Abstract
Post-kala-azar dermal Leishmaniasis (PKDL) is one of the important neglected tropical diseases, which has a tremendous epidemiological significance, being the reservoir of kala-azar. Relapse and resistance to treatment along with the lack of a drug of choice and consensus treatment guideline pose a significant problem in the management of PKDL. The aim of this article was to review the available therapeutic options for PKDL, with special emphasis on their pharmaco-dynamics, pharmaco-kinetics, effectiveness, safety, tolerability, and cost factor. A comprehensive English language literature search was done for therapeutic options in PKDL across multiple databases (PubMed, EMBASE, MEDLINE, and Cochrane) for keywords (alone and in combination). MeSH as well as non-MeSH terms such as “Kala-azar,” “Leishmaniasis” AND “Treatment,” “Management,” “Antimony Sodium Gluconate,” “Meglumine Antimoniate,” “Amphotericin B,” “Paromomycin,” “Miltefosine” were taken into consideration. Among 576 relevant articles, 15 were deemed relevant to this review. These articles were evaluated using “Oxford Centre for Evidence-Based Medicine (OCEBM)” AND “strength of recommendation taxonomy” (SORT) with respect to the level of evidence and grade of recommendation. The review includes 15 studies. The use of sodium stibogluconate is being discouraged because of multiple documented reports of treatment failure. Liposomal amphotericin B is emerging as a favorable option, owing to its superiority in terms of effectiveness and safety profile. Miltesfosine is the drug of choice in India because of the ease of oral administration and minimal risk of toxicity. Isolated Paromomycin alone is not effective in PKDL; however, combination therapy with sodium stibogluconate is found to be safe and effective. Combination of amphotericin B and miltefosine is one of the excellent options. Immunotherapy with combination of alum-precipitated autoclaved Leishmania major (Alum/ALM) vaccine + Bacille Calmette-Gu´erin (BCG) has shown promising results. Kala-azar continues to haunt the tropical countries and PKDL being its reservoir is threatening its elimination. With the availability of drugs such as liposomal amphotericin B and miltefosine, apart from the advent of immunotherapy, the future of treatment of this condition looks promising.
KEY WORDS: Amphotericin B, antifungal, antimonials, immunotherapy, miltefosine, paromomycin, Post Kala-azar Dermal Leishmaniasis, treatment
Introduction
Post-Kala-azar dermal leishmaniasis (PKDL) is one of the important neglected tropical diseases (NTDs) whose implication in the elimination of Kala-azar places it in a crucial position. Being a NTD, its management remains challenging, and the hindrances are recognized to range from funding issues to political commitment, apart from genuine lack of national and international cooperation and collaboration. Experts have also recognized the paucity of evidence-based therapeutic regimen and consensus guideline as another hurdle in its management.[1] A vital gray area remains the end point of therapy, which is yet to be defined; while recommends its continuation till the disappearance of lesions, other workers[2] including the authors are of the opinion that macular lesions persist for a long time even after completion of therapy. In deciding the therapeutic regime the natural course of the disease also needs to be considered; for example, in Sudan, spontaneous remission is seen in non-severe PKDL (84% within 1 year). Hence, it is suggested that mild form of Sudanese PKDL be left untreated under careful follow-up.[3]
The problem of PKDL therapy is compounded by the fact that asymptomatic nature of the disease limits the self-referral. Poor health-care seeking behavior of the affected individuals makes it imperative that active surveillance is essential for eliminating the hidden cases. Parasite becoming resistant to the drugs with passage of time poses another problem in its management; thus highlighting the need for the development of new drugs.
The choice of drug in endemic countries is decided by the national drug policy which is developed based on benefit–risk ratio, availability of anti-leishmanial medicines and other considerations such as drug resistance, health service setting, etc. [Table 1][1] The difference is also contributed by the difference in natural course of the disease. In Indian population PKDL develops in around 5%–10% cured patients of kala-azar after 6 months to many years and never resolves spontaneously; whereas in eastern Africa it develops in almost 50% of kala-azar patients sometimes concurrently or at least 6 months and demonstrates spontaneous recovery.[1] The recommended regime is bound to evolve with time with the advent of data from recent clinical trials, which this article will attempt to delve into.
Table 1.
Therapeutic recommendation of PKDL in different part of globe[1]
| Region | Recommended treatment regime | End point of therapy | |
|---|---|---|---|
| East Africa* | Pentavalent antimonials 20 mg Sb5+/kg per day intramuscularly or intravenously for 30-60 days, when indicated (C)† | Liposomal amphotericin B: 2.5 mg/kg per day by infusion for 20 days, when indicated (C)† | Flattening of lesions and improvement of dyschromia. |
| Bangladesh, India, Nepal | Amphotericin B deoxycholate | Miltefosine Orally for 12 weeks at dosage: | Disappearance of the lesion |
| Intermittent amphotericin B deoxycholate, 1 mg/kg per day by infusion, up to 60-80 doses over 4 months (20 days on, 20 days off) (C)† | Children aged 2-11 years: 2.5 mg/kg per day; People aged ≥12 years and <25 kg: 50 mg/day; 25-50 kg 100 mg/day; >50 kg body weight, 150 mg/day; (A)† | ||
*In East Africa: Patients with severe (grade 3) or disfiguring disease, those with lesions that have remained for >6 months, those with concomitant anterior uveitis and young children with oral lesions that interfere with feeding are treated. †Alphabet in parenthesis denotes the grade of recommendation
It needs to be emphasized that management of PKDL, does not only require pharmacotherapy but rather a multi-pronged approach including community participation for identification and referral of suspected cases to higher centers, ensuring compliance to therapy and monitoring of side-effects, development of sensitive and specific diagnostic tools which can be used in the field setting, control of vector and reservoir. This article will highlight on the different pharmacotherapeutic agents including immunotherapy.
Methodology for Literature Search in Preparation of the Review
A comprehensive English language literature search for management options in Post Kala-azar Dermal Leishmaniasis (PKDL) across multiple databases (PubMed, EMBASE, MEDLINE and Cochrane) for keywords (alone and in combination) and MeSH items as well as non-MeSH terms such as “Kala-azar,” “Leishmaniasis” AND “Treatment,” “Management,” “Antimony Sodium Gluconate,” “Meglumine Antimoniate,” “Amphotericin B,” “Paromomycin,” “Miltefosine” was undertaken. 576 results were obtained, of which 15 were noted to be relevant to this review.
