Abstract
Objectives
To review the pathophysiology of COVID-19 disease, potential aspirin targets on this pathogenesis and the potential role of aspirin in patients with COVID-19.
Design
Narrative review.
Setting
The online databases PubMed, OVID Medline and Cochrane Library were searched using relevant headlines from 1 January 2016 to 1 January 2021. International guidelines from relevant societies, journals and forums were also assessed for relevance.
Participants
Not applicable.
Results
A review of the selected literature revealed that clinical deterioration in COVID-19 is attributed to the interplay between endothelial dysfunction, coagulopathy and dysregulated inflammation. Aspirin has anti-inflammatory effects, antiplatelet aggregation, anticoagulant properties as well as pleiotropic effects on endothelial function. During the COVID-19 pandemic, low-dose aspirin is used effectively in secondary prevention of atherosclerotic cardiovascular disease, prevention of venous thromboembolism after total hip or knee replacement, prevention of pre-eclampsia and postdischarge treatment for multisystem inflammatory syndrome in children. Prehospital low-dose aspirin therapy may reduce the risk of intensive care unit admission and mechanical ventilation in hospitalised patients with COVID-19, whereas aspirin association with mortality is still debatable.
Conclusion
The authors recommend a low-dose aspirin regimen for primary prevention of arterial thromboembolism in patients aged 40–70 years who are at high atherosclerotic cardiovascular disease risk, or an intermediate risk with a risk-enhancer and have a low risk of bleeding. Aspirin’s protective roles in COVID-19 associated with acute lung injury, vascular thrombosis without previous cardiovascular disease and mortality need further randomised controlled trials to establish causal conclusions.
Keywords: cardiovascular diseases, global health, infectious disease medicine, respiratory system, COVID-19
Key points.
Question
What are the rationales for aspirin use in COVID-19 era?
Findings
COVID-19 progression is due to inflammation, coagulopathy and endotheliopathy.
Aspirin may potentially target COVID-19 pathogenesis through its anti-inflammatory, antiplatelet aggregation and anticoagulant properties as well as its pleiotropic effects on endothelial function.
Low-dose aspirin has multiple indications for use during COVID-19 era, including secondary prevention of cardiovascular disease, venous thrombophylaxis, pre-eclampsia prevention and paediatric multisystem inflammatory syndrome treatment.
Low-dose aspirin may be associated with reduced risk of intensive care unit admission, mechanical ventilation and mortality in hospitalised patients with COVID-19.
Meaning
Further randomised controlled trials are needed to establish the causal effect of low-dose aspirin in COVID-19 associated with acute lung injury, vascular thrombosis and mortality.
Introduction
Considering the heterogenicity of COVID-19 disease symptomatology and complications, a closer review of the pathogenesis of this global pandemic will help in a better understanding of this overwhelming illness and guide in avoiding its complications. Clinical deterioration in COVID-19 disease is attributed to the constellation of variable degrees of hyperinflammation, remarkable platelet activation, endothelial dysfunction, and coagulopathy.
Patients with severe/critical illness develop complications such as acute respiratory distress syndrome (ARDS), septic shock, thromboembolism and/or multiple organ failure (MOF), including acute cardiac and kidney injury.1 However, less information is available about the rate of complications in non-hospitalised patients with COVID-19 disease.
Of great interest, low-dose aspirin has anti-inflammatory, antiplatelet aggregation, anticoagulant effects as well as pleiotropic effects on endothelial function2; therefore, it might have a role in preventing COVID-19 complications. Also, aspirin has been shown to have antiviral activity against RNA viruses in the respiratory tract such as influenza A virus and human rhinoviruses, but its mode of action is still unknown and requires further research.3
Prehospital administration of aspirin might have a role in preventing COVID-19 complications, especially arterial thrombosis in both hospitalised and non-hospitalised patients. Throughout this narrative review, we will demonstrate in detail the effects of aspirin on four aspects of COVID-19 disease pathogenesis and list the current international recommendations about aspirin use in patients with COVID-19 disease.
Methods
The online databases PubMed, OVID Medline and Cochrane Reviews Library were searched using relevant headlines published from 1 January 2016 to 1 January 2021. Search queries were designed to address three central questions: “What is the current understanding of COVID-19 pathophysiology and its relationship to thrombus formation?”, “What research is available regarding COVID-19 and aspirin use?” and “What are the current guidelines or evidence supporting aspirin use for primary prevention of systemic diseases?” Only full-text articles available in English were included in the review. International guidelines from relevant societies, references from reviewed articles and forums were also assessed for relevance. Additionally, due to the nature of emerging evidence in the current pandemic, articles were identified from informal review during the drafting process of this article, which was deemed import for inclusion. Articles were excluded due to redundant data, the protocol of studies, articles in unranked journals or due to a limited number of required references. The initial search identified 854 articles, of which 59 articles were included in this review (online supplemental material 1).
