Abstract
Skin serves as the mirror of underlying systemic problems. The early diagnosis of subtle cutaneous clinical pointers often helps in identifying renal disorders, obviating the delay in diagnosis and treatment. Cutaneous changes can be observed from the beginning of renal impairment until the evolution to terminal stage, in uremia, hemodialysis, and after kidney transplantation. In the review, we have discussed the cutaneous changes, its implicated etiopathogenesis, and their treatment options, as encountered in chronic kidney disease, hemodialysis and post-renal transplantation.
KEY WORDS: Chronic kidney disease, dialysis, renal transplant, skin manifestations
Introduction
Chronic kidney disease (CKD) affects between 8% and 16% of the population worldwide. Defined by the presence of kidney damage or decreased kidney function for 3 or more months, irrespective of the cause, CKD is more prevalent in low- and middle-income than in high income nations and contributes to significant mortality and morbidity.[1,2,3] About 50–100% of patients with end-stage renal disease have at least one associated cutaneous change.[4]
Cutaneous changes in chronic kidney disease
-
Nonspecific manifestations:
The nonspecific manifestations associated with CKD include generalized pruritus, xerosis, acquired ichthyosis, pigmentation changes, purpuric spots, nail and mucosal changes [Figure 1], cutaneous findings due to hormonal changes [Table 1].[5,6,7,8,9,10,11,12,13,14,15] In patients with darker skin types, pallor and pigmentary changes (yellowish tinge) are less commonly perceived, possibly due to the masking effect of their darker complexion.[9]
Pruritus is a common symptom among patients with end-stage kidney disease (ESKD). About 90% of patients undergoing hemodialysis may experience pruritus, and patients receiving hemodialysis are more commonly affected in comparison to peritoneal dialysis.[16] It adversely affects the quality of life, and presence of pruritus is associated with a poor prognosis in ESKD. Risk factors associated with pruritus include inadequate dialysis, hyperparathyroidism, elevated calcium × phosphorus product, xerosis, elevated serum magnesium and aluminium, anemia, male sex, hypervitaminosis-A, increased beta-2 microglobulin levels, serotype human leukocyte antigen (HLA)-B35 and presence of comorbidities including congestive heart failure and neurologic disease. The pathogenesis of pruritus is not clear, however, several hypotheses have been proposed:[17,18]
- a) Immunohypothesis: Uremic pruritus is a systemic inflammatory disorder. Some studies show a direct role of proinflammatory T lymphocytes and cytokines. This is supported by higher levels of proinflammatory T helper-1 (TH1) cells, C-reactive protein, interleukin-6, and interleukin-2 levels among hemodialysis patients, in comparison to those without pruritus.
- b) Opioid hypothesis: The balance in the expression of mu and kappa opioid receptors is altered. Pruritus is aggravated by mu-receptor activation and kappa-receptor blockade; and treated by the reverse.
- c) Pruritogens: Release of pruritogens and histamine from mast cells has been thought to contribute towards uremic pruritus.
Therapeutic options for control of pruritus include optimal dialysis, treatment of hyperparathyroidism, hyperphosphatemia, and hypermagnesemia; and emollients. For cases of resistant pruritus (continued symptoms despite initial therapy for approximately four weeks), preferred modalities include oral antihistamines, gabapentin, pregabalin, sertraline, and montelukast. In cases of refractory uremic pruritus, kidney transplantation is considered to be the definitive therapy. Moreover, phototherapy can be tried. The beneficial effects of UVB therapy are due to a reduction in proinflammatory cytokines and induction of mast cell apoptosis.[19,20] However, prolonged phototherapy is considered to be associated with increased risk of carcinogenesis and should be avoided in patients who are on immunosuppressives. Experimental therapeutic options include thalidomide, opioid receptor agonists and antagonists, omega-6 fatty acids, charcoal, 5-hydroxytryptamine receptor inhibitors, cromolyn sodium, and dupilumab.[19,21]
-
Specific manifestations
The specific manifestations associated with CKD include acquired perforating dermatoses, calciphylaxis, calcinosis cutis, bullous diseases (porphyria cutanea tarda and pseudoporphyria), eruptive xanthoma, iododerma and nephrogenic systemic fibrosis. Among these, only nephrogenic systemic fibrosis is considered to be highly specific for CKD, other dermatoses may be noted in conditions apart from CKD as well.
Figure 1.
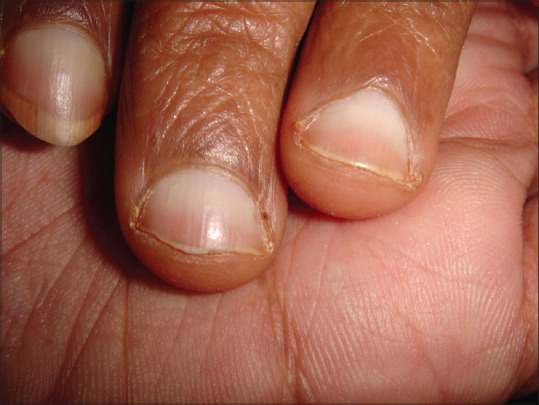
Half and half nail (Lindsay nail) in a patient of chronic kidney disease (Courtesy: Dr Suneil Gandhi, Surat, India)
Table 1.
