Abstract
In this study, 158 patients with different degrees of renal function were followed for 7 years to assess the prognostic value of various risk factors, including carotid intima-media thickness (cIMT) and biomarkers of renal function, for incident cardiovascular morbidity and mortality in patients with type 2 diabetes. The investigators found that estimated glomerular filtration rate, albuminuria, and history of cardiovascular disease (CVD) can be used for prognosis of CVD, whereas cIMT adds little to the accuracy of this prediction.
Type 2 diabetes, the leading cause of chronic kidney disease (CKD) and end-stage renal disease (ESRD), is characterized by a heavy cardiovascular (CV) burden. In large population-based epidemiological studies examining mortality and CV events, it has been shown that the risk of these outcomes is higher in patients with a combination of the two conditions (type 2 diabetes and CKD) compared with individuals with CKD but without diabetes and those with diabetes with normal renal function (1,2). Moreover, in the dialysis population, compared with people without diabetes, those with diabetes have a 1.6 times excess risk for CV mortality (3).
During the past few decades, novel risk factors have emerged, including factors triggered by the uremic or hyperglycemic environment such as anemia, hypoalbuminemia, albuminuria and reduction of renal function (4), oxidative stress (5), and inflammation (6). Based on the belief that the presence of calcium in any arterial wall of the human body is associated with a nearly fourfold increase in CV morbidity and mortality, it was considered that the presence of atherosclerosis in the carotid artery reflects general atherosclerosis (7). Carotid intima-media thickness (cIMT) has been proposed as a surrogate marker of subclinical atherosclerosis and a strong, independent predictor of CV events in patients with type 2 diabetes or predialysis CKD and those on dialysis (8).
Recently, scientific prognostic research has focused on developing risk-predictive models, especially in high-risk populations, such as people with diabetes or CKD. These prognostic models could provide an individualized risk prediction and stratification and thus improve patients’ outcomes and decision-making in clinical practice. An ideal risk model should be simple, quick, easy to assess, and accurate. Herein, we assessed the prognostic value of various risk factors, including cIMT and biomarkers of kidney function, to predict incident fatal and nonfatal CV events in a high-risk population such as patients with documented type 2 diabetes.
Materials and Methods
Study Population
The study was approved by the Ethics Committee of the Scientific Council of the University General Hospital of Alexandroupolis in Greece and was in accordance with the Helsinki Declaration of Human Rights. Informed consent was obtained from each patient.
A total of 158 adults, followed in the diabetic nephropathy clinic of the University General Hospital of Alexandroupolis were recruited in the study, including 30 patients in CKD stage 5D (patients with ESRD undergoing maintenance hemodialysis). All participants had a history of type 2 diabetes for at least 10 years. Exclusion criteria included urinary tract disease and acute illness.
At the first visit (baseline), anthropometric and clinical data, background clinical information regarding the history of CV events, blood and urine sampling, and measurement of cIMT were recorded. The definition of a CV event included a history of heart failure, coronary heart disease, angina, stroke, or peripheral artery disease. At baseline, estimated glomerular filtration rate (eGFR) was estimated by the CKD Epidemiology Collaboration (CKD-EPI) Equation (9), and every participant was classified in CKD stages according to the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (10).
All patients were followed for a period of 7 years (2008–2015) with fatal or nonfatal CV events as the primary outcome. Mortality from causes other than cardiovascular disease (CVD) was considered a competitive event. Follow-up data were obtained for the study population via death certificates, hospital medical records, regular follow-up visits, or an integrated telephone interview.
Laboratory Analyses
Blood was drawn from all participants in the fasting state to obtain whole blood, plasma, and serum, and the samples were immediately transferred to the laboratory for assay. Blood samples were collected from subjects with CKD stage 5D immediately before the start of a midweek dialysis session. Hemoglobin, serum albumin, creatinine, A1C, C-reactive protein (CRP), total cholesterol, LDL cholesterol, and HDL cholesterol were immediately assessed, as described elsewhere (11,12). For oxidized LDL, blood samples were immediately centrifuged, and plasma was stored at −20°C until analysis. Plasma levels of oxidized LDL were assessed by enzyme-linked immunosorbent assay (ELISA), according to the manufacturer’s instructions (human oxidized LDL ELISA kit, Mercodia, Sweden), as described before (12). Detection limits for oxidized LDL were 0.3 units/L, and intra-/interassay coefficients of variation were <10%, as described by the manufacturers. The presence of proteinuria (urine protein-to-creatinine ratio [UPCR]) and albuminuria (urine albumin-to-creatinine ratio [UACR]) were evaluated in a morning spot urine sample, as described elsewhere (13).