fmch-2020-000741supp001.pdf (7.3MB, pdf)
COVID-19-associated inflammation and aspirin
In most cases of COVID-19 disease, the stimulated immune system is capable of resolving the infection where it initially triggers a local immune response, followed by recruiting macrophages and monocytes that in turn release cytokines and prime adaptive T-cell and B-cell immune responses. Manjili et al suggest that the dysregulation of the inflammatory immune response, which is associated with severe COVID-19 disease, inhibits the development of protective immunity to the infection. They suggested that uncontrolled immune dysregulation, hypercytokinemia, ‘‘cytokine storm’ or macrophage activation syndrome is associated with ARDS, MOF and mortality in certain populations (men, elderly and individuals with comorbidities).4 Autoimmune conditions such as antiphospholipid syndrome (APS) and multisystem inflammatory syndrome in children (MIS-C) have been reported in patients with COVID-19.5 6
Cytokine storm in COVID-19 is associated with elevation of pro-inflammatory cytokines and chemokines. These cytokines include interleukin (IL)-6, IL-2, IL-7, IL-8, IL-1β, interferon (IFN)-γ, tumour necrosis factor-α (TNF-α), granulocyte colony-stimulating factors, chemokines including C-X-C motif chemokine ligand 10, C-X-C motif chemokine ligand 8 and chemokine (C-C motif) ligand 2.7 8
Because of hyperinflammation role in COVID-19, therapeutic agents that target the inflammatory pathway have been employed. Aspirin is used in moderate and high doses in children with MIS-C to treat inflammation in the acute stage,6 and it has already been listed in 14 studies on the clinical trials website, including 10 randomised controlled trials. Other immunomodulatory therapeutics were also used including steroids, intravenous immunoglobulin (IVIG), anticytokine agents (IL-1 antagonist anakinra, IL-6 receptor antagonists tocilizumab and sarilumab), antichemokine agents (eg, cenicriviroc or leronlimab) and Janus kinase (JAK) inhibitors (eg, baricitinib or ruxolitinib).8 Despite a strong rationale and several previous promising open studies, a randomised controlled study to evaluate the safety and efficacy of tocilizumab in patients with severe COVID-19 pneumonia (COVACTA) failed to meet its primary end point of improved clinical status or to improve patients’ mortality.9 Another prospective randomised controlled trial about the use of sarilumab, registered as (CORIMUNO-VIRO), was suspended for futility (NCT04341870).
COVID-19-associated endothelial dysfunction and aspirin
Teuwen et al postulated that endothelial cells play an important role in the pathogenesis of ARDS and MOF in patients with COVID-19. In other words, they contribute to the initiation and propagation of severe COVID-19 by inducing vascular endotheliitis, altering vessel barrier integrity and permeability, activating coagulation pathways and deregulating inflammatory cell infiltration. Host-dependent cardiovascular (CV) factors or established cardiovascular disease (CVD) in addition to viral factors could contribute to the severity of COVID-19 disease in these patients who have chronic endothelial dysfunction.10 Varga et al found endothelial cell involvement across vascular beds of different organs in three patients with COVID-19 with CV comorbidity, who developed respiratory failure and MOF. The histological findings showed the presence of viral bodies within endothelial cells and a responsive accumulation of inflammatory cells, with evidence of endothelial and inflammatory cell death. COVID-19 endotheliitis in several organs is suspected to be the result of direct viral infection, host inflammatory response, host apoptosis and pyroptosis.11 Pyroptosis and endothelial dysfunction were also demonstrated in the COVID-19 pulmonary samples,12 which may lead to systemic thrombosis as explained later in this article (figure 1).
Figure 1.
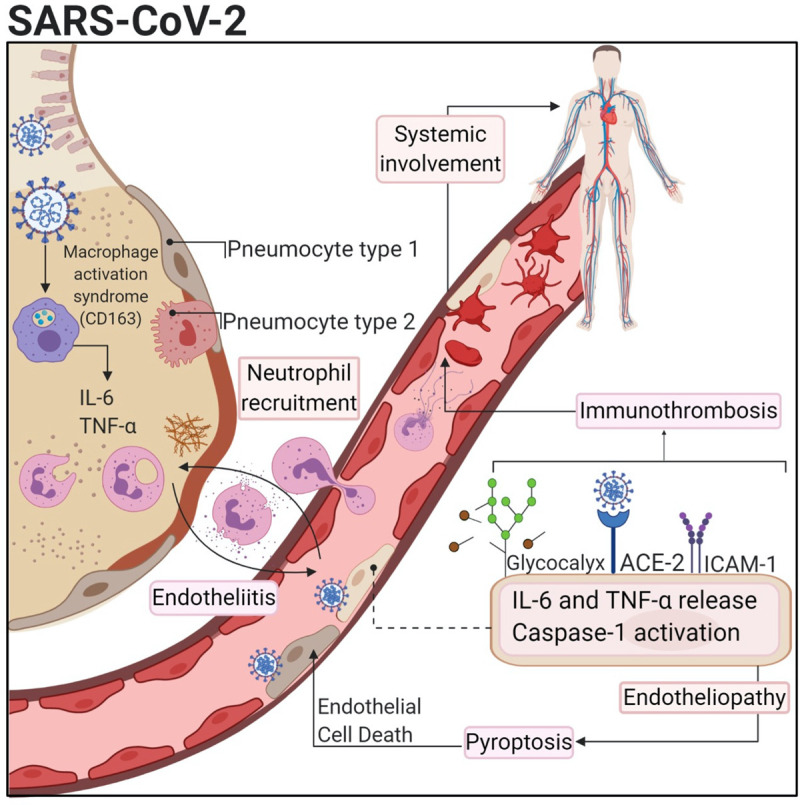
COVID-19-induced inflammation, endotheliopathy and thrombosis. Alveolar-capillary endothelial cells can be activated by SARS-CoV-2 infection leading to cytokine release and expression of vascular adhesion molecules. Also, endothelial cells express ACE which allows infection by SARS-CoV-2. This could trigger endothelial dysfunction and pyroptosis that also increase the pro-inflammatory stimuli and thrombogenic events.12 This figure was used with permission from the publisher Wolters Kluwer Health (license number: 4938390247706). ‘The Creative Commons license does not apply to this content. Use of the material in any format is prohibited without written permission from the publisher, Wolters Kluwer Health. Please contact permissions@lww.com for further information’. ICAM-1, intercellular adhesion molecule 1; IL, interleukin; TNF, tumour necrosis factor.
COVID-19-induced endotheliitis provides a reasonable sound for treatment modalities to stabilise the endothelium while tackling SARS-CoV-2 replication, especially with anti-inflammatory, anticytokine drugs, ACE inhibitors and statins.11 Florêncio et al13 formulated a hypothesis that aspirin and statins play an essential role in preventing COVID-19-induced endotheliitis, and progression to severe forms.
