Abstract
Background:
Optic nerve sheath diameter (ONSD) measurement is emerging as a noninvasive method to estimate raised ICP. It is helpful in situations where imaging of brain or direct ICP monitoring is not available or feasible. Use of ONSD is still limited, so this study was planned to determine whether the bedside sonographic measurement of ONSD can reliably predict elevated ICP in neuro-trauma patients.
Methodology:
After approval from Hospital Ethics Committee, this cross-sectional study was conducted in hundred traumatic brain injury (TBI) patients with suspected elevated ICP, admitted to neurosurgical ICU. The severity of brain injury was assessed according to Glasgow coma scale (GCS), initial CT scan findings, and revised trauma score (RTS). All patients underwent ONSD sonography of the eye and CT scan subsequently. ONSD of ≥5.0 mm was considered as a benchmark of raised ICP.
Results:
Mean ONSD of the study group with ONSD ≥5.0 mm was 5.6 ± 0.3 mm. ONSD was raised in 46% of patients, more so in patients with low GCS (3-6). The relationship of ONSD with GCS, CT scan findings, and RTS was highly significant. The sensitivity of the bedside sonographic measurement ONSD to detect raised ICP was 93.2% and specificity was 91.1% when compared with CT scan. Positive Predictive Value of the ONSD measurement was 89.1% and the negative predictive value was 94.4%.
Conclusion:
Ultrasonographic assessment of ONSD is a reliable modality to detect raised ICP in neurotrauma patients. It can be helpful in the early initiation of treatment of elevated ICP, thus preventing secondary brain damage.
Keywords: Computed tomography scan, Glasgow coma score, intracranial pressure, optic nerve sheath diameter, revised trauma score, traumatic brain injury
INTRODUCTION
It is important to determine raised intracranial pressure (ICP) in traumatic and nontraumatic neurological and neurosurgical patients as it may cause significant neurological deterioration due to secondary brain damage.[1,2] Hence, a quick evaluation of increased ICP is important for the management of patients with traumatic brain injury (TBI) to allow timely ICP lowering measures and maintain adequate cerebral perfusion.[3] Direct methods of ICP measurement, such as epidural bolt, microdialysis catheter, lumbar puncture (LP), and intraventricular catheterization are accurate but are invasive in nature and can result in complications such as hemorrhage and infection. Many noninvasive techniques for the detection of elevated ICP include computed tomography (CT) of the head, magnetic resonance imaging (MRI), and trans-cranial Doppler sonography (TCD). However, sometimes these tests are not readily available or the patient is nontransferable and along with this, there is a risk of radiation exposure with these techniques.[4] Ultrasonography-guided retro-bulbar optic nerve sheath diameter (ONSD) measurement by a trained Ultrasonologist is an alternative noninvasive method to estimate ICP in comparison to various invasive and other noninvasive ICP measurement techniques. Point of care ocular sonography is a quick, cost-effective, bedside and noninvasive method for measurement of ONSD and can lead to more timely interventions for raised ICP, especially in hemodynamically unstable patients in ICU, where CT scan is unavailable and invasive monitoring is contraindicated.[4] The optic nerve sheath is contiguous with the subarachnoid space so an increase in ICP results in similar pressure changes between intracranial and intraorbital subarachnoid spaces.[5] Ultrasound-guided ONSD measurement has been evaluated and proposed as a means of measuring ICP in the previous studies but its use is still limited to a few centers.[6,7,8] Therefore, the present study was planned to evaluate the accuracy of bedside ultrasound-guided ONSD to diagnose raised ICP and compare the results with CT findings of raised ICP in neuro-trauma patients. The aim of this study was to determine whether the bedside ultrasound-guided measurement of ONSD can reliably predict the elevated ICP in neuro-trauma patients.
MATERIALS AND METHODS
Study setting
This cross-sectional study was conducted in the Neurosurgery ICU of a tertiary care teaching hospital between October 2018 and July 2019, after obtaining approval from Hospital Ethics Committee.
Study population
After obtaining a written informed consent, hundred patients with TBI were enrolled in this study who had suspected elevated ICP. Patients consisted of heterogenous population including those with subdural hemorrhage (SDH), extradural hemorrhage (EDH), intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH), and diffuse cerebral edema due to TBI. In case of multiple diagnoses, patient was categorized with respect to the predominant manifestation of TBI. The severity of brain injury was assessed according to Glasgow coma scale (GCS), initial CT scan findings, and revised trauma score (RTS). All patients who were enrolled in the study underwent optic nerve sheath diameter (ONSD) sonography of both eyes followed by CT scan head subsequently.
