Case Presentation
C.R., a 59-year-old White man presented to the emergency room with fever, cough, shortness of breath, and diaphoresis for 4 days. His medical history was significant for hypertension treated with bisoprolol-hydrochlorothiazide 10 mg/6.25 mg daily and type 2 diabetes treated with metformin 1,000 mg nightly. He reported recent travel to Florida for 1 month before returning to Colorado 1 week before admission. His vital signs on admission (day 1), which took place 7 days after social distancing was instituted statewide, included blood pressure of 135/73 mmHg, pulse of 98 bpm, BMI of 38.46 kg/m2 (weight 126 kg, height 181 cm), oxygen saturation of 65% on room air, 76% on 3 L/min high-flow nasal cannula, and 85% on 15 L/min high-flow nasal cannula. His presenting blood glucose was 324 mg/dL, with an A1C of 9% on admission.
C.R. was admitted to the intensive care unit (ICU) after intubation. On hospital day 3, continuous renal replacement therapy (CRRT) was initiated because of worsening acute kidney injury (glomerular filtration rate on admission was >60 mL/min/1.73 m2 and decreased to a nadir of 14 mL/min/1.73 m2). He was also placed on veno-venous extracorporeal membrane oxygenation (VV ECMO) for worsening hypoxia despite proning, high positive end-expiratory pressure, and 100% fraction of inspired oxygen. C.R. remained on VV ECMO from day 3 to day 11 and on CRRT from day 3 to day 23. He intermittently required pressor therapy after discontinuation of ECMO from day 12 to day 19. In addition, nutritional therapy with enteral nutrition (EN), parenteral nutrition (PN), or a combination was started on day 3. There was an interruption of EN between day 9 and day 10 for ∼24 hours due to high volume residuals. C.R. did not receive corticosteroid therapy during his hospital stay. He was extubated successfully on day 22 and transferred to the floor on day 26.
C.R.’s insulin needs varied substantially during his ICU stay (Figure 1). He was placed on insulin via intravenous (IV) infusion at admission and continued this therapy through day 9. That day, biphasic human insulin 70/30 was added. From day 1 to day 9, C.R.’s total daily dose (TDD) of insulin ranged from 0.74 to 2.51 units/kg. On days 10 and 11, he did not require any insulin to maintain glycemic control. Insulin lispro correctional doses were started on day 12 when PN and trickle EN were started. By day 13, C.R.’s blood glucose started to rise to >250 mg/dL, so IV insulin infusion was reinitiated. His insulin infusion rate rose steadily to a maximum of 16 units/hour by day 15. At this time, biphasic human insulin 70/30 was restarted with the goal of reducing the insulin infusion rate and eventually discontinuing the insulin infusion to minimize staff exposure. Between days 15 and 18, C.R. had his highest insulin needs, with his TDD ranging from 338 to 353.6 units (2.7–2.8 units/kg) coinciding with a peak in his white blood cell (WBC) count (22.3–28.2 10*9/L). His blood glucose values stayed within the range of 120–180 mg/dL during most of his ICU stay. Occasional readings >180 mg/dL were associated with changes in nutritional therapy and were corrected with insulin adjustments. C.R. did not experience any hypoglycemia with close glucose monitoring and insulin adjustments while his severe insulin resistance improved.
FIGURE 1.