Low-dose aspirin has a putative role for secondary prevention of arterial thrombosis in patients with COVID-19 with established CVD,14 but its role in the primary prevention of atherosclerotic cardiovascular disease (ASCVD) with chronic endothelial dysfunction is controversial.15–19 Aspirin has pleiotropic effects on endothelial function and assessing these effects during COVID-19 era in further research will be very helpful for proper understanding and management of COVID-19 infection.
COVID-19-associated coagulopathy and aspirin
COVID-19-induced coagulopathy may, in part, be due to direct vascular damage induced by SARS-CoV-2 infection or ACE-2 inhibition, the latter is expressed on arterial and venous endothelial cells and plays an anti-inflammatory protective role. In severe and critical COVID-19, a bi-directional association of inflammation and coagulopathy may exist where persistent inflammatory status acts as an important trigger for the coagulation cascade and may promote an aggressive immune response. Certain cytokines, such as IL-6, could activate the coagulation system and suppress the fibrinolytic system. Inflammatory cytokines can contribute to a pro-coagulopathic state by several pathways, but the actual mechanisms of inflammatory-induced coagulopathy in severe COVID-19 remain to be determined.20
Since the emergence of COVID-19 pandemic, studies have shown an increased incidence of venous, arterial and microvascular thrombosis in this population of patients, with a higher prevalence in the severe form of the disease.21 22 A literature review by Obi et al reported a 21%–69% incidence rate of venous thromboembolism (VTE) in critically ill patients with COVID-19. In addition to deep venous thrombosis and acute pulmonary embolism (APE), patients developed circuit clotting of continuous renal replacement therapy and ECMO. They also described ‘breakthrough VTE’, where VTE develops despite receiving prophylactic or therapeutic doses of anticoagulation.23 In a cohort study of 198 hospitalised patients with COVID-19 in Amsterdam, where all patients had received prophylactic anticoagulation, the cumulative risk for developing VTE at 7, 14 and 21 days was 16%, 33% and 42%, respectively, with a higher incidence in intensive care unit (ICU) patients than non-ICU patients.24
Previous studies found that VTE was the predominant macrovascular complication.21 A Dutch study reported the frequency of arterial thrombosis (ischaemic stroke, myocardial infarction (MI) or systemic thromboembolism) as 3.7% in 184 ICU patients.25 Whereas in a health system in New York, where most patients were treated with low dose of anticoagulation, the incidence of arterial and venous thrombosis in 829 ICU patients were 18.6% and 13.6%, respectively, and among 2505 non-ICU patients, the incidence of arterial and venous thrombosis were 8.4% and 3.6% respectively.22
Gervaise et al concluded that the presence of APE was not just restricted to severe or critical COVID-19, in their study, 18% of non-hospitalised patients with COVID-19 were evaluated by CT pulmonary angiography in the emergency department were found to have APE.26 Multiple thromboembolic events have been also reported in outpatients with mild COVID-19 illness after developing extreme hypercoagulability status. Lack of universal guidelines for the early detection and treatment of COVID-19-associated coagulopathy in non-hospitalised patients might contribute to the scarcity of data about coagulation status in the outpatient setting.27
COVID-19 is also associated with antiphospholipid antibodies, which may manifest with macrovascular or microvascular thrombosis.5 Emergent evidence suggests that manifestations of severe COVID-19 mimic more the clinical phenotype and pathophysiology of complement-mediated thrombotic microangiopathies (TMA), rather than sepsis-induced coagulopathy or disseminated intravascular coagulation (DIC). COVID-19-associated coagulopathy presents initially with minimal abnormalities in PT and platelet count followed by increased D-dimer and fibrinogen. In DIC, D-dimer is minimally increased while the platelet count is markedly decreased than in patients with COVID-19. VTE and arterial thrombosis are more frequent in CAC compared with SIC/DIC. Clinical and laboratory features of CAC overlap somewhat with haemophagocytic syndrome (HPS), APS and TMA; however, patients with COVID-19 still might be at risk of developing DIC.5 28 To date, there is no evidence that aspirin was used to treat complement-mediated thrombotic microangiopathies. Nevertheless, early uncontrolled observations suggest good outcomes in patients with COVID-19 after the use of the C5 inhibitor eculizumab, the C3 inhibitor AMY-101 or anti-C5a antibody, which need further confirmation by randomised controlled trials.29