Inclusion criteria
All adult patients (=18 years) presenting to Neurosurgical ICU with TBI with a suspected rise in ICP were included in the study.
Exclusion criteria
Patients with penetrating trauma to head or significant ocular trauma, history of glaucoma, or optic nerve pathology were excluded from the study.
Procedure and Measurements
Ultra sonographic measurement of ONSD was done using a 10–13 MHz linear ultrasound probe. All patients were examined in the supine position. Conductive ultrasound gel was placed over a closed upper eyelid without any pressure. The probe was placed on the superior and lateral aspect of the orbit as is the recommended technique.[8] Optic nerve was visualized as a linear hypoechoic structure with defined margins posterior to the globe. The transverse ONSD was measured 3 mm behind the retina as shown in Image 1 and 2 and noted. Based on prior literature, binocular ONSD ≥5.0 mm was considered raised in this study.[9,10,11] The CT scan result was considered to be positive for elevated ICP if there was significant edema, midline shift of 3 mm or more, mass effect, effacement of sulci, collapse of ventricles, or compression of cisterns and its assessment was done by the radiologist blinded to the study.[7,11]
The RTS is a physiologic scoring system used in our study, based on the initial vital signs of a patient. A lower score indicates a higher severity of injury. Values for the RTS are in the range 0 to 7.84. We used an online calculator application to calculate RTS.[12]
Data collection
In a patient with suspected raised ICP, for which a CT was planned, bedside ONSD sonography of the eye was conducted by single well-trained investigator with the intention of minimizing interobserver variability. Investigator in the study had the experience of doing bedside ONSD examination of more than fifty abnormal ultrasounds in the past one year. Ultrasound-guided ONSD examination was quickly performed as it is available in our ICU block so that there is no time delay in shifting the patient to Radiology unit for CT scan subsequently.
The investigator performed three ONSD measurements on each eye of the patient and the mean of left and right eye ONSD measurements was calculated to minimize intraobserver variability. All patients underwent CT scan subsequently which was reviewed by a radiologist blinded to the ONSD sonography findings. CT measurements were performed and interpreted within 15–30 min of measurement of ONSD. ONSD readings and CT findings of the patient were then noted and analyzed. Other variables noted were age, sex, clinical diagnosis, GCS, hemodynamic parameters, RTS, mechanical ventilation, and comorbidities. Data collected was analyzed and was compared for any significant relation between various variables and ONSD measurements.
Statistical analysis
Data was recorded, tabulated, and statistically analyzed using SPSS version 21. Chi-square test was calculated and P value ≥0.05 was considered significant. ROC curve was plotted to determine optimal ONSD cutoff point that gives the highest sensitivity and specificity values for this modality.
RESULTS
In this study, we enrolled a hundred neuro-trauma patients who were admitted to Neurosurgery ICU and were detected to have raised ICP during the study period. ONSD measurement in these patients was done after taking informed consent from their relatives, prior to their shifting to Radiology suite for CT scan brain. Age distribution of the study group was 48.19 ± 14.99 years. Minimum age was 19 years and maximum age was 72 years. Maximum patients (42%) were of the age group 41–60 years as shown in Table 1. Study participants comprised of 81 male and 19 female patients. Age and gender had no significant relationship with ONSD measurements.
Table 1.
Age distribution of study participants with respect to ONSD measurements
| Age (years) | ONSD ≥5.0 mm | Total | Chi-square value | P | |
|---|---|---|---|---|---|
| No | Yes | ||||
| <20 | 1 (1.9) | 3 (6.5) | 4 | ||
| 21-40 | 20 (37.0) | 12 (26.1) | 32 | 3.970 | 0.265 |
| 41-60 | 24 (44.4) | 18 (39.1) | 42 | ||
| >60 | 9 (16.7) | 13 (28.3) | 22 | ||
| Total | 54 (100) | 46 (100) | 100 | ||
Figures in parenthesis indicate percentages
Raised ONSD was found in 46% of patients and mean ONSD of the study group with ONSD ≥5.0 mm was 5.6 ± 0.3 mm and with ONSD <5.0 mm was 4.1 ± 0.3 mm and the highest ONSD reading recorded was 5.9 mm [Table 2].
