Abstract
Background:
Identifying preterm infants at risk for mortality or major morbidity traditionally relies on gestational age, birthweight, and other clinical characteristics that offer underwhelming utility. We sought to determine whether a newborn metabolic vulnerability profile at birth can be used to evaluate risk for neonatal mortality and major morbidity in preterm infants.
Methods:
This was a population-based retrospective cohort study of preterm infants born between 2005 and 2011 in California. We created a newborn metabolic vulnerability profile wherein maternal/infant characteristics along with routine newborn screening metabolites were evaluated for their association with neonatal mortality or major morbidity.
Results:
9,639 (9.2%) preterm infants experienced mortality or at least one complication. Six characteristics and 19 metabolites were included in the final metabolic vulnerability model. The model demonstrated exceptional performance for the composite outcome of mortality or any major morbidity (AUC 0.923 (95% CI: 0.917–0.929). Performance was maintained across mortality and morbidity subgroups (AUCs 0.893–0.979).
Conclusion:
Metabolites measured as part of routine newborn screening can be used to create a metabolic vulnerability profile. These findings lay the foundation for targeted clinical monitoring and further investigation of biological pathways that may increase the risk of neonatal death or major complications in infants born preterm.
Introduction
In the United States, approximately 1 out of every 10 live born infants is delivered preterm (before 37 weeks)(1), and globally, the burden of preterm births stands at nearly 15 million per year(2). Preterm birth and related complications are the leading cause of death for children under 5 years of age(3–5), and neonatal deaths, specifically, account for 46% of mortality in this age group(5).
Beyond mortality, preterm infants are also at an increased risk for numerous complications including patent ductus arteriosus (PDA), respiratory distress syndrome (RDS), intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), necrotizing enterocolitis (NEC), jaundice, infections, sepsis, and longer term, cerebral palsy and neurodevelopmental disability(6–11). Cumulatively, these conditions result in increased health care resource utilization for infants born preterm costing at least 26.2 billion dollars each year in the United States(12). Additionally, infants continue to survive at increased frequency at lower gestational ages (GA) and birthweights (BW) due primarily to improvements in resuscitation and early neonatal care, which leads to reduced mortality but increased morbidity amongst extremely preterm infants(13–18). The convergence of human loss and economic cost have made the use of novel strategies – beyond insufficient models relying solely on gestational age, birthweight, and other common characteristics – to inform risk, minimize complications, and gain insight into underlying dysfunctional biological processes an increasingly critical priority(19, 20).
Routine newborn screening (NBS) is typically utilized for identifying infants at risk for rare metabolic disorders, but has also begun to shed light on the contribution of metabolomics in evaluating the complications associated with prematurity(21). For example, Ryckman et al. demonstrated associations between amino acid concentrations and RDS, as well as PDA in preterm infants(22). Fell et al. established associations between NBS metabolites and sepsis(23). Our group has previously demonstrated strong associations between measured NBS metabolites and persistent pulmonary hypertension, hyperbilirubinemia, NEC, and survival in extremely preterm (<26 GA weeks) infants(24–27). Further understanding of how metabolic dysfunction in preterm infants contributes to the development of common complications could direct preventative treatments in the future.
The primary objective of this study was to develop a metabolic vulnerability profile for neonatal mortality and major morbidity in preterm infants using metabolites measured as part of routine NBS and common maternal/infant characteristics. We also evaluated whether a newborn metabolic vulnerability profile could be applied effectively to specific major morbidities (RDS, BPD, NEC, IVH, PVL, ROP, and PDA) in preterm infants. To our knowledge, this is the first study to evaluate the efficacy of creating a newborn metabolic vulnerability profile that’s applicable to multiple neonatal outcomes.
Methods
This population-based retrospective cohort study included all infants born in California between 2005 and 2011. Birth and outcomes data were obtained through the California Office of Statewide Health Planning and Development (OSHPD). This database maintains maternal admission and discharge records up to one year prior to birth and maternal and infant admission and discharge records up to one year after birth including birth certificate and death certificates. Metabolic data were collected as a part of routine newborn screening conducted by the California Department of Public Health. This program requires all newborns to have a heel-stick bloodspot taken between 12 hours and 7 days after delivery and has been described in extensive detail elsewhere(28, 29). The newborn screening data was linked to the OSHPD data using common variables.
After linkage, 2,664,595 infants remained eligible for analysis. Exclusion criteria included term birth (≥37 GA weeks), birthweight outside of four standard deviations from the mean for gestational age and sex(30), non-singleton birth, incomplete metabolic data measured by newborn screening, and blood spot collection after 48 hours. The final study sample consisted of 104,907 preterm infants. The cohort was split into gestational age groups (the full cohort, infants born at 32–36 weeks GA, and infants born at <32 weeks GA) and each group was randomly divided into a training set (2/3 of sample) and a validation set (1/3 of sample). Randomization was performed after stratification in order to ensure stratum likeness between training and validation groups (Supplemental Figure 1).
Outcomes in our study were identified using the International Classification of Diseases 9th revision (ICD-9) diagnostic codes, linked infant death records, and hospital discharge status. Our primary outcome was a composite measure that included infant death and major preterm morbidity. Infant death included neonatal death (within 28 days of birth) and one year mortality. Preterm morbidities included: RDS (ICD-9 code 769.0), PDA (747.0), ROP (362.2), IVH (772.1), BPD (770.7), NEC (777.5), and PVL (779.7)]. We are confident that all deaths were captured given the linkage to death certificates and that ICD-9 codes captured ~90% of all outcomes associated with preterm birth. Explanatory variables evaluated included infant and maternal characteristics from birth certificate and hospital discharge records as well as routine metabolites and clinical factors measured as part of NBS. Specific characteristics evaluated included infant gestational age at birth, birthweight, sex, small for gestational age (SGA), administration of total parenteral nutrition (TPN, defined as receiving TPN prior to blood spot collection), age at NBS collection, cesarean delivery, Medi-Cal status (California’s Medicaid), adequacy of prenatal care(31, 32), maternal race/ethnicity, maternal education, maternal age, gestational diabetes, and gestational hypertension. Variables were selected based on occurrence before NBS, availability, and previous or suspected relationship with the primary or secondary outcomes. Metabolites included from NBS consisted of 12 amino acids, 26 acylcarnitines, and free carnitine measured by standardized tandem mass spectrometry (MS/MS); two hormones measured by high-performance liquid chromatography; and one enzyme measured with a fluorometric enzyme assay(28) (see Table 1 for complete list).
Table 1.
