Abstract
Background:
Discovery of serum myelin oligodendrocyte glycoprotein (MOG) antibody testing in demyelination segregated MOG-IgG disease from AQ-4-IgG positive NMOSD.
Aims:
To study clinico-radiological manifestations, pattern of laboratory and electrophysiological investigations and response to treatment through follow up in MOG-IgG positive patients.
Method:
Retrospective data of MOG-IgG positive patients was collected. Demographics, clinical manifestations at onset and at follow up and relapses, anti AQ-4-IgG status, imaging and all investigations were performed, treatment of relapses and further immunomodulatory therapy were captured.
Results:
In our 30 patients, F: M ratio was 2.75:1 and adult: child ratio 4:1. Relapses at presentation were optic neuritis {ON}(60%), longitudinally extensive transverse myelitis {LETM}(20%), acute disseminated encephalomyelitis {ADEM}(13.4%), simultaneous ON with myelitis (3.3%) and diencephalic Syndrome (3.3%). Salient MRI features were ADEM-like lesions, middle cerebellar peduncle fluffy infiltrates, thalamic and pontine lesions and longitudinally extensive ON {LEON} as well as non-LEON. Totally, 50% patients had a relapsing course. Plasma exchange and intravenous immunoglobulin worked in patients who showed a poor response to intravenous methylprednisolone. Prednisolone, Azathioprine, Mycophenolate and Rituximab were effective attack preventing agents.
Conclusions:
MOG-IgG related manifestations in our cohort were monophasic/recurrent/simultaneous ON, myelitis, recurrent ADEM, brainstem encephalitis and diencephalic Syndrome. MRI features suggestive of MOG-IgG disease were confluent ADEM-like lesions, middle cerebellar peduncle fluffy lesions, LETM, LEON and non-LEON. Where indicated, patients need to go on immunomodulation as it has a relapsing course and can accumulate significant disability. Because of its unique manifestations, it needs to be considered as a distinct entity. To the best of our knowledge, this is the largest series of MOG-IgG disease reported from India.
Keywords: Acute disseminated encephalomyelitis, longitudinally extensive transverse myelitis, myelin oligodendrocyte glycoprotein, Neuromyelitis optica spectrum disorders, optic neuritis
INTRODUCTION
AQ-4-IgG became a specific serological marker to diagnose Neuromyelitis optica spectrum disorders (NMOSD). However, 10–30% of patients presenting as NMOSD did not show AQ4-IgG positivity.[1,2] The pathology and pathogenesis behind the manifestations of these AQ-4-IgG negative patients remained unexplained. A subset of these AQ-4-IgG negative patients were found to have antibodies against myelin oligodendrocyte glycoprotein (MOG).[3,4] Thus, we have a new category of patients according to their immune status: AQ4-IgG-negative- MOG-IgG-positive. Some of the MOG-IgG positive patients had presentations fulfilling the criteria for NMOSD. So, to begin with, they got included as a subset of NMOSD. But, many MOG-IgG positive patients had distinctive clinical and radiological manifestations which neither satisfied the criteria for NMOSD nor MS.[4] We also learnt that, there are basic differences in its pathology and pathogenesis. So, MOG-IgG disease is now considered by most Neuroscientists as a distinct clinical entity, and no more, a subset of NMOSD.[5]
SUBJECTS AND METHODS
Retrospective data of serum MOG-IgG positive patients was collected from seven Neurology centers from Western India over a period of 3 years. Serum MOG-IgG as well as AQ-4 IgG were performed using a cell-based assay. Demographics, clinical manifestations at onset and at follow up were collected. Manifestations at relapses and modalities of treatment were noted. Imaging features, visual evoked potentials (VEPs), CSF studies, auto-immune profile and all relevant tests performed were recorded. Dose, duration of oral prednisolone and rate of tapering down was noted. Long-term immunomodulatory therapy (IMT) which was initiated was noted and any side effects or relapses whilst on IMT was also documented. Findings of all patients at follow up was taken. Mean duration for which follow up was possible was 3 years. The study was approved by the institutional ethics committee.
OBSERVATIONS AND RESULTS
Demographics
Thirty patients with relevant demyelinating events (total 52) were detected MOG-IgG antibody positive. There were 22 females and 8 males, with F: M ratio of 2.75:1 [Table 1]. Seven were in the age group 10–20, 7 in 21–30, 9 in 31–40, 1 in 41–50 and 6 above 50 years of age [Table 2]. The age of onset of disease ranged from 11-70 years with a median age of 34 years. The adult: child (<16 years) ratio was 4:1 (24:6).
Table 1.
Sex distribution in the cohort
| Sex | Number |
|---|---|
| Males | 8 |
| Females | 22 |
| Total | 30 |
Table 2.
Age-wise distribution of patients
| Age group (in years) | Number |
|---|---|
| 10-20 | 7 |
| 21-30 | 7 |
| 31-40 | 9 |
| 41-50 | 1 |
| >50 | 6 |
| Total | 30 |
AQ-4 IgG status and co-existing auto-immunity
No patient was AQ-4 antibody positive. Two patients had positive autoimmune blood test results. One had positive anti- nuclear antibodies and the other had anti-nRNP and anti-Sm antibodies (qualitative assay).