Measurement of cIMT
cIMT was determined in all patients by a single trained examiner using high-resolution, B-mode ultrasonography with a 7.5-MHz transducer (ATL Ultrasound HDI 1300; Phillips, Bothell, WA). cIMT was considered as the distance between the leading edge of the luminal-intimal interface and the leading edge of the media-adventitia interface (14). Three measurements were obtained for each of the common carotid arteries in three sites: at distances of 0.5, 1, and 1.5 cm from the carotid bulb. Mean cIMT was calculated as the mean of 18 values (nine for each carotid artery).
Statistical Analyses
Data were tested for normality by the Kolmogorov-Smirnov test. Normally distributed variables are presented as mean ± SD, nonnormally distributed variables are presented as median (interquartile range), and binary data are presented as percentage frequency. Subject characteristics were compared among groups according to previous history of CV events at baseline using a χ2 test for categorical variables and independent t tests and Mann-Whitney U tests for normally and nonnormally distributed variables, respectively, as appropriate.
By adopting the Fine and Gray model, which takes into account the competitive risk of death (15), we assessed the associations between fatal and nonfatal CV events and a series of candidate prognostic factors collected at baseline. By univariate survival analyses, we tested various risk factors, including age, sex, history of CV events, smoking, duration of type 2 diabetes, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), A1C, total/LDL/HDL cholesterol, triglycerides, eGFR, UACR, UPCR, albumin, hemoglobin, CRP, oxidized LDL, and a surrogate marker of subclinical atherosclerosis (cIMT). In multiple Cox Fine and Gray models, we included all variables that were correlated with the study outcome with P ≤0.05 in univariate analysis, which included sex, history of CV events, duration of type 2 diabetes, HDL cholesterol, eGFR, UACR, albumin, hemoglobin, and cIMT (full model). With both UPCR and UACR associated with the study outcome, we chose to use UACR in our analyses because it is more specific for glomerular disease.
The prognostic performance of the full model (including nine variables) was compared with that of a simpler (nested) model based only on three prognostic variables: history of CV events, eGFR, and UACR (simplified model). Because simulation studies indicate that the minimum observed interval coverage (91 vs. 91%) and maximum type I error rates (8.8 vs. 8.0%) were similar for 5–9 and >10 events per predictor variable in a Cox analysis, we introduced into the full model one prognostic factor for every eight patients with the event of interest, thus building a model of adequate statistical power (16). In Fine and Gray models, data were expressed as SEs, subhazard ratios (SHRs), 95% CIs, and P values.
To compare the data fitting of the full versus the simplified model, we used −2 log likelihood (−2 log L) statistics. This procedure compares two different models fitted to the same set of survival data, and the smaller the −2 log L value, the better the agreement between the model and the observed data. A difference in the −2 log L between two nested models that differ in “n” covariates has a χ2 distribution with n−1 df.
The calibration and the discrimination abilities of both full and simplified models were assessed by Hosmer-Lemeshow test and by receiver operating characteristic (ROC) curve, respectively. Briefly, calibration measures how much the outcome probability estimated by a predictive model matches the “real” probability of the same outcome. In calibration analysis, predicted and observed outcome probabilities are compared by the Hosmer-Lemeshow test. When not significant, this test provides statistical evidence that predicted and observed outcome probabilities do not differ, implying that the model is calibrated. The area under the ROC curve represents the proportion of all possible pairs of patients in which the risk of study outcome as estimated by the model agrees with the observed outcome. The concept underlying this index is that, under the assumption of random sampling, the predicted probabilities in patients who experience a given outcome should be systematically higher than those in patients who did not.
To assess the robustness of our results, a sensitivity analysis was conducted by comparing the prognostic accuracy of the full and simplified models by excluding patients with stage 5D CKD.
Statistical analyses were performed using two standard statistical packages (Stata 13.0 for Windows, College Station, TX, and IBM SPSS, v. 18.0, for Windows, Chicago, IL).
Results
Baseline Characteristics
Baseline anthropometric, clinical, and biochemical data in the whole group of patients with type 2 diabetes and separately according to background CVD are presented in Table 1. Patients with history of a CV event were more likely to be male, with a longer duration of type 2 diabetes, lower serum HDL cholesterol levels, and markedly higher cIMT values. Although glycemic control (assessed by serum A1C) was more impaired in the group with a previous CV event, this association was of marginal statistical significance (P = 0.052). No difference was found between groups for all other variables, including biomarkers of diabetic CKD (i.e., eGFR, UPCR, and UACR).
TABLE 1.