Crude analysis of maternal demographics, infant characteristics, and metabolites for infants with a morbidity or mortality and those without in the training sample.
| Variable | Morbidity or Mortality | No Morbidity or Mortality | OR (95% CI) | P-Value |
|---|---|---|---|---|
| n = 6,404 | n = 63,534 | |||
| Characteristics | ||||
| Gestational Age (weeks) | 31.3 (3.7) | 35.3 (1.2) | 0.48 (0.48–0.49) | <0.0001 |
| Birthweight (per 100g) | 17.9 (8.1) | 28.3 (5.2) | 0.77 (0.76–0.77) | <0.0001 |
| Age at Collection (hours) | 31.0 (9.5) | 29.0 (8.6) | 1.03 (1.02–1.03) | <0.0001 |
| Female | 2667 (41.65) | 28951 (45.57) | 0.85 (0.81–0.90) | <0.0001 |
| TPN | 3513 (54.86) | 2774 (4.37) | 26.61 (25.00–28.32) | <0.0001 |
| Small for GA | 802 (12.52) | 3784 (5.96) | 2.26 (2.09–2.45) | <0.0001 |
| Cesarean delivery | 3823 (59.7) | 19316 (30.4) | 3.39 (3.22–3.57) | <0.0001 |
| Medi-Cal | 3153 (49.23) | 31638 (49.8) | 0.98 (0.93–1.03) | 0.3912 |
| Prenatal Care | <0.0001 | |||
| Adequate or adequate plus | 4932 (77.01) | 49843 (78.45) | Reference | |
| Intermediate | 555 (8.67) | 4089 (6.44) | 1.37 (1.25–1.51) | |
| Inadequate | 676 (10.56) | 7728 (12.16) | 0.88 (0.81–0.96) | |
| Unknown | 241 (3.76) | 1874 (2.95) | 1.30 (1.13–1.49) | |
| Maternal Race | <0.0001 | |||
| White non-Hispanic | 1366 (21.33) | 12932 (20.35) | Reference | |
| Black | 389 (6.07) | 2724 (4.29) | 1.35 (1.20–1.52) | |
| Hispanic | 3357 (52.42) | 34429 (54.19) | 0.92 (0.86–0.99) | |
| Asian | 816 (12.74) | 9425 (14.83) | 0.82 (0.75–0.90) | |
| Other | 476 (7.43) | 4024 (6.33) | 1.12 (1.00–1.25) | |
| Maternal education | <0.0001 | |||
| <12 years | 1884 (29.42) | 20605 (32.43) | 0.88 (0.83–0.94) | |
| 12 years | 1486 (23.2) | 14427 (22.71) | 1.00 (0.93–1.06) | |
| >12 years | 2714 (42.38) | 26224 (41.28) | Reference | |
| unknown | 320 (5) | 2278 (3.59) | 1.36 (1.20–1.54) | |
| Maternal age | <0.0001 | |||
| <18 years | 247 (3.86) | 2497 (3.93) | 1.02 (0.90–1.17) | |
| 18–34 years | 4659 (72.75) | 48229 (75.91) | Reference | |
| >34 years | 1498 (23.39) | 12808 (20.16) | 1.21 (1.14–1.29) | |
| Gestational diabetes | 741 (11.57) | 6400 (10.07) | 1.17 (1.08–1.27) | 0.0002 |
| Gestational hypertension | 1088 (16.99) | 6583 (10.36) | 1.77 (1.65–1.90) | <0.0001 |
| Enzymes & Hormones | ||||
| 17 Hydroxyprogesterone | 4.27 (0.9) | 3.33 (0.65) | 6.00 (5.76–6.25) | <0.0001 |
| TSH | 0.74 (0.96) | 1.47 (0.61) | 0.24 (0.23–0.25) | <0.0001 |
| GALT | 5.43 (0.28) | 5.5 (0.23) | 0.37 (0.33–0.41) | <0.0001 |
| Amino Acids | ||||
| 5-Oxoproline | 3.37 (0.61) | 3.54 (0.55) | 0.65 (0.62–0.68) | <0.0001 |
| Alanine | 5.5 (0.42) | 5.48 (0.33) | 1.19 (1.10–1.28) | <0.0001 |
| Arginine | 2.66 (0.92) | 2.15 (0.68) | 2.62 (2.53–2.72) | <0.0001 |
| Citrulline | 2.75 (0.38) | 2.71 (0.28) | 1.66 (1.52–1.81) | <0.0001 |
| Glycine | 6.29 (0.33) | 6.35 (0.28) | 0.50 (0.46–0.55) | <0.0001 |
| Leucine/Isoleucine | 4.94 (0.49) | 4.65 (0.28) | 12.13 (11.24–13.09) | <0.0001 |
| Methionine | 3.55 (0.63) | 3.34 (0.33) | 3.92 (3.68–4.17) | <0.0001 |
| Ornithine | 4.69 (0.52) | 4.58 (0.35) | 2.23 (2.09–2.39) | <0.0001 |
| Phenylalanine | 4.37 (0.32) | 4.15 (0.23) | 25.55 (23.05–28.31) | <0.0001 |
| Proline | 5.24 (0.49) | 5.19 (0.34) | 1.50 (1.40–1.61) | <0.0001 |
| Tyrosine | 4.54 (0.65) | 4.66 (0.42) | 0.56 (0.53–0.59) | <0.0001 |
| Valine | 4.87 (0.46) | 4.67 (0.31) | 6.42 (5.94–6.94) | <0.0001 |
| Carnitines | ||||
| Free Carnitine | 3.77 (0.46) | 3.57 (0.41) | 3.01 (2.83–3.21) | <0.0001 |
| C-2 | 3.17 (0.37) | 3.2 (0.31) | 0.76 (0.70–0.83) | <0.0001 |
| C-3 | 0.89 (0.43) | 0.69 (0.4) | 3.29 (3.08–3.50) | <0.0001 |
| C-3DC | −2.57 (0.42) | −2.38 (0.39) | 0.29 (0.27–0.31) | <0.0001 |
| C-4 | −1.01 (0.51) | −1.33 (0.5) | 3.96 (3.75–4.19) | <0.0001 |
| C-5 | −1.37 (0.61) | −1.97 (0.54) | 10.18 (9.60–10.80) | <0.0001 |
| C-5:1 | −3.61 (0.86) | −3.78 (0.88) | 1.25 (1.21–1.29) | <0.0001 |
| C-5DC | −2.17 (0.48) | −2.06 (0.45) | 0.59 (0.56–0.63) | <0.0001 |
| C-5OH | −1.66 (0.51) | −1.72 (0.51) | 1.34 (1.27–1.42) | <0.0001 |
| C-6 | −3 (0.83) | −2.83 (0.79) | 0.79 (0.77–0.81) | <0.0001 |
| C-8 | −2.87 (0.77) | −2.73 (0.73) | 0.79 (0.77–0.82) | <0.0001 |
| C-8:1 | −2.45 (0.77) | −2.29 (0.62) | 0.71 (0.68–0.73) | <0.0001 |
| C-10 | −3.06 (0.78) | −2.48 (0.66) | 0.40 (0.39–0.41) | <0.0001 |
| C-10:1 | −3.1 (0.82) | −2.96 (0.7) | 0.79 (0.76–0.82) | <0.0001 |
| C-12 | −2.43 (0.75) | −1.8 (0.57) | 0.24 (0.24–0.26) | <0.0001 |
| C-12:1 | −3.24 (0.85) | −2.59 (0.83) | 0.48 (0.47–0.49) | <0.0001 |
| C-14 | −1.71 (0.47) | −1.44 (0.39) | 0.23 (0.21–0.24) | <0.0001 |
| C-14:1 | −2.36 (0.59) | −1.98 (0.55) | 0.36 (0.35–0.38) | <0.0001 |
| C-14OH | −4.05 (0.83) | −3.74 (0.84) | 0.67 (0.65–0.69) | <0.0001 |
| C-16 | 0.68 (0.47) | 0.98 (0.34) | 0.12 (0.11–0.13) | <0.0001 |
| C-16:1 | −1.77 (0.52) | −1.53 (0.46) | 0.39 (0.37–0.41) | <0.0001 |
| C-16OH | −3.73 (0.82) | −3.57 (0.81) | 0.79 (0.77–0.81) | <0.0001 |