Preceding infections, association with pregnancy and delivery
Ten patients (33%) had a preceding infection as a triggering factor for their first or a subsequent relapse. Two females suffered relapses in postpartum period. Both had ON. We had no relapses seen during pregnancy.
Clinical presentations and clinical course
Clinical presentations with number of patients of each type, percentage, number of relapses per patient, total number of relapses in category and average number of relapses in each category are displayed in the Table 3. The breakup of patients according to clinical manifestations was as follows: single episode of unilateral optic neuritis (SUON)- 5, recurrent episodes of unilateral ON (RUON)- 7, bilateral (simultaneous/sequential) ON (BON)- 6, simultaneous ON with myelitis- 1, longitudinally extensive transverse myelitis (LETM)- 6, recurrent ADEM- 2, ADEM followed by ON- 2 and Acute diencephalic Syndrome- 1. The total number of events in our patients were 52. Average number of relapses were 3 in the “ADEM followed by ON” category, 2.4 in the RUON, 2 in recurrent ADEM, 1.7 in BON, 1.3 in LETM and 1 each in SUON, simultaneous ON with myelitis and Diencephalic Syndrome categories.
Table 3.
Clinical presentations with number of patients of each type of presentation, percentage, number of relapses per patient, total number of relapses in category and average number of relapses in each category
| Presentation | Number of patients | Percentage | Number of relapses per patient | Total no. of relapses in category | Average no. of relapses |
|---|---|---|---|---|---|
| Unilateral ON | 12 | 40.0 | 22 | ||
| Single episode of unilateral optic neuritis (SUON) | 5 | 16.7 | 1, 1, 1, 1, 1 | 5 | 1 |
| Recurrent episodes of unilateral ON (RUON) | 7 | 23.3 | 2, 2, 2, 2, 2, 4, 3 | 17 | 2.4 |
| Bilateral (simultaneous/sequential) ON | 6 | 20.0 | 4, 1, 1, 1, 2, 1 | 10 | 1.7 |
| Simultaneous ON with myelitis | 1 | 3.3 | 1 | 1 | 1 |
| Longitudinally extensive transverse myelitis (LETM) | 6 | 20.0 | 1, 1, 2, 1, 1, 2 | 8 | 1.3 |
| Recurrent acute disseminated encephalomyelitis (ADEM) | 2 | 6.7 | 2, 2 | 4 | 2 |
| ADEM followed by ON | 2 | 6.7 | 2, 4 | 6 | 3 |
| Diencephalic Syndrome | 1 | 3.3 | 1 | 1 | 1 |
| Total | 30 | 100 | 52 |
Clinical phenotype at presentation was ON in 18 (60%), simultaneous ON with myelitis in 1 (3.3%), myelitis in 6 (20%), ADEM in 4 (13.4%) and Acute diencephalic Syndrome in 1 (3.3%) [Table 4].
Table 4.
Clinical phenotype at presentation, sub-categories in each type of presentation, total number of patients in each category and their percentages
| Clinical phenotype at presentation | Sub-categories | Total number of patients | Percentage |
|---|---|---|---|
| ON | 5- SUON, 7- RUON, 6- BON | 18 | 60.0 |
| Simultaneous ON with myelitis | - | 1 | 3.3 |
| Myelitis (LETM) | - | 6 | 20.0 |
| ADEM | 2- Recurrent ADEM, 2- ADEM followed by ON | 4 | 13.4 |
| Diencephalic Syndrome | - | 1 | 3.3 |
| Total | 30 | 100 |
Fifteen patients (50%) had more than one relapse. In these patients, the range of interval between the first and second relapses was 2 months to 10 years with a median time interval being 2 years. If we consider all relapses suffered by all relapsing patients (a total of 37 relapses in 15 patients), again the median time interval between relapses was 2 years.
Clinical phenotypes and change of phenotype
The clinical phenotypes of the patients who relapsed were RUON, BON, LETM, recurrent ADEM and ADEM followed by ON. Patients with RUON, BON, and LETM retained the same clinical phenotype in their subsequent relapses. However, out of the 4 patents who had ADEM as their initial relapse, 2 had ADEM again as their second relapse, whereas 2 had ON as their subsequent relapse. So, these 2 “ADEM followed by ON” patients were the only ones who had a change in their clinical presentation in the subsequent relapses and both had onset in childhood.
Description of each types of attacks
Optic neuritis (ON)
First of all, we categorize our ON patients broadly into two categories—unilateral and bilateral (simultaneous/sequential). Unilateral ON are the patients in whom a relapse of ON consisted of optic nerve involvement only on one side. Bilateral (simultaneous/sequential) ON (BON) are patients in whom both optic nerves got affected during the same relapse, either at the same time or second optic nerve got affected within days to weeks of the first one.
In our cohort, 21/30 (70%) had ON at some point during the course of illness (detail break up in Table 5). Of these, 18 (60%) had only ON and 1 had simultaneous ON with myelitis at presentation. The remaining 2 ON events were the subsequent relapses in the patients who had ADEM followed by ON. According to the categories described above, out of the 21 patients, 13 patients had unilateral ON while 8 had BON.
Table 5.
