Abstract
Background:
Minorities, including mainland Puerto Ricans, are impacted disproportionally by Alzheimer’s disease (AD), dementia, and cognitive decline. Studying blood metabolomics in this population has the potential to probe the biological underpinnings of this health disparity.
Objective:
We performed a comprehensive analysis of circulating plasma metabolites in relation to cognitive function in 736 participants from the Boston Puerto Rican Health Study (BPRHS) who underwent untargeted mass-spectrometry based metabolomics analysis and had undergone a battery of in-person cognitive testing at baseline.
Methods:
After relevant exclusions, 621 metabolites were examined. We used multivariable regression, adjusted for age, sex, education, apolipoprotein E genotype, smoking, and Mediterranean dietary pattern, to identify metabolites related to global cognitive function in our cohort. LASSO machine learning was used in a complementary analysis to identify metabolites that could discriminate good from poor extremes of cognition. We also conducted sensitivity analyses: restricted to participants without diabetes, and to participants with good adherence to Mediterranean diet.
Results:
Of 621 metabolites, FDR corrected (p <0.05) multivariable linear regression identified 3 metabolites positively, and 10 negatively, associated with cognitive function in the BPRHS. In a combination of FDR-corrected linear regression, logistic regression regularized via LASSO, and sensitivity analyses restricted to participants without diabetes, and with good adherence to the Mediterranean diet, β-cryptoxanthin plasma concentration was consistently associated with better cognitive function and N-acetylisoleucine and tyramine O-sulfate concentrations were consistently associated with worse cognitive function.
Conclusion:
This untargeted metabolomics study identified potential biomarkers for cognitive function in a cohort of Puerto Rican older adults.
Keywords: Cognitive function, diabetes, Puerto Ricans, metabolomics
INTRODUCTION
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disease in the world, affecting more than 5 million Americans and more than 35 million people worldwide [1]. To date, no therapy exists to prevent or cure AD [1]. Because individuals can benefit significantly from early detection and intervention, biomarkers for cognitive decline, potentially predictive of AD, are of intense interest. Studies have shown racial disparities in AD, potentially indicating that some of the mechanisms of the disorder may be race-specific [2]. US Latinos suffer a disproportionate burden of cognitive decline and are at approximately double the risk of AD, compared to non-Hispanic whites [3–6]. Further, within populations of Hispanic Americans, Puerto Ricans, the second largest Hispanic group in the United States, are particularly vulnerable to cognitive impairment and decline, with approximately twice the likelihood of Mexican Americans to suffer from impaired cognition [7]. In 2015, an Institute of Medicine report called for expansion of research on risk factors for cognitive aging, especially in high risk and underserved populations [8].
The blood metabolome is composed of metabolites including amino acids, fatty acids, lipo-proteins, and other small molecules. It is influenced by a combination of genetic predisposition, diet, environmental and lifestyle exposures, the gut microbiome, and medication use, among other factors. Prior studies have linked alterations in the blood metabolome to a variety of chronic conditions, including obesity [9], diabetes [10], cardiovascular disease [11], Parkinson’s disease [12], amyotrophic lateral sclerosis [13], and others [14, 15]. Several studies have investigated the role of blood metabolomics in AD, mild cognitive impairment, and cognitive decline [16–19]. While one study has been conducted in African Americans [16], we know of no study to date which has examined the association of blood metabolites with cognitive function in Puerto Ricans.
Therefore, we performed a comprehensive analysis of circulating plasma metabolites in relation to cognitive function in a cohort of Boston area Puerto Rican older adults. Examining the blood metabolome in relation to cognitive function may lead to the development of biomarkers for cognitive decline and/or AD. As mainland Puerto Ricans are impacted disproportionally by AD and dementia [3–6], studying blood metabolomics in this population also has the potential to probe the biological underpinnings of this health disparity.