| C-18 | −0.29 (0.37) | −0.23 (0.35) | 0.61 (0.57–0.66) | <0.0001 |
| C-18:1 | −0.05 (0.37) | 0.11 (0.32) | 0.25 (0.23–0.27) | <0.0001 |
| C-18:1OH | −4.02 (0.81) | −3.89 (0.83) | 0.83 (0.81–0.86) | <0.0001 |
| C-18:2 | −1.34 (0.63) | −1.6 (0.51) | 2.74 (2.60–2.89) | <0.0001 |
| C-18OH | −4.38 (0.79) | −4.19 (0.83) | 0.75 (0.73–0.78) | <0.0001 |
TPN: total parenteral nutrition; NBS: newborn screening; GALT: galactose-1-phosphate uridyl transferase; TSH: thyroid stimulating hormone. Continuous variables described using mean and SD and ORs by per unit increase. Categorical variables described using frequencies and proportions. T-tests and χ2 tests for continuous and categorical variables respectively
Statistical Analyses
In order to reduce skewness and minimize the influence of outliers, all metabolites were natural log transformed from their raw concentrations. Standardized growth curves by gestational age and sex were used to determine the 10th percentile cutoff to create SGA(30). Crude analyses to compare infants with any mortality or major morbidity to healthy infants relied on t-tests and χ2 tests for continuous and categorical variables, respectively.
Multivariable logistic regression utilizing stepwise selection in the training dataset was used to create a metabolic vulnerability model for the composite outcome of mortality or any major morbidity. Any variable that met a Bonferonni corrected p-value threshold of <0.000781 was permitted to enter the model with entry order determined by greatest significance and a p-value of <0.05 required to remain in the model. Age at NBS collection and TPN were included into the model a-priori as they are known to affect the concentration of metabolites(33–35). The established model was then applied and tuned to mortality (neonatal and 1 year) and to each major morbidity (RDS, PDA, ROP, IVH, BPD, NEC, PVL) in the training and validation subsets with further evaluation of performance in preterm infants born at <32 and 32–36 weeks in the validation sample. Model performance was assessed using area under the curve (AUC) from a receiver operating characteristic (ROC) curve wherein variable importance was evaluated using odds ratios (ORs) with 95% confidence intervals (95% CI) and standardized beta coefficients. Multicollinearity between metabolites was examined by calculating Pearson correlation coefficients (≥0.8 considered strong collinearity) and by assessing the tolerance and variable inflation factors within the multivariable model (<0.1 and ≥10 considered strong multicollinearity, respectfully).
All analyses were performed using SAS 9.4 (SAS institute, Cary, NC). Methods and protocols for the study were approved by the Committee for the Protection of Human Subjects within the Health and Human Services Agency of the State of California and the Institutional Review Board of the University of California, San Francisco.
Results
Within our population, 9,639 (9.2%) of infants with preterm birth experienced either mortality or at least one major complication. Training and validation samples had similar variable distributions. In univariable analysis, 13 of 14 infant and maternal characteristics as well as all 42 metabolites exhibited significant differences in preterm infants with mortality or major morbidity compared to those without (Supplemental Table 1; Table 1). Among characteristics, gestational age, birthweight, SGA, cesarean delivery, and TPN were all highly associated with mortality or major morbidity. Metabolites strongly associated with mortality or major morbidity included high levels of 17-hydroxyprogesterone, leucine/isoleucine, phenylalanine, valine, and acylcarnitine C-5 and low levels of TSH and acylcarnitines C-12, C-14, C-16, and C-18:1 (Table 1).
The final multivariable metabolic vulnerability model for the composite outcome of any mortality or major morbidity included 6 characteristics (infant sex, cesarean delivery, maternal education, maternal race/ethnicity, gestational age, & birthweight) and 19 metabolites (three enzymes & hormones [17-hydroxyprogesterone, TSH, & GALT], seven amino acids [5-oxoproline, glycine, leucine/isoleucine, ornithine, phenylalanine, proline, & tyrosine], and nine acylcarnitines [C-2, C-3, C-4, C-5, C-10, C-12, C-12:1, C-16:1, & C-18:2]). The variables most strongly associated with any mortality or major morbidity included cesarean delivery (OR: 1.79; 95% CI: 1.66–1.93), gestational age (OR: 0.75; 95% CI: 0.73–0.77), birthweight (OR: 0.94; 95% CI: 0.93–0.95), 17-hydroxyprogesterone (OR: 1.61; 95% CI: 1.52–1.70), TSH (OR: 0.62; 95% CI: 0.59–0.65), ornithine (OR: 0.36; 95% CI: 0.32–0.42), phenylalanine (OR: 11.19; 95% CI: 9.03–13.87), tyrosine (OR: 0.53; 95% CI: 0.49–0.57), C-3 (OR: 1.42; 95% CI: 1.26–1.60), and C-12 (OR: 0.68; 95% CI: 0.64–0.73) (Table 2).
Table 2.
Multivariable logistic regression model for any mortality or morbidity adjusted for total parenteral nutrition and age at NBS collection.