Baseline Anthropometric, Clinical, and Biochemical Data of Patients With Type 2 Diabetes According to Baseline CVD Status
| All Subjects(n = 158) | Subjects With No CV Events(n = 57) | Subjects With CV Events(n = 101) | P | |
|---|---|---|---|---|
| Age, years | 69 (45–90) | 66 (45–90) | 70 (47–86) | 0.24 |
| Female sex, % | 47.5 | 59.6 | 40.6 | 0.016 |
| Smoking habit, % | 19.6 | 15.8 | 21.8 | 0.24 |
| Diabetes duration, years | 14 (2–40) | 13 (2–25) | 15 (4–40) | 0.045 |
| BMI, kg/m2 | 30.7 (5.1) | 30.6 (5.0) | 30.8 (5.2) | 0.77 |
| SBP, mmHg | 137.4 (91–213) | 140 (98–213) | 135 (91–180) | 0.20 |
| DBP, mmHg | 80 (50–120) | 80 (60–120) | 80 (50–99) | 0.55 |
| Hemoglobin, g/dL | 12.4 (7.3–16.7) | 12.4 (9.3–15.9) | 12.4 (7.3–16.7) | 0.90 |
| A1C, % | 7.4 (5.0–11.6) | 7.2 (5.0–10.0) | 7.5 (5.6–11.6) | 0.052 |
| Albumin, g/dL | 4.2 (2.8–5.0) | 4.2 (2.9–4.9) | 4.1 (2.8–5.0) | 0.24 |
| CRP, mg/dL | 0.24 (0–14) | 0.20 (0–4.0) | 0.33 (0–14.0) | 0.12 |
| Total cholesterol, mg/dL | 168.5 (69–416) | 166 (100–416) | 169 (69–345) | 0.53 |
| LDL cholesterol, mg/dL | 93 (27–325) | 94 (30–325) | 92 (27–245) | 0.76 |
| HDL cholesterol, mg/dL | 45.0 (12.4) | 47.6 (11.3) | 43.5 (12.8) | 0.042 |
| Triglycerides, mg/dL | 142.5 (27–966) | 140 (27–551) | 147 (52–966) | 0.14 |
| eGFR, mL/min/1.73 m2 | 41.3 (4–106.3) | 57.3 (4.0–106.3) | 38.4 (4.0–106.2) | 0.14 |
| UPCR, g/g | 0.39 (0.01–9.7) | 0.25 (0.01–9.7) | 0.48 (0.01–7.0) | 0.50 |
| UACR, mg/g | 0.08 (0.001–65) | 0.04 (0.001–65) | 0.13 (0.02–70) | 0.16 |
| Oxidized LDL, units/L | 61.06 (17.9–123.4) | 61.06 (17.9–103.8) | 61.06 (22–123.4) | 0.23 |
| cIMT, mm | 0.86 (0.40–1.78) | 0.78 (0.40–1.78) | 0.90 (0.55–1.76) | <0.0001 |
Results for continuous variables are presented as mean (SD) or median (range). P values are from t test or Mann-Whitney U test for differences of variables and χ2 test for differences in frequencies among groups according to CVD status. Bold type indicates statistical significance.
Outcomes
During the follow-up period (median 57.5 months, range 7–84 months, total patient-time 6,263 months), 75 patients experienced CV events (33 fatal, 42 nonfatal), and 13 died of causes other than CV. In univariate competitive risk analysis, hemoglobin (SHR 0.85, 95% CI 0.74–0.98, P = 0.02), female sex (0.50, 0.30–0.82, P = 0.006), eGFR (0.98, 0.97–0.99, P <0.0001), serum albumin (0.43, 0.26–0.74, P = 0.002), and HDL cholesterol (0.97, 0.95–0.99, P = 0.02) were inversely associated with the incident rate of CV events, whereas the duration of type 2 diabetes (SHR 1.03, 95% CI 1.00–1.06, P = 0.04), background CVD (5.47, 2.45–12.23, P <0.0001), UPCR (1.36, 1.22–1.51, P <0.0001), UACR (1.01, 1.00–1.01, P <0.0001), and cIMT (7.45, 3.46–16.04, P <0.0001) were directly related to the same end point (Supplementary Table S1). In a multivariate Fine and Gray model including all univariate correlates of fatal and nonfatal CV events (full model), only history of CVD (SHR 5.86, 95% CI 2.15–13.36, P <0.0001), eGFR (0.99, 0.98–0.99, P = 0.009), UACR (1.01, 1.00–1.01, P <0.0001), and cIMT (3.92, 1.24–12.35, P = 0.02) remained significantly associated to the study outcome. In the simplified model (see Statistical Analysis), history of CVD (SHR 6.47, 95% CI 2.69–15.55, P <0.0001), UACR (1.01, 1.00–1.01, P <0.0001), and eGFR (0.98, 0.97–0.99, P <0.0001) (Table 2) all maintained an independent relationship with CV outcomes.
TABLE 2.