Diaz et al postulated that early administration of low-dose aspirin in patients with COVID-19 represents a pivotal pharmacological strategy for the prevention of arterial thromboembolism and VTE through targeting the thrombo-inflammatory process.30 Aspirin prevents and treats arterial thromboembolism and VTE through several mechanisms of action. The earliest events in thrombus formation are platelet adhesion followed by aggregation, platelet activation and granule release. Except for platelet adhesion, all of these platelet functions are inhibited by aspirin. Aspirin irreversibly inactivates cyclooxygenase-1 and suppresses the generation of prostaglandin H2 (a precursor of thromboxane A2), which results in inhibiting platelet aggregation. Other mechanisms of antithrombotic effects of aspirin include suppression of platelet activation and release reaction, inhibition of neutrophil recruitment on vascular endothelium, reducing thrombin generation, inhibition of factor XIII activation, increasing plasma clot permeability, altering fibrin clot structure and increasing its density, in addition to enhancing fibrinolysis by acetalisation of fibrinogen and fibrin.31
COVID-19-associated ARDS and aspirin
Acute respiratory failure resulting from ARDS is the leading cause of mortality in patients with COVID-19. There appear to be two distinct subphenotypes of COVID-19-induced acute lung injury (ALI) or ARDS-related COVID-19 described as pneumonia type ‘L’ and ‘H’. The ‘L’ refers to Low recruitability, lung weight, elastance and ventilation/perfusion (V/Q) ratio, and the ‘H’ refers to High recruitability, lung weight, elastance and right to left shunt. The L-type lung is not seen in ARDS caused by other mechanisms (bacterial sepsis, trauma, haemorrhage shock) and presents as a mostly open and relatively compliant lung that will not oxygenate the blood, while the H-type is considered typical ARDS with a large amount of collapsed non-compliant lung tissue. Loss of lung ability to match pulmonary blood flow (Q) with open ventilated (V) lung tissue using hypoxic pulmonary vasoconstriction results in very high pulmonary shunt fraction which is a hallmark of COVID-19 pathophysiology, even in a mostly inflated lung (L-type pneumonia). In ‘typical’ ARDS, V/Q mismatching is secondary to large volumes of oedema-filled and/or collapsed lung tissue. Although COVID-19 can also cause oedema and lung collapse, the severe hypoxia due to a low V/Q ratio in a lung with little to moderate collapse is a unique finding.32 The mechanisms for the loss of pulmonary perfusion control by COVID-19 are still unknown. Inflammation, endothelial damage, micro-embolisation, interference with the renin-angiotensin system or impairment of the hypoxic pulmonary venous system may all play a role. Eisenhut and Shin hypothesised that TNF affects alveolar epithelial sodium and chloride transport and disrupts the endothelial and epithelial cytoskeleton. They explained that in early COVID-19 airway disease, reduced airway liquid film causes tenacious airway secretions, atelectasis and dry cough. As the disease progresses, reduced alveolar fluid clearance and impaired cytoskeleton lead to pulmonary interstitial and alveolar fluid accumulation causing pulmonary oedema typical of ARDS. Aspirin reduces inflammatory mediators release including TNF and IL-6.33
Four of seven lungs of patients with COVID-19 at autopsy had thrombi in the pulmonary arteries, alveolar capillaries and postcapillary venules.34 Before the COVID-19 era, research demonstrated that platelets play a pivotal pro-inflammatory role in the pathogenesis of ALI, ARDS, sepsis and MOF, thus extending their role beyond thrombosis.35 Aspirin may manipulate the process of ARDS and sepsis (figure 2). Zhou et al propose that administration of aspirin before hospitalisation can prevent severe ARDS and decrease the risk of serious future complications of COVID-19 as a result of platelet inhibition, which may reduce intravascular fibrin and thrombus formation in the early phase of infection, thereby preventing the ensuing consequences.36
Figure 2.
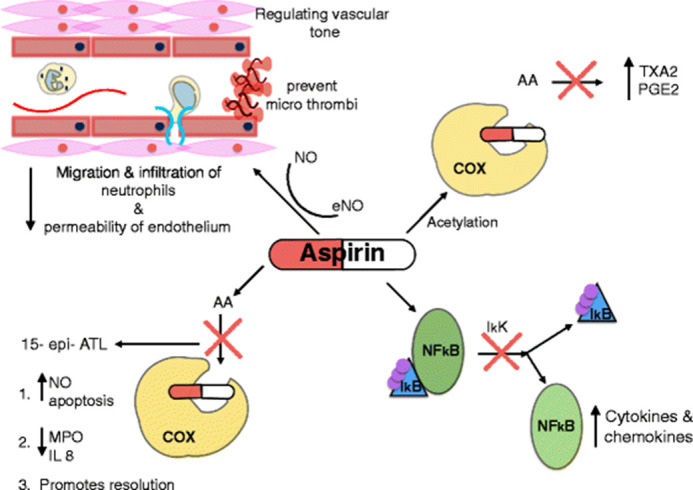
Mechanisms in which aspirin can manipulate the process in sepsis and acute respiratory distress syndrome: aspirin inhibits the enzyme COX, preventing the formation of pro-inflammatory thromboxane and prostaglandins. It also inhibits the release of NFκB from its inhibitor IkB, preventing the formation of pro-inflammatory cytokines and chemokines. Aspirin leads to the production of aspirin-triggered lipoxin, which induces the release of NO, inhibits the production of IL-8 and MPO, restores neutrophil apoptosis and promotes resolution. Aspirin increases the production of NO, resulting in reduced migration and infiltration of neutrophils and reduced permeability of the endothelium. 15-epi-ATL, aspirin-triggered 15-epi-lipoxinA4; AA, arachidonic acid; COX, cyclooxygenase; eNO, endothelial nitric oxide; IKK, IkB kinase; IL-8, interleukin 8; MPO, myeloperoxidase; NFκB, nuclear factor kappa B; NO, nitric oxide; PGE 2, prostaglandin E2; TXA 2, thromboxane.35 This image is licensed under the Creative Commons Attribution 4.0 International License found at http://creativecommons.org/licenses/by/4.0/.