Overview of non-specific skin changes in chronic kidney disease
| Non-specific skin changes | Etiopathogenesis | Clinical features | Treatment |
|---|---|---|---|
| Xerosis | Exact mechanism is not clear; proposed mechanisms are: a) dehydration of the stratum corneum b) abnormal eccrine gland function c) reduced sebum and sweat production due to gland atrophy. |
Skin appears dry, rough, or shiny. Xerotic skin may not be pruritic. Excoriations are associated with pruritus. Extensor surfaces of the lower extremities are most prominently involved. | Mainstay is hydration of the skin with moisturizing creams associated with 5-10% urea. |
| Acquired ichthyosis | Unknown | It is related to xerosis but is more than just dry skin, because the skin develops patterned scaly appearance. | Hydration of the skin. |
| Pigmentary alterations | a) Anemia of chronic disease b) Urochrome and carotenoid deposition in CKD c) Increased melanin production as a result of elevated levels of β-melanocyte-stimulating hormone. |
Pallor Yellowish discoloration (most common) Hyperpigmentation |
Erythropoiesis stimulating agents and iron. No treatment Sunscreen and photoprotection |
| Purpura | Qualitative platelet dysfunction due to uremia. | Purpura/ecchymoses can be spontaneous or in response to minor traumas. | Dialysis, desmopressin i.v estrogen |
| Nail changes: Lindsay (half-and-half) nail | Seen in approximately in 40% of uremic patients; likely due to melanin deposition in the nail bed and plate. | The distal portion is a pink-red, or brown horizontal band that occupies nearly half of the total nail length. Underlying nail bed changes give the proximal portion a dullwhite, ground-glass appearance. Other nail changes include absent, splinter hemorrhages, koilonychia, onycholysis, Mees’ lines, Muehrcke’s lines, Beau’s lines | No treatment. The nails may revert to normal following renal transplantation. |
| Mucosal changes | Nutritional deficiencies, candidiasis, poor oral hygiene, smoking, consumption of alcohol or hot/ spicy foods, dehydration, and mouth breathing remain the overall pathogenic triggers | Coated tongue, macroglossia with teeth markings, xerostomia, cheilitis and gingivitis | Maintenance of oral hygiene and eliminating potential triggers |
| Hair changes | Reduced sebum production and parathormone levels, anemia, stress of chronic disease/dialysis, or neglect | Sparse scalp and body hair, diffuse alopecia, lustreless hair | No specific therapy. Ensuring proper self-care is recommended |
| Cutaneous features due to hormonal changes | Elevated prolactin levels | Gynaecomastia in men and hirsutism and acne in women. Acanthosis nigricans resulting from insulin resistance may also be encountered. | Endocrinological consultation |
Acquired perforating dermatosis (APD): It is an umbrella term that describes the various types of perforating diseases and are often associated with an underlying systemic disease like diabetes mellitus and chronic renal failure. APD has been used in place of the previous nomenclature (e.g., perforating folliculitis, Kyrle's disease, reactive perforating collagenosis).[22] APD occurs in approximately 11% of patients receiving hemodialysis. In patients with chronic renal failure, APD most commonly arises after the initiation of dialysis and following renal transplantation, it almost always resolves. An interplay of various factors like chronic rubbing leading to epithelial hyperplasia and abnormal keratinization, reduced blood supply secondary to microangiopathy, over-expression of transforming growth factor beta-3 and elevated serum and tissue fibronectin leading to epidermal perforation are the main pathomechanisms implicated[22,23]
Umbilicated, hyperkeratotic pruritic papules, some with a central, white, keratotic crust characterizes APD [Figure 2]. Koebnerization may occur, and chronic scratching of pruritic areas may lead to coalescence of the papules into larger plaques, mostly located on the extensors of extremities.[23] Treatment of APD is difficult, although patients get some relief with topical steroids, keratolytics, lubrication, topical or oral retinoids and cryotherapy. Narrowband ultraviolet B light may be helpful in refractory cases. Recently, allopurinol at a dose of 100 mg/day helps in resolution of the pruritus and the papules.[24] It inhibits xanthine oxidase which decreases oxygen-free radicals that cause collagen damage and inhibits cross-linking of collagen by glycation end products due to an antioxidative effect.
Figure 2.