MATERIALS AND METHODS
The Boston Puerto Rican Health Study (BPRHS) is an ongoing longitudinal study of Puerto Rican adults, aged 45 to 75 years at baseline, residing in the greater Boston area [20]. Participants for the BPRHS were recruited from areas of high Hispanic density in the Boston metropolitan area, using year 2000 Census data. Households with at least one Puerto Rican adult, aged 45 to 75 years, were identified and one eligible adult per household was randomly selected for participation (specifics of the study and recruitment methodology are described in detail elsewhere) [20]. Briefly, recruitment occurred through door-to-door enumeration (84%), community activities (8%), and referrals from community partners and/or through media or flyers placed in the community (8%). Exclusion criteria included inability to answer questions due to serious health conditions, plans to move from the Greater Boston area within two years and/or a Mini-Mental State Examination (MMSE) score < 10. At baseline (2004-2009), 1,499 participants were enrolled, and participated in an at-home interview with bilingual interviewers. Study visits took ~4–5h, and were divided into two visits, when preferred (≈40%). Questionnaires included demographics, socioeconomic status (SES), health and health behaviors, functional limitations, and depressive symptomatology. A full battery of cognitive tests, described below, was administered. Dietary intake was interviewer administered using an ethnic-specific validated food frequency questionnaire (FFQ). All instruments were validated and translated for the Hispanic population, and were pretested and adapted before use. Blood pressure and anthropometrics (height, weight, waist circumference, and hip circumference) were measured. Blood samples were drawn after a 12 h fast, and immediately taken to the Human Nutrition Research Center on Aging at Tufts University in coolers with dry ice; cooled to 4°C and separated within 2 h in a refrigerated centrifuge. Plasma aliquots were saved in 1 mL cryogenic, screw-cap tubes, and stored at −80°C.
Cognitive assessment
A battery of cognitive tests was administered in Spanish or English (98% Spanish) at baseline by trained research assistants [21], including: 1) MMSE (a test of general cognition) [22]; a 16-word list learning test [23] that includes 2) word list learning (sum of words recalled over 5 attempts), 3) word recognition, and 4) percentage retention (number of words recalled after a delay relative to number of correct responses on the fifth word list learning trial); 5) digit span forward and backward [23] (a test of working memory); 6) Stroop test (executive function) [23]; 7) verbal fluency [23] (naming as many words as possible starting with a given letter); 8) clock drawing [24]; and 9) figure copying (visuo-spatial function and organization) [25]. A global cognitive function score was derived as the mean of the z-scores for each of the following cognitive scores: MMSE, world list learning, recognition, percentage retention, Stroop, letter fluency, digit span forward and backward, clock drawing, and weighted figure copying. The global cognitive function score was used as the primary outcome in the analyses.
Analytic cohort for untargeted metabolomic analysis
Metabolomic profiling was performed on plasma collected at baseline. Of the 1,449 participants in the baseline BPRHS cohort, 736 had metabolomic profiling performed (Supplementary Figure 1). Samples for metabolomic profiling were selected at random, with the exception of 240 samples which were selected as part of a diabetes case-control study (n = 120 diabetes cases and n = 120 controls), who were also included in this study. The missing indicator method [26] was used to account for missing APOE genotype, BMI, smoking, and Mediterranean diet in regression analyses. All participants had complete data on age, sex, and education.
Metabolomic profiling
Metabolomic profiling was performed by Metabolon (Metabolon, Inc., Morrisville, NC) using previously described proprietary procedures [27]. The Metabolon metabolomics platform uses liquid chromatography-MS/MS methods with positive ion and negative ion modes (Waters ACQUITY ultra-performance liquid chromatography; Thermo Scientific Q-Exactive high resolution/accurate mass spectrometer interfaced with a heated electrospray ionization source and Orbitrap mass analyzer operated at 35,000 mass resolution). Four sampling modes were used to quantify metabolites: 1) acidic positive ion (optimized for hydrophilic molecules); 2) acidic positive ion (optimized for hydrophobic molecules); 3) basic negative ion; and 4) negative ionization from eluent of a HILIC column. Raw data were extracted and proprietary methods used to identify metabolite peaks using over 3,300 commercially available purified molecules as reference. For each metabolite, relative metabolite concentration is reported as a normalized area under the curve. The median relative standard deviation for internal standards was 5%, this reflects a measure of instrument variability similar to a coefficient of variation.
In the dataset provided by Metabolon, 1,303 metabolites were identified, of which 943 were annotated and 360 were unknown. We restricted our analyses to the annotated metabolites (n = 943), then excluded xenobiotics (n = 229), to focus our conclusions on biological function. We further restricted our analyses to metabolites that had reported values for ≥80% of the participants, and then imputed missing values for individual metabolites as 50% of the minimum value of that metabolite across all other participants with non-missing values. After imputation, metabolite values were log-transformed and Pareto scaled [28]. 621 metabolites were included in the final analyses (Supplementary Figure 1).