Table 2.
Distribution of Mean±Standard deviation of Age and ONSD in study population
| ONSD ≥5.0 mm | t | P | ||||
|---|---|---|---|---|---|---|
| No | Yes | |||||
| Mean (cm) | SD | Mean (cm) | SD | |||
| Age | 46.98 | 13.79 | 48.24 | 16.00 | -0.422 | 0.674 |
| ONSD | 0.41 | 0.03 | 0.56 | 0.03 | -24.274 | 0.000 |
Most of the patients (86%) were involved in road traffic accidents, followed by those involved in fall from height (12%) and assault (2%). Distribution of study population with respect to clinical diagnosis is shown in Figure 1. Raised ONSD measurement was noted in 40% of patients with SDH, 70% of patients with ICH, and 36% of patients with SAH and ICH in this study population. The relationship of clinical diagnosis at admission and raised ONSD was not significant.
Figure 1.
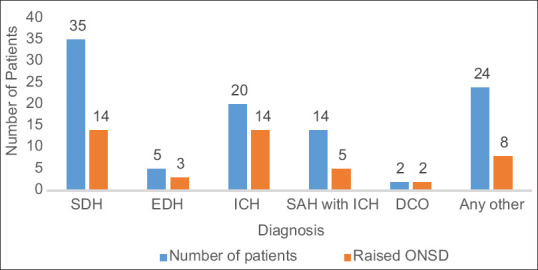
Distribution of patients with respect to diagnosis and ONSD
Among the study population, 49% of patients had comorbidities, 9% had chest injuries, 2% had abdominal injuries, and 22% had orthopedic injuries along with TBI. Out of 100 patients, 84 were mechanically ventilated and all the patients with raised ONSD were already on mechanical ventilation. Comorbidities, multiple injuries, and presence of mechanical ventilation did not have any significant relation with raised ONSD. Findings of ONSD were compared with GCS of the patient at the time of examination and it was observed that ONSD measurement was increased in patients with low GCS (3–8) and the relationship between GCS and ONSD readings was highly significant (P-value = 0.000) as shown in Table 3. Out of 46 patients with raised ONSD, 82.6% had RTS <4 and the relation between RTS and raised ONSD was statistically significant.
Table 3.
Relationship between GCS, RTS and ONSD measurements
| Parameter | ONSD ≥5.0 mm | Total | Chi-square value | P | |
|---|---|---|---|---|---|
| Yes | No | ||||
| GCS | |||||
| 3-5 | 24 (52.2) | 0 | 24 | ||
| 6-8 | 21 (45.7) | 21 (38.9) | 42 | 53.822 | 0.000 (HS) |
| >8 | 1 (2.2) | 33 (61.1) | 34 | ||
| Total | 46 (100) | 54 (100) | 100 | ||
| RTS | |||||
| <4 | 38 (82.6) | 2 (3.7) | 40 | 64.746 | 0.000 (HS) |
| 4-6 | 8 (17.4) | 48 (88.9) | 56 | ||
| >6 | 0 | 4 (7.4) | 4 | ||
| Total | 46 (100) | 54 (100) | 100 | ||
Figures in parenthesis indicate percentages
Hemodynamic parameters and ONSD findings were compared and it was observed that approximately 25 patients had systolic BP <110 and out of 46 patients with raised ONSD (ONSD ≥5.0 mm), SBP was lower in 43.5% of patients and 45.7% were on inotropic support [Table 4] and the relationship between systolic BP and inotropic support with respect to ONSD is highly significant.
Table 4.