The relevant articles were evaluated using two systems comprising “Oxford Centre for Evidence-Based Medicine (OCEBM)” AND “strength of recommendation taxonomy” (SORT). OCEBM[4] was based on evidence-based approach for treatment benefit depending upon the study quality, their imprecision and indirectness which is categorized into: Level 1 systematic review of randomized trials or n-of-1 trials, Level 2 randomized trial or observational study with dramatic effect, Level 3 nonrandomized controlled cohort/follow-up study, Level 4 Case-series, case-control studies, or historically controlled studies, and Level 5 Mechanism-based reasoning. The evidence in SORT developed by editors of the US Family Medicine and Primary Care journals (i.e., American Family Physician, Family Medicine, Journal of Family Practice and British Medical Journal USA)[5] is graded on 3point scale based on the quality of methodology: 1. Goodquality patientoriented evidence. 2. Limitedquality patientoriented evidence. 3. Other evidence including consensus guidelines, opinion or case studies. Clinical grade of recommendations were developed on the best available evidence and graded as follows: A. Recommendation based on consistent and good quality patientoriented evidence. B. Recommendation based on inconsistent or limitedquality patientoriented evidence. C. Recommendation based on consensus, opinion, or case studies.[5]
Antimonials
Pentavalent antimonials constitute two popular preparations––sodium stibogluconate (SSG) and meglumine antimoniate (MA). These drugs represent the earliest form of successful chemotherapy in almost all forms of leishmaniasis, dating back to 1940. However, recently their use has become restricted after several reports of drug resistance and treatment failure emerged in different parts of the world, including endemic areas of India, where treatment failure rates reached a dismal 30%.[6]
Mechanism of action
There are several proposed modalities of action including T-cell based mechanisms, cytokine alterations, intracellular signaling, and interference with reactive oxygen species (ROS) signaling. However, the most accepted theory involves conversion of pentavalent antimonials (Sb5+) to the more toxic trivalent form (Sb3+) by an enzyme As5+ reductase, present in the intracellular amastigote stage within the phagolysosome of the macrophage. Sb3+ interacts with sulfhydryl-containing molecules, for example, thiol groups (e.g., glutathione), proteins (e.g., enzymes) to exerts its effect. Sb3+ reduces thiol reduction potential of the amastigote by inducing efflux of trypanothione and glutathione, and by inhibiting trypanothione reductase; eventually exerting anti-parasitic action.[7]
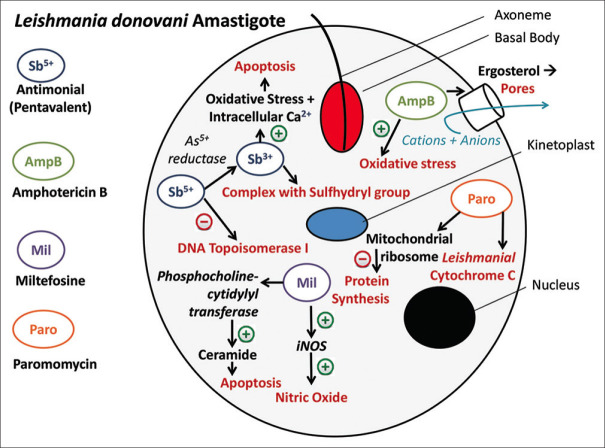
The two main models that explain the mechanism of action are the pro-drug model and the active Sb5+ model. In the active Sb5+ model, Sb5+ has intrinsic anti-leishmanial activity nally leading to the inhibition of DNA topoisomerase I[8] According to the pro-drug model, Sb5+ compounds are pro-drugs exerting their action on Leishmania parasite after reduction to Sb3+ in host cells[9] Sb3+ subsequently induces apoptosis by enhancing oxidative stress and intracellular Ca2+.[8,10] Interestingly, it has been observed that antimonials may be less effective and more toxic in immunosuppressed individuals compared to healthy controls[11] [Figure 1].
Figure 1.
Mechanism of action of the anti. leishmanial drugs used in Post Kala-azar Dermal Leishmaniasis
Pharmacokinetics
After IM injection, Sb is absorbed quickly. Highest accumulation has been observed in liver followed by thyroid and heart. No protein binding data is present. Renal clearance is consistently documented to be the main route of Sb excretion [Table 2].
Table 2.
Pharmacokinetic profile of systemically administered drugs used for PKDL[60]
| Amphotericin B | Miltefosine | Paromomycin | Antimonials | |
|---|---|---|---|---|
| Route of administration | IV | Oral | IM | IM/IV |
| Accumulation | Liver, spleen | Not reported | Not reported | Liver, thyroid, heart |
| Distribution in skin | Not reported (confirmed in rats) | Not reported (confirmed in rats) | Not reported | Confirmed |
| Metabolism | Reticuloendothelial system engulfs liposomes containing Amp B | Metabolized by Phospholipase D intracellularly | Not metabolized | Converted to Sb3+ intracellularly |
| Elimination | Urine and feces | Not excreted unchanged | Urine | Urine |
Dosage
It was noted that PKDL does not respond to the dose that is curative for VL (10 mg/Kg/day for 21 days), hence the dose was increased. SSG is typically administered intramuscularly (preferred) or slow intravenously, at a dose of 20 mg/kg/day up to a maximum of 850 mg/day (8.5 mL of SSG; or 10 mL of MA) for at least 120 days to obtain a favorable response in Indian PKDL.[1] However, for Sudanese PKDL, the optimum recommended duration is 2 months.[12] Injecting large volume (8.5 mL) intra-muscularly is painful and this issue can be ameliorated by intravenous administration, which is limited by the need for hospital admission. In case of patients intolerant to the daily injection, it is interrupted by drug holiday period; thus extending treatment duration.