We analyzed the cross-sectional association between each metabolite and linear global cognitive function in independent linear models, adjusting for age in years, sex, education (8th grade or below, 9th-12th grade, high school or above), BMI (normal, overweight, obese), smoking (never, past, current), APOE 4 (presence or absence of APOE ε4 allele), and Mediterranean diet score (1–9) among all 736 BPRHS participants with metabolite data. Dietary quality has been shown to be related to cognition in our [29] and other cohorts [30, 31] and impacts the composition of the metabolome [30]. Mediterranean diet was the dietary pattern most strongly related to cognition in our cohort [29]. We used the Benjamini-Hochberg false discovery rate (FDR), 5% threshold, to adjust for multiple hypothesis testing (type 1 error).
In complementary analyses, we conducted penalized logistic regression analyses to select a set of metabolites that could discriminate those with poor (<=1SD below the mean) versus good cognition (> = 1SD above the mean) in our cohort. All metabolites were included in the same model, penalized by a Least Absolute Shrinkage and Selection Operator (LASSO) penalty term. We computed the area under the curve in receiver operating characteristic analyses (AUROC) using unconditional logistic regression, including the metabolites that were retained in LASSO, performed with 10-fold cross-validation, after a training (50%) and validation (50%) test split.
Sensitivity analyses
We conducted additional sensitivity analyses for all main analyses: a) restricted to participants without diabetes (n = 453); b) restricted to participants who had higher levels of adherence to Mediterranean diet (Mediterranean diet score 4 or above) (n = 329); c) participants aged 60 or more years (n = 265) and d. those known to not carry the APOE ε4 genotype (n = 160, due to high extent of missing data on APOE in our cohort).
We also conducted sensitivity analyses among only participants who had complete data on all covariates (N = 578).
RESULTS
736 participants were included in the metabolomics study. The study population was 73.0% female, had mean age of 57.9 years, mean MMSE score of 23.3, and 23.0% were current smokers (Table 1). Participants were more likely to be carriers of at least one copy of the ε4 allele of the APOE gene, but were otherwise comparable to those who did not undergo profiling (Table 1).
Table 1.
Characteristics of the included BPRHS study subsample relative to the excluded subsample
| Study subsample (n = 736) |
Excluded subsample (n = 763) |
|||
|---|---|---|---|---|
| Mean (SD)/N (%) | N missing | Mean (SD)/N (%) | pc | |
| MMSE (mean) | 23.3 (3.5) | 0 | 23.2 (3.5) | 0.65 |
| Age (y) | 57.9 (7.5) | 0 | 57.2 (7.7) | 0.66 |
| Female sex % | 73.0% | 0 | 68.0% | 0.81 |
| Education (8th grade or below) | 48.8% | 0 | 44.3% | 0.09 |
| Dietary Quality (Mediterranean Score) | 4.4 (1.6) | 61 | 4.3 (1.6) | 0.33 |
| Physical Activity Scorea | 31.5 (4.6) | 0 | 31.6 (4.9) | 0.81 |
| Body mass index (kg/m2) | 32.2 (6.8) | 6 | 31.6 (6.6) | 0.11 |
| Type 2 diabetes | 38.1% | 0 | 41.8% | 0.16 |
| Hypertensionb | 70.2% | 0 | 66.1% | 0.11 |
| CRP (ng/mL) | 6.4 (8.6) | 2 | 6.1 (8.6) | 0.11 |
| APOE ε4 (presence of at least one allele) | 21.7% | 139 | 16.4% | 0.01 |
| CESDd | 20.3 (13.4) | 0 | 20.2 (12.9) | 0.97 |
| Current smoker | 23.0% | 2 | 26.1% | 0.18 |
Values are reported as mean and standard deviation (SD) or frequency.
derived physical activity score.
definition; Systolic blood pressure greater than 140mmHG or diastolic blood pressure greater or equal to 90mmHG or taking antihypertension medications.
p values reflect comparisons between study and excluded sub-cohort (Wilcoxon for continuous and chi-square for categorical.
Center for Epidemiologic Studies Depression Scale (range: 0–64).