Relationship between Hemodynamic parameters and ONSD
| Variable | Range | ONSD ≥5.0 mm | ONSD <5.0 mm | Total | Chi-square value | P |
|---|---|---|---|---|---|---|
| HR | <50 | 2 (4.3) | 1 (1.9) | 3 | ||
| 50-70 | 8 (17.4) | 6 (11.1) | 14 | 3.318 | 0.506 | |
| 70-90 | 14 (30.4) | 17 (31.5) | 31 | |||
| 90-110 | 13 (28.3) | 23 (42.6) | 36 | |||
| >110 | 9 (19.6) | 7 (13.0) | 16 | |||
| Total | 46 (100) | 54 (100) | 100 | |||
| SBP | <90 | 5 (10.9) | 0 | 5 | ||
| 90-110 | 15 (32.6) | 5 (9.3) | 20 | 17.885 | 0.001 | |
| 110-130 | 14 (30.4) | 20 (37.0) | 34 | |||
| 130-150 | 8 (17.4) | 22 (40.7) | 30 | |||
| >150 | 4 (8.7) | 7 (13.0) | 11 | |||
| Total | 46 (100) | 54 (100) | 100 | |||
| Inotropes | Yes | 21 (45.7) | 8 (14.8) | 29 | ||
| No | 25 (54.3) | 46 (85.2) | 71 | 11.472 | 0.001 | |
| Total | 46 (100) | 54 (100) | 100 |
Figures in parenthesis indicate percentages
On CT scan examination of brain, features of raised ICP were found in 44% of patients as confirmed by the radiologist and there were 41 patients in whom raised ICP findings on ONSD and CT scan corroborated and the relation between the two was highly significant [Figure 2]. In patients with normal CT scan (56 patients), only 5 patients showed increase in ONSD above threshold level in this study.
Figure 2.
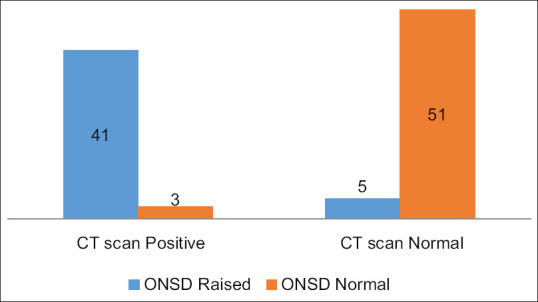
Relationship of ONSD and CT findings
Out of 46 patients with raised ONSD (≥5.0 mm), 41 patients (89%) had CT findings of raised ICP while 5 patients (11%) did not have finding suggestive of raised ICP [Table 5]. Some of the patients had to undergo neurosurgery (32 patients, 72.7%) and others (12 patients, 27.2%) were managed with pharmacological treatment of raised ICP.
Table 5.
Relationship of ONSD with respect to CT scan findings
| ONSD ≥5.0 mm | Total | Chi-square value | P | ||
|---|---|---|---|---|---|
| Yes | No | ||||
| CT Scan | |||||
| Positive | 41 (89.1) | 3 (5.6) | 44 | 70.415 | 0.000 (HS) |
| Normal | 5 (10.9) | 51 (94.4) | 56 | ||
| Total | 46 (100) | 54 (100) | 100 | ||
Figures in parenthesis indicate percentages
The sensitivity of the bedside sonographic measurement ONSD to detect raised ICP was found to be approximately 93.2% and specificity was 91.1% when compared with CT scan. Positive Predictive Value of the ONSD measurement to detect raised ICP was 89.1% and the negative predictive value was 94.4%. Accuracy of ONSD measurement was calculated as 92%.
As shown in Table 6, if the ONSD benchmark for raised ICP is increased to 0.51 cm or 0.52 cm, then the specificity increases but sensitivity decreases and if we lower the ONSD threshold to 0.48 cm or 0.47 cm, then the sensitivity increases but specificity decreases.
Table 6.
Different cut offs and their sensitivity and specificity calculations
| ONSD in cm | Sensitivity | Specificity |
|---|---|---|
| 0.46 | 0.95 | 0.88 |
| 0.47 | 0.95 | 0.89 |
| 0.48 | 0.93 | 0.89 |
| 0.49 | 0.93 | 0.91 |
| 0.50 | 0.93 | 0.93 |
| 0.51 | 0.91 | 0.96 |
| 0.52 | 0.89 | 0.98 |
| 0.52 | 0.86 | 0.98 |
The ROC for the mean ONSD had a high ability to discriminate between normal and high ICP, where the area under curve (AUC) value was 0.972 [Table 7] and shows a good separation between true positives and false positives. ONSD benchmark of ≥5.0 mm has a good negative as well as positive predictive value [Figure 3].
Table 7.
Area under the curve for ONSD
| Final ONSD | ||||
|---|---|---|---|---|
| Area | Sth. Error | P | Asymptomatic 95% confidence interval | |
| Lower bound | Upper bound | |||
| 0.972 | 0.020 | 0.000 | 0.000 | 1.000 |
Figure 3.