Category-wise distribution of ON patients, number of patients in each category, number of ON relapses per patient and total number of relapses in each category
| Distribution of ON patients | Number of patients | Number of ON relapses per patient | Total no. of ON relapses in category |
|---|---|---|---|
| Unilateral ON | 12 | 22 | |
| Single episode of unilateral optic neuritis (SUON) | 5 | 1, 1, 1, 1, 1 | 5 |
| Recurrent episodes of unilateral ON (RUON) | 7 | 2, 2, 2, 2, 2, 4, 3 | 17 |
| Bilateral (simultaneous/sequential) ON | 6 | 4, 1, 1, 1, 2, 1 | 10 |
| Simultaneous ON with myelitis | 1 | 1 | 1 |
| ADEM followed by ON | 2 | 1, 1 | 2 |
| Total | 21 | 35 | 35 |
Among the 13 unilateral ON, 1 patient had unilateral ON as the second episode 6 years after ADEM. This is one of the two patients listed in Table 4 as ADEM followed by ON. Of the remaining 12 unilateral ON patients, 5 had single episode of ON (SUON category), whereas 7 had recurrent episodes (RUON category).
In the 7 patients with RUON, the interval between first and second attack ranged between 2 months to 10 years, with a median interval of 2 years. Taking into account all relapses of RUON patients, (a total of 17 relapses in 7 patients), the median interval between two episodes of ON was 2½ years.
Eight patients had BON at some point in illness. Amongst them, one patient is the second of the two patients listed as ADEM followed by ON, and one is simultaneous ON with myelitis in Table 4. Six patients had only bilateral (simultaneous/sequential) ON as their manifestation. Of these, 4 had single episodes and 2 had multiple attacks. Of the two with multiple attacks, one had 4 relapses and the other had 2 relapses. In these 2 patients with multiple episodes, the interval between first and second attack ranged from 3 months to 4 years. In all of the 6 patients who suffered BON, the second eye got involved within 0-5 days of the first eye. In the patient who had simultaneous ON with myelitis, spinal cord got involved 2 weeks after the optic nerves.
The total number of ON relapses in all patients were 35, with break up as follows: 5 in 5 patients of SUON, 17 in 7 patients of RUON, 10 in 6 patients of BON, 1 in simultaneous ON with myelitis and 2 in 2 patients of ADEM followed by ON [Table 5].
Subacute onset loss of vision was the commonest mode of presentation. Impairment of vision between patients ranged from visual acuity of 6/18 to no perception of light. All patients with vision loss had scotoma with impaired color vision. Pupillary examination in patients with unilateral ON showed relative afferent pupillary defect (RAPD) in all except two who had sluggish reaction of affected pupil. Pupils in BON revealed sluggish reaction of pupils in all except one patient. This patient showed RAPD. She had severe involvement of vision in the eye with RAPD (finger counting from 2 feet) and moderate involvement in the other eye (visual acuity 6/18). Fundus examination showed disc oedema in 6, disc pallor or atrophy in 12 and normal optic disc in 3 cases.
Simultaneous ON with myelitis
In the one patient who had simultaneous ON with myelitis, the clinical manifestations of ON were similar to those who had BON. Myelitis presented with subacute paraparesis with moderate weakness at power grade 3-4/5, a sensory level and bladder involvement. The optic neuritis was simultaneous i.e. involved both eyes.
Myelitis
Seven patients suffered myelitis. Of these, 1 had simultaneous ON with myelitis and 6 had just LETM as presentations. Of the 6 patients with LETM, 4 had single attacks whereas two had 2 relapses. These patients had severe myelitis with power between 0 and 3/5, except one who had mild weakness at power 4/5. Two patients with 2 relapses each had attacks spaced by 6 months and 2 years. One of them with recurrent myelitis also had pruritus in the C2-5 dermatomes during his second attack.
Recurrent ADEM
Two patients had 2 relapses each of ADEM. The interval between relapses were 2 months and 6 months. Clinical features were seizures, hemiparesis, ataxia and confusion.
ADEM followed by ON
Two patients whose first relapse was ADEM, went on to suffer ON as a subsequent relapse. Of them, one had unilateral ON and the other had BON. The interval between ADEM and ON were 6 and 5 years, respectively.
Acute diencephalic syndrome
This 22-year-old female presented with excessive daytime sleepiness, reversal of sleep-wake cycle, hiccups and imbalance. She had vertical gaze palsy with nystagmus, gait as well as limb ataxia.
MRI features
Optic nerves
All but four patients with ON showed medium length hyperintense signal of the optic nerve. Four patients showed a long signal extending for almost the full length of the nerve, what is described as longitudinally extensive optic neuritis (LEON) as shown in Figure 1(a and b). Three ON patients showed asymptomatic brain/spine signals (bilateral thalami, dorsal spine, and parieto- temporal lobes).
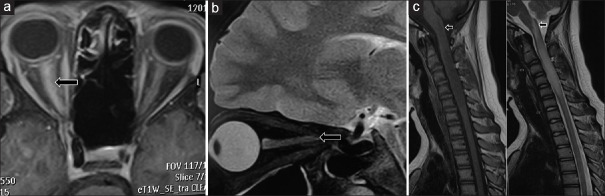
Figure 1.