Identification of individual metabolites associated with poor cognition
In linear models adjusted for age, sex, education, BMI, smoking, APOE genotype, and Mediterranean diet, 13 metabolites were significantly (p <0.05) associated with cognitive function as a continuous variable, after FDR correction for multiple comparisons. The top metabolites positively associated with cognition were the carbohydrate 1,5-anhydroglucitol (1,5-AG) (Coef: 0.078; 95% CI 0.041, 0.115; FDR adj. p: 0.00587), the carotenoid β-cryptoxanthin (Coef: 0. 0.079; 95% CI: 0.040, 0.117; FDR adj. p: 0.00859) and the lipid 1-stearoyl-GPI (18 : 0) (Coef: 0.108; 95% CI: 0.053, 0.164; FDR adj.p: 0.00921).
In sensitivity analyses restricted to participants without diabetes, of the above metabolites, only β-cryptoxanthin was significantly associated with cognitive function (Coef: 0.11; 95% CI: 0.062, 0.15; FDR adj. p: 0.003). No metabolites were positively associated with cognition when analyses were restricted to participants with a Mediterranean diet score of 5 or above.
Metabolites inversely associated with cognition in these analyses were primarily carbohydrates and amino acids and included the carbohydrates glucose (Coef: −0.159; 95% CI: −0.227, −0.091; FDR adj. p: 0.00188), mannose (Coef: −0.140; 95% CI: −0.203, −0.078; FDR adj.p: 0.00241), mannitol/sorbitol (Coef: −0.071; 95% CI: −0.111, −0.031; FDR adj.p: 0.0316), and ribitol (Coef: −0.108; 95% CI: −0.171, −0.046; FDR adj.p: 0.0368), and the amino acids and N-acetylisoleucine (Coef: −0.125; 95% CI: −0.178, −0.072; FDR adj.p: 0.00188), tyramine O-sulfate (Coef: −0.066; 95% CI: −0.099, −0.033; FDR adj.p: 0.00921), N-acetylleucine (Coef: −0.112; 95% CI: −0.169, −0.056; FDR adj.p: 0.00921), as well as vitamin E metabolite gamma-CEHC-glucuronide (Coef: −0.076; 95% CI: −0.115, −0.036; FDR adj.p: 0.0118). Glucose was the metabolite most strongly negatively associated with cognition among all participants, followed by N-acetylisoleucine. Supplementary Table 1 provides the full list of metabolite associations with cognitive function in this study.
Associations between the vitamin E metabolite gamma-CEHC-glucuronide and cognition remained significant in both the sensitivity analyses restricted to participants without diabetes (Coef: −0.094; 95% CI: −0.140, −0.047; FDR adj.p: 0.024), and in participants with high adherence to Mediterranean diet (Coef: −0.099; 95% CI: −0.147, −0.050; FDR adj.p: 0.020). Associations with N-actylisoleucine remained significant in participants with high adherence to Mediterranean diet (Coef: −0.17; 95% CI: −0.24, −0.010; FDR adj.p: 0.002). These sensitivity analyses are shown in Table 2.
Table 2.
Metabolites Associated with Global Cognitive Score at Baseline in the BPHRS in linear regression after FDR adjustment (N = 736)
| All participants |
Participants without diabetes |
Mediterranean diet score 5+ |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HMDB | Metabolite | Super Pathway | Sub Pathway | βa | 95% CI | p | Adj-pb | βa | 95% CI | p | Adj-pb | βa | 95% CI | p | Adj-pb |
| Positively associated metabolites | |||||||||||||||
| HMDB02712 | 1,5-anhydroglucitol (1,5-AG) | Carbohydrate | Glycolysis, Gluconeogenesis, and Pyruvate Metabolism | 0.078 | (0.041, 0.115) | 3.78*10−5 | 0.00587 | – | – | – | – | – | – | ||