Berthelot et al argued that binding of SARS-CoV2 to ACE2 results in ACE1/2 imbalance and overexpression of angiotensin II on the cell membrane, which leads to overactivation of stimulator of interferon genes (STING) pathways in COVID-19 promoting hypercoagulopathy through the release of IFN-β and the highly prothrombotic tissue factor by monocytes-macrophages.37 STING also activate coagulation through another independent pathway (STING-ITPR1-calcium release-gasdermin D-pyroptosis), which can lead to tissue factor deposition in various tissues.38 Tissue factor is expressed on the surface of damaged endothelial cells and leucocytes of patients with COVID-19, and it acts as a high-affinity receptor for coagulation factor VIIa which results in activating the coagulation pathway and fibrin deposition in the lungs.39 In addition to their antiplatelet activity, both aspirin and dipyridamole reduce tissue factor procoagulant activity; moreover, aspirin inhibits the STING pathway upstream.37
Role of aspirin in non-hospitalised patients with COVID-19
Symptomatic management of COVID-19 disease
The cornerstone therapy for patients with mild-to-moderate COVID-19 illness is still supportive care, which includes paracetamol and NSAIDs use, as they have analgesics and antipyretic effects. Paracetamol and ibuprofen are commonly used, but paracetamol is the first-line of treatment. WHO initially recommended against the use of ibuprofen, then relented.40 Previous studies revealed that NSAIDs use during episodes of acute respiratory infection were associated with a further increased risk of acute MI, stroke and complicated the course of community-acquired pneumonia (CAP).41–43 All of these claims could be applied to aspirin as it belongs to the NSAIDs group, but until now, there is no clinical evidence to support these speculations.40
A recent population-based cohort study in Denmark involved 9236 patients with confirmed COVID-19, among them NSAID users were 248 (2.7%). They found that the use of NSAIDs was not associated with 30-day mortality, hospitalisation, ICU admission, mechanical ventilation or renal replacement therapy in Danish patients who tested positive for COVID-19.44
Primary prevention of arterial thrombosis during COVID-19 pandemic
The COVID-19 Treatment Guidelines and European Society for Cardiology (ESC) Guidance for the Diagnosis and Management of CVD during the COVID-19 pandemic do not include any recommendations regarding aspirin for the primary prevention of arterial atherothrombosis during this pandemic.1 14 This role is controversial in literature before the COVID-19 era15–19 (table 1).
Table 1.
Primary prevention of arterial atherothrombosis events in guidelines
| Organisation | Year | Recommendation |
| The Canadian Stroke Best Practice18 | 2020 | Aspirin is no longer recommended for primary prevention in individuals without a history of symptomatic CVD, stroke or PAD; the harms of daily aspirin use could potentially outweigh the benefits. |
| American Diabetes Association17 | 2020 | Aspirin therapy (75–162 mg/day) may be considered as a primary prevention strategy in those with diabetes who are at increased cardiovascular risk, after a comprehensive discussion with the patient on the benefits versus the comparable increased risk of bleeding. |
| American College of Cardiology/American Heart Association Guideline on the primary prevention of CVD19 | 2019 | Low-dose aspirin (75–100 mg orally daily) might be considered for the primary prevention of ASCVD among select adults 40–70 years of age who are at higher ASCVD risk but not at increased bleeding risk. |
| Low-dose aspirin (75–100 mg orally daily) should not be administered on a routine basis for the primary prevention of ASCVD among adults >70 years of age. | ||
| Low-dose aspirin (75–100 mg orally daily) should not be administered for the primary prevention of ASCVD among adults of any age who are at increased risk of bleeding. | ||
| European Guidelines on CVD prevention in clinical practice16 | 2016 | Antiplatelet therapy is not recommended in individuals without CVD due to the increased risk of major bleeding. |
| US Preventive Services Task Force (USPSTF)15 | 2016 | The USPSTF recommends initiating low-dose aspirin use for the primary prevention of CVD in adults aged 50–59 years who have a 10% or greater 10-year CVD risk, are not at increased risk for bleeding, have a life expectancy of at least 10 years and are willing to take low-dose aspirin daily for at least 10 years. (Grade B recommendation) |
| The decision to initiate low-dose aspirin use for the primary prevention of CVD in adults aged 60–69 years who have a 10% or greater 10-year CVD risk should be an individual one. Persons who are not at increased risk for bleeding have a life expectancy of at least 10 years, and are willing to take low-dose aspirin daily for at least 10 years are more likely to benefit. Persons who place a higher value on the potential benefits than the potential harms may choose to initiate low-dose aspirin. (Grade C recommendation) | ||
| The current evidence is insufficient to assess the balance of benefits and harms of initiating aspirin use for the primary prevention of CVD and CRC in adults aged 70 years or older or in adults younger than 50 years. (Grade I recommendation) |
ASCVD, atherosclerotic cardiovascular disease; PAD, peripheral artery disease.
The Heart and Stroke Foundation of Canada in collaboration with the Canadian Stroke Consortium does not recommend the use of aspirin for primary prevention in individuals without a history of symptomatic CVD as the harms of daily aspirin use could potentially outweigh the benefits.18
Secondary prevention of arterial thrombosis during COVID-19 pandemic
The COVID-19 Treatment Guidelines Panel and the ESC recommend that patients who are receiving antiplatelet therapies (eg, aspirin) or anticoagulant for underlying conditions should continue these medications if they receive a diagnosis of COVID-19 unless there are contraindications such as the excessive risk of bleeding or planned invasive surgical procedures.1 14 These underlying conditions are acute coronary syndrome, unstable angina, previous MI or revascularisation, postpercutaneous coronary interventions, transient ischaemic attack and ischaemic stroke.16 18 A recent retrospective cohort study revealed that prehospital aspirin use in hospitalised patients with COVID-19 with established coronary artery disease was not associated with increased all-cause mortality.45
Prevention of venous thromboembolic events during COVID-19 pandemic
The COVID-19 Treatment Guidelines Panel does not recommend anticoagulant or antiplatelet therapy for VTE prophylaxis or at therapeutic doses in non-hospitalised patients with COVID-19 unless there are other indications.1 These indications may be for arterial thrombosis prevention as mentioned above or for the prevention of VTE following total hip or knee replacement46 after a risk assessment of postoperative VTE.47
Prevention of severe acute respiratory distress syndrome in COVID-19 disease
Before the COVID-19 era, several studies were conducted to investigate the role of aspirin in preventing ARDS in at-risk patients with conflicting results. A recent meta-analysis of 6764 at-risk patients across 7 studies concluded that prior aspirin use was associated with decreased incidence of ARDS in at-risk patients, but was not associated with decreased hospital mortality.48 Another meta-analysis concluded that antiplatelet therapy was not significantly associated with reducing hospital mortality in high-risk patients.49 The investigation of these effects in the COVID-19 era is an interesting area for researches.