A- T1W contrast axial MRI image in a patient with right ON showing contrast enhancement of full length of the right optic nerve suggestive of LEON. B- T2W sagittal MRI image in a patient with left ON showing hyperintense signal of full length of the left optic nerve suggestive of LEON. C- T1W and T2W sagittal sections of MRI cervical spine of a patient showing cervical cord LETM lesion upto C6 vertebra and also extending high up into medulla (black arrows)
Brain
ADEM patients showed medium size white matter lesions. Brain lesions of MOG-IgG disease were poorly demarcated while corpus callosal lesions were long, thick, occupying almost the whole breadth of callosum. Two ADEM patients showed bilateral middle cerebellar peduncle fluffy infiltrates with poorly defined margins. Distinctive feature in ADEM in the pediatric age group was bilateral thalamic and pontine involvement.
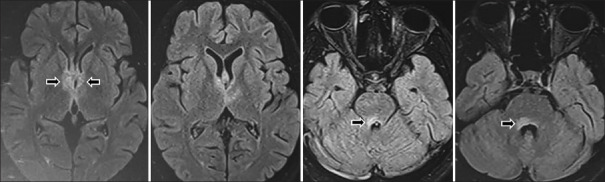
Acute diencephalic Syndrome patient showed hyperintensities involving bilateral medial thalami {around the 3rd ventricle} as well as around the 4th ventricle {periependymal} [Figure 2].
Figure 2.
FLAIR Axial MRI images in a patient who presented with diencephalic syndrome showing lesions involving bilateral medial thalami (around the 3rd ventricle) as well as around the 4th ventricle (periependymal)
Asymptomatic brain MRI lesions
Two patients of ON showed asymptomatic lesions, one had bilateral symmetrical thalami signal intensities and the other had a T2W signal in thoracic cord.
Asymptomatic brain MRI lesions and leptomeningeal enhancement
Another patient of ON showed hyperintense signals in the gyri of right parietal and temporal lobes on FLAIR and T2W sequences. She did not have any sensory or visual abnormalities on detailed neurological examination. Her contrast MRI also revealed focal leptomeningeal enhancement along right parieto-occipital region.
Spinal cord
Patients with LETM had classic LETM lesions. One patient had a very long lesion extending from C2 to conus medullaris. Another patient had a long cervical cord lesion also extending high up into medulla [Figure 1c]. Spinal cord lesions in ADEM patients were non-LETM, i.e. they were less than 3 vertebral segments in length.
Cerebrospinal fluid (CSF)
CSF was done in 16/30 patients. CSF protein ranged from 18 to 162 mg% with a mean of 54 mg% and sugar ranged from 40 to 156 mg% with a mean of 75 mg%. Only nine out of 16 patients had cell count above 5 cells/cu mm. Cell count ranged from 0 to 206 cells/cumm with a mean of 30 cells/cumm. Only 1/16 (6.3%) patients showed positive oligoclonal band (OCB).
Evoked potentials
Visual evoked potentials (VEP) were done in 13 patients. They showed prolonged latencies in all 10 patients who had suffered ON. It was normal in one patient who had only LETM as his illness. Two patients, one with recurrent ADEM and other with LETM, and never had any visual symptoms, also underwent VEP. It showed delayed P100 latencies.
MOG-IgG positive patients satisfying the criteria for NMOSD
Three out of 30 (10%) patients satisfied the IPND 2015 criteria for NMOSD.
Treatment and outcome of acute attacks
Most of the episodes showed a good recovery in function with intravenous methylprednisolone (IVMP) 1 gram daily for 5 days. Seven relapses in 7 different patients did not show a good recovery. These were: 1 ADEM, 4 LETM and 2 ON. One patient with ADEM received plasma exchange (PLEX) after IVMP to achieve remission from her first severe attack. Two patients with LETM received intravenous immunoglobulin (IVIG) after IVMP to improve their limb strength.
Four female patients, three with ON and the fourth with “ADEM followed by ON” suffered worsening of their deficits during their oral prednisolone taper, a phenomenon called steroid dependence. All were given IVMP again, and then, the second time, oral prednisolone was tapered gradually over 4 months.
Long-term immunomodulatory therapy (IMT)
In our series, all patients received bridging oral prednisolone treatment which was tapered down over a variable period between 2 to 6 months depending upon which steroid sparing IMT was initiated. Twenty-four (80%) patients were given long-term IMT. The break-up of the 24 patients who received IMT is as follows: 13 patients received Azathioprine (Aza) at 2-3 mg/kg/day in two divided doses, 4 patients received Mycophenolate mofetil (MMF) at 1.5 to 2 grams/day and 3 continued to get low dose oral prednisolone for attack prevention. Four patients received rituximab (RTX) cycles. RTX was initiated in these patients after the second attack and when one of the relapses was disabling. Out of the 6 patients who did not receive IMT, 4 had only a single episode of unilateral ON with very good recovery. One patient with 2 episodes of BON spaced by an interval of 4 years and another patient with 2 episodes of RUON with inter-attack interval of 2 years did not receive long-term IMT. They had very good recovery from all of their attacks.
