
Conclusion
Downregulation of Th2 cytokine IL-10 and upregulation of Th1 cytokines IL-1 and IFN-γ led improved cell immediate immunity which in-turn led to the clearance of warts. TNF-α was downregulated, thus was noncontributory in immunotherapy. Regarding the complete resolution of warts, IL-10 in PPD group played a key role and can be used as a biomarker for a complete response.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Horn TD, Johnson SM, Helm RM, Roberson PK. Intralesional immunotherapy of wars with mumps, candida and trichophyton skin test antigen: A single-blinded randomized, and controlled trial. Ach Dermatol. 2005;141:589–94. doi: 10.1001/archderm.141.5.589. [DOI] [PubMed] [Google Scholar]
- 2.Burns DA, Breathnach SM, Cox NH, Griffiths CEM. Rook's Textbook of Dermatology. 8th ed. Vol. 2. UK: John Wiley & Sons; 2010. pp. 33.37–33.39. [Google Scholar]
- 3.Chandrashekar L. Intralesional immunotherapy for the management of warts. Indian J Dermatol Venereol Leprol. 2011;77:261–3. doi: 10.4103/0378-6323.79694. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahum MA, Ahmed RE, Al Sadar M. Intradermal vs intralesional purified protein derivatives in treatment of warts. Gulf J Dermatol and Venereol. 2011;18:21–6. [Google Scholar]
- 5.Meena JK, Malhotra AK, Mathur DK, Mathur DC. Intralesional immunotherapy with Mycobacterium w vaccine in patients with multiple cutaneous warts: Uncontrolled open study. JAMA Dermatol. 2013;149:237–9. doi: 10.1001/jamadermatol.2013.866. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S, Malhotra AK, Verma KK, Sharma VK. Intralesional immunotherapy with killed Mycobacterium w vaccine for the treatment of ano-genital warts: An open label pilot study. J Eur Acad Dermatol Venereol. 2008;22:1089–93. doi: 10.1111/j.1468-3083.2008.02719.x. [DOI] [PubMed] [Google Scholar]
- 7.Park HJ, Choi YW, Kim SH, Shin MS, Lee SW, Oh MK, et al. Change in cytokines in patients with warts after contact immunotherapy with squaric acid dibutylester. Clin Exp Dermatol. 2013;38:775–81. doi: 10.1111/ced.12075. [DOI] [PubMed] [Google Scholar]
- 8.Nofal A, Salah E, Nofal E, Yosef A. Intralesional antigen immunotherapy for the treatment of warts: Current concepts and future prospects. Am J Clin Dermatol. 2013;14:253–60. doi: 10.1007/s40257-013-0018-8. [DOI] [PubMed] [Google Scholar]
- 9.Mitsuishi T, Iida K, Kawana S. Cimetidine treatment for viral warts enhances IL-2 and IFN-gamma expression but not IL-18 expression in lesional skin. Eur J Dermatol. 2003;13:445–8. [PubMed] [Google Scholar]
- 10.Azim AA, Said A, Swelim MM, el-Sheikh NA. Local expression of tumor necrosis factor-alpha and interleukin-4 mRNA in different types of warts. Egypt J Immunol. 2004;11:15–21. [PubMed] [Google Scholar]
- 11.Cao Y, Zhao J, Lei Z, Shen S, Liu C, Li D, et al. Local accumulation of FOXP3+regulatory T cells: Evidence for an immune evasion mechanism in patients with large condylomata acuminata. J Immunol. 2008;180:7681–6. doi: 10.4049/jimmunol.180.11.7681. [DOI] [PubMed] [Google Scholar]
- 12.Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012;32:23–63. doi: 10.1615/critrevimmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulhem E, Pinelis S. Treatment of nongenital cutaneous warts. Am Fam Physician. 2011;84:288–93. [PubMed] [Google Scholar]