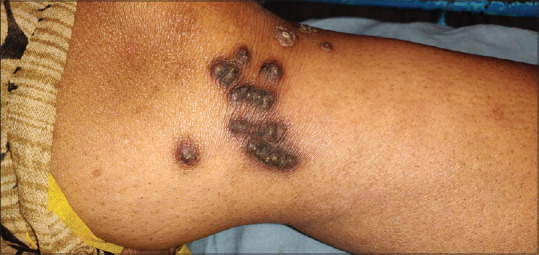
Umbilicated, hyperkeratotic papules, with a central, white, keratotic crust, in a patient of acquired perforating disorder, with long standing renal disease
Calcinosis cutis: Also known as benign nodular calcification, it comprises of calcium deposition within the cutaneous and subcutaneous tissues without tissue necrosis. In CKD, calcinosis cutis develops due to metastatic deposition of calcium salts into the skin and subcutaneous tissues as a result of elevated calcium phosphate product. Hyperparathyroidism may or may not be present. Clinically, firm white papules, plaques and nodules, most commonly over the periarticular areas and fingertips are observed.[25] Treatment revolves around normalizing calcium and phosphate levels. If hyperparathyroidism is present, parathyroidectomy may be beneficial. Use of phosphate binders and reduction of dietary phosphate are advised. Other medical therapies such as vinpocetine, sodium thiosulfate, and intravenous pamidronate have been used with variable success. Surgical removal may be considered if lesions are symptomatic[26]
Calciphylaxis: The term “calciphylaxis” is a misnomer because it implies a systemic hypersensitivity reaction. Calcific uremic arteriolopathy is a more rational term for this process in ESKD patients. It is characterized by calcification of arterioles and capillaries in the dermis and subcutaneous adipose tissue. It carries a high morbidity and mortality, with an estimated 6-month survival of approximately 50%.[27] Mostly, calciphylaxis occurs in end-stage kidney disease (ESKD) (those on dialysis), but it may even occur in non-ESKD patients and kidney transplant recipients. The pathogenesis of calciphylaxis is complicated and the salient features are:[28]
Increased NFkb activity leads to bone mineral loss and vascular calcification. Nuclear factor kb (NFkb) is a transcription factor activated by parathyroid hormone (PTH), aluminium, corticosteroids, chronic inflammatory states, and free radicals
Transformation of vascular smooth muscle cells into osteoblast-like cells is initiated by reactive oxygen species and imbalance between inhibitors and inducers of vascular calcification and metabolic disturbances (hyperparathyroidism, hyperphosphatemia, hypercalcemia, elevated calcium × phosphorus product)
Inducers of calcification like osteopontin and bone morphogenic protein 2 (BMP-2) are upregulated and inhibitors of calcification like matrix Gla protein (MGP), fetuin-A, and pyrophosphate are suppressed. Fetuin-A binds calcium and phosphate in the circulation, thereby forming “calciprotein particles.” This aids in clearing the circulation of excess Ca × P. Low fetuin-A level is associated with a chronic inflammatory state and cardiovascular calcification in hemodialysis patients. MGP, which is synthesized by vascular smooth muscle, endothelium, and chondrocytes inhibits calcification of arteries and cartilage. The activity of MGP activity depends upon carboxylation which is dependent on vitamin K. Warfarin inhibits of vitamin K-dependent carboxylation of MGP and thus increases the risk of calciphylaxis.
Progressive subintimal fibrosis and medial-arteriolar calcification lead to endothelial injury. The low-flow rate and luminal narrowing leads to decreased blood flow and a procoagulant state. It eventually leads to localized tissue ischemia and necrosis.
Clinically, most commonly affected areas are fatty areas of the thighs, abdomen, buttocks, and lower extremities. To begin with, lesions are firm with a pink or mottled color or a livedo reticularis like appearance; and progress to painful ulcers with a black eschar [Figure 3]. Surrounding the ulcers, there may be skin mottling with reticulate dyspigmentation. Histopathologic findings include calcification of medium-sized vessels with intimal hyperplasia and thrombosis. Radiologic investigations may also show linear calcium deposits in the skin.[27,28]
Figure 3.
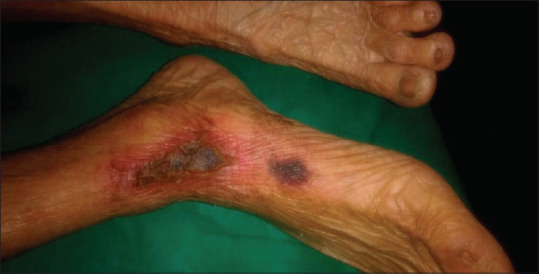
An ulcer on the leg with black leathery eschar surrounded by dyspigmentation and erythematous induration signifying calciphylaxis (Courtesy Dr Iti Varshney, Aligarh, India)
Treatment revolves around supportive and preventive measures including hyperbaric oxygen therapy, debridement, wound care, and antibiotic therapy. Prevention and/or reversal of calcium-phosphate precipitation is done by administration of sodium thiosulfate. Correction of calcium, phosphorus, and parathyroid hormone is carried out by using non-calcium-containing phosphate binders, such as sevelamer carbonate or lanthanum carbonate. Cinacalcet may be used to suppress PTH levels. Medications that may contribute to calciphylaxis, including vitamin D, calcium supplements and warfarin need to be withheld. Besides, dialysis optimization is always an option to be explored.[29]
Bullous diseases: These manifest with vesiculobullous eruptions on the skin and/or mucous membranes. In ESRD, porphyria cutanea tarda and pseudoporphyria may be seen. Porphyria cutanea tarda (PCT) is characterized by abnormalities in the enzyme uroporphyrinogen decarboxylase (URO-D). Approximately 18% of patients with renal failure may have porphyria cutanea tarda. These patients have elevated levels of urinary uroporphyrin and fecal isocoproporphyrin. The pathogenesis of PCT is not clear. Alcohol abuse, hepatitis C virus, HIV infection, and iron supplementation contribute to decreased URO-D activity. Porphyrins may form complexes with high-molecular weight proteins, which are poorly dialyzed by conventional methods. In hemodialysis (HD) patients, decreased URO-D activity and poor clearance of the plasma porphyrins, contribute to features of PCT. Cutaneous findings in PCT include non-inflamed blisters, erosions and crusts, especially over the dorsum of hands and forearms [Figure 4]. Blisters may heal with scarring or milia. Sclerodermatous plaques, hypertrichosis, and hyperpigmentation in sun-exposed areas are also seen. The essential part of treatment is avoidance of sunlight. Concomitant exacerbating factors (alcohol, estrogens, iron overload, hepatitis) should be avoided or treated. Small volume phlebotomy (50–100 ml once or twice weekly) to decrease hepatic iron stores can induce remission because iron overload is an inciting factor. Alternatively, desferrioxamine can be used. High-flux membrane hemodialysis, which removes plasma porphyrins effectively is also beneficial. Pseudoporphyria is clinically and histologically identical to PCT without the serum and urine porphyrin abnormalities. Concomitant use of drugs such as tetracycline, amiodarone, furosemide, naproxen, and nalidixic acid may be a precipitating factor. Treatment involves stopping/avoiding any of the drugs and photoprotection. N-acetylcysteine, an antioxidant is an effective treatment.[30]
Figure 4.