DISCUSSION
Demographics and associations
As far as we are aware, this is the largest series of MOG-IgG disease published from India to date. Our cohort has female preponderance (F: M ratio 2.75:1) as against majority of the studies showed a F: M ratio of 1 to 1.1:1.[6,7] The age of onset (11-70 years) and median age at presentation (34 years) of our cohort matches with series published so far.[6,8,9,10]
Aq-4-IgG negativity in all our patients goes with literature where all patients who were detected MOG-IgG positive were AQ-4-IgG negative.[1,6,11] Co-existing positive auto-immune blood tests found in our patients were also seen in previous series.[8,12,13]
33% of our patients suffered infections prior to their attacks which goes with previous studies which also showed 45–47% patients having a history of infection preceding their attacks.[10,14] Contrary to previous literature where relapses were seen in postpartum period as well as during pregnancy, two of our patients suffered attacks only during pregnancy.[8]
Clinical presentations and clinical course
Manifestations in our cohort matched with those described in literature showing ON (either monophasic/recurrent or simultaneous), myelitis, ADEM, and simultaneous ON with myelitis as the common features of MOG-IgG disease. Literature also states ON as the commonest presenting clinical symptom followed by myelitis, ADEM and then other presentations, a finding again seen in our study as well.[1,6,8,9,10,12,13,15]
In our series, 50% patients had a relapsing course which is less as compared to literature where more than 80% had a relapsing disease course when followed up over a mean duration of 8 years.[8] The study also observed that number of patients who relapsed went on increasing as the duration of follow up increased. In our series, the mean duration of follow up was 3 years. Longer follow up may show more relapsing patients.
The median time interval between first and second attack in our study was 2 years, which is much longer compared to literature, where the mean interval between such relapses was 5 months.[8]
Each types of attacks
ON
In our study, ON was the presenting feature in 60% and occurred at some point during the course of illness in 70% patients. Both unilateral as well as bilateral and monophasic as well as recurrent ON were seen. Similar findings have been reiterated in previous studies.[6,9,10,14]
Simultaneous ON with myelitis
In literature, all patients with simultaneous ON with myelitis had bilateral ON,[6] a finding replicated in our patient.
Myelitis
Myelitis was the second commonest manifestation, also observed by other authors.[6,9,10,13] One of our patents suffered pruritus during his relapse. Studies have reported pruritus as a manifestation of AQ4-IgG disease.[16] We did not find any study in literature reporting pruritus as a symptom of MOG-IgG disease.
Recurrent ADEM
Contrary to previous finding that ADEM was commoner in children, we now know that ADEM is reported frequently in adults as well,[6] as seen in our cohort.
ADEM followed by ON
Hacohen et al.[17] has described the same phenotype “ADEM followed by ON” seen in two of our patients. This phenotype seems to be suggestive of MOG-IgG disease.
Diencephalic syndrome
The features of our patient were same as in a previous publication.[8]
MRI features
ON
Medium length optic nerve signal and LEON, the features seen in our ON patients was supported by other studies as well.[8,18,19] None of our patients had optic chiasmal involvement. We did not come across perioptic nerve fat enhancement which is considered as a feature of MOG-IgG disease.[8]
Brain
The poorly demarcated morphology of white matter lesions and long, thick (occupying almost the whole breadth of corpus callosum) nature of callosal lesions distinguish them from MS {MS lesions have plaque like morphology}.[6,8,18,19,20] The bilateral middle cerebellar peduncle fluffy, poorly marginated infiltrates seen in our patients are also described in literature.[15,18,19,20] Such fluffy lesions are seen neither in AQ-4-IgG NMOSD nor in MS, and so are considered highly suggestive of MOG-IgG disease. Thalamic and pontine lesions seen in our children with ADEM have been described by Hacohen et al.[21] Leptomeningeal enhancement seen on contrast MRI of one of our patient has also been reiterated by Cobo- Calvo et al.[6]
Ramanathan et al.[14] has also shown presence of asymptomatic MRI lesions. Asymptomatic MRI lesions are not seen in AQ-4-IgG positive patients and are well known in MS. This issue of patients who develop asymptomatic lesions are at risk of developing disability progression independent of relapses has not been addressed in literature yet and needs to be studied further.
Spinal cord
LETM, long cord lesion extending from cervical cord to conus medullaris and LETM extending upto medulla, the spine MRI features of our patients, are also characteristics of AQ-4-IgG NMOSD.[19] In our study, we did not find preferential conus medullaris lesions which were described in previous literature.[13]
Other investigations
A 6.3% OCB positivity in our study matches with literature where majority (=90%) patients had a negative OCB.[1,8,13]
Delayed P100 latencies in asymptomatic eyes seen in two of our patients has also been found in a similar previous study.[8] This finding would suggest that MOG-IgG disease patients have asymptomatic optic nerve affection, similar to MS.
MOG-IgG positive patients satisfying the criteria for NMOSD
In studies by Jurynczyk et al.[9] and Jarius et al.,[8] 32% patients satisfied the IPND 2015 NMOSD criteria, whereas we had only 10% patients fulfilling the same.
Treatment and outcome of acute attacks
Our response to acute treatment co-relates with literature. Studies state that majority of relapses do well with IVMP, but 9–25% need further escalated treatment, either PLEX or IVIG, to accomplish improvement.[8,9,13,22] The steroid dependence seen in four of our patients is a feature of a disease called Chronic relapsing inflammatory Optic Neuropathy (CRION). Lee H et al.[23] has described CRION as a manifestation of MOG-IgG disease.