Effectiveness
SSG gives satisfactory results at a dose of 10-20 mg/kg IM daily, till clinical remission. In an assessor blind randomized study by Thakur et al. (1987), active treatment was administered intramuscularly in three dosages – 10 mg/kg/day, 15 mg/kg/day, 20 mg/kg/day for 120 days, followed by monthly follow-ups for 12 months. However, the cure rate has been maximum at a dose of 20 mg/kg/day. In rare circumstances, doses greater than 20 mg/kg/day are required.[13] (Level of evidence: SORT – 1, CEBM - 2) In another study by Thakur et al. (1997), 64% cure rate was observed in the patients receiving intramuscular injections of Sodium antimony gluconate (20 mg/kg/day), with a requirement of =6 courses of therapy.[14] (Level of evidence: SORT – 2, CEBM - 2) Rathi et al. in their non-blinded randomized trial demonstrated a better effectiveness of the combination of SAG 20 mg/kg IV daily and ketoconazole orally (200 mg twice daily), with 90%–95% improvement in nodular lesions and 25-30% improvement in macular lesions after 60 days of therapy.[15] (Level of evidence: SORT – 2, CEBM - 2) Besides, combination of SSG with allopurinol, rifampicin, and Mw vaccine has shown to reduce the number of injections required from 120 to 95, apart from providing better rates of cure.[16] (Ramesh V et al.) (Level of evidence: SORT – 2, CEBM - 3) Combination therapy with paromomycin has shown to provide very good results, in comparison to SSG alone (statistically significant, 7.6 times higher effectiveness) in a study by Abongomera et al.[17] (Level of evidence: SORT – 2, CEBM - 3).
Adverse effects/limitations
There are several adverse effects ranging from nausea, vomiting, abdominal pain, headache, fever, skin rash to the more sinister nephrotoxicity, neurotoxicity, pancreatitis and cardiac conduction defects. Thus, proper monitoring is essential to ensure an uneventful therapy. However, the major limitation is the emergence of drug resistance and declining cure rates over the last few decades. Possible mechanisms of resistance include diminished biological reduction of Sb5+ to Sb3+, decreased internalization of the drug, over-expression of ATP-binding cassette (ABC) transporters and MRPA (multi drug resistance protein A) and increased levels of trypanothione reductase, the target enzyme. Indiscriminate and inappropriate use of this drug for its low cost is considered to be the most important contributory factor for drug resistance and treatment failure.[7]
Price
Generic Stibogluconate € 5.65 per 30-mL vial of 100 mg/mL; For an individual weighing 35 kg, cost of therapy with generic SSG for 60 days is 88.1 USD (considering conversion rate of 1€=1.114 USD).
Final comments
SSGhas Grade A recommendation for use in PKDL. However, recently its use is being discouraged because of several reports of treatment failure from different corners of the world. Although still being used, liposomal amphotericin B (LAMB) has emerged as the preferable option. Notably, these complexes are no longer recommended in the Indian subcontinent for the treatment of visceral leishmaniasis.[18]
Amphotericin-B
Amphotericin B is a polyene antifungal agent, originally extracted from Streptomyces nodosus. It is available in several formulations- plain form, cholesteryl sulfated complex, lipid complex and a liposomal form. The liposomal form is preferred because of minimal side effects. It is currently the preferred treatment option for PKDL, especially in cases of SSG resistance.
Mechanism of action
The drug binds to the ergosterol component of parasite cell membrane,[19] leading to formation of pores. Eventually, there is increased permeability, leakage of anions and cations[7] from the cell leading to its death. Besides, it also induces oxidative stress through unknown mechanism, thereby depleting viable organisms [Figure 1].
Pharmacokinetics
Gastrointestinal absorption is minimal. In comparison to conventional amphotericin B deoxycholate, liposomal amphotericin B (L-AmB) has lower volume of distribution and reduced excretion of unchanged drug through urine and feces. Being liposomal, L-AmB has increased efficacy (targeted sequestration of drug into reticuloendothelial organs) with minimum toxicity. High initial doses (>5 mg/kg) of L-AmB provide better penetration and longer persistence in tissues than frequent low doses, favoring initial loading dose[20] [Table 2].
Dosage
Amphotericin B deoxycholate is administered as a slow intravenous infusion (IV) (over 2 hours on the initial day and then over 1 hour if tolerated) of 3-4 mg/Kg/day in 5% dextrose.[21] In a Sudanese study, 20 daily injections were effective.[22] However, in India the optimum dosage is 50 mg/day (dissolved in 500 mL 5% dextrose given slow i.v) for 3 cycles of 20 days course with 20 days of drug free interval for resolution of lesions.[14]
Effectiveness
Roustan et al.[23] reported clinical improvement in the plaques with daily dose of 100 mg liposomal Amphotericin B for 30 days. (Level of evidence: SORT – 3, CEBM - 4) Administration of 20 mg/kg Liposomal Amphotericin B in four divided doses on alternate days, followed by a repeat course after 4 weeks, delivered appreciable results in a case series published from Bangladesh.[24] (Level of evidence: SORT – 3, CEBM - 4) A Sudanese study published by Musa AM et al.[22] noted the effectiveness of 2.5 mg AmBisome/kg/day for 20 days, by intravenous infusion in 5% dextrose on 12 patients. Follow-up at 90 days had given wonderful results with complete resolution in 10 patients. (Level of evidence: SORT – 2, CEBM - 2) In an open-label randomized controlled trial from Bangladesh, Das et al. administered Amphotericin B in two different doses[25] 0.5 mg/kg in 5% dextrose daily (Group A) and 1 mg/kg in 5% dextrose on alternate days (Group B) with 25 patients in each treatment arm. Each regimen was delivered as 3 courses of 20 infusions each, with a 15-day gap in between each course and a follow-up period of one year. However, the cure rates in both the groups were comparable. [Intention-to-treat analysis – Group A - 92%, Group B – 88%; Per-protocol analysis – Group A -96.65%, Group B- 95.45%]. (Level of evidence: SORT – 1, CEBM - 2) In another study from Bangladesh, den Boer et al.[26] noted the effectiveness and safety of a short course of Amphotericin B (15 mg/kg/dose in 5 weekly injections) in a cohort of 280 patients. Patients were followed up for 12 months with clinical efficacy recorded at 1st, 3rd, 6th, and 12th months. Majority (89.7%) underwent marked clinical improvement with 78% showing clinical cure. However, 10.3% failed to show any response, while 4.8% reported appearance of new lesions. (Level of evidence: SORT – 2, CEBM - 2)
Adverse effects/limitations
Conventional amphotericin B has several notable adverse effects such as nephrotoxicity and hypokalemia. Infusion site reactions include fever with chill and rigor, headache, rash, nausea, vomiting, skin exanthema, hypotension, and drowsiness. Fortunately, liposomal amphotericin B (LAMB) is much safer as it is packaged into lipid vesicles, thus showing higher affinity for macrophages. However dose-related (30 mg/kg cumulative dose) hypokalemia-induced rhabdomyolysis has been reported in six patients[27] and one Sudanese patient of PKDL experienced anaphylactoid reaction- characterized by breathlessness, wheezing and asthmatic attack after the 5th dose of liposomal Amphotericin B (2.5 mg/kg in 5% dextrose).[28] Reports of treatment failure with LAMB are rare, especially in immunocompetent individuals.[18] However, its use needs to be monitored in the coming days especially in the Indian subcontinent to prevent the development of resistance.