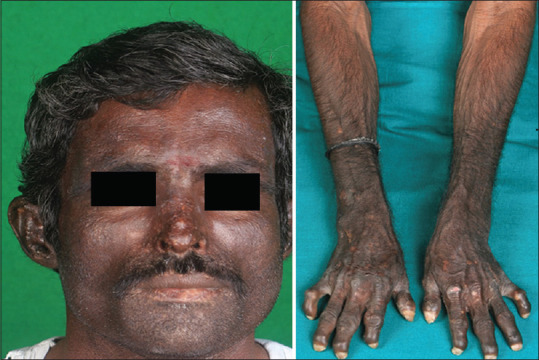
Hyperpigmentation, scarring and milia, over the sun-exposed areas in a patient of porphyria cutanea tarda, with chronic kidney disease (Courtesy: Dr PVK Chaitanya, Puducherry, India)
Eruptive xanthomas: These are clinically characterized by 1–5 mm yellow or erythematous papules that appear in crops and have an abrupt onset. The most common sites are the extensor surfaces of the extremities and buttocks [Figure 5]. Koebnerization can occur. The lesions are associated with hyperlipidemia, either familial or due to nephrotic syndrome. In nephrotic syndrome, the xanthomas are secondary to hyperlipidemia resulting from compensatory increased lipoprotein synthesis due to hypoproteinemia. Generally, xanthomas resolve spontaneously once lipid levels are normalized.[31]
Figure 5.
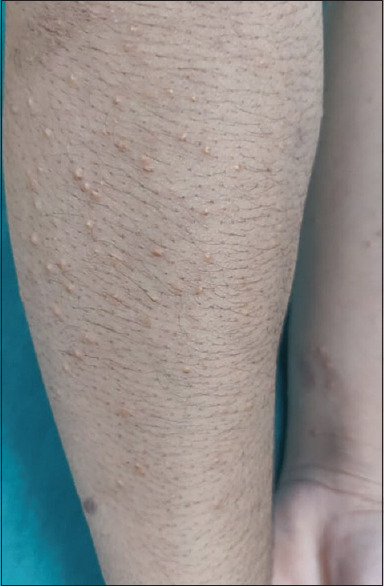
Yellowish papules over the forearms in a patient of eruptive xanthoma with recently diagnosed renal disease (Courtesy: Dr Ashish Amrani, New Delhi, India)
Nephrogenic systemic fibrosis (NSF): It is a scleroderma-like disorder caused by exposure to gadolinium-based contrast agents (GBCAs) in patients with renal insufficiency. NSF was originally named nephrogenic fibrosing dermopathy (NFD) because of the predominant skin findings. It is seen in patients with advanced kidney disease and is characterized by thickening and hardening of the skin, mainly over the extremities and trunk. It leads to marked expansion and fibrosis of the dermis, associated with CD34+ fibrocytes.
The pathogenesis of NSF revolves around Gadolinium (Gd), cytokines and immunological events. Gd is renally eliminated in normal persons with a half-life of 1–2 h. In patients with CKD or on dialysis, the half-life of Gd is increased (up to 60 h). During this period, Gd dissociates into its toxic ionic form (Gd3+), following which, Gd3+ precipitates with anions, like phosphates and leads to tissue deposition. After deposition, dissociated Gd is phagocytized by macrophages, activating and recruiting fibroblasts. Fibrogenic cytokines are secreted by the macrophages and fibroblasts lead to a cascade of exaggerated fibrogenic response characterized by activation of the transforming growth factor (TGF) beta-1 pathway, increase in circulating fibrocytes and secretion of cytokines and growth factors by monocytes.[32,33]
Other important pathogenic factors include:
Patients on peritoneal dialysis, which is less effective in removing Gd (in comparison to hemodialysis), are at an increased risk of developing NSF
High dose (0.2 mmol/kg) or multiple cumulative doses of GBCA versus a single standard dose of GBCA (0.1 mmol/kg)
Presence of concomitant triggering factors like vascular injury (such as thrombosis and acute ischemia), infections and high-dose erythropoietin therapy
Often, the type of GBCA determines the risk of developing NSF:
The American College of Radiology (ACR) has classified the GBCAs into three categories, with group I being the most likely to cause NSF, group II being the least likely to cause it, and group III having limited data:
Group I – Gadodiamide, Gadopentetate dimeglumine
Group II - Gadobenate dimeglumine, Gadobutrol
Group III – Gadofosveset trisodium, Gadoxetate disodium.