Long-term immunomodulatory therapy (IMT)
Knowledge about MOG-IgG disease is still evolving. Most of the researchers establish the fact, that the disease is not benign and not that non-disabling. It does accrue disability.[8,9,22] A recent study followed up a cohort of 125 patients to find that, 84% patients relapsed at 5 years.[22] Many studies also report a high disability rate. After a mean follow up of 4½ years, visual acuity is still reduced in half, whereas one third have significant paresis in a large study.[8] In a recent study, only 42% of patients had no residual disability.[14]
The same study evaluated risk factors for poor outcomes and severe disability.[14] The proportion of relapses with complete recovery was higher with ON and ADEM and lower with LETM, and patients who had ON have less long-term disability. Patients who had myelitis had more severe residual disability. Data on how many patients have a relapsing course and what proportion stay monophasic for a long time is also variable in different publications.
Our study also showed that not all patients were relapsing, and only some of them continued to accumulate disability. First of all, some of the patients have had a monophasic course so far and the single relapse has improved remarkably with acute therapy. Secondly, number of relapses were not as high as other demyelinating diseases like MS or AQ-4 IgG disease. Some of the patients had 2, or at the most 4 relapses over a total of 3–10 years duration. So, there were relapses spaced by large intervals. And majority of these relapses responded very well to standard acute therapy. Third point is that, in spite of suffering multiple relapses, some of the patients were not left with much residual disability.
Criteria as to which patients should go on long-term IMT are still in the process of being formulated. After extensive review, we came to certain conclusions in relation to which category of patients should go on IMT. Some of the points we used to make our decision to initiate IMT are: two or more frequent relapses, single relapse with residual disability, poor response to acute relapse therapy, appearance of attack on tapering oral steroid and if the index event was myelitis. On the other hand, those patients who had a single non- disabling relapse, even those patients who had two or more relapses occurring at long intervals and left no disability, and those who had ON with good recovery and no functional impairment did not receive long-term IMT. They only received a course of oral prednisolone only, tapered gradually over a period of 4–6 months. However, these patients are being regularly followed up to pick up new symptoms/signs and review the need for long-term treatment. In addition, we are periodically reviewing literature in relation to the evolving trends in therapy for MOG-IgG disease. As and when literature comes up with any new concepts, we may review our decisions about IMT for our cohort.
So, the salient outcomes of our study were a F: M ratio of 2.75:1, a much less female involvement than AQ-4-positive NMOSD and MS. Attacks were triggered by an infectious prodrome in 33% and the disease had a relapsing course in 50%. ON was the commonest presenting feature (60%) followed by myelitis (20%) and ADEM (13.4%). ON was monophasic/recurrent and unilateral as well as bilateral. ADEM was seen in both children as well as adults. All patients stuck to the same clinical phenotype in their subsequent relapses except two ADEM patients who changed to ON. Classical MRI features were lesions in middle cerebellar peduncle (fluffy), thalami and pons, callosum (long thick) and LEON as well as non-LEON. Asymptomatic MRI lesions and VEP abnormalities was a unique feature. Relapses generally showed a good response to IVMP and 80% went on long-term IMT with a good response.
MOG-IgG disease—A separate entity
AQ-4-IgG NMOSD is an astrocytopathy. With MOG-IgG disease, the antigenic substrate MOG is a structural glycoprotein expressed on the outer lamella of the myelin sheath.[24] So, MOG-IgG disease is pathologically a myelinopathy. Although AQ-4 IgG disease and MOG-IgG disease have similar clinical and radiological features, they are different entities in terms of pathogenesis as their antigenic substrates are different.[25] At present, a subset of MOG-IgG disease patients whose clinico-radiological phenotype satisfies the IPND 2015 criteria for NMOSD, are categorized as NMOSD. However, many Neuroscientists now consider it as a separate entity [8,26,27] Passos et al.[28] has even suggested a new name to this disease as MOG associated optic neuritis and encephalomyelitis (MONEM). Jarius et al.[5] has not only given it a new name “MOG-IgG-Associated Encephalomyelitis (MOG- EM)”, but also devised diagnostic criteria.