| HMDB33844 | beta-cryptoxanthin | Cofactors and Vitamins | Vitamin A Metabolism | 0.079 | (0.040, 0.117) | 6.92*10−5 | 0.00859 | 0.11 | (0.062, 0.15) | 5.30*10−6 | 0.003 | – | – | – | |
| HMDB61696 | 1-stearoyl-GPI (18:0) | Lipid | Lysophospholipid | 0.108 | (0.053, 0.164) | 1.19*10−4 | 0.00921 | – | – | – | – | – | – | ||
| Negatively-associated metabolites | |||||||||||||||
| HMDB00122 | glucose | Carbohydrate | Glycolysis, Gluconeogenesis, and Pyruvate Metabolism | −0.159 | (−0.227, −0.091) | 6.06*10−6 | 0.00188 | – | – | – | – | – | – | – | – |
| HMDB61684 | N-acetylisoleucine | Amino Acid | Leucine, Isoleucine and Valine Metabolism | −0.125 | (−0.178, −0.072) | 3.78*10−6 | 0.00188 | – | – | – | – | −0.17 | (0.24, −0.10) | 3.62*10−6 | 0.002 |
| HMDB00169 | mannose | Carbohydrate | Fructose, Mannose and Galactose Metabolism | −0.140 | (−0.203, −0.078) | 1.17*10−5 | 0.00241 | – | – | – | – | – | – | – | |
| HMDB06409 | tyramine O-sulfate | Amino Acid | Tyrosine Metabolism | −0.066 | (−0.099 −0.033) | 1.11*10−4 | 0.00921 | – | – | – | – | – | – | – | |
| HMDB11756 | N-acetylleucine | Amino Acid | Leucine, Isoleucine and Valine Metabolism | −0.112 | (−0.169, −0.056) | 1.11*10−4 | 0.00921 | – | – | – | – | – | – | – | |
| – | gamma-CEHC glucuronide* | Cofactors and Vitamins | Tocopherol Metabolism | −0.076 | (−0.115, −0.036) | 1.71*10−4 | 0.0118 | −0.094 | (−0.140, −0.047) | 7.78*10−5 | 0.024 | −0.099 | (−0.147, −0.050) | 6.49*10−5 | 0.020 |
| HMDB00854 | formiminoglutamate | Amino Acid | Histidine Metabolism | −0.090 | (−0.139, −0.042) | 2.81*10−4 | 0.0175 | – | – | – | – | – | – | ||
| HMDB00247 | mannitol/sorbitol | Carbohydrate | Fructose, Mannose and Galactose Metabolism | −0.071 | (−0.111, −0.031) | 5.59*10−4 | 0.0316 | – | – | – | – | – | – | ||
| HMDB00508 | ribitol | Carbohydrate | Pentose Metabolism | −0.108 | (−0.171, −0.046) | 7.12*10−4 | 0.0368 | – | – | – | – | – | – | ||
| HMDB01173 | 5-methylthioadenosine (MTA) | Amino Acid | Polyamine Metabolism | −0.113 | −(0.178, −0.047) | 7.81*10−4 | 0.0373 | – | – | – | – | – | – | ||
beta coefficient from a regression of the metabolite on linear global cognitive score adjusted for age, sex, education (8th grade or below/9th–12th grade/high school or above), BMI (normal/overweight/obese), smoking (never/past/current), and APOE allele (presence or absence).
multiple-correction (FDR_adjusted) p-value from a regression of the metabolite on linear global cognitive score adjusted for age, sex, education (8th grade or below/9th–12th grade/high school or above),) and APOE allele (presence or absence).
Results are only presented with FDR adjusted p-value <0.05.
A heatmap in Fig. 1 shows the inter-correlations between the metabolites associated with cognition in FDR adjusted analyses. The strongest positive correlations were observed between glucose and mannose (r = 0.75 (p <0.05)) which, as expected, were significantly inversely correlated with the top metabolite positively associated with cognition in these analyses:1,5-anhydroglucitol (1,5-AG) (r = −0.71 for glucose and −0.61 for mannose; p < 0.05 for both). The second metabolite, after glucose, mostly strongly negatively associated with cognition in these analyses, N-acetylisoleucine, was correlated with other negatively associated metabolites, N-acetylleucine (r = 0.54, p < 0.05), and 5-methylthioadenosine (MTA) (r = 0.30, p < 0.05).
Fig. 1.