Clinical features of NSF include fibrotic, indurated papules, plaques, or subcutaneous nodules (mostly bilaterally symmetrical). The disease starts from ankles, lower legs, feet, and hands, and then progress proximally to involve the thighs and forearms. The lesions begin with edema of the region, often mimicking cellulitis. Upon resolution of edema, the involved skin retains a thickened and indurated texture. The skin may have a “cobblestone,” or peau d'orange appearance (orange peel-like). The lesions may be pruritic or painful or have a burning sensation. Confluent dermal plaques with thickening and hardening may also occur. Joint contractures and yellow sclera plaques are common. Fibrosis has also been identified in a variety of internal organs, including the lungs (with reduced diffusion capacity) There may be sclerodactyly or loss of skin folds over the dorsum of the hands and lower extremities. Diagnosis of NSF is confirmed by combining clinical features and histopathology. A deep incisional or a punch biopsy is obtained. Histopathologically, increased dermal and/or subcutaneous fibroblast-like cells are seen. The cells stain with procollagen I and CD34 by immunohistochemical methods. Mucin deposition may also be observed. The best method to prevent nephrogenic systemic fibrosis (NSF) is the avoidance of gadolinium (Gd) in patients with acute kidney injury (AKI) or chronic kidney disease (CKD). If Gd is necessary, then group I and group III agents should be avoided. Hemodialysis may be performed for its removal, however this practice is controversial and the ability of post-Gd hemodialysis session to prevent NSF is unproven. No medical therapy for NSF has been found to be effective. Recovery of kidney function may lead to stabilization, improvement, or resolution of NSF. Kidney transplantation is sometimes helpful in curbing progression and occasionally reverses the disease. A trial of extracorporeal photopheresis with ultraviolet A (ECP; also called photochemotherapy), which involves extracorporeal exposure of peripheral blood mononuclear cells to photoactivated 8-methoxypsoralen, followed by reinfusion of the treated cells, can be done.[34] ECP induces monocyte-derived tumor necrosis factor (TNF)-alpha, which, in turn, suppresses collagen synthesis and enhances collagenase production. Alternatively, treatment with imatinib mesylate is also reasonable.[35]
Iododerma: Patients receiving iodide in the form of intravenous contrast may develop certain skin lesions, in the setting of renal insufficiency or failure. A study documented that the plasma half-life of the contrast was increased to 23 h in patients with end-stage renal disease, compared with 2 h in those with normal kidney function.[36] The pathophysiology is not clear, however, it is believed to be a result of a delayed hypersensitivity reaction to iodine[37] (after binding to serum proteins). Clinically, patients present with acneiform eruptions confined to the regions of the body rich in sebaceous glands. Advanced stages may demonstrate the presence of edematous bullous and hemorrhagic lesions. Histologic findings are non-specific. Treatment relies on excretion of the accumulated iodine and avoidance of further exposure. Supportive treatment is usually satisfactory. Lesions heal with post inflammatory hyperpigmentation.[38]
Cutaneous changes in patients undergoing dialysis
Dialysis-related amyloidosis: This develops due to deposition of beta2-microglobulin amyloid fibrils in bones, joints, and other soft tissues. Scapulohumeral periarthritis, flexor tenosynovitis of the hand and carpal tunnel syndrome constitute the characteristic triad. Rarely it can also present as immobile dermal and subcutaneous nodules, mostly in buttocks[39]
-
Dialysis access steal syndrome: Symptomatic steal syndrome is a rare complication of vascular access for hemodialysis. The risk factors for the development of steal are diabetes, female sex, coronary heart disease, and age over 60. Common locations of arteriovenous (AV) fistula prone to developing steal are proximal sites like brachiocephalic AV fistula and brachiobasilic AV fistula. Decreased blood flow to the distal extremity resulting from blood steal through the proximal AV fistula causes hand pain, coldness, cyanosis or pallor of the digits, and decreased pulses or loss of sensation and motor function., There may be associated pallor or a pink to blue discoloration of the skin surrounding necrosis or ulceration. Symptoms worsening during hemodialysis sessions improving with compression of the AV access is the clinical diagnostic clue. Treatment options include fistula ligation and/or banding[40]
It may be difficult to implicate either CKD or hemodialysis alone for any particular cutaneous manifestation as many of them (non-specific cutaneous features, bullous dermatoses) are associated with both[41]
Pseudo Kaposi sarcoma: It is a reactive angiodysplasia of cutaneous vasculature, seen in association with venous insufficiency, chronic kidney failure undergoing dialysis (secondary to arterio-venous shunt) and other conditions.[42] It presents with multiple well-defined reddish brown papulo-nodules and plaques, on the dorsum of feet and the lower aspect of the shin. Histology shows proliferation of endothelial cells, neovascularisation, and surrounded by pericytes in the dermis. Correction of the underlying pathology is the mainstay of treatment, apart from using compression stockings to reduce the circulatory disturbances.
Renal transplant related skin conditions
Drug-induced side effects: They have been summarised in Table 2.[43,44]
-
Infections[45]
- Bacterial Infections: Wound infections, folliculitis, impetigo, cellulitis and erysipelas are observed in immunosuppressed transplant recipients. Group A streptococci and Staphylococcus aureus are the commonest causes. However the possibility of unusual pathogens should be kept in mind
- Viral Infections: Reactivation of latent HSV and varicella zoster virus infections may occur in kidney transplant recipients due to their immunosuppressed state. Herpes zoster may be multi-dermatomal and generalised. Human herpesvirus type 8 infection may cause Kaposi's sarcoma, and it is commonly seen in patients from the Mediterranean, Middle East and parts of Africa. Trichodysplasia spinulosa is a rare cutaneous condition caused by polyoma virus that has been exclusively described in immunosuppressed patients (organ transplant recipients on immunosuppressives). Cutaneous cytomegalovirus infection can give rise to non-specific skin findings, including ulceration. Moreover, human papilloma virus induced warts are common in kidney transplant recipients. They are often multiple, usually of cosmetic concern and resistant to treatment in allograft recipients
- Fungal Infections: In kidney transplant recipients, the frequencies of reported cutaneous fungal infections range from 7% to 75%. Pityriasis versicolor, onychomycosis, candidiasis have been described in transplant recipients. Factors such as gender, time since transplantation, immunosuppressive medications, skin type, tropical environment and UV exposure may be associated with cutaneous fungal infection in kidney transplant recipients.