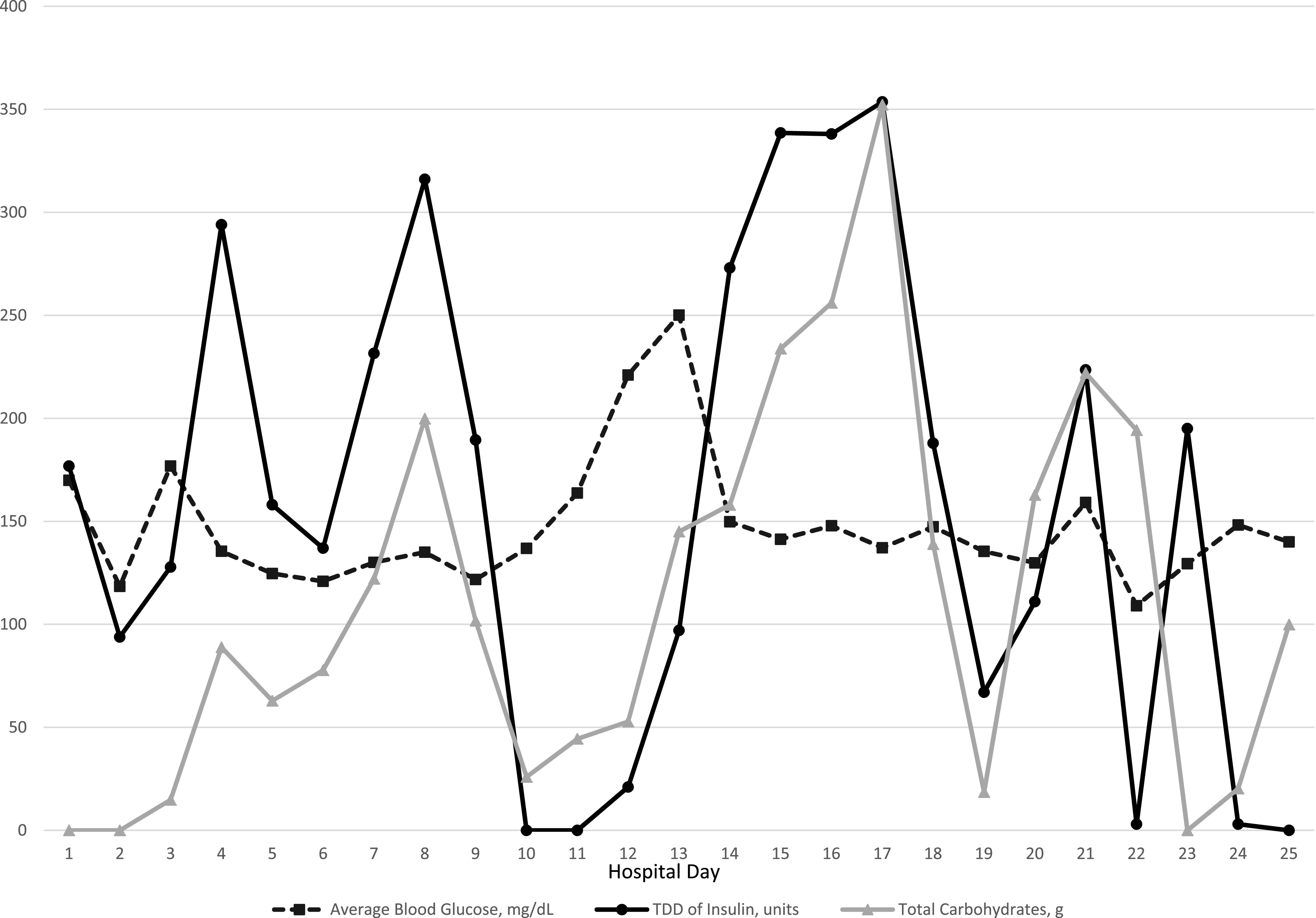
Daily comparisons of average blood glucose, insulin, and total carbohydrates.
C.R. was transferred to the coronavirus 2019 (COVID-19) medical floor on day 26 of his hospital stay. Despite remaining on EN for a number of days, he did not require supplemental insulin to maintain glycemic control. C.R. was transferred to our acute rehabilitation unit for treatment of deconditioning from his prolonged hospital stay. He was discharged home 44 days after his initial presentation with COVID-19 and resumed metformin, with a dose change to 500 mg twice daily.
Questions
What comorbidities can adversely affect the outcomes of patients with COVID-19?
What factors may contribute to insulin resistance and hyperglycemia observed in patients with COVID-19?
What insulin regimen may be used for optimization of glycemic control in patients with COVID-19?