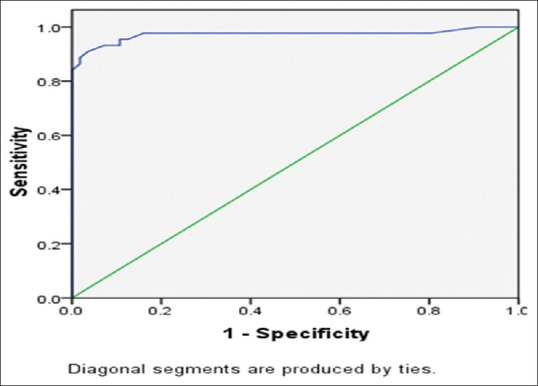
Receiver Operating Characteristic curve analysis of ONSD
DISCUSSION
The optic nerve sheath is contiguous with the duramater, and its contents are contiguous with the subarachnoid space [Figure 1]. Thus, raised ICP leads to an increase in the ONSD, which can further result in papilloedema.[13] It was observed that the ONSD increased by up to 60% at a distance of 3 mm behind the globe as compared to other distances.[14] Studies have also shown that there was no significant difference in measurement of ONSD by ultrasound in lateral, axial, or transverse projection.[14] High-frequency transducers (=7.5 MHz) available in ultrasound machines have made the measurements easier and more accurate for measuring ONSD.[15] The technique used in determining ONSD in our study was similar to the standard technique described in the literature in various studies.[16,17] Ultrasonographic measurement of ONSD is a noninvasive, bedside technique to measure increased ICP in the hands of an experienced operator. It is difficult to quantify experience, but it has been suggested that ten scans with three abnormal scans should be sufficient training for an experienced sonologist, whereas in new ultrasound operators, 25 scans may be needed and measurement of both eyes should be performed in less than 4 min.[18] We studied 100 patients and observed that age and gender of the group had no significant relation with ONSD findings. We also observed that raised ONSD findings had significant relation with hemodynamic parameters, RTS and GCS of the patient at the time of examination, which can be explained by the pathophysiological effects of these variables on intracranial pressure and ONSD. For comparing results of ONSD, we chose CT as our reference standard for detection of raised ICP, as CT scan of head is used on a daily basis in clinical practice to detect raised ICP in many health care facilities. Some other studies have also taken CT scan as the reference diagnostic test.[7,18] Invasive monitoring of ICP is the gold standard, but it needs neurosurgical expertise, requires time for insertion and is not practiced in all institutions.[19]
Comorbidities, multiple injuries, and presence of mechanical ventilation did not have any significant relation with raised ONSD in our study. As compared to other studies, the present study included a larger number of patients enrolled with traumatic brain injury (n = 100) as well as maximum number of ONSD readings concurrently. It was observed that ultrasound-based ONSD estimation had a good sensitivity (approximately 93.2%) and specificity (91.1%) when compared with CT scan and can reliably predict raised ICP in neuro-trauma patients. Positive predictive value of the ONSD measurement to detect raised ICP was 89.1% and negative predictive value was 94.4% with an accuracy of 92%. Other studies have also demonstrated significant correlation between ultrasonographic measurement of ONSD and the presence of intracranial hypertension.[16,18] In the study by Kimberly et al., direct measurement of ICP compared with ultrasonographic assessment through ONSD demonstrated significant correlation between values of ICP catheter and ONSD with a sensitivity of 88% and specificity of 93%.[16] Tayal et al. in their study reported that sensitivity of ONSD for detecting elevated ICP was 100% and specificity was 63%.[18] In another study on comparing ONSD measurements with CT Head findings of raised ICP, a sensitivity of 100% with specificity of 95% for an upper limit cut off value of 5 mm for normal ONSD was reported.[11] These studies also emphasized that ONSD =5 mm correlates well with ICP more than 20 cm of water.[16,18] Rajajee et al. reported that for the detection of ICP =20 mmHg, the optimal ONSD was ≥0.48 cm with a sensitivity of 96% and specificity of 94%.[9]
In our study, we also took binocular ONSD <5.0 mm as a reference standard and ONSD ≥5.0 mm was considered abnormal. As shown in Table 5, when ONSD benchmark for raised ICP is increased to 0.51 cm or 0.52 cm, then the specificity increases but sensitivity decreases and if we lower the ONSD threshold to 0.48 cm or 0.47 cm, then the sensitivity increases but specificity decreases. The ROC for the ONSD readings had an AUC value of 0.972 and showed a good separation between true positives and false positives.