-
Cutaneous malignancies: Skin is the most frequent site of malignancy accounting for nearly 40% of all post-transplant malignancies. Squamous cell carcinoma and basal cell carcinoma (BCC) account for over 90% of post-transplant skin malignancies, but melanoma, merkel cell carcinoma, and kaposi sarcoma are also observed frequently. In the general population, the frequency of BCCs is greater than SCCs, whereas the ratio of SCC/BCC may be as high as 5 in transplant recipients.[42] The risk factors for development of malignancies are:
- Fitzpatrick skin type I–III
- Cumulative sun exposure and skin type: Increasing age is also associated with the development of skin cancers, mostly due to the cumulative UV radiation exposure in the elderly. Forearms and bald scalp are common locations
-
Intensity and duration of immunosuppression:
- Azathioprine photosensitizes human skin to UVA radiation. 6-thioguanine, the content of azathioprine, is a strong UV chromophore that results in abnormal photosensitivity and accelerated carcinogenesis
- Both calcineurin inhibitors cause decreased production of IL-2 leading to expression of TGF-β and decreased tumor surveillance
- Induction therapy with anti thymocyte globulin, OKT3, or monoclonal anti-IL-2 receptor antibodies is also linked to skin cancers
- Studies have shown that triple immunosuppression (with prednisolone, cyclosporine, and azathioprine or sirolimus) correlates with higher risk of developing skin cancer versus patients on dual therapy (prednisolone and azathioprine or sirolimus).
- Geographic location (skin cancer is common in Australia)
- HPV infection
- History of lymphoma or leukemia
- Presence of prior skin cancer or precancerous lesions.
- History of voriconazole use.
Table 2.
Cutaneous side-effects of immunosuppressive drugs used post renal transplant
| Immunosuppresive drug | Cutaneous side-effects |
|---|---|
| Cyclosporine | Hypertrichosis, gingival and sebaceous hyperplasia, trichodysplasia spinulosa, non-melanoma skin cancer |
| Mycophenolate mofetil | Increased risk of herpes simplex, herpes zoster and and CMV infections; cutaneous side-effects are extremely rare |
| Tacrolimus | Non-melanoma skin cancer, virus-associated trichodysplasia |
| Sirolimus | Acneiform eruption, scalp folliculitis, inflammatory facial papules and nodules, aphthous ulceration, impaired wound healing, onychopathy, periungual infections, chronic gingivitis |
The clinical features of these malignancies have been summarised in Table 3. The key to the management of post-transplant dermatologic malignancies is prevention by awareness, and observation. Annual self-examination and examination by a physician are warranted. Suspicious lesions should undergo biopsy. Avoidance of excessive sun exposure and use of sunscreens must be advocated. Retinoids are sometimes used in high-risk patients. Suspicious skin lesions should be surgically excised. In patients with post-transplant squamous cell carcinoma, switching from a calcineurin inhibitor to sirolimus reduces the risk further.[46]
Table 3.
Clinical presentation of cutaneous malignancies in renal transplant recipients
| Tumour | Clinical presentation |
|---|---|
| Squamous cell carcinoma (most frequent skin cancer in transplant recipients) | Enlarging, raised, keratotic (often ulcerated) lesions on an indurated, erythematous base. It has a high risk of local and distant spread and recurrence |
| Keratoacanthoma | Solitary firm dome-shaped tumor on sun-exposed skin. Due to the central keratin core, it is referred to as “crateriform” lesion. It can undergo ulceration. |
| Basal cell carcinoma | Flesh-colored pearly papule with a characteristic rolled-out beaded margin, often with central ulceration and overlying telangiectasias. It is a locally aggressive malignancy, with rare chances of metastases |
| Melanoma | Development of a new pigmented lesion or recent-onset change in colour, shape or size of a pigmented skin lesion is the most sensitive clinical sign for melanoma. |
| Merkel cell carcinoma | Usually presents as pink-red to violaceous, firm, dome-shaped solitary nodule that has rapidly grown; shows prediliction for head and neck region of older adults and demonstrates aggressive malignant behaviour. |
| Kaposi sarcoma | Cutaneous lesions vary from pink patches to dark violet plaques, nodules, or polyps, depending on clinical variant and stage. |
Conclusion
Cutaneous manifestations are quite frequent in patients with CKD. Sometimes, these skin signs and symptoms may be the first obvious clue to underlying kidney disease. Recent advances in the treatment have improved the quality of life and life expectancy of these patients, resulting in changes in the frequency and types of cutaneous disorders observed in conjunction with CKD. Some prophylactic measures can prevent some of the cutaneous manifestations, such as emollients for xerosis and pruritus, sunscreens, avoidance of sun exposure and adequate clothing for pigmentary changes, and cutaneous malignancies. Prompt recognition and management of some of these dermatological manifestations by treating physicians, in close liaison with a dermatologist, will vastly reduce the morbidity and improve the quality of life of suffering patients.