Commentary
Anecdotal findings of extreme insulin resistance in patients with COVID-19, the infection caused by the SARS-CoV-2 virus, have been reported (1–3). C.R. had diabetes, obesity, and hypertension, which are conditions associated with hospitalization for COVID-19 and have been correlated with a more severe disease course (4–7). Chronic inflammation from these conditions, combined with severe acute inflammation in response to COVID-19 infection, can lead to extreme insulin resistance (4–7). Guan et al. (4) noted elevated C-reactive protein (CRP), creatinine kinase (CPK), lactate dehydrogenase (LDH), and WBC count in patients with COVID-19.
Our patient demonstrated elevated CRP, CPK, LDH, and WBC count on admission (Table 1). Of note, on days 15–18, when C.R.’s insulin needs were the highest, there was a corresponding peak in WBC count (Table 2). C.R.’s admission CRP level was highest at 455.7 mg/dL and then trended down, except for a slight increase during days 15 to 18. While C.R.’s CPK was elevated on admission, it trended down during his ICU stay and was within normal limits on days 15–18. Unfortunately, interleukin-6 drawn close to admission could not be interpreted because of interfering substances and was not redrawn.
TABLE 1.
C.R.’s Data, Day 1 Through Day 12
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average blood glucose, mg/dL | 169.9 | 118.4 | 176.8 | 135.5 | 124.6 | 120.9 | 130.1 | 135.0 | 121.7 | 136.9 | 163.7 | 221.0 |
| Insulin TDD, units | 176.8 | 93.8 | 127.8 | 294.0 | 158.0 | 137.0 | 231.5 | 316.0 | 189.5 | 0 | 0 | 21.0 |
| Insulin drip, gtt rate range/day | 3.2–11.2 | 3.2–4.2 | 3.2–11 | 11–14 | 5–12 | 4.5–6.5 | 5.5–7 | — | 0–5 | — | — | — |
| Biphasic 70/30 insulin TDD, units | — | — | — | — | — | — | — | 153 | 138 | — | — | — |
| Rapid-acting correctional insulin TDD, units | — | — | — | — | — | — | — | — | — | — | — | 21 |
| Regular insulin in PN daily total, units | — | — | — | — | — | — | — | — | — | — | — | — |
| GFR, mL/min/1.73 m2 | 58 | 33 | 28 | 16 | 11 | 15 | 18 | 20 | 21 | 22 | 23 | 22 |
| CRP, mg/L | — | — | 455.7 | 398.7 | 211.1 | — | 121.4 | 79.8 | 46.8 | 25.5 | 21.8 | 22.0 |
| CPK, units/L | — | — | 954 | 750 | 2212 | 2970 | 1497 | 1405 | 964 | 542 | 364 | 261 |
| LDH, units/L | — | — | — | 819 | 802 | 923 | 807 | 673 | 688 | 648 | 580 | 528 |
| WBC count, 10*9/L | 12.5 | 12.2 | 13.6 | 15.3 | 12.5 | 13.2 | 16.2 | 17.3 | 17.3 | 16.8 | 14.9 | 13.2 |
| Platelet count, 10*9/L | 211 | 218 | 165 | 201 | 189 | 190 | 174 | 184 | 134 | 124 | 107 | 106 |
| Prothrombin time, seconds | — | — | 15.3 | — | — | — | — | — | — | — | — | 15.8 |
| Partial thromboplastin time, seconds | — | — | 104.2 | 61.6 | 70.0 | 64.9 | 78.3 | 88.5 | 87.5 | 94.3 | 84.7 | 27.7 |
| D-dimer, FEU | — | — | — | — | — | — | — | — | — | — | — | — |
| Fibrinogen, mg/dL | — | — | 740 | — | — | — | — | — | — | — | — | — |
| Intubation | X | X | X | X | X | X | X | X | X | X | X | X |
| ECMO | — | — | X | X | X | X | X | X | X | X | X | — |
| CRRT | — | — | X | X | X | X | X | X | X | X | X | X |
| Pressor therapy | — | — | — | — | — | — | — | — | — | — | — | X |
| EN, hours/24 hours | — | — | 4 | 24 | 24 | 24 | 24 | 24 | 9 | 14 | 24 | 22 |
| EN, carbohydrates/24 hours | — | — | 14.8 | 88.8 | 62.9 | 77.7 | 122.1 | 199.8 | 101.8 | 25.9 | 44.4 | 40.7 |
| PN, hours/24 hours | — | — | — | — | — | — | — | — | — | — | — | 2 |
| PN, dextrose/24 hours | — | — | — | — | — | — | — | — | — | — | — | 12.1 |
Boldface type indicates laboratory values that were significantly out of range. FEU, fibrinogen equivalent units; gtt, drops.