This reflects that ultrasonographic measurement of ONSD may be a good surrogate of invasive ICP measurement or other imaging methods. This noninvasive method may be an alternative and quick approach to predict the ICP value of patients whose ICP measurement via lumbar puncture is difficult (in high risk such as hemodynamically unstable patient).[19] It was reported in a study that both ONSD ultrasonography and straight sinus systolic flow velocity are strongly correlated with invasive ICP. Robba et al. also remarked that ultrasonographic measurement of ONSD is noninvasive, safe, and quick as the orbital window is easily seen and has no complications.[20]
CT scan of brain requires time for transferring the patient to the radiology department and resources and in certain emergency situations, there is lack of time. Rapid diagnosis of elevated ICP through ultrasound-guided ONSD measurement can result in early detection in TBI and will further prevent secondary brain injury. A large study showed that management based on measurement of ICP by intracranial catheter had no additional benefit compared to management based on clinical and imaging findings alone.[21] At our institute, we do not practice invasive monitoring and in suspicion of raised ICP in patients, repeated CT scans are done. In such health care facilities, ONSD can be very helpful in early detection of raised ICP so that measures to reduce ICP can be initiated quite early. Therefore, ONSD is a valuable modality in determining the severity of raised ICP, deciding next line of treatment, referring to higher centers, in disasters, or when CT scan is unavailable and where the referral center or a tertiary care center is at longer distance.
Limitations
The main limitation of this modality is that there is wide interobserver variability in measurements though this can be minimized with training and experience. The investigator was blinded to the CT findings, but knew about the clinical condition of the patient or diagnosis. It essentially is an indirect assessment of raised ICP rather than actual measurement of ICP. Ultrasonographic assessment of ONSD is not a continuous assessment of raised ICP and needs to be repeated in patients who are at increased risk of raised ICP. Ocular trauma, optic nerve injury, and neuritis were few of the conditions where this modality was not used.
CONCLUSIONS
ONSD assessment through ultrasound has good sensitivity and specificity and has good correlation with CT scan brain for determining raised ICP. It has a good accuracy and it can be used in screening for raised ICP in patients with TBI, especially in hemodynamically unstable nontransferable patients. It is also helpful in early initiation of treatment of raised ICP. It is a quick, bedside, noninvasive, cost-effective method and is devoid of ionizing radiation. It can be used for repeated re-evaluation of cases and in other patients with neurological illnesses and hepatic encephalopathy, and also in situations where CT scan is not available or shifting the patient is not possible.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We are grateful to the Neurosurgery consultant and Neuro-radiologist for giving important inputs. We are also thankful to our statistician for her unparalleled contribution and data analysis.
REFERENCES
- 1.Zanier ER, Ortolano F, Ghisoni L, Colombo A, Losappio S, Stocchetti N. Intracranial pressure monitoring in intensive care: Clinical advantages of a computerized system over manual recording? Crit Care. 2007;11:R7. doi: 10.1186/cc5155. doi: 10.1186/cc5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koziarz A, Sne N, Kegel F, Alhazzani W, Nath S, Badhiwala JH, et al. Optic nerve sheath diameter sonography for the diagnosis of increased intracranial pressure: A systematic review and meta-analysis protocol. BMJ Open. 2017;7:e016194. doi: 10.1136/bmjopen-2017-016194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joseph B, Haider AA, Pandit V, Tang A, Kulvatunyou N, O’Keeffe T, et al. Changing paradigms in the management of 2184 patients with traumatic brain injury. Ann Surg. 2015;262:440–8. doi: 10.1097/SLA.0000000000001418. [DOI] [PubMed] [Google Scholar]