Clinical features i.e. acute relapses of MOG-IgG disease can be similar to AQ-4-IgG disease, and at times also like MS. Optic neuritis is usually moderate with good response to steroids, thus appearing similar to MS. But it can also be severe, simultaneous in both eyes, can have chiasmal involvement like AQ-4-IgG NMOSD and show poor recovery. Recurrent ON is however a very common manifestation of MOG disease as compared to even AQ-4-IgG NMOSD and MS. Simultaneous ON with myelitis is also seen frequently in MOG-IgG disease, as is also seen in AQ-4-IgG NMOSD.[29] ADEM like presentation also appears to be a common feature of MOG-IgG disease, more frequent than AQ-4-IgG NMOSD.[9,14] Acute brainstem Syndrome and brainstem involvement as a part of ADEM is also one of the protean manifestations of MOG-IgG disease, like AQ-4-IgG NMOSD.[6,15]
On MRI, optic neuritis of MOG disease can have the standard medium length involvement and can be LEON as well. Likewise, in spinal cord, MRIs of MOG disease patients may show LETM as well as non-LETM lesions. Other MRI features strongly suggestive of MOG-IgG disease are confluent ADEM-like lesions, middle cerebellar peduncle lesions, fluffy poorly marginated nature of lesions and perioptic nerve fat involvement.[8,9,15,18,19,20]
After following up MOG-IgG disease patients over many years, it is established that majority relapse.[8,9] Many relapses are known to leave behind residual disability.[9,14] MOG-IgG disease is certainly not as aggressive as AQ-4-IgG disease, but definitely not benign. So, it mandates IMT for attack prevention. Answers to issues such as drug of choice, dose and duration of IMT needs more randomized comparative studies. Oral therapies used have been low dose prednisolone, Aza, MMF and methotrexate, and have been found to be effective.[8] On Rituximab (RTX), the mean ARR was reduced from 1.08 to 0.43.[22] Our experience also showed a good response to oral prednisolone, Aza and MMF. Four of our patients received RTX after they suffered second relapse, and since then, they have had no attacks. Further systematic studies of above drugs need to be undertaken to confirm their efficacy shown in earlier studies.
Another important issue we want to bring up is that, now we know that some of the clinico-radiological features of MOG-IgG disease may also mimic MS. However, pathology and pathogenesis of MOG-IgG disease is completely different from MS. Disease modifying therapies for MS such as interferons and natalizumab have been shown to worsen MOG-IgG disease, whereas glatiramer is not effective in MOG-IgG disease.[8] So, it is imperative to be able to distinguish between MS and MOG-IgG disease, so that we choose the right IMT.
CONCLUSION
To conclude, MOG-IgG disease is a distinct entity from AQ-4-IgG NMOSD and MS.
It often presents as monophasic/recurrent/simultaneous ON, myelitis, ADEM/brainstem encephalitis and less frequently, as diencephalic Syndrome. Brain MRI features suggestive of MOG-IgG disease are non-MS medium to large fluffy/confluent lesions, with particular predilection for upper brainstem and middle cerebellar peduncles. Optic nerve imaging clues to MOG-IgG disease are LEON and perioptic nerve fat enhancement. Spinal cord lesions in MOG disease can be LETM or non-LETM. Asymptomatic brain lesions are seen in MOG-IgG disease unlike AQ-4 IgG disease where lesions are seen on MRI only during relapses. Both moderate and severe relapses are seen, with an overall good response to IVMP. MOG-IgG disease usually responds well to maintenance oral steroids and gentle immunosuppression is often adequate to prevent relapses. Some patients are “steroid dependent”, i.e. relapse on steroid tapering/discontinuation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sato D, Callegaro D, Lana-Peixoto M, Waters PJ, de Haidar Jorge FM, Takahashi T, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. 2014;82:474–81. doi: 10.1212/WNL.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato D, Callegaro D, Lana-Peixoto M, Nakashima I, Fujihara K. Seronegative Neuromyelitis Optica Spectrum-the challenges on disease definition and Pathogenesis. Arq Neuropsiquiatr. 2014;72:445–50. doi: 10.1590/0004-282x20140032. [DOI] [PubMed] [Google Scholar]
- 3.Kitley J, Woodhall M, Waters P, Leite MI, Devenney E, Craig J, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. 2012;79:1273–7. doi: 10.1212/WNL.0b013e31826aac4e. [DOI] [PubMed] [Google Scholar]
- 4.Hamid SH, Whittam D, Mutch K, Samantha Linaker, Tom Solomon, Kumar Das, et al. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are MOG-IgG positive.A cross sectional study of 132 patients? J Neurol. 2017;264:2088–94. doi: 10.1007/s00415-017-8596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarius S, Paul F, Aktas O, Asgari N, Dale RC, de Seze J, et al. MOG encephalomyelitis: International recommendations on diagnosis and antibody testing. J Neuroinflammation. 2018;15:134–43. doi: 10.1186/s12974-018-1144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cobo-Calvo A, Ruiz A, Maillart E, Audoin B, Zephir H, Bourre B, et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: MOGADOR study. Neurology. 2018;90:1858–69. doi: 10.1212/WNL.0000000000005560. [DOI] [PubMed] [Google Scholar]
- 7.Höftberger R, Sepulveda M, Armangue T, Blanco Y, Rostásy K, Calvo AC, et al. Antibodies to MOG and AQP4 in adults with neuromyelitis optica and suspected limited forms of the disease. Mult Scler. 2015;21:866–74. doi: 10.1177/1352458514555785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13:280. doi: 10.1186/s12974-016-0718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jurynczyk M, Messina S, Woodhall MR, Raza N, Everett R, Roca-Fernandez A, et al. Clinical presentation and prognosis in MOG-antibody disease: A UK study. Brain. 2017;140:3128–38. doi: 10.1093/brain/awx276. [DOI] [PubMed] [Google Scholar]