Price
Amphotericin B deoxycholate US $ 7.5 per 50-mg vial and Liposomal amphotericin B US $ 18 per 50-mg vial.
For an individual weighing 35 kg, cost of therapy with Liposomal amphotericin B is 630 USD.
Final comments
Amphotericin B has Grade A recommendation for use in PKDL. The liposomal variant (LAMB) is the preferred formulation, because of its increased efficacy and favorable safety profile. However, proper monitoring is needed to curb it's inappropriate and injudicious use to prevent the emergence of drug resistance.
Miltefosine
Miltefosine, also called hexadecylphosphocholine, is the first oral drug used for the treatment of post-kala-azar dermal leishmaniasis (PKDL). It was originally developed as an anticancer agent and its anti-leishmanial properties have been subsequently demonstrated.[29] Development of an oral therapy provided a significant breakthrough in anti-leishmanial chemotherapy, especially in endemic areas such as India, due to its ease of administration and increased patient compliance.[30]
Mechanism of action
Although the exact mechanism of action is unclear, it has been proposed that miltefosine leads to apoptotic changes in the amastigote form of Leishmania donovani, after intracellular accumulation.[31] Inhibition of phosphocholine-cytidylyl transferase disrupts and alters phosphatidylcholine and sphingomyeline biosynthesis, respectively. This leads to increased intracellular ceramide that triggers apoptosis.[32] Two membrane transporters have been identified viz. LdMT and its β-subunit LdRos3, a P-type ATPase (aminophospholipidase family) which regulate its intracellular transportation.[33] Recently Losieau et al.[34] have demonstrated that miltefosine also reduces the lipid content in promastigote cell membrane, thus limiting its proliferation. This drug also exerts its effect by enhancing the synthesis of inducible nitric oxide synthetase 2 (iNOS2), nitric oxide (NO) and IFN-gamma, all being lethal for the parasite[35] [Figure 1].
Pharmacokinetics
Miltefosine is slowly,[36] but well absorbed orally and distributed throughout the human body. It has a long t1/2 (30.9 days) in humans. Plasma concentrations are proportional to the dose. Metabolism is mediated by phospholipases into choline which is likely used in the physiological biosynthesis of cell membranes or as an important source for the synthesis of, for example, acetylcholine or lecithin. The extremely slow elimination of miltefosine is manifested by the long elimination half-life and high accumulation during treatment [Table 2].
Effectiveness
Patients (both immunocompetent and seropositive) not responding to SSG, show good response to oral miltefosine at a dose of 100 mg/day in divided doses.[37,38,39,40,41] (Sundar et al., Dejenie Belay et al., Rihl et al., Salam et al., Abongomera et al.) (Level of evidence: SORT – 3, CEBM - 4) In an open-label single-arm study by Ramesh et al.,[42] 26 patients treated with miltefosine 50 mg thrice daily for 60 days provided a cure rate of 96%. (Level of evidence: SORT – 2, CEBM – 2) A multicentric parallel-group trial from India explored the usefulness of Miltefosine in PKDL at 2.5 mg/kg/day for 8 and 12 weeks, respectively, and concluded both regimens to be effective in the Indian context.[43] (Level of evidence: SORT – 2, CEBM - 2) Ghosh and colleagues obtained excellent results after 16 weeks of therapy in their open-label trial[44] but severe abdominal pain, nausea, and vomiting were some of the notable adverse effects, on account of which, treatment had to be stopped. (Level of evidence: SORT – 2, CEBM - 4) In another open-label trial from India, Sundar et al. recorded admirable efficacy with minimal adverse effects.[45] (Level of evidence: SORT – 2, CEBM - 2) Ramesh et al.[46] conducted a non-randomized non-blinded trial to compare the effectiveness of Miltefosine 50 mg twice daily for 90 days and thrice daily for 60 days with a follow-up period of 18 months. Clinico-pathological correlation and assessment of parasitic load observed relapse to be significantly higher in the second group (P < 0.005) (Level of evidence: SORT – 2, CEBM - 3) A meta-analysis by Pijpers et al.[47] on 324 patients from eight studies on effectiveness of miltefosine in PKDL (at 2.5 mg/kg/day for a duration 6-16 weeks) provided an initial cure rate of 95.2% and definite cure rate of 90%. (Level of evidence: SORT – 2, CEBM - 1).
Adverse effects/limitations
Gastrointestinal symptoms including nausea, vomiting, pain abdomen, diarrhea, raised bilirubin levels were reported from majority of the published studies. Despite having several operational advantages and no significant toxicity, teratogenicity and abortifacient property limits its use during pregnancy[33] Another dreaded concern is the possible emergence of drug-resistance due to its long half-life (152 hours) and improper or indiscriminate usage in highly endemic countries such as India. Some cases have been reported to develop re-emergence of symptoms about 9-12 months after successful treatment with miltefosine. However, more studies are needed to highlight its true nature- relapse, re-infection, or resistance.[33] Several theories have been proposed to explain the development of drug resistance such as single-point mutation in LdMT and LDRos3 genes, overexpression of multidrug-resistant MDR1 gene, alterations, and reduced amount of unsaturated phospholipid alkyl chains in the parasite cell membrane or alterations in the mitochondrial HSP70.[48] Alarmingly, laboratory-confirmed cases of miltefosine resistance and declining clinical response have been recently reported from India.[45,49]
Ophthalmic complications were reported from Bangladesh with miltefosine use for PKDL, characterized by the development of Mooren's ulcer or marginal keratitis in 5 out of 194 PKDL patients.[50]
Price
For adults: € 45.28–54.92 for 56 (50-mg) capsules and For children: € 34.36–39.3 for 56 (10-mg) capsules.