Multiple Cox Proportional Analysis (Fine-Gray SHR Model) Showing Predictors for Fatal or Nonfatal CV Events in Multivariate Models in Patient With Type 2 Diabetes
| Variables | SHR | 95% CI | P |
|---|---|---|---|
| Full model (nine variables) | |||
| Hemoglobin, g/dL | 0.93 | 0.79–1.09 | 0.37 |
| eGFR, mL/min/1.73 m2 | 0.99 | 0.98–0.99 | 0.009 |
| Female sex | 0.63 | 0.38–1.06 | 0.08 |
| Diabetes duration, years | 0.98 | 0.95–1.02 | 0.51 |
| Positive history of CV events | 5.86 | 2.15–13.36 | <0.0001 |
| Albumin, g/dL | 0.92 | 0.44–1.91 | 0.82 |
| HDL cholesterol, mg/dL | 1.00 | 0.98–1.02 | 0.98 |
| UACR, mg/g | 1.01 | 1.00–1.01 | <0.0001 |
| cIMT, mm | 3.92 | 1.24–12.35 | 0.02 |
| Simplified model (three variables) | |||
| eGFR, mL/min/1.73 m2 | 0.98 | 0.97–0.99 | <0.0001 |
| Positive history of CV events | 6.47 | 2.69–15.55 | <0.0001 |
| UACR, mg/g | 1.01 | 1.00–1.01 | <0.0001 |
Bold type indicates statistical significance.
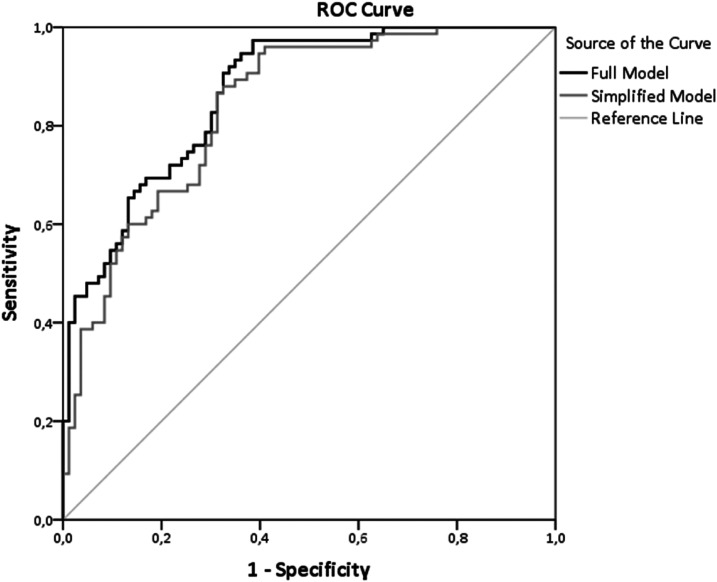
The comparison of the full (including nine variables) versus the simplified (including only three variables) model by −2 log L statistics showed that the data fitting of the two models did not significantly differ (χ2 = 9.48, 6 df, P = 0.15). Furthermore, the assessment of the areas under the ROC curves (Figure 1) confirmed that the two models had almost identical accuracy to predict the study outcome (full model 87%, 95% CI 0.81–0.92, P <0.001; simplified model 84%, 95% CI 0.78–0.90, P <0.001). Moreover, the Hosmer-Lemeshow test showed that the simplified model was better calibrated than the full model, because the P value of this test in the simplified model is farther from statistical significance (χ2 = 9.24, P = 0.32) than that of the full model (χ2 = 11.09, P = 0.20).
FIGURE 1.
ROC curves showing the performance of the full model and the simplified model in predicting CV events in patients with type 2 diabetes.
The sensitivity analysis excluding patients in stage 5D CKD (n = 30) confirmed that the full and simplified models did not significantly differ (χ2 = 11.8, 6 df, P = 0.07). The areas under the ROC curves were quite similar (full model 83%, 95% CI 0.76–0.09, P <0.001; simplified model 79%, 95% CI 0.72–0.87, P <0.001), and, once again, calibration of the simplified model (χ2 = 4.97, P = 0.76) was better than that of the full model (χ2 = 5.85, P = 0.60).
Discussion
In this study, we found that, among various risk factors, biomarkers of kidney function (eGFR and urinary albumin) and background CVD have an adequate prognostic value to predict CV mortality and morbidity in patients with documented type 2 diabetes. Patients with diabetes and CKD are considered one of the highest risk groups for CV events. In our study, oxidative stress, inflammation, and Framingham risk score (FRS) risk factors failed to show any predictive value.
Kidney disease progression measures such as eGFR and albuminuria—independent of each other—have been repeatedly highlighted as strong predictors of CV mortality and morbidity in the general population, people with diabetes, and those with both early and advanced CKD (17–19). Reduction of eGFR via various patterns (slow or abrupt) has been shown to independently predict early death in people with ESRD and predialysis CKD (20). Moreover, albuminuria confers a high CV risk on people with type 2 diabetes via three pathways. First, it is an independent predictor of deterioration of renal function; second, it has been associated with progression of prediabetes to type 2 diabetes (21); and, finally, it predicts CVD independently of diabetes and eGFR.
