Abstract
Background
Irisin (a glycosylated protein) is cleaved from fibronectin type III domain-containing protein 5 (FNDC5), which is expressed mainly in animal muscle tissues and has multiple metabolic regulatory activities. However, their roles in controlling myofiber types in skeletal muscle remain unclear.
Methodology
Two different commercial hybridized pigs, LJH (a crossed pig containing Chinese native pig genotypes) and DLY (Duroc × Landrace × Yorkshire) were selected to analyze FNDC5 mRNA expression and the mRNA composition of four adult myosin heavy chain (MyHC) isoforms (IIIaIIxIIb) in the longissimus dorsi (LD) muscle. C2C12 myoblasts were cultured to investigate the effects of FNDC5 on the four MyHCs mRNA expressive levels, using small interfering RNA for depletion and a eukaryotic expression vector carrying FNDC5 for overexpression. ZLN005 (a small molecule activator of FNDC5’s upstream control gene PGC1α) or recombinant human irisin protein were also used.
Results
In LD muscle, LJH pigs had the higher FNDC5 mRNA level, and MyHC I or IIa proportion than DLY pigs (P < 0.05). For C2C12 cells in vitro, small interfering RNA (si-592) silencing of FNDC5 expression markedly reduced MyHC IIa mRNA levels (P < 0.05), while FNDC5 overexpression significantly increased MyHC IIa mRNA levels (P < 0.05). Exogenous irisin increased the mRNA levels of PGC1α (peroxisome proliferator-activated receptor gamma coactivator 1-alpha), FNDC5, MyHCI, MyHCIIa, NRF1 (nuclear respiratory factor 1), VEGF (vascular endothelial growth factor), and TFAM (mitochondrial transcription factor A,) (P < 0.05), and the enzyme activities of SDH (succinate dehydrogenase), CK (creatine kinase), and MDH (malate dehydrogenase) in C2C12 myotubes (P < 0.05). These results showed that FNDC5 mRNA expression had a significant association with the characteristics of myofiber types in porcine muscle, and participated in regulating MyHCs mRNA expression of C2C12 myogenic differentiation cells in vitro. FNDC5 could be an important factor to control muscle fiber types, which provides a new direction to investigate pork quality via muscle fiber characteristics.
Keywords: FNDC5, Pig, Irisin, MyHC, C2C12
Introduction
Muscle fiber is the basic unit of skeletal muscle in animals (Choi, Ryu & Kim, 2007). According to the contraction properties, mammalian skeletal muscle fibers are classified as slow-oxidative (type I), fast-oxidative (type IIa), intermediate (type IIx) and fast-glycolytic (type IIb) muscle fibers, and their myosin heavy chains (MyHCs) are mainly MyHC I, MyHCIIa, MyHC IIx and MyHC IIb isforms, respectively. Compared with glycolytic (type IIb) or intermediate (type IIx) muscle fibers, oxidative (type I and IIa) muscle fibers are richer in mitochondria and capillaries, have the stronger resistance to fatigue and rely more on oxidative phosphorylation to generate energy (Schiaffino & Reggiani, 2011). In contrast, glycolytic (type IIb) muscle fibers are prone to fatigue due to their rapid contraction (Schiaffino & Reggiani, 2011). Muscle fiber-types composition not only has a direct effects on the morphological characteristics and contractile function of skeletal muscle (Lee, Joo & Ryu, 2010; Ryu & Kim, 2005), but also affects meat water-holding capacity, tenderness, meat colour, pH and flavor in postmortem muscle through the different metabolic active factors, protein composition, fiber diameter and density among different muscle fiber-types. Thus, it has become a consensus that pork quality can be controlled through changing muscle fiber-types.
Irisin, as a glycosylated protein cleaved from fibronectin type III domain-containing protein 5 (FNDC5), was found to play certain roles in the processes of brown adipose cells and showed anti-obesity (Boström et al , 2012). Later, it was demonstrated that irisin could also improve multiple diseases, such as diabetes mellitus, chronic kidney disease, alcoholic fatty liver disease, metabolic syndrome, and neurological diseases (Cao et al., 2019). Irisin secretion or FNDC5 gene expression can be regulated by exercise-activated and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α ) pathways (Boström et al , 2012; Cao et al., 2019). In the present study, the objective was to investigate the relationship between FNDC5 gene expression and the mRNA composition of four adult MyHC isoforms(I, IIa, IIx and IIb) in porcine muscle, and the effects of FNDC5-irisin on the four MyHCs mRNA expression in C2C12 myoblast cells in vitro. The results could provide the new idea for us to control pork quality by regulating muscle-fiber types.
Materials & Methods
Animal feeding, muscle sampling, FNDC5 expression, and MyHC mRNA composition analysis
We selected 16 LJH (a crossed pigs containing Chinese native pig genotypes) and DLY (Duroc × Landrace × Yorkshire) weanling castrated pigs (eight pigs each hybrid combination), fed in Lvjia Yuan Livestock Industry Co., Ltd, Zhejiang Province, China. All animal feeding management and slaughtering processes were approved by the Laboratory Animal Management Committee of Zhejiang Academy of Agricultural Sciences in China (No. 2018110). At final body weights of 100–110 kg, these pigs were transferred to an abattoir by ordinary commercial truck (journey time ∼2.5 h) according to the commercial standard slaughtering process in China. The longissimus dorsi (LD) muscle located in the last 3–4 thoracic vertebrae was immediately collected after slaughter.
The total RNA in muscle was isolated using the Trizol method, and cDNA was synthesized using a ReverTra Ace qPCR RT Kit (Toyobo, Osaka, Japan). Quantitative real-time PCR (qPCR) was performed for the FNDC5 mRNA level, with the 18s rRNA gene as the reference gene. The FNDC5 primer sequences were as follows: F-5′-tgcaggccatccattcag-3′, R-5′-ccacagagaccacga-3′, generating a 182 bp amplicon (Cai et al., 2017); 18s primer sequences were as follows: F-5′-cccacggaatcgagaaagag-3′, R-5′-ttgacggaagggcacca-3′(Men et al., 2017).The relative quantification of FNDC5 mRNA was calculated using the 2 −ΔΔCT method (Livak & Schmittgen, 2002).