For adults (25 – 50 kg), cost of therapy with miltefosine is 195-450 USD and for children, cost of therapy with miltefosine (10-mg capsules daily) is 57.4 – 65.7 USD (considering conversion rate of 1€=1.114 USD).
Final comment
Miltefosine has Grade A recommendation for use in PKDL. Oral administration and limited toxicity have made this drug the first-line treatment in the Indian subcontinent. Although the standard duration of therapy is 3 months, recent reports suggest 4 months is a better option to avoid relapses.
Paromomycin
Paromomycin is an aminoglycoside antibiotic, having both anti-leishmanial and antibacterial properties. Its curative role has already been established for visceral and cutaneous leishmaniasis;[31] recently the same is being explored for post kala-azar dermal leishmaniasis (PKDL) as well. Several studies are highlighting the role of this drug in PKDL, both alone and in combination with SSG, which have been discussed below.
Mechanism of action
Although the exact mechanism of action is unclear, it probably acts by inhibiting the Leishmanial cytochrome C enzyme.[31] Recently Jhingran et al.[51] have suggested parasitic mitochondria as the primary target of action, as cationic paromomycin binds to the negatively charged glycocalyx. Additionally, it also inhibits the translocation (translocation initiation factor-3) and recycling of cytoplasmic and mitochondrial ribosomes by interacting with both 30S and 50S subunits, thus inhibiting protein synthesis[52] [Figure 1].
Pharmacokinetics
Oral bioavailability of paromomycin is extremely low (0.3%) when administered in 0.1% CMC, suggesting permeability limited absorption, thus intramuscular administration is recommended. Paromomycin demonstrated 2.5-fold high volume of distribution compared to total body water (0.7 L/kg), suggesting that it will be extensively distributed into tissues where the parasites are residing. Tissue distribution is critical for paromomycin treatment since the parasite resides in tissues such as liver, spleen, and pancreas.[53] Paromomycin being a substrate for both CYP enzymes and P-gp efflux transporter, low oral bioavailability has been reported even for a high dose of 500 mg/kg [Table 2].
Effectiveness
In an open-label single-arm study by Sundar et al.,[54] paromomycin at 11 mg/kg daily was given intramuscularly, for 45 days, but the patient compliance was poor. (Level of evidence: SORT – 2, CEBM - 3) In another interesting RCT by Pandey et al.,[32] a combination of paromomycin and miltefosine for two courses of 10 days duration was compared with the same combination for three courses of 10 days duration. Each group was followed up for up to one year. The final cure rate was found to be 83%, with a relapse rate of 17%. (Level of evidence: SORT – 2, CEBM - 3). Similar combination of paromomycin (15mg/kg/day for 17 days) with sodium stibogluconate was found to be more effective than sodium stibogluconate alone in a retrospective cohort study by Abongomera et. al. in 201617 with benefits of shorter spells of therapy, reduced hospitalisation and subsequent reduction in dose-dependent toxicity of SSG. (Level of evidence: SORT – 2, CEBM - 3)
Adverse effects/Limitations
Paromomycin is a relatively safe drug, occasional adverse effects have been reported including, pain at injection site, reversible ototoxicity, reversible nephrotoxicity, hypokalemia, and occasional liver function problems.[55] Due to its reserved usage, clinical resistance has not yet been reported; however, laboratory resistance (in vitro) has been reported in Leishmania donovani and Leishmania tropica.[31] Hence, cautious use is recommended in the days to come.
Price
USD 15 per adult course of 21 days
Final comment
Paromomycin has Grade A recommendation for use in PKDL. Although monotherapy has unacceptably low cure rate, combination therapy with SSG is safe, effective and time-saving.
Other agents
Antifungal drugs
There are sporadic reports regarding the use of some antifungal drugs for the treatment of PKDL viz. ketoconazole, itraconazole and terbinafine. Studies have been conducted both in India and Sudan with varying rates of efficacy, as discussed below:
Effectiveness
In an isolated case series published by Ramesh et al.,[56] only 1 out of 4 PKDL patients who were administered ketoconazole at a dose of 800 mg/day, achieved clinical cure at the end of 9 months. (Level of evidence: SORT – 3, CEBM - 4) In another case series by Khalil et al.,[57] 9 PKDL patients were treated with terbinafine (250 mg/day)+ itraconazole (200 mg/day) for 4 weeks. Although there was an improvement initially, but the patients returned to pre-treatment condition, once the drugs were stopped. (Level of evidence: SORT – 3, CEBM - 4) Ketoconazole (200 twice daily) given along with SSG (20 mg/Kg/day) showed better result than SSG alone.
Final comment
Systemic antifungals are not effective in the treatment of PKDL (Level C recommendation). However, ketoconazole has shown better efficacy in Indian PKDL than the Sudanese variety, probably due to different causative species.
Immunochemotherapy
Recently immunotherapy has been proposed as a novel form of therapy for recalcitrant PKDL. Immunotherapy has been found to be safe and effective when used in combination with SSG.
Mechanism of action
Immunochemotherapy or vaccination probably acts by enhancing IFN-γ production in the host thus expediting the healing process. It may also increase the IL-10 level which accelerates the healing process.[58]
Effectiveness
In a randomized double-blind placebo-controlled trial by Musa et al.,[58] the group receiving alum -precipitated autoclaved Leishmania major (Alum/ALM) vaccine + Bacille Calmette-Gu´erin (BCG) and SSG, showed far better results than placebo group 3 months after treatment (cure rate of 87% vs 53%) (Level of evidence: SORT – 1, CEBM - 2). Adverse effects were limited to injection site induration and ulceration.
Limitations
As PKDL is primarily restricted to the economically starved areas of Asia and Africa, this form of therapy is too expensive to become sustainable. Also, the cost-effectiveness is doubtful as prevalence of PKDL is not much high. However, the search for cheaper vaccines continues, which may be administered to endemic populations to achieve a favorable cost–benefit outcome.