Financial support and sponsorship
Institutional.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 2.Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: A review. JAMA. 2019;322:1294–304. doi: 10.1001/jama.2019.14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prabhakar MR, Chandrasekaran V, Soundarajan P. Epidemic of chronic kidney disease in India-what can be done? Saudi J Kidney Dis Transpl. 2008;19:847–53. [PubMed] [Google Scholar]
- 4.Pico MR, Lugo Somolinos A, Sanchez JL, Burgos Calderon R. Cutaneous alterations in patients with chronic renal failure. Int J Dermatol. 1992;31:860–3. doi: 10.1111/j.1365-4362.1992.tb03543.x. [DOI] [PubMed] [Google Scholar]
- 5.Morton CA, Lafferty M, Hau C, Henderson I, Jones M, Lowe JG. Pruritus and skin hydration during dialysis. Nephron Dial Transplant. 1996;11:2031–6. doi: 10.1093/oxfordjournals.ndt.a027092. [DOI] [PubMed] [Google Scholar]
- 6.Guptha AK, Guptha MA, Cardella CJ, Haberman HF. Cutaneous associations of chronic renal failure and dialysis. Int J Dermatol. 1986;25:498–504. doi: 10.1111/j.1365-4362.1986.tb00858.x. [DOI] [PubMed] [Google Scholar]
- 7.Etter L, Myers SA. Pruritus in systemic disease: Mechanisms and management. Dermatol Clin. 2002;20:459–72. doi: 10.1016/s0733-8635(02)00011-6. [DOI] [PubMed] [Google Scholar]
- 8.Thomas EA, Pawar B, Thomas A. A prospective study of cutaneous abnormalities in patients with chronic kidney disease. Indian J Nephrol. 2012;22:116–20. doi: 10.4103/0971-4065.97127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Udayakumar P, Balasubramanian S, Ramalingam KS, Lakshmi C, Srinivas CR, Mathew AC. Cutaneous manifestations in patients with chronic renal failure on hemodialysis. Indian J Dermatol Venereol Leprol. 2006;72:119–25. doi: 10.4103/0378-6323.25636. [DOI] [PubMed] [Google Scholar]
- 10.Park TH, Park CH, Ha SK, Lee SH, Song KS, Lee HY, et al. Dry skin (xerosis) in patients undergoing maintenance haemodialysis: The role of decreased sweating of the eccrine sweat gland. Nephrol Dial Transplant. 1995;10:2269–73. doi: 10.1093/ndt/10.12.2269. [DOI] [PubMed] [Google Scholar]
- 11.Smith AG, Shuster S, Comaish JS, Plummer NA, Thody AJ, Alvarez-Ude F, et al. Plasma immunoreactive beta-melanocyte-stimulating hormone and skin pigmentation in chronic renal failure. Br Med J. 1975;1:658–9. doi: 10.1136/bmj.1.5959.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comaish JS, Ashcroft T, Kerr DN. The pigmentation of chronic renal failure. J Am Acad Dermatol. 1975;55:215–7. [Google Scholar]
- 13.Remuzzi G. Bleeding in renal failure. Lancet. 1988;28:1205–8. doi: 10.1016/s0140-6736(88)92019-3. [DOI] [PubMed] [Google Scholar]
- 14.Lindsay RM, Briggs JD, Luke RG, Boyle IT, Kennedy AC. Gynaecomastia in chronic renal failure. Br Med J. 1967;4:779–80. doi: 10.1136/bmj.4.5582.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathew MT, Rajarathnam K, Rajalaxmi PC, Jose L. The tongue sign of CRF: Further clinical and histopathological features of this new clinical sign of chronic renal failure. J Assoc Phy Ind. 1986;34:52. [Google Scholar]
- 16.Zucker I, Yosipovitch G, David M, Gafter U, Boner G. Prevalence and characterization of uremic pruritus in patients undergoing hemodialysis: Uremic pruritus is still a major problem for patients with end-stage renal disease. J Am Acad Dermatol. 2003;49:842–6. doi: 10.1016/s0190-9622(03)02478-2. [DOI] [PubMed] [Google Scholar]
- 17.Meyer TW, Hostetter TH. Uremia. N Engl J Med. 2007;357:1316–25. doi: 10.1056/NEJMra071313. [DOI] [PubMed] [Google Scholar]
- 18.Mettang T. Chronic kidney disease-associated pruritus. In: Misery L, Ständer S, editors. Hypertension. London: Springer-Verlag; 2010. pp. 166–75. [Google Scholar]
- 19.Tey HL, Yosipovitch G. Targeted treatment of pruritus: A look into the future. Br J Dermatol. 2011;165:5–17. doi: 10.1111/j.1365-2133.2011.10217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feramisco JD, Berger TG, Steinhoff M. Innovative management of pruritus. Dermatol Clin. 2010;28:467–78. doi: 10.1016/j.det.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Zhai LL, Savage KT, Qiu CC, Jin A, Valdes-Rodriguez R, Mollanazar NK. Chronic pruritus responding to dupilumab - A case series. Medicines (Basel) 2019;6:72. doi: 10.3390/medicines6030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morton CA, Henderson IS, Jones MC, Lowe JG. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996;135:671–7. [PubMed] [Google Scholar]
- 23.Saray Y, Seçkin D, Bilezikçi B. Acquired perforating dermatosis: Clinicopathological features in twenty-two cases. J Eur Acad Dermatol Venereol. 2006;20:679–88. doi: 10.1111/j.1468-3083.2006.01571.x. [DOI] [PubMed] [Google Scholar]