According to the description in Men et al. (2017), the MyHC mRNA composition was analyzed using qPCR. The relative ratio of each MyHC-type mRNA was calculated as the corresponding copy number per mg of muscle sample divided by the sum of four MyHC-types mRNA, multiplied by 100.
Culture, differentiation, and transfection of C2C12 myoblast cells
According to Zhou et al. (2013), C2C12 cells (an immortalized mouse myoblast cell line from the Shanghai Institute of Biochemistry and Cell Biology of Chinese Academy of Sciences, Shanghai, China) were cultured and induced to differentiate into myotubes in Dulbecco’s modified Eagle’s medium (DMEM) with 2% horse serum. Mycoplasma detection on C2C12 myoblast cells was carried out using a MycAway™ -Color One-Step Mycoplasma Detection Kit by YEASEN Biotech Co. Ltd., Shanghai city, China.
The small interfering RNA targeting FNDC5 (si-592), the native oligonucleotide fragments (3′-uucuccgaacgugucacgutt-5′, 3′-acgugacacguucggagaatt-5′) for siRNA transfection, and the eukaryotic expression vector overexpressing FNDC5 were supplied by GenePharma Biotechnology Co. Ltd., Shanghai city, China, according to the coding region sequence of FNDC5 (NM_027402.4). The si-592 sequences included 3′-gccaguaugauaugaucaat-5′and 3′-uugaugaugauaucauacuggctt-5′and were transfected into C2C12 cells twice at day 0 and day 2 post inductive differentiation, respectively. The FNDC5 eukaryotic expression vector was constructed using plasmid pcDNA3.1(+), and transfected into C2C12 cells once at day 0 post inductive differentiation.
The transfection experiment was grouped as follows: nuclease-free water as the blank group, transfection reagent as the mock group, pcDNA3.1(+) plasmid the as vector-NC group, native oligonucleotide fragments as the si-NC group, si-592 fragment as FNDC5(-) group, and pcDNA3.1(+)-FNDC5 vector as FNDC5(+) group. According to the instructions of GP-RNA-mate kit supplied by GenePharma Biotechnology Co. Ltd, 0.4 mL of each transfection mixture was added into each well of a 6-well plate with 1.6 mL of fresh serum-free DMEM medium, and incubated at 37 °C for 5 h. After 4 days of incubation, the cultured cells were harvested for RNA or protein determination.
ZLN005 or irisin treatment on C2C12 myoblasts
ZLN005, a small molecule activator of PGC-1α, was purchased from Cayman Chemical Company (Ann Arbor city, Michigan, USA). C2C12 cells (70–80% confluent) were induced in differentiation medium (DMEM+2% horse serum) containing various concentrations of ZLN005 (0, 5, and 10 µmol/mL) for 4 days and then collected. For the ZLN005-10 group, some cells were transfected with si-592, and harvested for RNA extraction. Recombinant human irisin protein was purchased from Pepro Tech Inc. Rocky Hill, NJ, USA. C2C12 cells were incubated in differentiation medium for 4 days, cultured in medium containing 0, 20, or 200 ng/mL irisin for 5 h, and then harvested for RNA extraction and metabolic enzyme activity assays.
Quantitative real-time PCR (qPCR) and western blotting of cells
The RNA extraction, cDNA synthesis, and qPCR for C2C12 cells were performed similarly to the above descriptions for muscle tissue. All data were normalized against the mRNA expression of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene. Determinations were performed in triplicate. The primer sequences in Table 1 were designed and synthesized by GenePharma Biotechnology Co. Ltd, Shanghai city, China.
Table 1. List of genes and sequences of the primers for real-time quantitative PCR in vitro C2C12 cells.
| Genes | Primer | Sequence 5′–3′ | References |
|---|---|---|---|
| FNDC5 | Forward Reverse | AGCTCAGAAGTAGAATGCGAGAG GGTGATAGGAGAAGATGGTGGTG |
Chen et al. (2019) |
| GAPDH | Forward Reverse | AGACAGCCGCATCTTCTTGT CTTGCCGTGGGTAGAGTCAT |
Chen et al. (2019) |
| MyHCI | Forward Reverse | TTGCTGTTATTGCCGCCATTG GAGTTGTCATTCCGAACTGTCTTG |
Li et al. (2019) |
| MyHCIIx | Forward Reverse | CGAAGTTGCATCCCTAAAGGCAG CGAAAACGGCCATCTCGGC |
Li et al. (2019) |
| MyHCIIb | Forward Reverse | GAAGGAGGGCATTGATTGGGAG TGTTCTTGAAGGAGGTGTCTGTCGC |
Li et al. (2019) |
| MyHCIIa | Forward Reverse | TTCCAGAAGCCTAAGGTGGTC GCCAGCCAGTGATGTTGTAAT |
Chen et al. (2018) |
| PGC1 α | Forward Reverse | CCAGTACAACAATGAGCCTGC CAATCCGTCTTCATCCACG |
Chen et al. (2018) |
| NRF1 | Forward Reverse | GCTGCTTTCAGTCCTTCTGG GTGTTCAGTTTGGGTCACTCC |
Wadley, Choate & Mcconell (2007) |
| IL15 | Forward Reverse | AATCCACCTTGACACATGGC AGGCTGGTTATCTGCTGACA |
Waickman et al. (2017) |
| VEGF | Forward Reverse | CGTTTAACTCAAGCTGCCTCGC CTTCCAGGAGTACCCCGACGAGATA |
Tang et al. (2010) |
| TFAM | Forward Reverse | CACCCAGATGCAAAACTTTCAG CTGCTCTTTATACTTGCTCACAG |
Makiko et al. (2015) |
Total protein extraction, SDS-PAGE gel electrophoresis, and western blotting for cells were performed according to Cai et al. (2017). The FNDC5 protein was separated using 12% SDS electrophoresis, transferred onto a cellulose acetate membrane, and then incubated with recombinant rabbit anti-FNDC5 monoclonal antibodies (ab174833, Abcam, Cambridge, UK), or a monoclonal anti- β-Actin antibody produced in mouse (A5441, Merck Life Science Co., Ltd, Shanghai city, China. Chemiluminesence detection was performed on an FR-1800 Luminescent and Fluorescent Biological Image Analysis System of Furi company, Shanghai city, China. The scanned images were processed and analyzed using Gel-Pro analyzer software from Media Cybernetics, Rockville, MD, USA. The relative content of FNDC5 protein in a sample was calculated through the ratio of the gray value to that of the internal reference actin.