- 10.Mariotto S, Ferrari S, Monaco S, Benedetti MD, Schanda K, Alberti D, et al. Clinical spectrum and IgG subclass analysis of anti-myelin oligodendrocyte glycoprotein antibody associated syndromes: A multicenter study. J Neurol. 2017;264:2420–30. doi: 10.1007/s00415-017-8635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Piarokoili K, et al. MOG-IgG in NMO and related disorders: A multicentre study of 50 patients. Part 1: Frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J Neuroinflammation. 2016;13:279. doi: 10.1186/s12974-016-0717-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandit L, Sato DK, Mustafa S, Takahashi T, D'Cunha A, Malli C, et al. Serological markers associated with neuromyelitis optica spectrum disorders in South India. Ann Indian Acad Neurol. 2016;19:505–9. doi: 10.4103/0972-2327.192389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitley J, Woodhall M, Waters P, Leite MI, Murchison A, George J, et al. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: A comparative study. JAMA Neurol. 2014;71:276–83. doi: 10.1001/jamaneurol.2013.5857. [DOI] [PubMed] [Google Scholar]
- 14.Ramanathan S, Mohammad S, Tantsis E, Nguyen TK, Merheb V, Fung VSC, et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry. 2018;89:127–37. doi: 10.1136/jnnp-2017-316880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarius S, Kleiter I, Ruprecht K, Asgari N, Pitarokoili K, Borisow N, et al. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients. Part 3: Brainstem involvement- frequency, presentation and outcome. J Neuroinflammation. 2016;13:281. doi: 10.1186/s12974-016-0719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Netravathi M, Saini J, Mahadevan A, Hari-Krishna B, Yadav R, Pal PK, et al. Is pruritus an indicator of aquaporin-positive neuromyelitis optica? Mult Scler. 2017;23:810–7. doi: 10.1177/1352458516665497. [DOI] [PubMed] [Google Scholar]
- 17.Hacohen Y, Wong YY, Lechner C, Jurynczyk M, Wright S, Konuskan B, et al. Disease course and treatment responses in children with relapsing myelin oligodendrocyte glycoprotein antibody-associated disease. JAMA Neurol. 2018;75:478–87. doi: 10.1001/jamaneurol.2017.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michaela C, Vincenza F, Matteo C, Marta P, Giancarlo O. Anti-myelin oligodendrocyte glycoprotein antibodies: Magnetic resonance imaging findings in a case series and a literature review. Neuroradiol J. 2018;31:69–82. doi: 10.1177/1971400917698856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akaishi T, Nakashima I, Sato DK, Takahashi T, Fujihara K. Neuromyelitis optica spectrum disorders. Neuroimaging Clin N Am. 2017;27:251–65. doi: 10.1016/j.nic.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Jurynczyk M, Geraldes R, Probert F, Woodhall MR, Waters P, Tackley G, et al. Distinct brain imaging characteristics of autoantibody-mediated CNS conditions and multiple sclerosis. Brain. 2017;140:617–27. doi: 10.1093/brain/aww350. [DOI] [PubMed] [Google Scholar]
- 21.Hacohen Y, Banwell B. Treatment approaches for MOG-Ab-associated demyelination in children. Curr Treat Options Neurol. 2019;21:2–15. doi: 10.1007/s11940-019-0541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cobo-Calvo, Sepúlveda M, Rollot F, Armangué T, Ruiz A, Maillart E, et al. Evaluation of treatment response in adults with relapsing MOG-Ab-associated disease. J Neuroinflammation. 2019;16:134–45. doi: 10.1186/s12974-019-1525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee H, Kim B, Waters P, Woodhall M, Irani S, Ahn S, et al. Chronic relapsing inflammatory optic neuropathy (CRION): A manifestation of myelin oligodendrocyte glycoprotein antibodies. J Neuroinflammation. 2018;15:302–10. doi: 10.1186/s12974-018-1335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peschl P, Bradl M, Höftberger R, Berger T, Reindl M. Myelin oligodendrocyte glycoprotein: Deciphering a target in inflammatory demyelinating diseases. Front Immunol. 2017:29–43. doi: 10.3389/fimmu.2017.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JJ, Jaunmuktane Z, Mummery C, Brandner S, Leary S, Trip SA. Inflammatory demyelination without astrocyte loss in MOG antibody- positive NMOSD. Neurology. 2016;87:229–31. doi: 10.1212/WNL.0000000000002844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernard-Valnet R, Liblau RS, Vukusic S, Marignier R. Neuromyelitis optica: A positive appraisal of seronegative cases. Eur J Neurol. 2015;22:1511–8. doi: 10.1111/ene.12679. [DOI] [PubMed] [Google Scholar]
- 27.Weber M, Derfuss T, Metz I, Brück W. Defining distinct features of anti-MOG antibody associated central nervous system demyelination. Ther Adv Neurol Disord. 2018;11:1–15. doi: 10.1177/1756286418762083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Passos GR, Oliveira LM, da Costa BK, Apostolos-Pereira SL, Callegaro D, Fujihara K, et al. MOG-IgG-associated optic neuritis, encephalitis, and myelitis: Lessons learned from neuromyelitis optica spectrum disorder. Front Neurol. 2018;9:217–26. doi: 10.3389/fneur.2018.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pache F, Zimmermann H, Mikolajczak J, Schumacher S, Lacheta A, Oertel FC, et al. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients. Part 4: Afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients. J Neuroinflammation. 2016;13:282. doi: 10.1186/s12974-016-0720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]