- 24.Tilz H, Becker JC, Legat F, Schettini AP, Inzinger M, Massone C. Allopurinol in the treatment of acquired reactive perforating collagenosis. An Bras Dermatol. 2013;88:94–7. doi: 10.1590/S0365-05962013000100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takashima S, Sakamoto T, Ota M. Tumoral calcinosis in chronic renal failure. Lancet Diabetes Endocrinol. 2014;2:852. doi: 10.1016/S2213-8587(14)70193-7. [DOI] [PubMed] [Google Scholar]
- 26.Fathi I, Sakr M. Review of tumoral calcinosis: A rare clinico-pathological entity. World J Clin Cases. 2014;2:409–14. doi: 10.12998/wjcc.v2.i9.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazhar AR, Johnson RJ, Gillen D. Risk factors and mortality associated with calciphylaxis in end-stage renal disease. Kidney Int. 2001;60:324–32. doi: 10.1046/j.1523-1755.2001.00803.x. [DOI] [PubMed] [Google Scholar]
- 28.Rogers NM, Teubner DJO, Coates PTH. Calcific uremic arteriolopathy: Advances in pathogenesis and treatment. Semin Dial. 2007;20:150–7. doi: 10.1111/j.1525-139X.2007.00263.x. [DOI] [PubMed] [Google Scholar]
- 29.Udomkarnjananun S, Kongnatthasate K, Praditpornsilpa K, Eiam-Ong S, Jaber BL, Susantitaphong P. Treatment of calciphylaxis in CKD: A systematic review and meta-analysis. Kidney Int Rep. 2018;4:231–44. doi: 10.1016/j.ekir.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryali ME, Whittier WL. Bullous skin lesions in a patient undergoing chronic hemodialysis. Semin Dial. 2010;23:83–7. doi: 10.1111/j.1525-139X.2009.00672.x. [DOI] [PubMed] [Google Scholar]
- 31.Naik NS. Eruptive xanthomas. Dermatol Online J. 2001;7:11. [PubMed] [Google Scholar]
- 32.Jiménez SA, Artlett CM, Sandorfi N, Derk C, Latinis K, Sawaya H, et al. Dialysis-associated systemic fibrosis (nephrogenic fibrosing dermopathy): Study of inflammatory cells and transforming growth factor beta1 expression in affected skin. Arthritis Rheum. 2004;50:2660–6. doi: 10.1002/art.20362. [DOI] [PubMed] [Google Scholar]
- 33.Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35:238–49. doi: 10.1016/j.semarthrit.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathur K, Morris S, Deighan C, Green R, Douglas KW. Extracorporeal photopheresis improves nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: Three case reports and review of literature. J Clin Apher. 2008;23:144–50. doi: 10.1002/jca.20170. [DOI] [PubMed] [Google Scholar]
- 35.Chandran S, Petersen J, Jacobs C, Fiorentino D, Doeden K, Lafayette RA. Imatinib in the treatment of nephrogenic systemic fibrosis. Am J Kidney Dis. 2009;53:129–32. doi: 10.1053/j.ajkd.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 36.Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast media. Br J Dermatol. 2014;170:1377–9. doi: 10.1111/bjd.12852. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg FR, Einbinder J, Walzer RA. Vegetating iododerm: An immunologic mechanism. Arch Dermatol. 1972;105:900–5. [PubMed] [Google Scholar]
- 38.Hesseler MJ, Clark MR, Zacur JL, Rizzo JM, Hristov AC. An acneiform eruption secondary to iododerma. JAAD Case Rep. 2018;4:468–70. doi: 10.1016/j.jdcr.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimizu S, Yasui C, Yasukawa K, Nakamura H, Shimizu H, Tsuchiya K. Subcutaneous nodules on the buttocks as a manifestation of dialysis-related amyloidosis: A clinicopathological entity? Br J Dermatol. 2003;149:400–4. doi: 10.1046/j.1365-2133.2003.05466.x. [DOI] [PubMed] [Google Scholar]
- 40.Malik J1, Tuka V, Kasalova Z, Chytilova E, Slavikova M, Clagett P, et al. Understanding the dialysis access steal syndrome. A review of the etiologies, diagnosis, prevention and treatment strategies. J Vasc Access. 2008;9:155–66. [PubMed] [Google Scholar]
- 41.Abdelbaqi-Salhab M, Shalhub S, Morgan MB. A current review of the cutaneous manifestations of renal disease. J Cutan Pathol. 2003;30:527–38. doi: 10.1034/j.1600-0560.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 42.Nakanishi G, Tachibana T, Soga H, Fujimoto N, Tanaka T. Pseudo-Kaposi's sarcoma of the hand associated with acquired iatrogenic arteriovenous fistula. Indian J Dermatol. 2014;59:415–6. doi: 10.4103/0019-5154.135511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghaninejad H, Ehsani AH, Ghiasi M, Noormohammadpour P, Najafi E, Naderi G. Benign and malignant skin lesions in renal transplant recipients. Indian J Dermatol. 2009;54:247–50. doi: 10.4103/0019-5154.55634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warino L, Libecco J. Cutaneous effects of sirolimus in renal transplant recipients. J Drugs Dermatol. 2006;5:273–4. [PubMed] [Google Scholar]
- 45.Sandhue K, Gupta S, Kumar B, Dhandha R, Udigiri NK, Minz M. The pattern of mucocutaneous infections and infestations in renal transplant recipients. J Dermatol. 2003;30:590–5. doi: 10.1111/j.1346-8138.2003.tb00440.x. [DOI] [PubMed] [Google Scholar]
- 46.Euvard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681–5. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]