Detection of metabolic enzyme activity in cells
The harvested cell cultures were homogenized in one mL of phosphate buffered saline (pH 7.4), and then centrifuged at 1,000 rpm for 10 min at 4 °C. The supernatant was collected to detect the activities of succinate dehydrogenase (SDH), malate dehydrogenase (MDH), lactate dehydrogenase (LDH) and creatine kinase (CK), and protein concentration using the standard commercial kits from Nanjing Jiancheng Biochemical Institute, Nanjing city, China.. Enzyme activities were expressed as U/mg protein.
Statistical analysis
All data are presented as the mean ± standard deviation (SD) and all statistical analysis programs were operated in SPSS 16.0 (IBM Corp., Armonk, NY, USA). Data from porcine muscle tissue were analyzed using the t-test (P < 0.05) between LJH and DLY pig groups. Data from the cell experiments were analyzed using one-way analysis of variance and Duncan’s test (P < 0.05) among multiple treatment groups.
Results
The variation of FNDC5 mRNA levels and MyHC mRNA composition in porcine LD muscle and their correlations
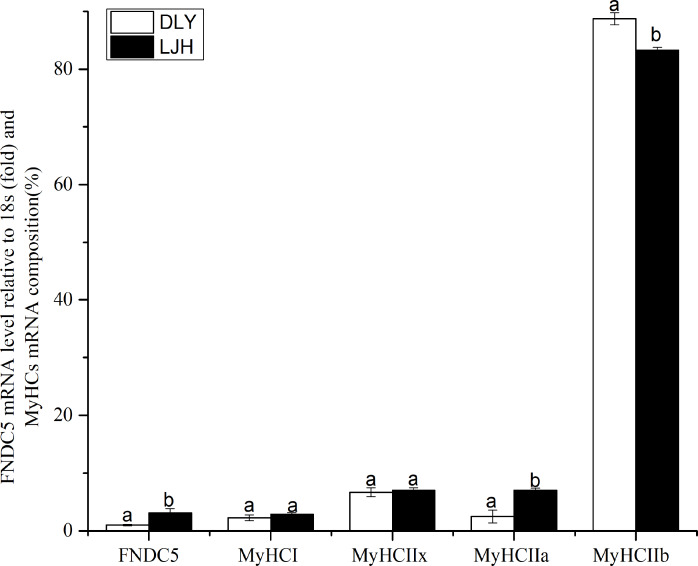
As shown in Fig. 1, LJH pigs had a higher FNDC5 mRNA level and MyHCI or IIa mRNA proportions, and a lower MyHCIIb mRNA proportion in LD muscle than DLY pigs (P < 0.05).
Figure 1. Difference of FNDC5 mRNA level and MyHCs mRNA composition in porcine LD muscle between LJH and DLY crossed pigs.
Data are shown as means ± sd from eight pigs each crossed combination. The same letter on the label means P > 0.05; a different letter means P < 0.05. FNDC5, fibronectin type III domain-containing protein 5. LJH means a crossed pigs containing Chinese native pig genotypes, DLY means Duroc × Landrace × Yorkshire crossed pigs.
Knockdown or up expression of FNDC5 gene in C2C12 myoblast cells
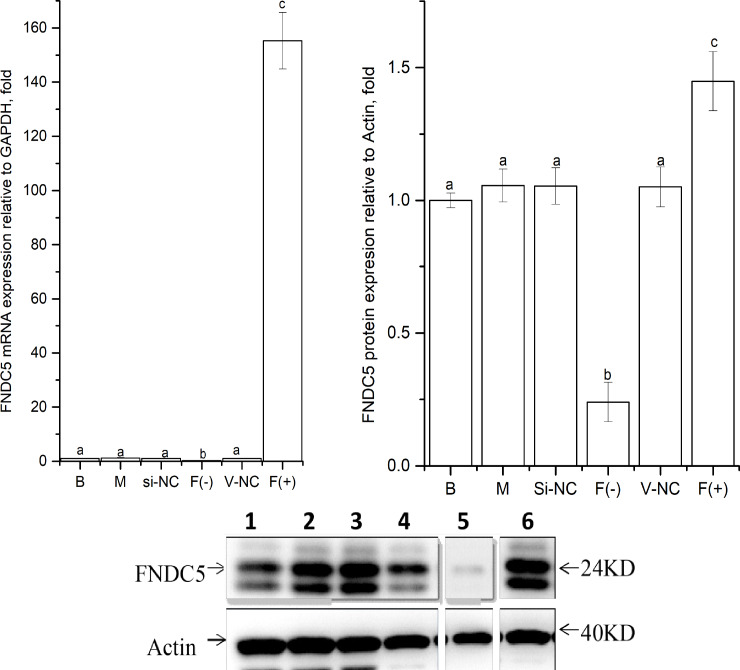
As shown in Fig. 2, the expression levels of FNDC5 mRNA and protein were not significantly different among the blank, mock, si-NC, and vector-NC groups (P > 0.05). In the FNDC5(-)group, the expression levels of FNDC5 mRNA and protein were decreased significantly compared with those in the blank group by si-592 (P < 0.05). In the FNDC5(+) group, the expression levels of FNDC5 mRNA and protein were increased significantly compared those in the blank group by pcDNA3.1(+)-FNDC5 vector (P < 0.05).