| Variable | Any Morbidity or Mortality | No Morbidity or Mortality | Parameter Estimate (Std Error) | OR (95% CI) | Standardized Estimate |
|---|---|---|---|---|---|
| Female | 2,667 (41.65) | 28,951 (45.57) | −0.31 (0.04) | 0.74 (0.68–0.80) | −0.084 |
| C-Section | 3,823 (59.70) | 19,316 (30.40) | 0.58 (0.04) | 1.79 (1.66–1.93) | 0.151 |
| Race/Ethnicity | |||||
| White non-Hispanic | 1,366 (21.33) | 12,932 (20.35) | Ref | Ref | Ref |
| Asian | 816 (12.74) | 9,425 (14.83) | −0.35 (0.06) | 0.71 (0.62–0.80) | −0.068 |
| Black | 389 (6.07) | 2,724 (4.29) | −0.13 (0.09) | 0.87 (0.73–1.05) | −0.015 |
| Hispanic | 3,357 (52.42) | 34,429 (54.19) | −0.18 (0.05) | 0.83 (0.75–0.92) | −0.051 |
| Other | 476 (7.43) | 4,024 (6.33) | −0.12 (0.09) | 0.89 (0.75–1.05) | −0.017 |
| Education (years) | |||||
| <12 | 1884 (29.42) | 20,605 (32.43) | −0.22 (0.05) | 0.80 (0.73–0.89) | −0.057 |
| High School | 1,486 (23.20) | 14,427 (22.71) | −0.15 (0.05) | 0.87 (0.78–0.96) | −0.034 |
| >12 | 2,714 (42.38) | 26,224 (41.28) | Ref | Ref | Ref |
| Unknown | 320 (5.00) | 2,278 (3.59) | −0.02 (0.10) | 0.98 (0.81–1.19) | −0.002 |
| Birthweight (per 100g) | 17.9 (8.10) | 28.3 (5.20) | −0.06 (0.01) | 0.94 (0.93–0.95) | −0.216 |
| Gestational age (weeks) | 31.3 (3.70) | 35.3 (1.20) | −0.29 (0.01) | 0.75 (0.73–0.77) | −0.311 |
| 17 Hydroxyprogesterone | 4.27 (0.90) | 3.33 (0.65) | 0.47 (0.03) | 1.61 (1.52–1.70) | 0.191 |
| TSH | 0.74 (0.96) | 1.47 (0.61) | −0.48 (0.04) | 0.62 (0.59–0.65) | −0.179 |
| GALT | 5.43 (0.28) | 5.50 (0.23) | −0.47 (0.08) | 0.63 (0.53–0.74) | −0.060 |
| 5-Oxoproline | 3.37 (0.61) | 3.54 (0.55) | −0.12 (0.03) | 0.89 (0.83–0.95) | −0.037 |
| Glycine | 6.29 (0.33) | 6.35 (0.28) | 0.41 (0.09 | 1.51 (1.27–1.78) | 0.064 |
| Leucine/Isoleucine | 4.94 (0.49) | 4.65 (0.28) | −0.54 (0.11) | 0.59 (0.48–0.72) | −0.093 |
| Ornithine | 4.69 (0.52) | 4.58 (0.35) | −1.01 (0.07) | 0.36 (0.32–0.42) | −0.205 |
| Phenylalanine | 4.37 (0.32) | 4.15 (0.23) | 2.42 (0.11) | 11.19 (9.03–13.87) | 0.330 |
| Proline | 5.24 (0.49) | 5.19 (0.34) | 0.31 (0.07) | 1.37 (1.19–1.57) | 0.062 |
| Tyrosine | 4.54 (0.65) | 4.66 (0.42) | −0.64 (0.04) | 0.53 (0.49–0.57) | −0.159 |
| C-2 | 3.17 (0.37) | 3.20 (0.31) | −0.71 (0.08) | 0.49 (0.42–0.58) | −0.121 |
| C-3 | 0.89 (0.43) | 0.69 (0.40) | 0.35 (0.06) | 1.42 (1.26–1.60) | 0.078 |
| C-4 | −1.01 (0.51) | −1.33 (0.50) | 0.29 (0.05) | 1.33 (1.22–1.45) | 0.081 |
| C-5 | −1.37 (0.61) | −1.97 (0.54) | 0.25 (0.05) | 1.29 (1.17–1.41) | 0.079 |
| C-10 | −3.06 (0.78) | −2.48 (0.66) | −0.13 (0.03) | 0.87 (0.83–0.92) | −0.051 |
| C-12 | −2.43 (0.75) | −1.80 (0.57) | −0.38 (0.03) | 0.68 (0.64–0.73) | −0.130 |
| C-12:1 | −3.24 (0.85) | −2.59 (0.83) | −0.10 (0.03) | 0.90 (0.86–0.95) | −0.048 |
| C-16:1 | −1.77 (0.52) | −1.53 (0.46) | 0.25 (0.05) | 1.28 (1.17–1.42) | 0.064 |
| C-18:2 | −1.34 (0.63) | −1.60 (0.51) | −0.23 (0.04) | 0.80 (0.74–0.86) | −0.066 |
All metabolites are natural log transformed. Original units – umol/L
GALT: galactose-1-phosphate uridyl transferase; TSH: thyroid stimulating hormone; OR: Odds Ratio; CI: confidence interval.
All variables have model p-value of <0.001
Continuous variables described using mean and SD and ORs by per unit increase. Categorical variables described using frequencies and proportions. T-tests and χ2 tests for continuous and categorical variables respectively
Overall, the metabolic vulnerability profile demonstrated exceptional performance in the validation sample (AUC 0.923 (0.917–0.929); Table 3), and at a probability cut point of 0.5, had 52.2% (95% CI: 50.5–53.9%) sensitivity, 98.4% (95% CI: 98.4–98.5%) specificity, 76.7% (95% CI: 74.9–78.5%) positive predictive value, and 95.3% (95.1–95.5%) negative predictive value (Supplemental Table 2). The model also displayed robust performance when tuned to and evaluated by mortality and major complication subgroups (AUCs 0.893–0.977 across training and validation samples, Supplemental Tables 3 & 4). Performance was maintained in groupings by gestational weeks at birth (<32 and 32–36 weeks, AUCs 0.682–0.929) with generally stronger performance observed in late preterm infants (Table 3 and Supplemental Figure 2). Models relying solely on the metabolic portion of the final model outperformed those relying only on characteristics for all outcomes except ROP and PVL (Table 4). Finally, the model remained robust when stratified by TPN (No TPN: AUC 0.880 (95% CI: 0.872–0.889); with TPN: AUC 0.821 (95% CI: 0.811–0.832)).
Table 3.
Performance of the full model stratified by gestational age categories for individual outcomes within the validation population.