TABLE 2.
C.R.’s Data, Day 13 Through Day 24
| Day 13 | Day 14 | Day 15 | Day 16 | Day 17 | Day 18 | Day 19 | Day 20 | Day 21 | Day 22 | Day 23 | Day 24 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average blood glucose, mg/dL | 250.1 | 149.8 | 141.3 | 147.8 | 137.1 | 147.3 | 135.4 | 129.8 | 159.1 | 109.0 | 129.4 | 148.2 |
| Insulin TDD, units | 97.0 | 273.0 | 338.5 | 338.0 | 353.6 | 187.9 | 67.0 | 111.0 | 223.5 | 3.0 | 195.0 | 3.0 |
| Insulin drip, gtt rate range/day | 3–9 | 9–11 | 11–45 | 8–15 | 0–19 | 1.3–12.5 | 0.5–5 | 3.5–7 | 0–10.5 | — | — | — |
| Biphasic 70/30 insulin TDD, units | — | — | 5 | 35 | 60 | — | — | — | 130 | — | 195 | — |
| Rapid-acting correctional insulin TDD, units | 27 | — | — | — | — | — | — | — | — | 3 | — | 3 |
| Regular insulin in PN daily total, units | 14 | 26 | 26 | 29 | 29 | 29 | — | — | — | — | — | — |
| GFR, mL/min/1.73 m2 | 12 | 10 | 15 | 21 | 21 | 24 | 24 | 15 | 23 | 38 | 47 | 51 |
| CRP, mg/L | 22.1 | 20.4 | 29.6 | 66.7 | 44.5 | 27.0 | 19.1 | — | — | 46.8 | — | 23.6 |
| CPK, units/L | 235 | 148 | 88 | — | — | — | — | — | — | — | — | — |
| LDH, units/L | 459 | 453 | 463 | — | — | — | — | — | — | — | — | — |
| WBC, 10*9/L | — | 18.5 | 22.3 | 25.1 | 28.2 | 20.3 | 19.5 | 10.7 | 9.3 | 9.1 | 10.4 | 10.3 |
| Platelet count, 10*9/L | 126 | 64 | 57 | 66 | 46 | 53 | 97 | 74 | 103 | 136 | 262 | 324 |
| Prothrombin time, seconds | — | — | — | 13.8 | — | 15.3 | — | 15.4 | 14.3 | 14.8 | — | — |
| Partial thromboplastin time, seconds | 35.0 | 49.4 | 74.0 | 38.9 | 37.3 | 34.6 | 31.2 | 30.7 | 31.4 | 32.8 | 43.3 | |
| D-dimer, FEU | — | 35,210 | — | — | — | — | — | — | — | — | — | — |
| Fibrinogen, mg/dL | — | — | — | — | — | 289 | — | — | — | — | — | — |
| Intubation | X | X | X | X | X | X | X | X | X | X | — | — |
| ECMO | — | — | — | — | — | — | — | — | — | — | — | — |
| CRRT | X | X | X | X | X | X | X | X | X | X | X | — |
| Pressor therapy | X | X | X | X | X | X | X | X | X | X | X | — |
| EN, hours/24 hours | — | 4 | 24 | 24 | 24 | — | 10 | 24 | 24 | 20 | — | 8 |
| EN, carbohydrates/24 hours | — | 13.0 | 88.8 | 111.0 | 207.2 | — | 18.5 | 162.8 | 222.0 | 194.3 | — | 20.3 |
| PN, hours/24 hours | 24 | 24 | 24 | 24 | 24 | 23 | — | — | — | — | — | — |
| PN, dextrose/24 hours | 145 | 145 | 145 | 145 | 145 | 138.9 | — | — | — | — | — | — |
Boldface type indicates laboratory values that were significantly out of range. FEU, fibrinogen equivalent units; gtt, drops.