Final comments
Immunotherapy/vaccine is a promising tool for PKDL as therapeutic agent. Prophylactic role of PKDL vaccine is not yet established
Conclusion
PKDL is asymptomatic, so patients seldom report for treatment; and this negative treatment-seeking behavior is a major hurdle in eliminating the reservoir of kala-azar. The need for active surveillance to detect the hidden cases will be of paramount importance for the successful implementation of 'elimination of kala-azar by 2020 in south-east Asia.[59] The cost of therapy is another deterrent for successful treatment, but fortunately the medicines are being made available free-of-cost by the Governments of the affected countries to make them accessible to the needy population. Miltefosine has the advantage of ambulatory treatment but development of resistance has ignited the search for other agents. The use of other available agents is limited by the need for hospitalization, subsequently leading to loss-of-wage thus impacting their rate of treatment completion adversely. Development of side-effects to therapy also make patients non-compliant, thus emphasizing the need for developing a safe, effective and short-course therapeutic modality and treatment regime.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.WHO Expert Committee on the Control of the Leishmaniases & World Health Organization. (2010). Control of the leishmaniases: Report of a meeting of the WHO Expert Commitee on the Control of Leishmaniases, Geneva, 22-26 March 2010. World Health Organization [Google Scholar]
- 2.Ramesh V, Mukherjee A. Post kala-azar dermal leishmaniasis. Int J Dermatol. 1995;34:85–91. doi: 10.1111/j.1365-4362.1995.tb03584.x. [DOI] [PubMed] [Google Scholar]
- 3.Zijlstra EE, Musa AM, Khalil EAG, el-Hassan IM, el-Hassan AM. Post-kala-azar dermal leishmaniasis. Lancet Infect Dis. 2003;3:87–98. doi: 10.1016/s1473-3099(03)00517-6. [DOI] [PubMed] [Google Scholar]
- 4.OCEBM Levels of Evidence Working Group*. “The Oxford Levels of Evidence 2”. [[Last accessed on 2020 Jul 03]];Oxford Centre for Evidence-Based Medicine. https://www.cebm.net/index.aspx?o=5653 . [Google Scholar]
- 5.Ebell MH, Siwek J, Weiss BD, Woolf SH, Susman J, Ewigman B, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. Am Fam Physician. 2004;69:548–56. [PubMed] [Google Scholar]
- 6.Tiuman TS, Santos AO, Ueda-Nakamura T, Dias Filho BP, Nakamura CV. Recent advances in leishmaniasis treatment. Int J Infect Dis. 2011;15:e525–32. doi: 10.1016/j.ijid.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Ramesh V, Verma P. Treatment of post-kala-azar dermal Leishmaniasis. In: Noiri E, Jha TK, editors. Kala Azar in South Asia: Current Status and Sustainable Changes. 2nd ed. Springer; 2016. pp. 67–77. [Google Scholar]
- 8.Fre´zard F, Demicheli C, Ribeiro RR. Pentavalent antimonials: New perspectives for old drugs. Molecules. 2009;14:2317–36. doi: 10.3390/molecules14072317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baiocco P, Colotti G, Franceschini S, Ilari A. Molecular basis of antimony treatment in leishmaniasis. J Med Chem. 2009;52:2603–12. doi: 10.1021/jm900185q. [DOI] [PubMed] [Google Scholar]
- 10.Mookerjee Basu J, Mookerjee A, Sen P, Bhaumik S, Sen P, Banerjee S, et al. Sodium antimony gluconate induces generation of reactive oxygen species and nitric oxide via phosphoinositide 3-kinase and mitogen-activated protein kinase activation in Leishmania donovani-infected macrophages. Antimicrob Agents Chemother. 2006;50:1788–97. doi: 10.1128/AAC.50.5.1788-1797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diro E, Lynen L, Mohammed R, Boelaert M, Hailu A, van Griensven J. High parasitological failure rate of visceral leishmaniasis to sodium stibogluconate among HIV co-infected adults in Ethiopia. PLoS Negl Trop Dis. 2014;8:e2875. doi: 10.1371/journal.pntd.0002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musa AM, Khalil EA, Younis BM, Elfaki ME, Elamin MY, Adam AO, et al. Treatment-based strategy for the management of post-kala-azar dermal leishmaniasis patients in the Sudan? J Trop Med. 2013;2013:708391. doi: 10.1155/2013/708391. doi: 10.1155/2013/708391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thakur CP, Kumar K, Sinha PK, Mishra BN, Pandey AK. Treatment of Post kala-azar dermal leshmaniasis with sodium stibogluconate. Br Med J. 1987;295:886–7. doi: 10.1136/bmj.295.6603.886-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thakur CP, Narain S, Kumar N, Hassan SM, Jha DK, Kumar A. Amphotericin B is superior to sodium antimony gluconate in the treatment of Indian post-kala-azar dermal leishmaniasis. Ann Trop Med Parasitol. 1997;91:611–6. doi: 10.1080/00034989760707. [DOI] [PubMed] [Google Scholar]
- 15.Rathi SK, Pandhi RK, Khanna N, Chopra P. Therapeutic trial of sodium antimony gluconate alone and in combination with ketoconazole in post-kala-azar dermal leishmaniasis (PKDL) Indian J Dermatol Venereol Leprol. 2003;69:392–3. [PubMed] [Google Scholar]
- 16.Ramesh V, Kumar J, Kumar D, Salotra P. A retrospective study of intravenous sodium stibogluconate alone and in combinations with allopurinol, rifampicin, and an immunomodulator in the treatment of Indian post-kala-azar dermal leishmaniasis. Indian J Dermatol Venereol Leprol. 2010;76:138–44. doi: 10.4103/0378-6323.60553. [DOI] [PubMed] [Google Scholar]
- 17.Abongomera C, Gatluak F, Buyze J, Ritmeijer K. A comparison of the effectiveness of sodium stibogluconate monotherapy to sodium stibogluconate and paromomycin combination for the treatment of severe post kala azar dermal leishmaniasis in South Sudan: A retrospective cohort study. PLoS One. 2016;11:e0163047. doi: 10.1371/journal.pone.0163047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sundar S, Chakravarty J, Meena LP. Leishmaniasis: Treatment, drug resistance and emerging therapies. Expert Opin Orphan Drugs. 2019;7:1–10. [Google Scholar]