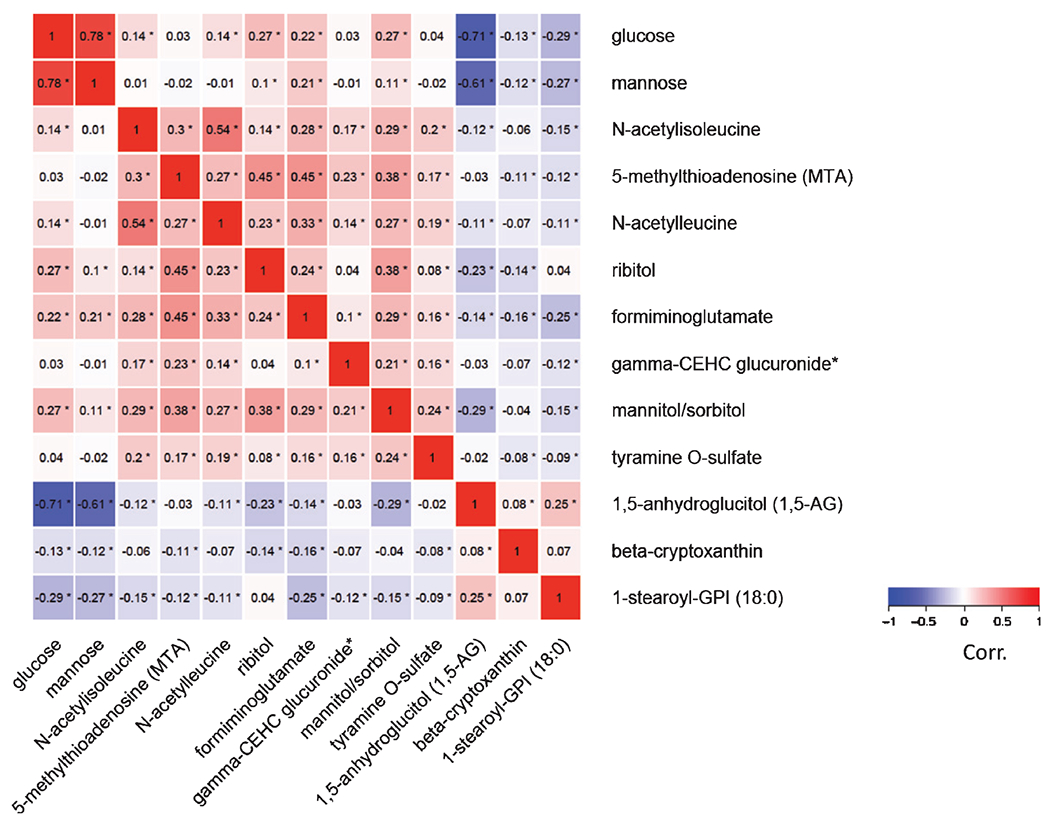
Correlation matrix of cognition–associated metabolites identified among all BPHRS participants (n = 736).
LASSO penalized conditional logistic regression
In complementary penalized logistic regression analyses, with a LASSO penalty term, 18 metabolites were positively associated with good (≥1SD above the mean, n = 112) versus poor (≤1SD below the mean, n = 104) cognition among all participants with metabolomic data (Table 3). The the amino acid 4-methoxyphenol sulfate was most strongly associated with cognition (βall: 0.631), followed by the lipids N-stearoylserine (βall: 0.582), and eicosapentaenoate (EPA; 20 : 5n3) (βall: 0.494). Of the metabolites identified as significantly positively associated with cognition in our FDR-corrected linear models, the carotenoid beta-cryptoxanthin, was also identified via LASSO. LASSO retained 33 metabolites associated with poor (≤1SD below the mean) cognition. The metabolite most strongly associated with poor cognition via LASSO was the amino acid N-acetylisoleucine (βall: −0.660). This metabolite had also been identified as associated with worse cognitive function in our FDR-corrected linear regression. Other metabolites identified by LASSO and also significantly associated with cognitive function in FDR-corrected regression included tyramine O-sulfate (βall: −0.256), N-acetylleucine (βall: −0.080), and glucose (βall: −0.063). In the AUROC analysis, including all metabolites as individual variables in the model, with a 50% validation/training split, the AUC of the model was 0.754 (95% CI: 0.662, 0.845). The ROC curve is shown in Supplementary Figure 2.
Table 3.