Medical nutrition therapy (MNT) is a contributing factor to hyperglycemia in the hospital setting. C.R. was receiving both EN and PN when he exhibited higher insulin needs. During his hospital course, we accounted for these forms of nutrition by calculating total nutritional carbohydrates administered daily, including the carbohydrate content of specific tube feed formulas and dextrose from his PN (Figure 1). On days 10–12, when there was a notable decrease in insulin requirement, there was an associated decrease in carbohydrate intake, since the EN was being infused at a trickle rate of only 10 mL/hour (an 80% decrease in carbohydrate content).
The use of ECMO and continuous veno-venous hemofiltration CRRT can influence glycemia. A study by Ciapetti et al. (8) showed an increase in insulin resistance in patients on ECMO for treatment of influenza A H1N1. C.R.’s insulin needs decreased on day 12 when ECMO was stopped for clinical improvement, which likely contributed to his decreased insulin needs. Additionally, CRRT has been shown to increase blood glucose levels through increased glucose absorption from dialysate in addition to increased insulin resistance (9,10). C.R. was on CRRT from day 3 to day 23. Another potential mechanism for hyperglycemia in COVID-19 patients is the effect of SARS‐CoV‐2 on pancreatic β-cells (11). SARS‐CoV‐2 and SARS‐CoV, the virus that causes severe acute respiratory syndrome, preferentially bind angiotensin-converting enzyme 2 receptors, which are widely expressed throughout the body, including in pancreatic β-cells. β-Cells are damaged by SARS‐CoV, leading to impaired insulin secretion (11), which can lead to hyperglycemia during the acute phase of the illness. This damage may have played a role in our patient’s stress hyperglycemia. Additionally, RNA viruses such as influenza have been shown to increase glycolysis leading to hyperglycemia (12).
Regarding hyperglycemia management, IV insulin infusion is the recommended approach in critically ill patients and reduces both morbidity and mortality (3,13). Basal-bolus insulin or biphasic human insulin can also be used. However, the use of a nonpeaking basal insulin can increase the risk of hypoglycemia with quickly changing insulin needs in critical illness. On day 9, C.R. required a TDD of 189.5 units of insulin in contrast to day 10, when he required no insulin. The approach used for C.R. was dosing of biphasic human insulin 70/30 three times daily to cover EN.
The standard of care for patients receiving MNT in our institution is the use of either an insulin infusion if critically ill or biphasic human insulin 70/30 dosed three times a day with a rapid-acting insulin correctional scale for blood glucose levels >180 mg/dL dosed as needed every 4 hours. This approach may reduce the risk of hypoglycemia in patients compared with long-acting basal insulin (14). However, to avoid hypoglycemia, it is crucial that a dextrose-containing IV infusion such as D10 W be started if the EN is interrupted for any reason.
Finally, an additional method for management of PN-associated hyperglycemia is the addition of regular insulin to the PN solution (13). Insulin was added to C.R.’s PN infusion using a ratio of 1 unit of regular insulin for every 10 g dextrose. A combination of these insulin therapies was used successfully to manage C.R.’s hyperglycemia while avoiding hypoglycemia.
On day 21, C.R. was successfully transitioned off of an IV insulin infusion rate of 10.5 units/hour with biphasic human insulin 70/30 80 units three times daily, which allowed for decreased frequency of blood glucose monitoring and decreased staff exposure. The combined use of IV insulin infusion and biphasic 70/30 insulin is not standard practice for the treatment of hyperglycemia in critically ill patients but has been used successfully by the authors for patients with COVID-19 with extreme insulin resistance.