The risk model proposed in this study contains only three simple variables (albuminuria, eGFR, and previous CVD). Because it requires a very short interview, a blood sample for serum creatinine, and a urine spot test for UΑCR, it is simple, quick, and inexpensive.
The performance of a risk prediction model is evaluated by assessment of its discrimination and calibration (22). Discrimination measures the ability of a prognostic model to distinguish (discriminate) individuals who develop the outcome (event) of interest from those who do not (23), whereas calibration measures the agreement between real (observed) and predicted event rates (24). In our study the simplified three-variable model had a high discriminative power (area under the ROC curve: 84%), which was almost identical to that of the full nine-variable model (area under the ROC curve: 87%) and was better calibrated. These results remained almost unchanged in a sensitivity analysis excluding patients with stage 5D CKD. Unfortunately, in most studies, the issue of competitive risk is overlooked, and calibration values are usually omitted, leading to poorly validated models (25).
cIMT is considered a noninvasive surrogate marker for atherosclerosis. Its measurement is time-consuming and requires a B-mode sonograph and a trained technician or physician. Moreover, to eliminate examiner bias in a specific cohort of patients, it is preferable that all measurements should be done by a single examiner, which is difficult to schedule in everyday clinical practice.
The clinical utility of cIMT for CV risk prognosis remains controversial. Although the American Heart Association (AHA) and the American College of Cardiology (ACC) produced guidelines recommending evaluation of cIMT for CV risk assessment in asymptomatic patients at intermediate risk or having other CVD risk factors (26), 3 years later, the AHA and ACC published new guidelines that discouraged the use of cIMT for CV risk prediction in everyday clinical practice (27). Moreover, the data regarding the association between carotid atherosclerosis and CVD in the special population of people having CKD and diabetes remain controversial. In this population, some studies showed that cIMT was a predictor of CV morbidity (28,29), but other investigators failed to show any predictive value of carotid atherosclerosis (30,31). Despite the initial enthusiasm regarding the clinical predictive value of cIMT, a meta-analysis of 14 population-based studies and 45,828 subjects showed that the addition of cIMT to the FRS conferred a slight, clinically insignificant improvement in the 10-year prediction of stroke or myocardial infraction (similar C statistic for both models and 3.6% reclassification improvement in the cIMT + FRS model) (32). Similar results in patients with type 2 diabetes were published by den Ruijter et al. (33); the FRS and the expanded model with cIMT had similar predictive value for 10-year development of CV events, as assessed by similar C statistic and no net reclassification improvement among groups. Therefore, although cIMT remains a fundamental treatment target in patients with type 2 diabetes, its measurement solely for risk stratification is unwarranted.
Tangri et al. (25) performed a systematic review to identify risk prognostic models for CKD patients and found three studies (six models) involving CV events (34–36). The first study aimed to compare the predictive value of traditional versus novel risk factors using data from the community-based Cardiovascular Health Study cohort (1,249 subjects ≥65 years of age with CKD defined as eGFR <60 mL/min/1.73 m2) followed for an average period of 8.6 years (34). The authors found that FRS risk factors such as diabetes, SBP, physical inactivity, smoking, and use of alcohol were stronger predictors than novel factors such as CRP, lipoprotein, interleukin, and fibrinogen. However, the authors did not include in their study eGFR, albuminuria, and history of CVD. Moreover, their model had a modest discriminative power (0.73), whereas no calibration and data-fitting assessments were reported. Therefore, Tangri et al. (25) concluded that this proposed model had no clinical utility or usability. The second study, by Weiner et al. (35), reported that the FRS underpredicted CV events in a population with CKD (934 patients aged 45–74 years), with poor discriminatory power (0.60) and calibration. The third study was an analysis of TREAT (Trial to Reduce CV Events with Aranesp Therapy), aiming to establish predictors of fatal and nonfatal CV events in a cohort of 3,847 patients with type 2 diabetes, anemia, and CKD (stages 2–4) (36). In agreement with our results, the authors found that history of heart failure, coronary heart disease, arrythmia, serum albumin, and proteinuria (assessed by UPCR) were strong independent factors for their end point. Using these variables, the authors formed a risk model with modest discriminatory power (0.72), although no calibration or data-fitting assessments were reported. Therefore, Tangri et al. (25) suggested that this model might not be clinically useful. None of the three studies was designed to focus on creating clinical risk prognostic models, and none included an easy-to-use model that could aid everyday clinical decision-making. The authors of the systematic review (25) concluded that a quick and accurate bedside risk model should be developed for this high-risk population.