Figure 2. Effects of nucleic acid transfection on FNDC5 mRNA (A) and protein (B) expressions in vitro in C2C12 myotubes.
Each data point are shown as means ± sd from three repeated cell treatments. The same letter on the label means P > 0.05; a different letter means P < 0.05. In the images (C) of Western-Blot for FNDC5 protein expression, 1–6 means B, M, Si-NC, Vector-NC, si592/FNDC5(-),FNDC5(+).
Effects of down or up regulating FNDC5 expression on MyHCs mRNA levels in C2C12 myoblast cells
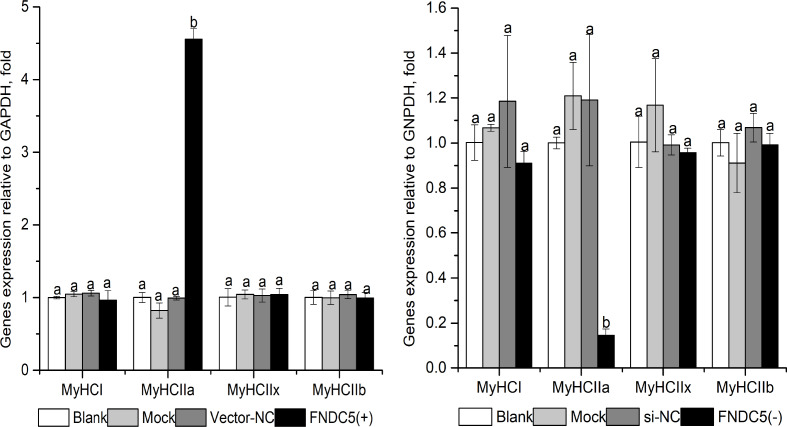
As shown in Fig. 3, MyHCI, MyHCIIx and MyHCIIb mRNA levels were not affected significantly in the FNDC5 (-) or FNDC5 (+)group (P > 0.05). MyHCIIa mRNA expression in the FNDC5 (-) group were decreased significantly compared with that in other groups (P < 0.05), and increased significantly in the FNDC5 (+) group compared that in other groups (P < 0.05).
Figure 3. Effect of FNDC5. gene knockdown (A) or up-regulated (B) expression on MyHCs mRNA levels during C2C12 myogenic differentiation.
Each data point are shown as means ± sd from three repeated cell treatments. The same letter on the label means P > 0.05; a different letter means P < 0.05. FNDC5, fibronectin type III domain-containing protein 5; MyHCs, myosin heavy-chains. FNDC5 (-) means FNDC5 down-regulation by si592, FNDC (+) means FNDC5 up-regulation by over-expression vector.
Effects of FNDC5 knockdown expression on PGC1α-FNDC5-MyHCs mRNA expression under ZLN005 in C2C12 myoblast cells
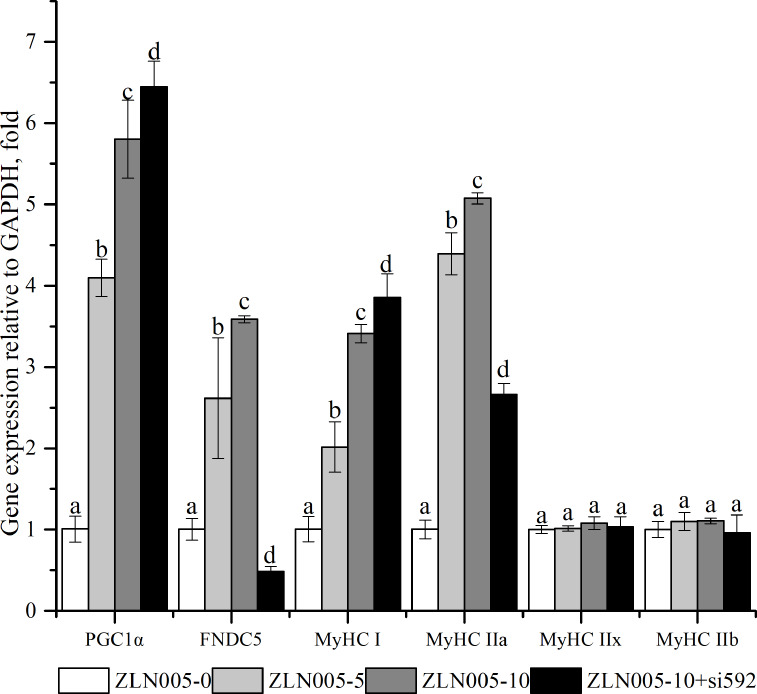
As shown in Fig. 4, compared with the ZLN005-0 group, the ZLN005-5 and ZLN005-10 groups showed significantly the increased expressive levels of PGC1α, FNDC5, MyHCI and MyHCIIa mRNA (P < 0.05). Compared with that in the ZLN005-10 group, ZLN005-10 + si592 group showed significantly the decreased expressive levels of FNDC5 and MyHCIIa mRNA (P < 0.05).
Figure 4. PGC1α-FNDC5-MyHC mRNA expressions in C2C12 myotubes with different amounts of ZLN005 and FNDC5(-) (si592) transfection.
Each data point are shown as means ± sd from three repeated cell treatments. The same letter on the label means P > 0.05; a different letter means P < 0.05. FNDC5, fibronectin type III domain-containing protein 5; MyHC, myosin heavy-chain; PGC1 α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha.