A recent single-centre case-control phase IIB study examined the effect of intensive antiplatelet administration, in addition to anticoagulation therapy, on five patients with severe COVID-19 and hypercoagulopathy with promising results of improved ventilation-perfusion ratio in comparison to the control group with similar baseline characteristics treated with anticoagulation only.50 Of note, dipyridamole, an antiplatelet agent that inhibits phosphodiesterase, has a potential role in the treatment of COVID-19. It is known to have antiviral, anti-inflammatory and antifibrotic properties and was investigated in a proof-of-concept randomised controlled trial in China. Although this study was conducted on a small number of patients, it resulted in a remarkably improved outcome in severely ill patients with COVID-19.51 52
Most recently, the results of a multicentre cohort study of 412 patients with COVID-19 in the USA were released that support the potential adjunctive therapeutic role of aspirin in COVID-19. The authors found that after adjustment for confounding variables, aspirin use was independently associated with a lower risk of mechanical ventilation (adjusted HR 0.56, 95% CI 0.37 to 0.85, p=0.007), ICU admission (adjusted HR 0.57, 95% CI 0.38 to 0.85, p=0.005) and in-hospital mortality (adjusted HR 0.53, 95% CI 0.31 to 0.90, p=0.02).53
Treatment of paediatric multisystem inflammatory syndrome or MIS-C
Patients with paediatric multisystem inflammatory syndrome should receive IVIG at 2 g/kg and high-dose aspirin (50–80 mg/kg/day) if their Kobayashi scores were below 5 for 5 days or moderate-dose aspirin (30 mg/kg/day) plus methylprednisolone at 2 mg/kg/day for 5 days if their Kobayashi scores were ≥5, followed by tapering of methylprednisolone over 2 weeks. Aspirin therapy should be maintained until 48 hours after defervescence and then should be continued at a low-dose aspirin (3–5 mg/kg/day) for 8 weeks to prevent acute coronary complications.6 Interestingly, Loo et al have successfully used dipyridamole (5 mg/kg in three divided doses) in addition to IVIG to treat two siblings with familial Kawasaki disease in whom aspirin therapy was contraindicated due to glucose-6-phosphate dehydrogenase deficiency.54
Prevention of pre-eclampsia during COVID-19 pandemic
Before the COVID-19 era, both the US Preventive Services Task Force and the American College of Obstetricians and Gynaecologists recommended low-dose aspirin (81 mg/day) as pre-eclampsia prophylaxis in pregnant women with high risk for pre-eclampsia after 12 weeks of their gestation.55 56 During the current COVID-19 pandemic, Kwiatkowski et al concluded that women who are at high-risk for pre-eclampsia and fetal growth restriction should still receive low-dose aspirin for pre-eclampsia prevention during this pandemic.57 On the other hand, Gavillet et al recommend immediate cessation of aspirin prophylaxis for pre-eclampsia after diagnosis of COVID-19, and restarting it after a full recovery, especially in women in the third trimester of pregnancy as the benefit of aspirin is minimal and could contribute to severe bleeding in thrombocytopaenic patients with COVID-19 or if emergency caesarean delivery is indicated by the maternal condition.58 Therefore, physicians should take into consideration the gestational age and platelet count before starting aspirin in pregnant women.59
Conclusion
Hospitalised patients with COVID-19 often suffer from pneumonia with hypoxia, ALI/ARDS, macrovascular/microvascular thrombosis, sepsis, MOF or death. Arterial thrombosis has been repeatedly reported in these patients despite the use of anticoagulants. The data and information about acute COVID-19 complications in non-hospitalised patients seem to be scarce. Thromboembolic events may be the first manifestation of COVID-19 in outpatients. The pathogenesis of these COVID-19 complications reflects the interplay between inflammation, endotheliopathy and coagulopathy. Platelet activation might play an essential role in the pathogenesis of ALI, ARDS, thrombosis, sepsis and MOF. Low-dose aspirin is an inexpensive drug and may manipulate the process of these complications through its anti-inflammatory, antiplatelet aggregation and anticoagulant properties.
During the COVID-19 pandemic, low-dose aspirin is used effectively in secondary prevention of ASCVD, prevention of VTE after total hip or knee replacement, prevention of pre-eclampsia and postdischarge treatment for MIS-C. Prehospital low-dose aspirin therapy may reduce the risk of ICU admission and mechanical ventilation in hospitalised patients with COVID-19, whereas aspirin association with mortality is still debatable. The authors recommend the use of a low-dose aspirin regimen for primary prevention of arterial thromboembolism in patients aged 40–70 years who are at high ASCVD risk, or intermediate ASCVD risk with a risk enhancer such as the family history of premature CVD and have a low risk of bleeding. Aspirin protective roles in COVID-19-associated ALI/ARDS, vascular thrombosis without previous CVD and mortality needs further investigation to establish guidelines.
Acknowledgments
The authors would like to thank Wolters Kluwer Health and Springer Nature for allowing to use figure (1) and (2), respectively in the article.