| All Gestational Ages (n=34,969) | Gestational ages <32 (n=1,924) | Gestational ages 32–36 (n=33,045) | ||||
|---|---|---|---|---|---|---|
| Model | n (%) | AUC (95% CI) | n = (%) | AUC (95% CI) | n = (%) | AUC (95% CI) |
| Morbidity or Mortality | 3,235 (9.25) | 0.923 (0.917–0.929) | 1,485 (77.18) | 0.841 (0.819–0.862) | 1,769 (5.35) | 0.881 (0.871–0.891) |
| Neonatal Mortality | 157 (0.45)* | 0.948 (0.928–0.967) | 104 (5.56)* | 0.843 (0.803–0.883) | 67 (0.20)* | 0.872 (0.819–0.924) |
| 1-Year Mortality | 266 (0.76) | 0.913 (0.893–0.934) | 159 (8.26) | 0.830 (0.797–0.864) | 120 (0.36) | 0.815 (0.767–0.863) |
| RDS | 2,367 (6.77) | 0.954 (0.950–0.958) | 1,243 (64.60) | 0.787 (0.766–0.809) | 1,165 (3.53) | 0.928 (0.919–0.936) |
| PDA | 1,367 (3.91) | 0.893 (0.882–0.904) | 767 (39.86) | 0.783 (0.762–0.803) | 578 (1.75) | 0.814 (0.794–0.835) |
| ROP | 616 (1.76) | 0.977 (0.971–0.984) | 592 (30.77) | 0.756 (0.734–0.779) | 69 (0.21) | 0.929 (0.894–0.964) |
| IVH | 554 (1.58) | 0.953 (0.944–0.963) | 431 (22.40) | 0.741 (0.715–0.767) | 131 (0.40) | 0.878 (0.841–0.916) |
| BPD | 423 (1.21) | 0.953 (0.941–0.966) | 366 (19.02) | 0.806 (0.784–0.828) | 66 (0.20) | 0.815 (0.756–0.874) |
| NEC | 182 (0.52) | 0.952 (0.939–0.964) | 150 (7.80) | 0.694 (0.655–0.734) | 62 (0.19 | 0.908 (0.863–0.954) |
| PVL | 36 (0.10) | 0.938 (0.887–0.989) | 32 (1.66) | 0.682 (0.602–0.762) | 9 (0.03) | 0.844 (0.691–0.997) |
Full sample n=34,860, n=32,992, & n=1,869 for gestational age categories respectivelyAUC: area under the curve; RDS: respiratory distress syndrome; PDA: patent ductus arteriosus; ROP: retinopathy of prematurity; IVH: intraventricular hemorrhage; BPD: bronchopulmonary dysplasia; NEC: necrotizing enterocolitis; PVL periventricular leukomalacia
Table 4.
Performance of the full model as compared to a model using only metabolites and a model using only characteristics in the validation population
| AUC (95% CI) |
||||
|---|---|---|---|---|
| Model | n (%) | Full | Metabolites | Characteristics |
| Morbidity or Mortality | 3,235 (9.25) | 0.923 (0.917–0.929) | 0.911 (0.905–0.917) | 0.871 (0.863–0.879) |
| Neonatal Mortality | 157 (0.45)* | 0.948 (0.928–0.967) | 0.938 (0.917–0.958) | 0.900 (0.871–0.930) |
| 1-Year Mortality | 266 (0.76) | 0.913 (0.893–0.934) | 0.907 (0.887–0.928) | 0.873 (0.847–0.898) |
| RDS | 2,367 (6.77) | 0.954 (0.950–0.958) | 0.943 (0.938–0.948) | 0.904 (0.897–0.911) |
| PDA | 1,367 (3.91) | 0.893 (0.882–0.904) | 0.877 (0.865–0.889) | 0.853 (0.839–0.866) |
| ROP | 616 (1.76) | 0.977 (0.971–0.984) | 0.964 (0.957–0.972) | 0.977 (0.971–0.984) |
| IVH | 554 (1.58) | 0.953 (0.944–0.963) | 0.945 (0.935–0.954) | 0.943 (0.931–0.955) |
| BPD | 423 (1.21) | 0.953 (0.941–0.966) | 0.949 (0.937–0.961) | 0.947 (0.933–0.961) |
| NEC | 182 (0.52) | 0.952 (0.939–0.964) | 0.944 (0.929–0.959) | 0.931 (0.910–0.952) |
| PVL | 36 (0.10) | 0.938 (0.887–0.989) | 0.934 (0.883–0.985) | 0.942 (0.903–0.982) |
Full n=34,860 (other deaths not included)
AUC: area under the curve; RDS: respiratory distress syndrome; PDA: patent ductus arteriosus; ROP: retinopathy of prematurity; IVH: intraventricular hemorrhage; BPD: bronchopulmonary dysplasia; NEC: necrotizing enterocolitis; PVL periventricular leukomalacia
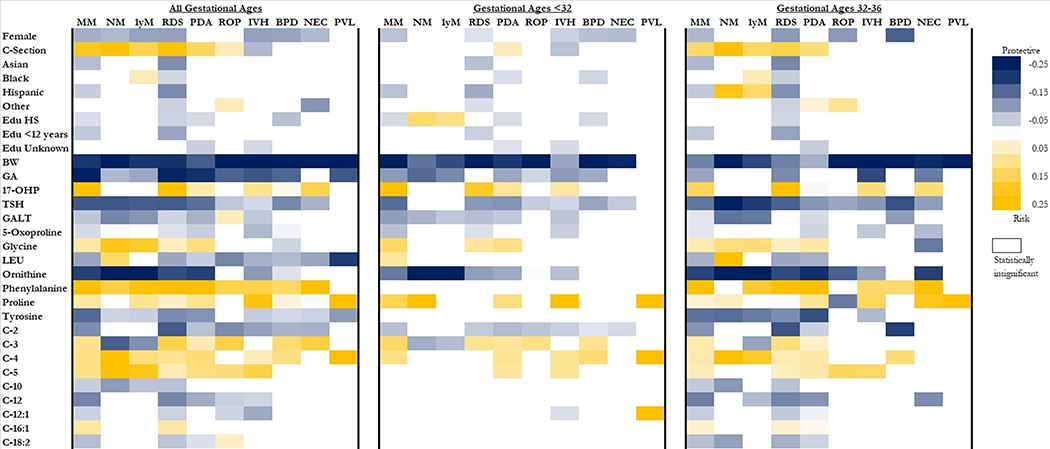
The importance of individual variables fluctuated within gestational age stratifications and by outcome (Figure 1). Birthweight was the only variable with ubiquitous importance across gestational age groups and outcomes as larger infants were less likely to experience morbidity or mortality. Older gestational age was also generally protective against morbidities and mortality. Female sex was associated with lower risk of mortality and most morbidities, and delivering via cesarean section was associated with increased risk of mortality, RDS, PDA, and ROP but decreased risk of IVH. Infants born between 32 and 36 weeks were at increased risk of mortality or morbidity if they had increased concentrations of phenylalanine, glycine, 17-OHP, proline, C-4, and C-5, and decreased concentrations of TSH, GALT, 5-oxoproline, ornithine, tyrosine, C-2, and C-12. For infants born before 32 weeks, increased risk for morbidity or mortality was associated with increased concentrations of 17-OHP, glycine, proline, and C-4 as well as decreased concentrations of TSH, GALT, 5-oxoproline, ornithine, and C-2 (Figure 1).
Figure 1.
Reference for race/ethnicity is white and the reference for education is >12 years. For continuous variables, risk/protective effect corresponds to increasing amounts of the variable. MM: any mortality or morbidity; NM: neonatal mortality; 1-yr M: 1-year mortality; RDS: respiratory distress syndrome; PDA: patent ductus arteriosus; ROP: retinopathy of prematurity; IVH: intraventricular hemorrhage; BPD: bronchopulmonary dysplasia; NEC: necrotizing enterocolitis; PVL periventricular leukomalacia; BW: birthweight; GA: gestational age; Edu: education; HS: high school; GALT: galactose-1-phosphate uridyl transferase; TSH: thyroid stimulating hormone; 17-OHP: 17 hydroxyprogesterone; LEU: leucine/isoleucine ratio
Discussion
Our study examined the relationship between initial metabolic profile and mortality and major morbidity in preterm infants. We built a newborn metabolic vulnerability profile that was able to identify preterm infants more likely to experience at least one of these outcomes, and that outperformed models based only on clinical characteristics like GA and BW. Furthermore, we identified metabolic markers that may be especially useful for follow-up investigation into etiologic drivers of mortality and of specific complications. This information has the potential to be used for targeted long-term clinical monitoring and interventions that are specific to a newborn’s metabolic profile to improve both survival rates and long term health outcomes for this highly vulnerable population.