Effects of adding irisin on PGC1α-FNDC5-MyHCs mRNA expression in C2C12 myoblast cells
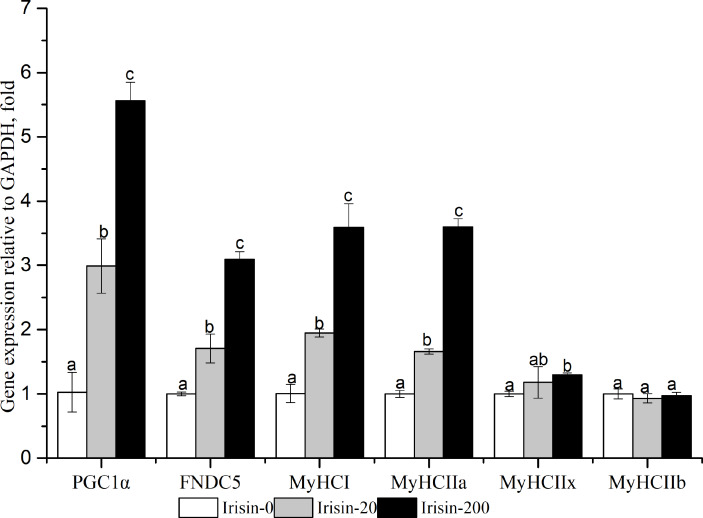
As shown in Fig. 5, PGC1α , FNDC5, MyHCI, and MyHCIIa mRNA expressions were significantly upregulated with the increasing irisin addition (P < 0.05).The Irisin-200 group also showed significantly increased MyHCIIx mRNA expression compared with that in the irisin-0 group (P < 0.05).
Figure 5. Effects of adding irisin on PGC1α, FNDC5 and MyHC genes expressions in C2C12 myotubes with different amounts of irisin.
Each data point are shown as means ± sd from three repeated cell treatments. The same letter on the label means P > 0.05; a different letter means P < 0.05. FNDC5, fibronectin type III domain-containing protein 5; MyHCs, myosin heavy-chain; PGC1 α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha.
Effects of adding irisin on metabolic enzyme activities and other downstream gene expression levels in C2C12 myoblast cells
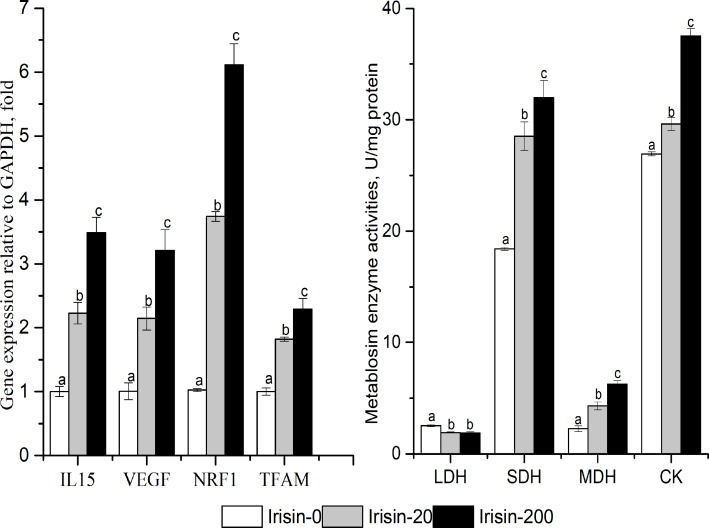
As shown in Fig. 6, the enzyme activities of SDH, MDH and CK, and the mRNA expression levels of IL15 (interleukin 15), VEGF (vascular endothelial growth factor), NRF1 (nuclear respiratory factor 1), and TFAM (transcription factor A, mitochondrial) increased with increasing irisin addition in C2C12 myotubes (P < 0.05). The activity of LDH was significantly lower in the irisin-20 and irisin-200 groups than that in the Irisin-0 group (P < 0.05).
Figure 6. Effects of adding irisin the metabolic enzyme activities(B) and other downstream genes expressions (A) in C2C12 myotubes.
Each data point are shown as means ± sd from three repeated cell treatments. The same letter on the label means P > 0.05; a different letter means P < 0.05. CK, creatine kinase; SDH, Succinate dehydrogenase; MDH, Malate dehydrogenase; LDH, lactate dehydrogenase; IL15, interleukin 15; VEGF, vascular endothelial growth factor; NRF1, nuclear respiratory factor 1; TFAM, transcription factor A mitochondrial.
Discussion
To date, various cellular signaling pathway factors mediating muscle fiber-types conversion have been identified, such as the Ca 2+/calcineurin, NFAT, AMP-activated protein kinase (AMPK), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α), mitogen-activated protein kinase, myocyte enhancer factor 2 (MEF2), Wnts, forkhead transcriptional factor (FoxO1) and myofiber regulation factors (MRFs) (Li et al., 2019; Rockl et al., 2007; Handschin et al., 2007a; Handschin et al., 2007b; Scharf et al., 2013; Anderson et al., 2015; Cisternas et al., 2014; Yuan et al., 2011; Li et al., 2014).
As a key factor in regulating skeletal muscle fiber type transformation (especially oxidative fiber formation), PGC1α can induced FNDC5 expression and irisin secretion in skeletal muscle (Boström et al , 2012; Svensson & Handschin, 2014; Bai et al., 2019). PGC1α, FNDC5 and oxidative-type muscle fiber can be induced some the same factors, such as endurance training, stretching, mechanical load, outdoor exercise and cold environment (Men, Tao & Xu, 2016). In addition, the increasing mitochondrion is an important marker of oxidative-type muscle fibers formation, also the significant result of FNDC5 or irisin action. FNDC5 also plays a regulatory role in fatty acid metabolism and glucose utilization (Guo et al., 2019; Cao et al., 2019), and interacts with myogenic regulatory factors (Bai et al., 2019). Ellefsen et al. (2014) found that FNDC5 gene expression was correlated with proportions of aerobic muscle fibers in untrained women, but the relationship disappeared in trained ones. These existed researches suggested a closely association between FNDC5 and muscle fiber-types. The study found that FNDC5 mRNA expression and MyHC I and IIa mRNA percentage exhibited the same direction difference in LD muscle from different genotype pigs, which confirmed the closely association between FNDC5 gene and muscle fiber types.