The clinical value of albuminuria, eGFR, and history of CVD as predictors of CV events has been highlighted in other risk prediction models. In agreement with our results, Bansal et al. (37) developed a prediction model for 5-year risk for all-cause mortality in 789 older adults with predialysis CKD (stages 3–5). They found that, in univariate Cox models with all tested variables, type 2 diabetes, UACR, history of CV events, sex, and eGFR were the strongest predictors of death. The model also included age, race, and smoking and had an acceptable discrimination and calibration. Moreover, one of the major advantages of this risk model was that it included variables that were easy to measure. In another 16-variable risk model developed to predict mortality in 4,054 elderly (aged 65–80 years) patients with moderate to severe CKD (eGFR <30 mL/min/1.73 m2), sex, type 2 diabetes, history of CVD, and eGFR were reported as independent risk factors (38).
Grams et al. (39) enrolled 1,798 patients with advanced CKD (eGFR <30 mL/min/1.73 m2) and found that age, sex, race, eGFR, albuminuria, type 2 diabetes, BMI, smoking, background CVD, SBP, and ejection fraction were independent predictors of CV event occurrence over a follow-up period of 5.5 years.
Similarly, the predictive value of eGFR and proteinuria was evaluated in an observational, retrospective study including 447 patients with diabetes and advanced CKD (40). The authors reported that, among all tested variables, eGFR and proteinuria were the strongest predictors of CV events, whereas the effect of age, SBP, and A1C were minimal. The authors proposed a risk model for mortality/progression to ESRD and CVD based on these variables.
Schlackow et al. (41) developed a risk prognostic model for CV events in patients with moderate-to-severe CKD (stages 3–5) using the 5-year follow-up data of the 9,270 patients in SHARP (Study of Heart and Renal Protection). Similar to our findings, the authors reported that previous CVD and advanced CKD were the main contributors to increased individual risk for future CV events. Moreover, this was the first prognostic study to include duration of CKD as a risk factor. All variables of our full model were included in their risk models except cIMT, which was not measured.
A very recent article reported on a risk model for CV events using data from 264,296 patients with severe CKD (stages 4–5) from 30 countries participating in the CKD Prognosis Consortium (42). The authors included in their model similar variables to those in our study: sex, UACR, type 2 diabetes, history of CVD, eGFR, age, race, smoking, and SBP. Moreover, they identified eGFR and albuminuria as the factors that could dramatically affect the probability of adverse outcomes in this cohort.
It has been reported that eGFR and albuminuria promote atherosclerosis and the development of CVD through entirely different mechanisms. Population-based studies in large cohorts of people with type 2 diabetes suggest that reduced eGFR and increased UACR are strong, independent risk factors for both renal and CVD outcomes (43,44). Given that, in our study, we found that eGFR and albuminuria were associated with CV outcomes independently of each other, we hypothesize that these two factors might increase CV risk by partially different pathogenetic pathways.
It is believed that low eGFR might represent local and systemic atherosclerosis (45,46), and high albuminuria may indicate endothelial dysfunction (47,48). Indeed, it has been suggested that endothelial dysfunction, inflammation, and increased transvascular leakage of albumin might be the pathophysiologic processes underlying the strong association between albuminuria and CVD (47,49). On the other hand, it has been suggested that decline in eGFR might represent duration of exposure to FRS risk factors, whereas others suggest that the uremic environment triggers development and progression of inflammation, oxidative stress, abnormal calcium/phosphorus metabolism, and secondary hyperparathyroidism (50).
To the best of our knowledge, this is the first study aiming to develop a risk model for prediction of CV events in a cohort of patients with long-term type 2 diabetes and different degrees of renal function. Moreover, it is the first study in this population to compare risk models with and without cIMT, a surrogate marker for atherosclerosis with controversial clinical utility. However, our study had certain limitations. First, the observational design cannot establish causality for our findings. However, the demographic characteristics of our cohort and the outcomes were consistent with published data in large populations. Second, our cohort was composed of White only; therefore, race could not be examined, and our results were based on a single cohort. For generalization of our findings, further studies are needed in multiple cohorts that include patients of various races.
Conclusion
Based on this study, we propose a simple model for CV event prediction that includes only three easy-to-measure variables. eGFR, albuminuria, and history of CVD can be used for prognosis of CVD, whereas cIMT adds little to the accuracy of this prediction.
Article Information
Duality of Interest
No potential conflicts of interest related to this article were reported.
Author Contributions
S.R. searched the literature, wrote the first draft of the manuscript, recruited patients, assessed patients’ files, and collected data. V.L. and S.P. designed the study and reviewed/edited the manuscript. A.R. recruited patients and collected and assessed data. A.S. performed all laboratory analyses and collected and assessed data. G.D and G.T. performed the statistical analysis and reviewed the final version of the manuscript. S.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.13519793.