- 19.Rogers PD, Krysan DJ. Antifungal agents. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman's The Pharmacological basis of Therapeutics. 13th ed. New York: McGraw-Hill; 2018. pp. 1087–104. [Google Scholar]
- 20.Sundar S, Chakravarty J. Liposomal amphotericin B and leishmaniasis: Dose and response. J Glob Infect Dis. 2010;2:159–66. doi: 10.4103/0974-777X.62886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett JE. Antifungal drugs. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman's The Pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill; 2010. pp. 1571–92. [Google Scholar]
- 22.Musa AM, Khalil EAG, Mahgoub FA, Hamad S, Elkadaru AM, El Hassan AmM. Efficacy of liposomal amphotericin B (AmBisomeH) in the treatment of persistent post-kala-azar dermal leishmaniasis (PKDL) Ann Trop Med Parasitol. 2005;99:563–9. doi: 10.1179/136485905X514127. [DOI] [PubMed] [Google Scholar]
- 23.Roustan G, Jimenez JA, Gutierrez-Solar B, Gallego JL, Alvar J, Patron M. Post-kala-azar dermal leishmaniasis with mucosal involvement in a kidney transplant recipient: Treatment with liposomal amphotericin B. Br J Dermatol. 1998;138:526–8. doi: 10.1046/j.1365-2133.1998.02139.x. [DOI] [PubMed] [Google Scholar]
- 24.Basher A, Maruf S, Nath P, Hasnain MG, Mukit MA, Anuwarul A, et al. Case report: Treatment of widespread nodular post kala-azar dermal leishmaniasis with extended-dose liposomal amphotericin B in Bangladesh: A series of four cases. Am J Trop Med Hyg. 2017;97:1111–5. doi: 10.4269/ajtmh.16-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das VNR, Siddiqui NA, Pal B, Lal CS, Verma N, Kumar A, et al. To evaluate efficacy and safety of amphotericin B in two different doses in the treatment of post kala-azar dermal leishmaniasis (PKDL) PLoS One. 2017;12:e0174497. doi: 10.1371/journal.pone.0174497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Den Boer, Das AK, Akhter F, Burza S, Ramesh V, Ahmed BN, et al. Safety and effectiveness of short-course AmBisome in the treatment of Post-Kala-azar Dermal Leishmaniasis (PKDL): A prospective cohort study in Bangladesh. Clin Infect Dis. 2018;67:667–75. doi: 10.1093/cid/ciy172. [DOI] [PubMed] [Google Scholar]
- 27.Marking U, den Boer M, Das AK, Ahmed EM, Rollason V, Ahmed B, et al. Hypokalaemia-Induced Rhabdomyolysis after Treatment of Post-Kala-azar Dermal Leishmaniasis (PKDL) with High-Dose AmBisome in Bangladesh—A Case Report. PLoS Negl Trop Dis. 2014;8:e2864. doi: 10.1371/journal.pntd.0002864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukhtar M, Aboud M, Kheir M, Bakhiet S, Abdullah N, Ali A, et al. First report on ambisome-associated allergic reaction in two Sudanese leishmaniasis patients. Am J Trop Med Hyg. 2011;85:644–5. doi: 10.4269/ajtmh.2011.10-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Croft SL, Coombs GH. Leishmaniasis–current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003;19:502–8. doi: 10.1016/j.pt.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Jha TK, Sundar S, Thakur CP, Bachmann P, Karbwang J, Fischer C, et al. Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N Engl J Med. 1999;341:1795–800. doi: 10.1056/NEJM199912093412403. [DOI] [PubMed] [Google Scholar]
- 31.Singh N, Kumar M, Singh RK. Leishmaniasis: Current status of available drugs and new potential drug targets. Asian Pac J Trop Med. 2012;5:485–97. doi: 10.1016/S1995-7645(12)60084-4. [DOI] [PubMed] [Google Scholar]
- 32.Pandey K, Pal B, Das VNR, Murti K, Lal CS, Verma N, et al. Safety and efficacy of a combination of paromomycin and miltefosine for two vs. Three courses in patients with post-kala-azar dermal leishmaniasis: An observational pilot study. British J Dermatol. 2017;177:557–9. doi: 10.1111/bjd.15119. [DOI] [PubMed] [Google Scholar]
- 33.Pérez-Victoria FJ, Gamarro F, Ouellette M, Castanys S. Functional cloning of the miltefosine transporter A novel P-type phospholipid translocase from leishmania involved in drug resistance. J Biol Chem. 2003;278:49965–71. doi: 10.1074/jbc.M308352200. [DOI] [PubMed] [Google Scholar]
- 34.Loiseau PM, Bories C. Mechanisms of drug action and drug resistance in Leishmania as basis for therapeutic target identification and design of antileishmanial modulators. Curr Top Med Chem. 2006;6:539–50. doi: 10.2174/156802606776743165. [DOI] [PubMed] [Google Scholar]
- 35.Wadhone P, Maiti M, Agarwal R, Kamat V, Martin S, Saha B. Miltefosine promotes IFN-γ-dominated anti-leishmanial immune response. J Immunol. 2009;182:7146–54. doi: 10.4049/jimmunol.0803859. [DOI] [PubMed] [Google Scholar]
- 36.Dorli TPC, Balasegaram M, Beijen JH, de Vries PJ. Miltefosine: A review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother. 2012;67:2576–97. doi: 10.1093/jac/dks275. [DOI] [PubMed] [Google Scholar]
- 37.Sundar S, Kumar K, Chakravarty J, Agrawal D, Agrawal S, Chhabra A, et al. Cure of antimony-unresponsive Indian post-kala-azar dermal leishmaniasis with oral miltefosine. Trans R S Trop Med Hyg. 2006;100:698–700. doi: 10.1016/j.trstmh.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 38.Dejenie Belay A, Asafa Y, Mesure J, Davidson RN. Successful miltefosine treatment of post-kala-azar dermal leishmaniasis occurring during antiretroviral therapy. Ann Trop Med Parasitol. 2006;100:203–6. doi: 10.1179/136485906X91440. [DOI] [PubMed] [Google Scholar]