It is well known that preterm infants are at increased risk for mortality and major morbidity compared to their term counterparts(36, 37). Even within preterm cohorts, there are infants who thrive and those who do not(13, 14, 25, 38, 39). These differences are often not explained by gestational age, birth weight, or other characteristics of the mother or infant alone(13, 14, 19, 25, 38, 39). Our study found that metabolic profile as measured by NBS can help identify preterm infants at more or less risk for mortality or major morbidity. Further, our study demonstrated that a metabolic vulnerability profile can identify distinct metabolic pathways associated with specific outcomes.
To our knowledge, no other published study has investigated building a model to assess the potential comprehensive metabolic vulnerability of preterm infants. There have, however, been a handful of studies showing associations between metabolite and specific outcomes. Our previous work within this dataset has revealed links between metabolite concentrations and survival, persistent pulmonary hypertension, hyperbilirubinemia, and NEC(24–27). Other studies have demonstrated relationships between metabolites and PDA, RDS, sepsis, and childhood wheezing(22, 23, 40). Combined, this evidence suggests that while the degree of association of particular metabolites fluctuates between complications, there are underlying dysfunctional metabolic patterns that are shared.
Our findings make it clear that NBS metabolites provide additional utility beyond gestational age and birthweight. While older gestational age and increased birthweight were consistently associated with decreased risk for morbidity or mortality, the metabolic-only model generally outperformed the clinical characteristics model (GA and BW included) across groups. Gestational age and birthweight’s association with morbidity and mortality is overwhelmingly supported by previous research(13, 14, 39, 41), and a relationship between metabolic patterns, gestational age, and birthweight has also been well demonstrated(42–47). Nevertheless, the role of these two variables as confounders doesn’t sufficiently explain our results, which supports the concept that preterm infants who suffer complications have metabolic patterns distinct from their preterm peers who also have immature metabolic processes.
In addition to the identification capacity of NBS metabolites demonstrated by the present study, the observed metabolite patterns appear to point to several potentially important etiologic pathways that could prove important for further clinical and investigative follow-up aimed at preventing mortality or major morbidity in infants born preterm. For example, in the present study elevated levels of TSH, a hormone indicative of thyroid function, were found to be associated with a lower risk of morbidity and mortality. This finding is supported by other studies examining TSH levels in preterm infants(48, 49) wherein it has been as suggested that higher TSH levels may be related to a greater production of surfactant, which has been shown to be crucial in preventing or minimizing the severity of RDS(50–52). TSH and thyroid hormones are important in normal neonatal physiology, influencing everything from metabolic homeostasis to proper neurodevelopment (53, 54). The importance of TSH in multiple physiologic pathways likely explains the significant role that TSH played in our metabolic vulnerability model and in observed patterning across outcomes.
The confluence of raised concentrations of phenylalanine and lower levels of tyrosine being associated with increased risk of morbidity and mortality in our model may suggest a dysfunction in the biosynthesis of tyrosine from phenylalanine by phenylalanine hydroxylase (PAH) in these infants. In order to function properly, PAH relies on the cofactor tetrahydrobiopterin (BH4), but BH4 becomes depleted in situations of high oxidative stress due to inflammation and immune activation(55, 56). Oxidative stress, in particular has been implicated in a number of neonatal outcomes including IVH, BPD, and RDS(57–59).
Lower levels of the amino acid ornithine were also associated with increased risk of preterm complications. Ornithine, citrulline and arginine are all important intermediates within the urea cycle, which has been connected to neonatal outcomes including NEC and persistent pulmonary hypertension(26, 60–62). It’s hypothesized that reduced levels of urea cycle enzymatic activity, especially in carbamoyl-phosphate synthetase (responsible for catalyzing the rate dependent step) results in diminished concentrations of citrulline, arginine, and ornithine. Without these intermediates, ammonia can begin to accumulate and precipitate nervous and respiratory sequelae and potentially death(61, 63, 64).
Another significant metabolic pattern demonstrated within this study was the relevance of short-chain acylcarnitines to the measured infant outcomes. Specifically, high levels of C-2 were generally associated with lower risk of morbidity and mortality while high levels of C-3, C-4, and C-5 were consistently associated with higher risk. Acylcarnitines are heavily involved in the process of shuttling fatty acids across the mitochondrial membrane in order to be utilized in β-oxidation. Therefore, abnormal levels of acylcarnitines may suggest systemic dysfunction of fatty acid oxidation(65, 66).
Several strengths and limitations exist within our study. An important asset was that our sample was derived from an extensive and diverse population-based data set, minimizing the potential for significant selection bias. Furthermore, we split our data into training and validation sets to ensure our model was not over-fitted. These analyses, however, were performed on a retrospective data set, which limited the potential variables to what was available in records or could be captured by ICD-9 codes. This may have restricted our ability to sufficiently control for confounding. For example, we were unable to capture antenatal corticosteroid use, which is well known to help prevent RDS, IVH, and mortality(67–69). Using ICD-9 codes also likely hindered our ability to precisely capture diagnoses as criteria for diagnosis varies between hospitals and providers, though our rates of morbidity are similar to other studies (13). This diagnostic error would have biased our study toward the null as it is non-differential, meaning our metabolic associations would likely have been more significant in an unbiased situation. Finally, temporality of association between metabolites and any given outcome is difficult to evaluate since newborn screening is often administered during or after complications are diagnosed, which prevented us from being able to make any causal assertions.
Validating the model in external population-based data sets should be the primary focus of future studies. The state of California is exceptionally diverse, with the majority of infants in our study population of Hispanic descent. External generalizability, therefore, would be enhanced by future testing in unique populations. Additionally, it would be useful to examine the study’s identified metabolic pathways in more detail (e.g. TSH, phenylalanine, ornithine, & acylcarnitines) to determine explicit etiologies resulting in morbidity or mortality. Given that newborn screening is limited to measuring metabolites at one point in time, with a significant delay between testing and results, it would be advantageous to develop point of care technology that could assess metabolic changes over time and deliver expedited results for potential real-time clinical decision-making. Models may also be improved by employing techniques such as untargeted metabolomics and machine learning with expanded sets of metabolites. Finally, we limited our study to a subset of major morbidities associated with preterm birth, but given the significant associations of metabolites shown here, investigations into additional morbidities are justified.