Footnotes
Contributors: Conceptualisation: HASA and HA. Methodology: HASA and EM. Drafting of the manuscript: HASA, EM, MI, AIJ, HH, RAG, MHS, GFN and HA. Data acquisition: all authors. Data interpretation: HASA, MI, JBR, AS, ED, HA, GFN and HA. Critical appraisal and review: all authors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: GFN reports grants from Drager Medial, outside the submitted work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.COVID-19 Treatment Guidelines Panel . Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. Available: https://www.covid19treatmentguidelines.nih.gov/ [Accessed 24 Jan 2021]. [PubMed]
- 2.Dzeshka MS, Shantsila A, Lip GYH. Effects of aspirin on endothelial function and hypertension. Curr Hypertens Rep 2016;18:83. 10.1007/s11906-016-0688-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glatthaar-Saalmüller B, Mair KH, Saalmüller A. Antiviral activity of aspirin against RNA viruses of the respiratory tract-an in vitro study. Influenza Other Respir Viruses 2017;11:85–92. 10.1111/irv.12421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manjili RH, Zarei M, Habibi M, et al. COVID-19 as an acute inflammatory disease. J Immunol 2020;205:12–19. 10.4049/jimmunol.2000413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 2020;382:e38. 10.1056/NEJMc2007575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020;395:1771–8. 10.1016/S0140-6736(20)31103-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coperchini F, Chiovato L, Croce L, et al. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev 2020;53:25–32. 10.1016/j.cytogfr.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–4. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furlow B. COVACTA trial raises questions about tocilizumab's benefit in COVID-19. Lancet Rheumatol 2020;2:e592. 10.1016/S2665-9913(20)30313-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teuwen L-A, Geldhof V, Pasut A, et al. COVID-19: the vasculature unleashed. Nat Rev Immunol 2020;20:389–91. Erratum in: Nat Rev Immunol. 2020;20(7):448.10.1038/s41577-020-0356-8. 10.1038/s41577-020-0343-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417–8. 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagashima S, Mendes MC, Camargo Martins AP, et al. Endothelial dysfunction and thrombosis in patients with COVID-19-Brief report. Arterioscler Thromb Vasc Biol 2020;40:2404–7. 10.1161/ATVBAHA.120.314860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Florêncio FKZ, Tenório MdeO, Macedo Júnior ARA, et al. Aspirin with or without statin in the treatment of endotheliitis, thrombosis, and ischemia in coronavirus disease. Rev Soc Bras Med Trop 2020;53:e20200472. 10.1590/0037-8682-0472-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The European Society for Cardiology . ESC guidance for the diagnosis and management of CV disease during the COVID-19 pandemic. Available: https://www.escardio.org/Education/COVID-19-and-Cardiology/ESCCOVID-19-Guidance [Accessed 3 Aug 2020].
- 15.Bibbins-Domingo K, U.S. Preventive Services Task Force . Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. preventive services Task force recommendation statement. Ann Intern Med 2016;164:836–45. 10.7326/M16-0577 [DOI] [PubMed] [Google Scholar]
- 16.Authors/Task Force Members:, Piepoli MF, Hoes AW, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016;252:207–74. 10.1016/j.atherosclerosis.2016.05.037 [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association . Standards of Medical Care in Diabetes-2020 Abridged for Primary Care Providers. Clin Diabetes 2020;38:10–38. 10.2337/cd20-as01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wein T, Lindsay MP, Gladstone DJ, et al. Canadian stroke best practice recommendations, seventh edition: acetylsalicylic acid for prevention of vascular events. CMAJ 2020;192:E302–11. 10.1503/cmaj.191599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American heart association Task force on clinical practice guidelines. Circulation 2019;140:e596–646. 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 2020;20:355–62. Erratum in: Nat Rev Immunol. 2020;20(7):448. 10.1038/s41577-020-0353-y. 10.1038/s41577-020-0331-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020;191:9–14. 10.1016/j.thromres.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilaloglu S, Aphinyanaphongs Y, Jones S, et al. Thrombosis in hospitalized patients with COVID-19 in a new York City health system. JAMA 2020;324:799–801. 10.1001/jama.2020.13372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obi AT, Barnes GD, Napolitano LM, et al. Venous thrombosis epidemiology, pathophysiology, and anticoagulant therapies and trials in severe acute respiratory syndrome coronavirus 2 infection. J Vasc Surg Venous Lymphat Disord 2021;9:23–35. 10.1016/j.jvsv.2020.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 2020;18:1995–2002. 10.1111/jth.14888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–7. 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gervaise A, Bouzad C, Peroux E, et al. Acute pulmonary embolism in non-hospitalized COVID-19 patients referred to CTPA by emergency department. Eur Radiol 2020;30:6170–7. 10.1007/s00330-020-06977-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shawkat A, Merrell ET, Fadel GA, et al. Multiple thrombotic events in a 67-year-old man 2 weeks after testing positive for SARS-CoV-2: a case report. Am J Case Rep 2020;21:e925786. 10.12659/AJCR.925786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iba T, Levy JH, Connors JM, et al. The unique characteristics of COVID-19 coagulopathy. Crit Care 2020;24:360. 10.1186/s13054-020-03077-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merrill JT, Erkan D, Winakur J, et al. Emerging evidence of a COVID-19 thrombotic syndrome has treatment implications. Nat Rev Rheumatol 2020;16:581–9. 10.1038/s41584-020-0474-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diaz T, Trachtenberg BH, Abraham SJK, et al. Aspirin bioactivity for prevention of cardiovascular injury in COVID-19. Front Cardiovasc Med 2020;7:562708. 10.3389/fcvm.2020.562708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mekaj YH, Daci FT, Mekaj AY. New insights into the mechanisms of action of aspirin and its use in the prevention and treatment of arterial and venous thromboembolism. Ther Clin Risk Manag 2015;11:1449–56. 10.2147/TCRM.S92222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 2020;46:1099–102. 10.1007/s00134-020-06033-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisenhut M, Shin JI. Pathways in the pathophysiology of coronavirus 19 lung disease accessible to prevention and treatment. Front Physiol 2020;11:872. 10.3389/fphys.2020.00872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular Endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020;383:120–8. 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toner P, McAuley DF, Shyamsundar M. Aspirin as a potential treatment in sepsis or acute respiratory distress syndrome. Crit Care 2015;19:374. 10.1186/s13054-015-1091-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou X, Li Y, Yang Q. Antiplatelet therapy after percutaneous coronary intervention in patients with COVID-19: implications from clinical features to pathologic findings. Circulation 2020;141:1736–8. 10.1161/CIRCULATIONAHA.120.046988 [DOI] [PubMed] [Google Scholar]