References
- 1.Collins AJ, Li S, Gilbertson DT, Liu J, Chen S-C, Herzog CA. Chronic kidney disease and cardiovascular disease in the Medicare population: management of comorbidities in kidney disease in the 21st century: anemia and bone disease. Kidney Int 2003;64(Suppl. 87):S24–S31 [DOI] [PubMed] [Google Scholar]
- 2.Tonelli M, Muntner P, Lloyd A, et al.; Alberta Kidney Disease Network . Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet 2012;380:807–814 [DOI] [PubMed] [Google Scholar]
- 3.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998;32(Suppl. 3):S112–S119 [DOI] [PubMed] [Google Scholar]
- 4.Zoccali C. Traditional and emerging cardiovascular and renal risk factors: an epidemiologic perspective. Kidney Int 2006;70:26–33 [DOI] [PubMed] [Google Scholar]
- 5.Liakopoulos V, Roumeliotis S, Gorny X, Dounousi E, Mertens PR. Oxidative stress in hemodialysis patients: a review of the literature. Oxid Med Cell Longev 2017;2017:3081856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spoto B, Mattace-Raso F, Sijbrands E, et al. Association of IL-6 and a functional polymorphism in the IL-6 gene with cardiovascular events in patients with CKD. Clin J Am Soc Nephrol 2015;10:232–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag 2009;5:185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palanca A, Castelblanco E, Betriu À, et al. Subclinical atherosclerosis burden predicts cardiovascular events in individuals with diabetes and chronic kidney disease. Cardiovasc Diabetol 2019;18:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey AS, Coresh J, Balk E, et al.; National Kidney Foundation . National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137–147 [DOI] [PubMed] [Google Scholar]
- 11.Roumeliotis S, Roumeliotis A, Panagoutsos S, et al. Matrix Gla protein T-138C polymorphism is associated with carotid intima media thickness and predicts mortality in patients with diabetic nephropathy. J Diabetes Complications 2017;31:1527–1532 [DOI] [PubMed] [Google Scholar]
- 12.Tavridou A, Georgoulidou A, Roumeliotis A, et al. Association of plasma adiponectin and oxidized low-density lipoprotein with carotid intima-media thickness in diabetic nephropathy. J Diabetes Res 2015;2015:507265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Eckardt K-U, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005;67:2089–2100 [DOI] [PubMed] [Google Scholar]
- 14.Naqvi TZ, Lee M-S. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging 2014;7:1025–1038 [DOI] [PubMed] [Google Scholar]
- 15.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509 [Google Scholar]
- 16.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007;165:710–718 [DOI] [PubMed] [Google Scholar]
- 17.Nitsch D, Grams M, Sang Y, et al.; Chronic Kidney Disease Prognosis Consortium . Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ 2013;346:f324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Velde M, Matsushita K, Coresh J, et al.; Chronic Kidney Disease Prognosis Consortium . Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality: a collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011;79:1341–1352 [DOI] [PubMed] [Google Scholar]
- 19.Mahmoodi BK, Matsushita K, Woodward M, et al.; Chronic Kidney Disease Prognosis Consortium . Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet 2012;380:1649–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu RK, Chai B, Roy JA, et al.; CRIC Study Investigators . Abrupt decline in kidney function before initiating hemodialysis and all-cause mortality: the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 2016;68:193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neves JS, Correa S, Baeta Baptista R, Bigotte Vieira M, Waikar SS, Mc Causland FR. Association of prediabetes with CKD progression and adverse cardiovascular outcomes: an analysis of the CRIC study. J Clin Endocrinol Metab 2020;105:e1772–e1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tripepi G, Heinze G, Jager KJ, Stel VS, Dekker FW, Zoccali C. Risk prediction models. Nephrol Dial Transplant 2013;28:1975–1980 [DOI] [PubMed] [Google Scholar]
- 23.Tripepi G, Jager KJ, Dekker FW, Zoccali C. Statistical methods for the assessment of prognostic biomarkers (part I): discrimination. Nephrol Dial Transplant 2010;25:1399–1401 [DOI] [PubMed] [Google Scholar]
- 24.Tripepi G, Jager KJ, Dekker FW, Zoccali C. Statistical methods for the assessment of prognostic biomarkers (part II): calibration and re-classification. Nephrol Dial Transplant 2010;25:1402–1405 [DOI] [PubMed] [Google Scholar]
- 25.Tangri N, Kitsios GD, Inker LA, et al. Risk prediction models for patients with chronic kidney disease: a systematic review. Ann Intern Med 2013;158:596–603 [DOI] [PubMed] [Google Scholar]
- 26.Greenland P, Alpert JS, Beller GA, et al.; American College of Cardiology Foundation; American Heart Association . 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010;56:e50–e103 [DOI] [PubMed] [Google Scholar]