Metabolites associated with cognition that were retained in LASSO penalized regression models (N = 736)
| HMBD | Biochemical | Super Pathway | Sub Pathway | βwhole a cohort | βwithoutb diabetesb | βmedDietc |
|---|---|---|---|---|---|---|
| POSITIVELY ASSOCIATED METABOLITES | ||||||
| – | 4-methoxyphenol sulfate | Amino Acid | Tyrosine Metabolism | 0.631 | 0.073 | 0.228 |
| – | N-stearoylserine* | Lipid | Endocan-binoid | 0.582 | – | 0.479 |
| HMDB01999 | eicosapentaenoate (EPA; 20 : 5n3) | Lipid | Polyunsaturated Fatty Acid (n3 and n6) | 0.494 | 0.051 | 0.104 |
| – | 3-hydroxyhexanoate | Lipid | Fatty Acid, Monohydroxy | 0.346 | – | – |
| HMDB02329 | oxalate (ethanedioate) | Cofactors and Vitamins | Ascorbate and Aldarate Metabolism | 0.328 | – | – |
| HMDB07218 | oleoyl-oleoyl-glycerol (18 : 1/18 : 1) [1]* | Lipid | Diacylglycerol | 0.288 | – | – |
| – | lignoceroyl sphingomyelin (d18 : 1/24 : 0) | Lipid | Sphingolipid Metabolism | 0.188 | – | – |
| HMDB00574 | Cysteine | Amino Acid | Methionine, Cysteine, SAM and Taurine Metabolism | 0.152 | – | – |
| HMDB04705 | 12,13-DiHOME | Lipid | Fatty Acid, Dihydroxy | 0.128 | 0.088 | – |
| HMDB02823 | docosatrienoate (22 : 3n3) | Lipid | Polyunsaturated Fatty Acid (n3 and n6) | 0.118 | – | – |
| HMDB33844 | beta-cryptoxanthin | Cofactors and Vitamins | Vitamin A Metabolism | 0.108 | 0.195 | – |
| HMDB08141 | 1-linoleoyl-2-linolenoyl-GPC (18:2/18:3)* | Lipid | Phosphatidylcholine (PC) | 0.089 | – | 0.002 |
| HMDB00345 | 3-hydroxyadipate* | Lipid | Fatty Acid, Dicarboxylate | 0.065 | – | – |
| – | 1-stearoyl-2-oleoyl-GPG (18 : 0/18 : 1) | Lipid | Phosphatidylglycerol (PG) | 0.065 | – | – |
| HMDB00017 | Pyridoxate | Cofactors and Vitamins | Vitamin B6 Metabolism | 0.048 | – | – |
| HMDB00661 | glutarate (C5-DC) | Lipid | Fatty Acid, Dicarboxylate | 0.045 | – | – |
| HMDB61115 | tryptophan betaine | Amino Acid | Tryptophan Metabolism | 0.025 | – | 0.240 |
| HMDB00202 | methylmalo-te (MMA) | Lipid | Fatty Acid Metabolism (also BCAA Metabolism) | 0.013 | – | 0.046 |
| HMDB00760 | Hyocholate | Lipid | Secondary Bile Acid Metabolism | – | – | 0.002 |
| NEGATIVELY ASSOCIATED METABOLITES | ||||||
| HMDB61684 | N-acetylisoleucine | Amino Acid | Leucine, Isoleucine and Valine Metabolism | −0.660 | −0.287 | −0.824 |
| – | sphingomyelin (d18:2/16: 0, d18 : 1/16 : 1)* | Lipid | Sphingolipid Metabolism | −0.546 | – | −0.339 |
| – | (N(1)+N(8))-acetylspermidine | Amino Acid | Polyamine Metabolism | −0.405 | – | −0.169 |
| – | arabote/xylote | Carbohydrate | Pentose Metabolism | −0.351 | – | – |
| HMDB00190 | lactate | Carbohydrate | Glycolysis, Gluconeogenesis, and Pyruvate Metabolism | −0.281 | – | – |
| – | glycosyl-N-(2-hydroxynervonoyl)-sphingosine (d18 : 1/24: 1(2OH))* | Lipid | Ceramides | −0.277 | −0.051 | −0.071 |
| HMDB06409 | tyramine O-sulfate | Amino Acid | Tyrosine Metabolism | −0.256 | −0.053 | −0.086 |
| HMDB00063 | Cortisol | Lipid | Corticosteroids | −0.237 | – | – |
| – | 3-methylcytidine | Nucleotide | Pyrimidine Metabolism, Cytidine containing | −0.237 | – | – |
| – | gamma-glutamylcitrulline* | Peptide | Gamma-glutamyl Amino Acid | −0.231 | – | – |
| HMDB07257 | linoleoyl-arachidonoyl-glycerol (18:2/20 : 4) [1]* | Lipid | Diacylglycerol | −0.228 | −0.168 | – |