In our population-based retrospective cohort analysis, we built a newborn metabolic vulnerability profile that demonstrated excellent performance. Utilizing this profile for precision clinical monitoring, targeted investigation of etiologic pathways, and development of specific interventions could lead to reductions in mortality as well as the incidence and severity of major morbidities associated with preterm birth.
Supplementary Material
Flow chart of infants in California between 2005 and 2011 eligible for analysis
Impact:
We built a newborn metabolic vulnerability profile that could identify preterm infants at risk for major morbidity and mortality.
Identifying high risk infants by this method is novel to the field and outperforms models currently in use that rely primarily on infant characteristics.
Utilizing the newborn metabolic vulnerability profile for precision clinical monitoring and targeted investigation of etiologic pathways could lead to reductions in the incidence and severity of major morbidities associated with preterm birth.
Acknowledgements:
Data from the California Prenatal and Newborn Screening Programs were obtained through the California Biobank Program (Screening Information System request no. 476). Data were obtained with an agreement that the California Department of Public Health is not responsible for the results or conclusions drawn by the authors of this publication.
Funding: This work was supported by the California Preterm Birth Initiative within the University of California, San Francisco (UCSF).
Consent Statement: The State of California granted a waiver of consent for this study.
Footnotes
Disclosure Statement: No authors have any financial ties to products in the study or potential/perceived conflicts of interest.
References
- 1.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P 2018. Births: Final Data for 2016. Natl Vital Stat Rep 67:1–55. [PubMed] [Google Scholar]
- 2.Chawanpaiboon S, et al. 2018. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, et al. 2016. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 388:3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L, et al. 2015. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 385:430–440. [DOI] [PubMed] [Google Scholar]
- 5.2017. Levels & Trends in Child Mortality: Report 2017. UN Inter-Agency Group for Child Mortality Estimation, New York. [Google Scholar]
- 6.Benitz WE, Committee on F, Newborn AAoP 2016 Patent Ductus Arteriosus in Preterm Infants. Pediatrics 137. [DOI] [PubMed] [Google Scholar]
- 7.Platt MJ 2014. Outcomes in preterm infants. Public Health 128:399–403. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, et al. 2017. Association between perinatal hypoxic-ischemia and periventricular leukomalacia in preterm infants: A systematic review and meta-analysis. PLoS One 12:e0184993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang LW, Lin YC, Wang ST, Huang CC, on behalf of the Taiwan Premature Infant Developmental Collaborative Study G 2018. Identifying Risk Factors Shared by Bronchopulmonary Dysplasia, Severe Retinopathy, and Cystic Periventricular Leukomalacia in Very Preterm Infants for Targeted Intervention. Neonatology 114:17–24. [DOI] [PubMed] [Google Scholar]
- 10.Saigal S, Doyle LW 2008. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 371:261–269. [DOI] [PubMed] [Google Scholar]
- 11.Mwaniki MK, Atieno M, Lawn JE, Newton CR 2012. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet 379:445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purisch SE, Gyamfi-Bannerman C 2017. Epidemiology of preterm birth. Semin Perinatol 41:387–391. [DOI] [PubMed] [Google Scholar]
- 13.Ancel PY, et al. 2015. Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr 169:230–238. [DOI] [PubMed] [Google Scholar]
- 14.Anderson JG, et al. 2016. Survival and Major Morbidity of Extremely Preterm Infants: A Population-Based Study. Pediatrics 138. [DOI] [PubMed] [Google Scholar]
- 15.Hintz SR, et al. 2005. Changes in mortality and morbidities among infants born at less than 25 weeks during the post-surfactant era. Arch Dis Child Fetal Neonatal Ed 90:F128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kyser KL, Morriss FH Jr., Bell EF, Klein JM, Dagle JM 2012. Improving survival of extremely preterm infants born between 22 and 25 weeks of gestation. Obstet Gynecol 119:795–800. [DOI] [PubMed] [Google Scholar]
- 17.Rysavy MA, et al. 2015. Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med 372:1801–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Younge N, et al. 2017. Survival and Neurodevelopmental Outcomes among Periviable Infants. N Engl J Med 376:617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tyson JE, et al. 2008. Intensive care for extreme prematurity--moving beyond gestational age. N Engl J Med 358:1672–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawn JE, et al. 2014. Every Newborn: progress, priorities, and potential beyond survival. Lancet 384:189–205. [DOI] [PubMed] [Google Scholar]
- 21.Bennett MJ 2014. Newborn screening for metabolic diseases: saving children’s lives and improving outcomes. Clin Biochem 47:693–694. [DOI] [PubMed] [Google Scholar]
- 22.Ryckman KK, et al. 2013. Association of amino acids with common complications of prematurity. Pediatr Res 73:700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fell DB, et al. 2017. Using newborn screening analytes to identify cases of neonatal sepsis. Sci Rep 7:18020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarthy ME, et al. 2018. Newborn Metabolic Profile Associated with Hyperbilirubinemia With and Without Kernicterus. Clin Transl Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oltman SP, et al. 2018. Initial Metabolic Profiles Are Associated with 7-Day Survival among Infants Born at 22–25 Weeks of Gestation. J Pediatr 198:194–200 e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steurer MA, et al. 2018. Altered metabolites in newborns with persistent pulmonary hypertension. Pediatr Res 84:272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sylvester KG, et al. 2016. Acylcarnitine Profiles Reflect Metabolic Vulnerability for Necrotizing Enterocolitis in Newborns Born Premature. J Pediatr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feuchtbaum L, Carter J, Dowray S, Currier RJ, Lorey F 2012. Birth prevalence of disorders detectable through newborn screening by race/ethnicity. Genet Med 14:937–945. [DOI] [PubMed] [Google Scholar]
- 29.Jelliffe-Pawlowski LL, Norton ME, Baer RJ, Santos N, Rutherford GW 2016. Gestational dating by metabolic profile at birth: a California cohort study. Am J Obstet Gynecol 214:511 e511–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talge NM, Mudd LM, Sikorskii A, Basso O 2014. United States birth weight reference corrected for implausible gestational age estimates. Pediatrics 133:844–853. [DOI] [PubMed] [Google Scholar]