- 27.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2935–2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szeto CC, Chow KM, Woo KS, et al. Carotid intima media thickness predicts cardiovascular diseases in Chinese predialysis patients with chronic kidney disease. J Am Soc Nephrol 2007;18:1966–1972 [DOI] [PubMed] [Google Scholar]
- 29.Roumeliotis A, Roumeliotis S, Panagoutsos S, et al. Carotid intima-media thickness is an independent predictor of all-cause mortality and cardiovascular morbidity in patients with diabetes mellitus type 2 and chronic kidney disease. Ren Fail 2019;41:131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcos AG, Watanabe R, Lemos MM, Canziani MEF. Evaluation of intima-media thickness in patients with chronic kidney disease not on dialysis: a prospective study of 24 months. J Bras Nefrol 2014;36:35–41 [in Portuguese] [DOI] [PubMed] [Google Scholar]
- 31.Zoungas S, Cameron JD, Kerr PG, et al. Association of carotid intima-medial thickness and indices of arterial stiffness with cardiovascular disease outcomes in CKD. Am J Kidney Dis 2007;50:622–630 [DOI] [PubMed] [Google Scholar]
- 32.den Ruijter HM, Peters SA, Anderson TJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA 2012;308:796–803 [DOI] [PubMed] [Google Scholar]
- 33.den Ruijter HM, Peters SA, Groenewegen KA, et al. Common carotid intima-media thickness does not add to Framingham risk score in individuals with diabetes mellitus: the USE-IMT initiative. Diabetologia 2013;56:1494–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA 2005;293:1737–1745 [DOI] [PubMed] [Google Scholar]
- 35.Weiner DE, Tighiouart H, Elsayed EF, et al. The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol 2007;50:217–224 [DOI] [PubMed] [Google Scholar]
- 36.McMurray JJ, Uno H, Jarolim P, et al. Predictors of fatal and nonfatal cardiovascular events in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia: an analysis of the Trial to Reduce cardiovascular Events with Aranesp (darbepoetin-alfa) Therapy (TREAT). Am Heart J 2011;162:748–755.e3 [DOI] [PubMed] [Google Scholar]
- 37.Bansal N, Katz R, De Boer IH, et al. Development and validation of a model to predict 5-year risk of death without ESRD among older adults with CKD. Clin J Am Soc Nephrol 2015;10:363–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss JW, Platt RW, Thorp ML, et al. Predicting mortality in older adults with kidney disease: a pragmatic prediction model. J Am Geriatr Soc 2015;63:508–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grams ME, Yang W, Rebholz CM, et al.; CRIC Study Investigators . Risks of adverse events in advanced CKD: the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 2017;70:337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen PM, Wada T, Chiang CK. Prognostic value of proteinuria and glomerular filtration rate on Taiwanese patients with diabetes mellitus and advanced chronic kidney disease: a single center experience. Clin Exp Nephrol 2017;21:307–315 [DOI] [PubMed] [Google Scholar]
- 41.Schlackow I, Kent S, Herrington W, et al.; SHARP Collaborative Group . A policy model of cardiovascular disease in moderate-to-advanced chronic kidney disease. Heart 2017;103:1880–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grams ME, Sang Y, Ballew SH, et al. Predicting timing of clinical outcomes in patients with chronic kidney disease and severely decreased glomerular filtration rate. Kidney Int 2018;93:1442–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minutolo R, Gabbai FB, Provenzano M, et al. Cardiorenal prognosis by residual proteinuria level in diabetic chronic kidney disease: pooled analysis of four cohort studies. Nephrol Dial Transplant 2018;33:1942–1949 [DOI] [PubMed] [Google Scholar]
- 44.Ninomiya T, Perkovic V, de Galan BE, et al.; ADVANCE Collaborative Group . Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 2009;20:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bigazzi R, Bianchi S, Nenci R, Baldari D, Baldari G, Campese VM. Increased thickness of the carotid artery in patients with essential hypertension and microalbuminuria. J Hum Hypertens 1995;9:827–833 [PubMed] [Google Scholar]
- 46.de Zeeuw D, Parving H-H, Henning RH. Microalbuminuria as an early marker for cardiovascular disease. J Am Soc Nephrol 2006;17:2100–2105 [DOI] [PubMed] [Google Scholar]
- 47.Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol 2006;17:2106–2111 [DOI] [PubMed] [Google Scholar]
- 48.Jefferson JA, Shankland SJ, Pichler RH. Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int 2008;74:22–36 [DOI] [PubMed] [Google Scholar]
- 49.Roumeliotis S, Mallamaci F, Zoccali C. Endothelial dysfunction in chronic kidney disease, from biology to clinical outcomes: a 2020 update. J Clin Med 2020;9:2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macisaac RJ, Ekinci EI, Jerums G. Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis 2014;63(Suppl. 2):S39–S62 [DOI] [PubMed] [Google Scholar]