- 39.Rihl M, Stoll M, Ulbricht K, Bange FC, Schmidt RE. Successful treatment of post-kala-azar dermal leishmaniasis (PKDL) in a HIV infected patient with multiple relapsing leishmaniasis from Western Europe. J Infect. 2006;53:e25–7. doi: 10.1016/j.jinf.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Salam MA, Siddiqui MA, Nabi SG, Bhaskar KRH, Mondal D. Post-lala-azar dermal leishmaniasiswith mucosal involvement: An unusual case presentation including successful treatment with miltefosine. J Health Popul Nutr. 2013;31:294–7. doi: 10.3329/jhpn.v31i2.16395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abongomera C, Battagliolia T, Aderaa C, Ritmeijer K. Severe post-kala-azar dermal leishmaniasis successfully treated with miltefosine in an Ethiopian HIV patient. Int J Infect Dis. 2019;81:221–4. doi: 10.1016/j.ijid.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Ramesh V, Singh R, Avishek K, Verma A, Deep DK, Verma S, et al. Decline in Clinical efficacy of oral miltefosine in treatment of post kala-azar dermal leishmaniasis (PKDL) in India. PLoS Negl Trop Dis. 2015;9:e0004093. doi: 10.1371/journal.pntd.0004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sundar S, Sinha P, Jha TK, Chakravarty J, Rai M, Kumar N, et al. Oral miltefosine for Indian post-kala-azar dermal leishmaniasis: A randomised trial. Trop Med Int Health. 2013;18:96–100. doi: 10.1111/tmi.12015. [DOI] [PubMed] [Google Scholar]
- 44.Ghosh S, Das NK, Mukherjee S, Mukhopadhyay D, Barbhuiya JN, Hazra A, et al. Inadequacy of 12-week Miltefosine treatment for Indian post-Kala-Azar dermal leishmaniasis. Am J Trop Med Hyg. 2015;93:767–9. doi: 10.4269/ajtmh.14-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sundar S, Singh A, Chakravarty J, Rai M. Efficacy and safety of miltefosine in treatment of post-kala-azar dermal leishmaniasis. ScientificWorldJournal. 2015;2015:414378. doi: 10.1155/2015/414378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramesh V, Singh R, Avishek K, Verma A, Deep DK, Verma S. Decline in clinical efficacy of oral miltefosine in treatment of post kalaazar dermal leishmaniasis (PKDL) in India. PLoS Negl Trop Dis. 2015;9:e0004093. doi: 10.1371/journal.pntd.0004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pijpers J, den Boer ML, Essink DR, Ritmeijer K. The safety and efficacy of miltefosine in the long-term treatment of post-kala-azar dermal leishmaniasis in South Asia-A review and meta-analysis. PLoS Negl Trop Dis. 2019;13:e0007173. doi: 10.1371/journal.pntd.0007173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vacchina P, Norris-Mullins B, Carlson ES, Morales MA. A mitochondrial HSP70 (HSPA9B) is linked to miltefosine resistance and stress response in Leishmania donovani. Parasit Vectors. 2016;9:621. doi: 10.1186/s13071-016-1904-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srivastava S, Mishra J, Gupta AK, Singh A, Shankar P, Singh S. Laboratory confirmed miltefosine resistant cases of visceral leishmaniasis from India. Parasit Vectors. 2017;10:49. doi: 10.1186/s13071-017-1969-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maruf S, Nath P, Islam MR, Aktar F, Anuwarul A, Mondal D, et al. Corneal complications following post kala-azar dermal leishmaniasis treatment. PLoS Negl Trop Dis. 2018;12:e0006781. doi: 10.1371/journal.pntd.0006781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jhingran A, Chawla B, Saxena S, Barrett MP, Madhubala R. Paromomycin: Uptake and resistance in Leishmania donovani. Mol Biochem Pasrasitol. 2009;164:111–7. doi: 10.1016/j.molbiopara.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirokawa G, Kaji H, Kaji A. Inhibition of antiassociation activity of translation initiation factor 3 by paromomycin. Antimicrob Agents Chemother. 2007;51:175–80. doi: 10.1128/AAC.01096-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pinjari MJSK, Somani R, Gilhotra RM. Investigation of in vitro absorption, distribution, metabolism, and excretion and in vivo pharmacokinetics of paromomycin: Influence on oral bioavailability. Indian J Pharmacol. 2017;49:297–303. doi: 10.4103/ijp.IJP_651_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sundar S, Singh A, Tiwari A, Shukla S, Chakravarty J, Rai M. Efficacy and safety of paromomycin in treatment of post-kala-azar dermal leishmaniasis? ISRN Parasitol. 2014;2014:548010. doi: 10.1155/2014/548010. doi: 10.1155/2014/548010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sundar S, Singh A. Recent developments and future prospects in the treatment of visceral leishmaniasis. Ther Adv Infect Dis. 2016;3:98–109. doi: 10.1177/2049936116646063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramesh V, Saxena U, Misra RS. Efficacy of Ketoconazole in Post—kala-azar Dermal Leishmaniasis. Arch Dermatol. 1992;128:411–2. [PubMed] [Google Scholar]
- 57.Khalil EA, Nur NM, Zijlstra EE, El-Hassan AM, Davidson RN. Failure of a combination of two antifungal drugs, terbinafine plus itraconazole, in Sudanese post kala-azar dermal leishmaniasis. Trans R Soc Trop Med Hyg. 1996;90:187–8. doi: 10.1016/s0035-9203(96)90134-0. [DOI] [PubMed] [Google Scholar]
- 58.Musa AM, Khalil EA, Mahgoub FA, Elgawi SH, Modabber F, Elkadaru AE, et al. Immunochemotherapy of persistent post-kala-azar dermal leishmaniasis: A novel approach to treatment. Trans R S Trop Med Hyg. 2008;102:58–63. doi: 10.1016/j.trstmh.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 59.Zijlstra EE, Alves F, Rijal S, Arana B, Alvar J. (2017) Post-kala-azar dermal leishmaniasis in the Indian subcontinent: A threat to the South-East Asia Region Kala-azar Elimination Programme. PLoS Negl Trop Dis. 2017;11:e0005877. doi: 10.1371/journal.pntd.0005877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kip AE, Schellens JHM, Beijnen JH, Dorlo TPC. Clinical pharmacokinetics of systemically administered antileishmanial drugs. Clin Pharmacokinet. 2018;57:151–76. doi: 10.1007/s40262-017-0570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]