To further explore the underlying mechanism of FNDC5-irisin effects on muscle fiber types, the skeletal muscle model cells C2C12 were used. We found that MyHCIIa mRNA and FNDC5 mRNA or protein expression levels were increased by transfection of the FNDC5-vector during C2C12 myogenic differentiation in vitro. By contrast, MyHCIIa mRNA, and FNDC5 mRNA or protein expression levels were decreased after transfection with si-FNDC5 (si-592). These results suggested that FNDC5 might be involved in the formation of fast-oxidative muscle fibers during the myogenic differentiation of C2C12 cells in vitro.
In previous reports, PGC1α was identified as the upstream dependent factor controlling FNDC5 expression and irisin secretion (Boström et al , 2012). For instance, the PGC1α-FNDC5-UCP1 axis functions in regulating metabolism in adipose tissue or cells (Sanchez-Delgado et al., 2015). In the present study, the addition of ZLN005 increased the mRNA levels of PGC1α, FNDC5, MyHCI, and MyHCIIa in C2C12 myoblast cells. This phenomenon accorded with the transcriptional activation by ZLN005 on PGC1α (Zhang et al., 2013), and the promoting effects of PGC1α on oxidative-type myofiber formation were verified in mouse skeletal muscle (Handschin, 2009) and porcine skeletal muscle (Lin, Hangschin & Spiegelman, 2005; Ying et al., 2016). Therefore, the PGC1α-FNDC5-MyHCs pathway of C2C12 cells was activated by ZLN005.
To further reveal the effects of FNDC5 gene on PGC1α-regulated muscle fiber-types, we knocked down FNDC5 gene expression in the context of ZLN005-mediated activation of the PGC1α signaling pathway. The result showed that FNDC5 knockdown expression significantly suppressed MyHCIIa mRNA expression under PGC1α activation, but had no effects on MyHCI, MyHCIIx or MyHCIIb mRNA levels. FNDC5 might be directly involved in PGC1α-induced MyHCIIa expression rather than MyHCI expression in C2C12 myoblast cells.According to the previous reports, irisin has a wide range of biological functions on various tissues or organs with the help of circulation system (Polyzos et al., 2013; Hawley et al., 2014; Cao et al., 2019). Our results confirmed that irisin could activate the upstream PGC1α-FNDC5 pathway in C2C12 cells. According to Ye et al. (2019), irisin could promote glucose uptake in C2C12 cells by activating the AMP-activated protein kinase (AMPK) signaling pathway (Ye et al., 2019). At the same time, the AMPK pathway can activate mitochondrial biogenesis through activating PGC1α (Liang et al., 2018). These observations indicated that irisin functions via an autocrine regulatory mechanism, which would provide a theoretical explanation for irisin’s effects on the differentiation of muscle fiber-types, mitochondrial synthesis, and tissue metabolism.
Our results supported the functions of PGC1α gene. First, the activities of the oxidative metabolic enzymes (SDH and MDH) and the expression of oxidative muscle-fiber genes (MyHC I and MyHC IIa) were increased by irisin treatment in C2C12 myotubes. Second, irisin treatment increased the expressions of IL15, NRF1, VEGF, and TFAM in a dose-dependent manner. FNDC5, IL15, NRF1, VEGF, and TFAM are important downstream target genes of PGC1α (Cao et al., 2019; Jessica et al., 2009; Chen et al., 2018; Chen et al., 2019). In muscle cells, IL15 can stimulate glucose transport and oxidation (Scharf et al., 2013), NRF1 and TFAM participate in mitochondrial synthesis (Yu et al., 2014; Makiko et al., 2015), and VEGF can stimulate angiogenesis and is associated with the formation of oxidative muscle-fibers (Li, 2014).
Conclusions
This study investigate the intermediate effects of FNDC5 gene on muscle fiber types. In porcine muscle, the differences between two different porcine populations was uniform in FNDC5 mRNA level, the mRNA proportions of MyHC I, MyHCIIa, or MyHCIIx, but contrasted in MyHCIIb mRNA proportion.. In C2C12 myoblast cells in vitro, FNDC5 gene was demonstrated to be directly involved in MyHCIIa mRNA expression.Irisin could activate PGC1α gene expression, a upstream dependent gene of FNDC5, and further play a more extensive role in skeletal muscle cells, including the autocrine regulation on FNDC5 expression, mitochondrial functions, energy metabolic enzyme activities. FNDC5 gene could be an important factor to control muscle fiber types, which would provide the newdirection to investigate pork quality by muscle fiber characteristics.
Supplemental Information
Each data point indicates the value from one pig.
Each data point indicates the average performance of three repeats measures of cells
The instructions of MycAway™ -Color One-Step Mycoplasma Detection Kit and detection results.
Funding Statement
This work was supported by grants from the Science and Technology Projects in Zhejiang Province of China (grant numbers 2021C02007, LY17C170005, 2016C02054). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Xiao-Ming Men conceived and designed the experiments, performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Zi-Wei Xu conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Xin Tao performed the experiments, prepared figures and/or tables, and approved the final draft.
Bo Deng analyzed the data, prepared figures and/or tables, and approved the final draft.
Ke-Ke Qi performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
Laboratory Animal Management Committee of Zhejiang Academy of Agricultural Sciences provided full approval for this research (No. 2018110).
Data Availability
The following information was supplied regarding data availability:
Raw data showing MyHCs mRNA composition in porcine muscle and various genes expressions in C2C12 cells are available in the Supplementary Files.