- 4.Širanovi M, Turkovi TM, Gopevi A, Kelei M, Kova N, Kova J, et al. Comparison of ultra sonographic measurement of optic nerve sheath diameter (ONSD) versus direct measurement of intracranial pressure (ICP) in traumatic brain injury patients. Signa Vitae. 2011;6:33–5. [Google Scholar]
- 5.Hansen HC, Helmke K. The subarachnoid space surrounding the optic nerves: An ultrasound study of the optic nerve sheath. Surg Radiol Anat. 1996;18:323–8. doi: 10.1007/BF01627611. [DOI] [PubMed] [Google Scholar]
- 6.Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: A systematic review and meta-analysis. Intensive Care Med. 2011;37:1059–68. doi: 10.1007/s00134-011-2224-2. [DOI] [PubMed] [Google Scholar]
- 7.Ohle R, McIsaac SM, Woo MY, Perry JJ. Sonography of the optic nerve sheath diameter for detection of raised intracranial pressure compared to computed tomography: A systematic review and meta-analysis. J Ultrasound Med. 2015;34:1285–94. doi: 10.7863/ultra.34.7.1285. [DOI] [PubMed] [Google Scholar]
- 8.Raffiz M, Abdullah JM. Optic nerve sheath diameter measurement: A means of detecting raised ICP in adult traumatic and non-traumatic neurosurgical patients. Am J Emerg Med. 2017;35:150–3. doi: 10.1016/j.ajem.2016.09.044. [DOI] [PubMed] [Google Scholar]
- 9.Rajajee V, Vanaman M, Fletcher J, Jacobs T. Optic nerve ultrasonography for detection of raised intracranial pressure. Neurocrit Care. 2011;15:506–15. doi: 10.1007/s12028-011-9606-8. [DOI] [PubMed] [Google Scholar]
- 10.Moretti R, Pizzi B. Optic nerve ultrasound for detection of intracranial hypertension in intracranial hemorrhage patients: Confirmation of previous findings in a different patient population. Neurosurg Anesthesiol. 2009;21:16–20. doi: 10.1097/ANA.0b013e318185996a. [DOI] [PubMed] [Google Scholar]
- 11.Blavias M, Theodoro D, Sierzenski PR. Elevated intracranial pressure detected by bedside emergency ultrasonography of the optic nerve sheath. Acad Emerg Med. 2003;10:376–81. doi: 10.1111/j.1553-2712.2003.tb01352.x. [DOI] [PubMed] [Google Scholar]
- 12.Revised Trauma Score calculator. Available from: https://wwwmdcalccom/revised-trauma-score . Last accessed on 2019 Jun 12.
- 13.Hansen HC, Helmke K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: Ultrasound findings during intrathecal infusion tests. J Neurosurg. 1997;87:34–40. doi: 10.3171/jns.1997.87.1.0034. [DOI] [PubMed] [Google Scholar]
- 14.Helmke H, Hansen HC. Fundamentals of transorbital sonographic evaluation of optic nerve sheath expansion under intracranial hypertension.I Experimental study. Paediatr Radiol. 1996;26:701–5. doi: 10.1007/BF01383383. [DOI] [PubMed] [Google Scholar]
- 15.Bergès O, Koskas P, Lafitte F, Piekarski JD. Sonography of the eye and orbit with a multipurpose ultrasound unit. J Radiol. 2006;87:345–53. doi: 10.1016/s0221-0363(06)74012-4. [DOI] [PubMed] [Google Scholar]
- 16.Kimberly HH, Shah S, Marill K, Noble V. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. 2008;15:201–4. doi: 10.1111/j.1553-2712.2007.00031.x. [DOI] [PubMed] [Google Scholar]
- 17.Ballantyne SA, O'Neill G, Hamilton R, Hollman AS. Observer variation in the sonographic measurement of optic nerve sheath diameter in normal adults. Eur J Ultrasound. 2002;15:145–9. doi: 10.1016/s0929-8266(02)00036-8. [DOI] [PubMed] [Google Scholar]
- 18.Tayal VS, Neulander M, Norton HJ, Foster T, Saunders T, Blaivas M. Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med. 2007;49:508–14. doi: 10.1016/j.annemergmed.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 19.Liu D, Li Z, Zhang X, Zhao L, Jia J, Sun F, et al. Assessment of intracranial pressure with ultrasonographic retrobulbar optic nerve sheath diameter measurement. BMC Neurol. 2017;17:188. doi: 10.1186/s12883-017-0964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robba C, Cardim D, Tajsic T, Pietersen J, Bulman M, Donnelly J, et al. Ultrasound non-invasive measurement of intracranial pressure in neurointensive care: A prospective observational study. PLoS Med. 2017;14:e1002356. doi: 10.1371/journal.pmed.1002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soliman I, Johnson GG, Gillman LM, Zeiler FA, Faqihi F, Aletreby WT, et al. New optic nerve sonography quality criteria in the diagnostic evaluation of traumatic brain injury? Crit Care Res Pract. 2018;2018:3589762. doi: 10.1155/2018/3589762. doi: 10.1155/2018/ [DOI] [PMC free article] [PubMed] [Google Scholar]