In summary, there is an important link between COVID-19 infection and metabolic control in patients with diabetes that may contribute to worsening outcomes (2,15,16). Insulin is the preferred treatment for hyperglycemia in hospital patients. C.R. had markedly variable insulin needs during his hospital stay and required different types of insulin therapies. In addition, MNT contributed to his hyperglycemia, so insulin dosing was adjusted to account for provision of carbohydrates. This case study presents a unique view of a patient with COVID-19 and the dramatic variation in insulin needs seen in patients with diabetes and COVID-19.
Clinical Pearls
Diabetes, obesity, and hypertension can worsen the severity of COVID-19 infection.
Critically ill patients with COVID-19 infection can acutely develop transient extreme insulin resistance.
Insulin requirements can vary dramatically from day to day and must be monitored closely.
MNT must be accounted for in insulin regimens.
-
Strategies for treating extreme insulin resistance in critically ill COVID-19 patients include:
○ IV insulin infusion, allowing insulin dosages to be titrated rapidly for varying needs
○ Biphasic human insulin 70/30 dosed three times daily with MNT
○ Regular insulin added to the PN solution
Article Information
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
S.A.S. wrote the manuscript and analyzed the data. C.C.I., S.C., and C.C.L.W. contributed to discussion and reviewed/edited the manuscript. S.A.S. is the guarantor of this work and, as such, had full access to all the patient data and takes responsibility for the integrity and accuracy of the case presentation.
References
- 1.Richardson S, Hirsch JS, Narasimhan M, et al.; the Northwell COVID-19 Research Consortium . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020;323:2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sardu C, D’Onofrio N, Balestrieri ML, et al. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control? Diabetes Care 2020;43:1408–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korytkowski M, Antinori-Lent K, Drincic A, et al. A pragmatic approach to inpatient diabetes management during the COVID-19 pandemic. JCEM 2020;105:dgaa342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Liang WH, Zhao Y, et al.; China Medical Treatment Expert Group for COVID-19 . Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 2020;55:2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med 2020;8:e35. [PMC free article] [PubMed] [Google Scholar]
- 6.Schiffrin EL, Flack JM, Ito S, Muntner P, Webb RC. Hypertension and COVID-19. Am J Hypertens 2020;33:373–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiappetta S, Sharma AM, Bottino V, Stier C. COVID-19 and the role of chronic inflammation in patients with obesity. Int J Obes 2020;44:1790–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciapetti M, Mancinelli P, Cecchi A, Borrelli E, Bocci V, Peris A. Reduction of non-enzymatic antioxidants in plasma during ECMO-treatment in ARDS by influence A H1N1. J Crit Care 2018;43:220–224 [DOI] [PubMed] [Google Scholar]
- 9.Bellomo R, Colman PG, Caudwell J, Boyce N. Acute continuous hemofiltration with dialysis: effect on insulin concentrations and glycemic control in critically ill patients. Crit Care Med 1992;20:1672–1676 [PubMed] [Google Scholar]
- 10.Geronemus R, Bosch JP, Thornton J, Rayfield EJ. Studies of carbohydrate metabolism after hemodialysis and hemofiltration in uremic patients. Arch Intern Med 1982;142:707–710 [PubMed] [Google Scholar]
- 11.Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol 2010;47:193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thaker SK, Ch’ng J, Christofk HR. Viral hijacking of cellular metabolism. BMC Biol 2019;17:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association . 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S193–S202 [DOI] [PubMed] [Google Scholar]
- 14.Hsia E, Seggelke SA, Gibbs J, Rasouli N, Draznin B. Comparison of 70/30 biphasic insulin with glargine/lispro regimen in non-critically ill diabetic patients on continuous enteral nutrition therapy. Nutr Clin Pract 2011;26:714–717 [DOI] [PubMed] [Google Scholar]
- 15.Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab 2020;318:E736–E741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev 2020;e3319. [DOI] [PMC free article] [PubMed] [Google Scholar]