References
- Anderson et al. (2015).Anderson CM, Hu J, Barnes RM, Heidt AB, Cornelissen I, Black BL. Myocyte enhancer factor 2C function in skeletal muscle is required for normal growth and glucose metabolism in mice. Skeletal muscle. 2015;5:7. doi: 10.1186/s13395-015-0031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai et al. (2019).Bai Y, Bi H, Li L, Li J, Yu X, Ren H, Li Y, Ji Y, Hi Li, Wang H. Effects of myostatin deficiency on PGC-1α and FNDC5 expression in three different murine muscle types. Acta Histochemica. 2019;121:323–329. doi: 10.1016/j.acthis.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Boström et al (2012).Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. A PGC1- α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai et al. (2017).Cai CB, Xiao GJ, Qian LL, Jiang SW, Li B, Xie SW, Gao T, An XR, Cui WT, Li K. Gene location, expression, and function of FNDC5 in Meishan pigs. Scientific Reports. 2017;7:7886. doi: 10.1038/s41598-017-08406-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao et al. (2019).Cao RY, Zheng HC, Damian R, Yang J. FNDC5: a novel player in metabolism and metabolic syndrome. Biochimie. 2019;158:111–116. doi: 10.1016/j.biochi.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2019).Chen SQ, Ding LN, Zeng NX, Liu HM, Zheng SH, Xu JW, Li RM. Icariin induces irisin/FNDC5 expression in C2C12 cells via the AMPK pathway. Biomedicine and Pharmacotherapy. 2019;115:108930. doi: 10.1016/j.biopha.2019.108930. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2018).Chen ZB, Tao SW, Li XH, Yao QH. Resistin destroys mitochondrial biogenesis by inhibiting the PGC-1α/NRF1/TFAM signaling pathway. Biochemical and Biophysical Research Communications. 2018;504:13–18. doi: 10.1016/j.bbrc.2018.08.027. [DOI] [PubMed] [Google Scholar]
- Choi, Ryu & Kim (2007).Choi YM, Ryu YC, Kim BC. Influence of myosin heavy and light chain iso-forms on early postmortem glycolytic rate and pork quality. Meat Science. 2007;76:281e288. doi: 10.1016/j.meatsci.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Cisternas et al. (2014).Cisternas P, Henriquez JP, Brandan E, Inestrosa NC. Wnt signaling in skeletal muscle dynamics: myogenesis, neuromuscul, synap, fibros. Molecular neurobiology. 2014;49:574–589. doi: 10.1007/s12035-013-8540-5. [DOI] [PubMed] [Google Scholar]
- Ellefsen et al. (2014).Ellefsen S, Vikmoen O, Slettalkken G, Whist JE, Nygaard H, Hollan I, Rauk I, Vegge G, Strand TA, Raastad T, Rannestad BR. Irisin and FNDC5: effects of 12-week strength training, and relations to muscle phenotype and body mass composition in untrained women. European Journal of Apply Physiology. 2014;114:1875–1888. doi: 10.1007/s00421-014-2922-x. [DOI] [PubMed] [Google Scholar]
- Guo et al. (2019).Guo Q, Wei X, Hu H, Yang DQ, Zhang B, Fan X, Liu J, He H, Oh Y, Wu Q, Zhang Y, Wang C, Liu C, Gu N. The saturated fatty acid palmitate induces insulin resistance through Smad3-mediated down-regulation of FNDC5 in myotubes. Biochemical and Biophysical Research Communications. 2019;520:619–626. doi: 10.1016/j.bbrc.2019.10.077. [DOI] [PubMed] [Google Scholar]
- Handschin (2009).Handschin C. The biology of PGC-1a and its therapeutic potential. Cell. 2009;30:322–329. doi: 10.1016/j.tips.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Handschin et al. (2007a).Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelma BM. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. Journal of Biological Chemistry. 2007a;282:30014–30021. doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- Handschin et al. (2007b).Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM. Skeletal muscle fiber-type switching, exercise in tolerance, and myopathy in PGC-1α muscle-specific knock-out animals. Journal of Biological Chemistry. 2007b;282:30014–30021. doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- Hawley et al. (2014).Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative Biology of Exercise. Cell. 2014;159:738–749. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- Jessica et al. (2009).Jessica C, Jorge R, Gupta RK, Thom R, Shoag J, Rowe GC, Sawada N, Raghuram S, Arany Z. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21401–21406. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Joo & Ryu (2010).Lee SH, Joo ST, Ryu YC. Skeletal muscle fiber type and myofibrillar proteins in relation to meat quality. Meat Science. 2010;86:166e170. doi: 10.1016/j.meatsci.2010.04.040. [DOI] [PubMed] [Google Scholar]
- Li et al. (2014).Li BJ, Li PH, Wu WJ, Li QF, Huang RH, Liu HL. Progresses in research of the mechanisms of skeletal muscle fiber formation. Scientia Agricultura Sinica. 2014;47:1200–1207. (in Chinese) [Google Scholar]
- Li et al. (2019).Li LX, Wang J, Bai Y, Li JW, Yu XJ, Luo XM, Zhu ZW, He XY, Dong YJ, Li HQ, Wang HD. Effect of hypoxia on the muscle fiber switching signal pathways CnA/NFATc1 and myostatin in mouse myocytes. Acta Histochemica. 2019;121:539–545. doi: 10.1016/j.acthis.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Li (2014).Li Y. The effect of VEGF activating transcription factor in therapeutic angiogenesis and skeletal muscle fiber in the mouse with hindlimb ischemia. Journal of Vascular Surgery. 2014;59:81S-81S. doi: 10.1016/j.jvs.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Liang et al. (2018).Liang TY, Wu JP, Liu T, Bai Y, Zhang R. Recent progress in classification and transformation mechanism of muscle fiber types. Meat Research. 2018;32:55–61. (in Chinese) [Google Scholar]