- 31.Kotelchuck M 1994. The Adequacy of Prenatal Care Utilization Index: its US distribution and association with low birthweight. Am J Public Health 84:1486–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kotelchuck M 1994. An evaluation of the Kessner Adequacy of Prenatal Care Index and a proposed Adequacy of Prenatal Care Utilization Index. Am J Public Health 84:1414–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin HJ, et al. 2011. Extremely high phenylalanine levels in a newborn on parenteral nutrition: phenylketonuria in the neonatal intensive care unit. J Perinatol 31:507–510. [DOI] [PubMed] [Google Scholar]
- 34.Morris M, et al. 2014. Reduction in newborn screening metabolic false-positive results following a new collection protocol. Genet Med 16:477–483. [DOI] [PubMed] [Google Scholar]
- 35.Ryckman KK, Berberich SL, Shchelochkov OA, Cook DE, Murray JC 2013. Clinical and environmental influences on metabolic biomarkers collected for newborn screening. Clin Biochem 46:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Natarajan G, Shankaran S 2016. Short- and Long-Term Outcomes of Moderate and Late Preterm Infants. Am J Perinatol 33:305–317. [DOI] [PubMed] [Google Scholar]
- 37.Patel RM 2016. Short- and Long-Term Outcomes for Extremely Preterm Infants. Am J Perinatol 33:318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baer RJ, et al. 2016. Population-based risks of mortality and preterm morbidity by gestational age and birth weight. J Perinatol 36:1008–1013. [DOI] [PubMed] [Google Scholar]
- 39.Horbar JD, et al. 2012 Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics 129:1019–1026. [DOI] [PubMed] [Google Scholar]
- 40.Donovan BM, et al. 2018. Association of newborn screening metabolites with risk of wheezing in childhood. Pediatr Res 84:619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costeloe KL, et al. 2012. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ 345:e7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jelliffe-Pawlowski LL, Norton ME, Baer RJ, Santos N, Rutherford GW 2015. Gestational dating by metabolic profile at birth: A California cohort study. Am J Obstet Gynecol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryckman KK, Berberich SL, Dagle JM 2016. Predicting gestational age using neonatal metabolic markers. Am J Obstet Gynecol 214:515 e511–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson K, et al. 2016. Accurate prediction of gestational age using newborn screening analyte data. Am J Obstet Gynecol 214:513 e511–519. [DOI] [PubMed] [Google Scholar]
- 45.Atzori L, et al. 2011. 1H NMR-based metabolomic analysis of urine from preterm and term neonates. Front Biosci (Elite Ed) 3:1005–1012. [DOI] [PubMed] [Google Scholar]
- 46.Wilson K, et al. 2014. Metabolomics of prematurity: analysis of patterns of amino acids, enzymes, and endocrine markers by categories of gestational age. Pediatr Res 75:367–373. [DOI] [PubMed] [Google Scholar]
- 47.Clark RH, Kelleher AS, Chace DH, Spitzer AR 2014. Gestational age and age at sampling influence metabolic profiles in premature infants. Pediatrics 134:e37–46. [DOI] [PubMed] [Google Scholar]
- 48.Ryckman KK, Spracklen CN, Dagle JM, Murray JC 2014. Maternal factors and complications of preterm birth associated with neonatal thyroid stimulating hormone. J Pediatr Endocrinol Metab 27:929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kantor MJ, Leef KH, Bartoshesky L, Getchell J, Paul DA 2003. Admission thyroid evaluation in very-low-birth-weight infants: association with death and severe intraventricular hemorrhage. Thyroid 13:965–969. [DOI] [PubMed] [Google Scholar]
- 50.Redding RA, Pereira C 1974. Thyroid function in respiratory distress syndrome (RDS) of the newborn. Pediatrics 54:423–428. [PubMed] [Google Scholar]
- 51.Hitchcock KR 1980. Lung development and the pulmonary surfactant system: hormonal ifluences. Anat. Rec. 198:13–34. [DOI] [PubMed] [Google Scholar]
- 52.Cuestas RA, Lindall A, Engel RR 1976. Low thyroid hormones and respiratory-distress syndrome of the newborn. Studies on cord blood. N Engl J Med 295:297–302. [DOI] [PubMed] [Google Scholar]
- 53.Panicker V 2011. Genetics of thyroid function and disease. Clin Biochem Rev 32:165–175. [PMC free article] [PubMed] [Google Scholar]
- 54.Simpson J, et al. 2005. Serum thyroid hormones in preterm infants and relationships to indices of severity of intercurrent illness. J Clin Endocrinol Metab 90:1271–1279. [DOI] [PubMed] [Google Scholar]
- 55.Thony B, Auerbach G, Blau N 2000. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J. 347 Pt 1:1–16. [PMC free article] [PubMed] [Google Scholar]
- 56.Ploder M, et al. 2008. Serum phenylalanine in patients post trauma and with sepsis correlate to neopterin concentrations. Amino Acids 35:303–307. [DOI] [PubMed] [Google Scholar]
- 57.Gitto E, Pellegrino S, D’Arrigo S, Barberi I, Reiter RJ 2009. Oxidative stress in resuscitation and in ventilation of newborns. Eur Respir J 34:1461–1469. [DOI] [PubMed] [Google Scholar]
- 58.Tataranno ML, Perrone S, Buonocore G 2015. Plasma Biomarkers of Oxidative Stress in Neonatal Brain Injury. Clin Perinatol 42:529–539. [DOI] [PubMed] [Google Scholar]
- 59.Vento M, et al. 2009. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics 124:e439–449. [DOI] [PubMed] [Google Scholar]
- 60.Becker RM, et al. 2000. Reduced serum amino acid concentrations in infants with necrotizing enterocolitis. J Pediatr 137:785–793. [DOI] [PubMed] [Google Scholar]
- 61.Pearson DL, et al. 2001. Neonatal pulmonary hypertension--urea-cycle intermediates, nitric oxide production, and carbamoyl-phosphate synthetase function. N Engl J Med 344:1832–1838. [DOI] [PubMed] [Google Scholar]
- 62.Celik IH, Demirel G, Canpolat FE, Dilmen U 2013. Reduced plasma citrulline levels in low birth weight infants with necrotizing enterocolitis. J Clin Lab Anal 27:328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hediger N, Landolt MA, Diez-Fernandez C, Huemer M, Haberle J 2018. The impact of ammonia levels and dialysis on outcome in 202 patients with neonatal onset urea cycle disorders. J Inherit Metab Dis 41:689–698. [DOI] [PubMed] [Google Scholar]
- 64.Ruder J, Legacy J, Russo G, Davis R 2014. Neonatal citrullinemia: novel, reversible neuroimaging findings correlated with ammonia level changes. Pediatr Neurol 51:553–556. [DOI] [PubMed] [Google Scholar]
- 65.Longo N, Frigeni M, Pasquali M 2016. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta. 1863:2422–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sauer SW, Okun JG, Hoffmann GF, Koelker S, Morath MA 2008. Impact of short- and medium-chain organic acids, acylcarnitines, and acyl-CoAs on mitochondrial energy metabolism. Biochim. Biophys. Acta. 1777:1276–1282. [DOI] [PubMed] [Google Scholar]
- 67.Deshmukh M, Patole S 2017. Antenatal corticosteroids for neonates born before 25 Weeks-A systematic review and meta-analysis. PLoS One 12:e0176090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Travers CP, et al. 2017. Exposure to any antenatal corticosteroids and outcomes in preterm infants by gestational age: prospective cohort study. BMJ 356:j1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Travers CP, et al. 2018. Mortality and pulmonary outcomes of extremely preterm infants exposed to antenatal corticosteroids. Am J Obstet Gynecol 218:130 e131–130 e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow chart of infants in California between 2005 and 2011 eligible for analysis