- 37.Berthelot J-M, Drouet L, Lioté F. Kawasaki-like diseases and thrombotic coagulopathy in COVID-19: delayed over-activation of the STING pathway? Emerg Microbes Infect 2020;9:1514–22. 10.1080/22221751.2020.1785336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Zeng L, Xie M, et al. TMEM173 drives lethal coagulation in sepsis. Cell Host Microbe 2020;27:556–70. 10.1016/j.chom.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whyte CS, Morrow GB, Mitchell JL, et al. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J Thromb Haemost 2020;18:1548–55. 10.1111/jth.14872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore N, Carleton B, Blin P, et al. Does ibuprofen worsen COVID-19? Drug Saf 2020;43:611–4. 10.1007/s40264-020-00953-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wen Y-C, Hsiao F-Y, Chan KA, et al. Acute respiratory infection and use of nonsteroidal anti-inflammatory drugs on risk of acute myocardial infarction: a nationwide case-crossover study. J Infect Dis 2017;215:503–9. 10.1093/infdis/jiw603 [DOI] [PubMed] [Google Scholar]
- 42.Wen Y-C, Hsiao F-Y, Lin Z-F, et al. Risk of stroke associated with use of nonsteroidal anti-inflammatory drugs during acute respiratory infection episode. Pharmacoepidemiol Drug Saf 2018;27:645–51. 10.1002/pds.4428 [DOI] [PubMed] [Google Scholar]
- 43.Voiriot G, Philippot Q, Elabbadi A, et al. Risks related to the use of non-steroidal anti-inflammatory drugs in community-acquired pneumonia in adult and pediatric patients. J Clin Med 2019;8:786. 10.3390/jcm8060786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lund LC, Kristensen KB, Reilev M, et al. Adverse outcomes and mortality in users of non-steroidal anti-inflammatory drugs who tested positive for SARS-CoV-2: a Danish nationwide cohort study. PLoS Med 2020;17:e1003308. 10.1371/journal.pmed.1003308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan S, Chen P, Li H, et al. Mortality and pre-hospitalization use of low-dose aspirin in COVID-19 patients with coronary artery disease. J Cell Mol Med 2021;25:1263–73. 10.1111/jcmm.16198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matharu GS, Kunutsor SK, Judge A, et al. Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med 2020;180:376–84. 10.1001/jamainternmed.2019.6108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krauss ES, Cronin M, Dengler N, et al. Lessons learned: using the Caprini risk assessment model to provide safe and efficacious thromboprophylaxis following hip and knee arthroplasty. Clin Appl Thromb Hemost 2020;26:107602962096145. 10.1177/1076029620961450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang H, Ding X, Li H, et al. Association between prior aspirin use and acute respiratory distress syndrome incidence in at-risk patients: a systematic review and meta-analysis. Front Pharmacol 2020;11:738. 10.3389/fphar.2020.00738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, Zhong M, Wang Z, et al. The preventive effect of antiplatelet therapy in acute respiratory distress syndrome: a meta-analysis. Crit Care 2018;22:60. 10.1186/s13054-018-1988-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Viecca M, Radovanovic D, Forleo GB, et al. Enhanced platelet inhibition treatment improves hypoxemia in patients with severe Covid-19 and hypercoagulability. A case control, proof of concept study. Pharmacol Res 2020;158:104950. 10.1016/j.phrs.2020.104950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X, Li Z, Liu S, et al. Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19. Acta Pharm Sin B 2020;10:1205–15. 10.1016/j.apsb.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanthi Y, Knight JS, Zuo Y, et al. New (re)purpose for an old drug: purinergic modulation may extinguish the COVID-19 thromboinflammatory firestorm. JCI Insight 2020;5:e140971. 10.1172/jci.insight.140971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chow JH, Khanna AK, Kethireddy S, et al. Aspirin use is associated with decreased mechanical ventilation, ICU admission, and in-hospital mortality in hospitalized patients with COVID-19. Anesth Analg 2020;Publish Ahead of Print. 10.1213/ANE.0000000000005292 [DOI] [PubMed] [Google Scholar]
- 54.Loo SK-F, Hon KL, Leung AK. Kawasaki disease in siblings and a review of drug treatment. Drugs Context 2020;9:1–5. 10.7573/dic.2020-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LeFevre ML. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. preventive services Task force recommendation statement. Ann Intern Med 2014;161:819–26. 10.7326/M14-1884 [DOI] [PubMed] [Google Scholar]
- 56.ACOG practice Bulletin No. 202: gestational hypertension and preeclampsia. Obstet Gynecol 2019;133:1. 10.1097/AOG.0000000000003018 [DOI] [PubMed] [Google Scholar]
- 57.Kwiatkowski S, Borowski D, Kajdy A, et al. Why we should not stop giving aspirin to pregnant women during the COVID-19 pandemic. Ultrasound Obstet Gynecol 2020;55:841–3. 10.1002/uog.22049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gavillet M, Rolnik DL, Hoffman MK, et al. Should we stop aspirin prophylaxis in pregnant women diagnosed with COVID-19? Ultrasound Obstet Gynecol 2020;55:843–4. 10.1002/uog.22063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bianconi V, Violi F, Fallarino F, et al. Is Acetylsalicylic Acid a Safe and Potentially Useful Choice for Adult Patients with COVID-19 ? Drugs 2020;80:1383–96. 10.1007/s40265-020-01365-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
fmch-2020-000741supp001.pdf (7.3MB, pdf)