- Lin, Hangschin & Spiegelman (2005).Lin J, Hangschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metabolism. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Makiko et al. (2015).Makiko Y, Ayano N, Yuri S, Ayako S, Mariko T, Sakuka T, Kazuo K, Kaoruko I. Dietary isoflavone daidzein promotes Tfam expression that increases mitochondrial biogenesis in C2C12 muscle cells. The Journal of Nutritional Biochemistry. 2015;26:1193–1199. doi: 10.1016/j.jnutbio.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Men et al. (2017).Men XM, Deng B, Tao X, Qi KK, Xu ZW. Wnt gene expression in adult porcine longissimus dorsi and its association with muscle fiber type, energy metabolism, and meat quality. Journal of Integrative Agriculture. 2017;16:144–150. doi: 10.1016/S2095-3119(16)61451-X. [DOI] [Google Scholar]
- Men, Tao & Xu (2016).Men XM, Tao X, Xu ZW. Irisin-processor gene: molecular structure, expressive regulation, biological functions and the associations with skeletal muscle fiber types. Chinese Journal of Animal Nutrition. 2016;28:310–318. (In Chinese) [Google Scholar]
- Polyzos et al. (2013).Polyzos SA, Kountouras J, Shields K, Mantzoros CS. Irisin: a renaissance in metabolism? Metabolism: Clinical and Experimental. 2013;62:1037–1044. doi: 10.1016/j.metabol.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Rockl et al. (2007).Rockl KSC, Hirshman MF, Brandauer J, Fujii N, Witters LA, Goodyear LJ. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes. 2007;56:2062–2069. doi: 10.2337/db07-0255. [DOI] [PubMed] [Google Scholar]
- Ryu & Kim (2005).Ryu YC, Kim BC. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus-dorsi muscle. Meat Science. 2005;71:351e357. doi: 10.1016/j.meatsci.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Sanchez-Delgado et al. (2015).Sanchez-Delgado G, Martinez-Tellez B, Olza J, Aguilera CM, Á Gil, Ruiz JR. Role of exercise in the activation of brown adipose tissue. Annals of Nutrition and Metabolism. 2015;67:21–32. doi: 10.1159/000437173. [DOI] [PubMed] [Google Scholar]
- Scharf et al. (2013).Scharf M, Neef S, Freund R, Geers-Knorr C, Franz-Wachtel M, Brandis A, Krone D, Schneider H, Groos S, Menon MB, Chang KC, Kraft T, Meissner JD, Boheler KR, Maier LS, Gaestel M, Scheibe RJ. Mitogen-activated protein kinase-activated protein kinases 2 and 3 regulate SERCA2a expression and fiber type composition to modulate skeletal muscle and cardiomyocyte function. Molecular and Cellular Biology. 2013;33:2586–2602. doi: 10.1128/MCB.01692-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino & Reggiani (2011).Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiological Reviews. 2011;91:1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- Svensson & Handschin (2014).Svensson K, Handschin C. Modulation of PGC-1a activity as a treatment for metabolic and muscle-related diseases. Drug Discovery Today. 2014;19:1024–1029. doi: 10.1016/j.drudis.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Tang et al. (2010).Tang K, Feng CX, Wagner PD, Breen EC. Exercise-induced VEGF transcriptional activation in brain, lung and skeletal muscle. Respiratory Physiology & Neurobiology. 2010;170(1):16–22. doi: 10.1016/j.resp.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadley, Choate & Mcconell (2007).Wadley GD, Choate J, Mcconell GK. NOS isoform-specific regulation of basal but not exercise-induced mitochondrial biogenesis in mouse skeletal muscle. Journal of Physiology. 2007;585(1):253–262. doi: 10.1113/jphysiol.2007.141309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waickman et al. (2017).Waickman AT, Ligons DL, Hwang SJ, Park JY, Lazarevic V, Sato N, Hong C, Park JH. CD4 effector T cell differentiation is controlled by IL-15 that is expressed and presented in trans. Cytokine. 2017;99:266–274. doi: 10.1016/j.cyto.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye et al. (2019).Ye X, Shen Y, Ni C, Ye J, Xin Y, Zhang W, Ren Y. Irisin reverses insulin resistance in C2C12 cells via the p38-MAPK-PGC-1α pathway. Peptides. 2019;119:17020. doi: 10.1016/j.peptides.2019.170120. [DOI] [PubMed] [Google Scholar]
- Ying et al. (2016).Ying F, Zhang L, Bu G, Xiong YZ, Bo Zuo. Muscle fiber-type conversion in the transgenic pigs with overexpression of PGC1α gene in muscle. Biochemical and Biophysical Research Communications. 2016;480:669–674. doi: 10.1016/j.bbrc.2016.10.113. [DOI] [PubMed] [Google Scholar]
- Yu et al. (2014).Yu J, Xiao Y, Liu J, Ji Y, Liu H, Xu J, Jin X, Liu L, Guan MX, Jiang P. Loss of MED1 triggers mitochondrial biogenesis in C2C12 cells. Mitochondrion. 2014;14:18–25. doi: 10.1016/j.mito.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Yuan et al. (2011).Yuan Y, Shi XE, Liu YG, Yang GS. FoxO1 regulates muscle fiber-type specification and inhibits calcineurin signaling during C2C12 myoblast differentiation. Molecular and Cellular Biochemistry. 2011;348:77–87. doi: 10.1007/s11010-010-0640-1. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2013).Zhang LN, Zhou HY, Fu YY, Li YY, Wu F, Gu M, Wu LY, Xia CM, Dong TC, Li JY, Shen JK, Li J. Novel small-molecule PGC-1alpha transcriptional regulator with beneficial effects on diabetic db/db mice. Diabetes. 2013;62:1297–1307. doi: 10.2337/db12-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou et al. (2013).Zhou LN, Wang Y, Li QY, Xu HJ, Lan XF, Zhang XJ. Culture and differentiation of C2C12 cells for identification of skeletal muscular fibers. Journal of Shanghai Jiaotong University(Medical Science) 2013;33:1423–1427. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Each data point indicates the value from one pig.
Each data point indicates the average performance of three repeats measures of cells
The instructions of MycAway™ -Color One-Step Mycoplasma Detection Kit and detection results.
Data Availability Statement
The following information was supplied regarding data availability:
Raw data showing MyHCs mRNA composition in porcine muscle and various genes expressions in C2C12 cells are available in the Supplementary Files.