| – | sphingomyelin (d18 : 2/18 : 1)* | Lipid | Sphingolipid Metabolism | −0.224 | – | – |
| HMDB03464 | 4-guanidinobutanoate | Amino Acid | Guanidino and Acetamido Metabolism | −0.201 | – | – |
| HMDB02802 | cortisone | Lipid | Corticosteroids | −0.174 | – | – |
| HMDB00258 | sucrose | Carbohydrate | Disaccharides and Oligosaccharides | −0.162 | −0.070 | −0.160 |
| HMDB07228 | oleoyl-arachidonoyl-glycerol (18:1/20:4) [2]* | Lipid | Diacylglycerol | −0.151 | – | – |
| HMDB00126 | glycerol 3-phosphate | Lipid | Glycerolipid Metabolism | −0.113 | – | −0.169 |
| HMDB09069 | 1-oleoyl-2-arachidonoyl-GPE (18:1/20:4)* | Lipid | Phosphatidylethanolamine (PE) | −0.106 | −0.092 | −0.108 |
| HMDB00012 | 2’-deoxyuridine | Nucleotide | Pyrimidine Metabolism, Uracil containing | −0.100 | – | – |
| HMDB11745 | N-acetylmethionine | Amino Acid | Methionine, Cysteine, SAM and Taurine Metabolism | −0.097 | – | – |
| – | acisoga | Amino Acid | Polyamine Metabolism | −0.095 | −0.372 | – |
| HMDB00522 | 3-methylglutaco-te | Amino Acid | Leucine, Isoleucine and Valine Metabolism | −0.089 | – | – |
| HMDB01547 | corticosterone | Lipid | Corticosteroids | −0.082 | – | −0.195 |
| – | cis-4-decenoylcarnitine (C10: 1) | Lipid | Fatty Acid Metabolism (Acyl Carnitine) | −0.082 | – | −0.085 |
| HMDB11756 | N-acetylleucine | Amino Acid | Leucine, Isoleucine and Valine Metabolism | −0.080 | −0.122 | – |
| HMDB00631 | glycodeoxycholate | Lipid | Secondary Bile Acid Metabolism | −0.066 | – | – |
| HMDB00122 | glucose | Carbohydrate | Glycolysis, Gluconeogenesis, and Pyruvate Metabolism | −0.063 | – | – |
| HMDB00754 | beta-hydroxyisovalerate | Amino Acid | Leucine, Isoleucine and Valine Metabolism | −0.062 | – | – |
| HMDB02820 | 1-methyl-4-imidazoleacetate | Amino Acid | Histidine Metabolism | −0.044 | – | – |
| HMDB09789 | 1-palmitoyl-2-arachidonoyl-GPI (16 : 0/20 : 4)* | Lipid | Phosphatidylinositol (PI) | −0.035 | – | – |
| HMDB00669 | 2-hydroxyphenylacetate | Amino Acid | Tyrosine Metabolism | −0.016 | – | −0.295 |
| HMDB04089 | N-formylanthranilic acid | Amino Acid | Tryptophan Metabolism | −0.012 | – | – |
| HMDB10395 | 1-arachidonoyl-GPC (20 : 4n6)* | Lipid | Lysophospholipid | −0.005 | – | – |
| HMDB01173 | 5-methylthioadenosine (MTA) | Amino Acid | Polyamine Metabolism | – | −0.034 | – |
| HMDB02331 | 1-ribosyl-imidazoleacetate* | Amino Acid | Histidine Metabolism | – | −0.133 | – |
| – | N-linoleoyltaurine* | Lipid | Endocanbinoid | – | −0.112 | – |
| – | sphingomyelin (d18 : 2/24 : 2)* | Lipid | Sphingolipid Metabolism | – | −0.197 | – |
| HMDB00232 | quinolite | Cofactors and Vitamins | Nicotite and Nicotimide Metabolism | – | – | −0.163 |
| HMDB00709 | cysteinylglycine disulfide* | Amino Acid | Glutathione Metabolism | – | – | −0.078 |
| HMDB00730 | isobutyrylglycine | Amino Acid | Leucine, Isoleucine and Valine Metabolism | – | – | −0.015 |
| HMDB00854 | formiminoglutamate | Amino Acid | Histidine Metabolism | – | – | −0.320 |
| – | glyco-beta-muricholate** | Lipid | Primary Bile Acid Metabolism | – | – | −0.219 |
| – | hexadecenedioate (C16 : 1-DC)* | Lipid | Fatty Acid, Dicarboxylate | – | – | −0.089 |
| – | N-palmitoyl-sphingadienine (d18 : 2/16 : 0)* | Lipid | Sphingolipid Metabolism | – | – | −0.186 |
beta coefficient from a LASSO regression that initially included all the metabolites among all partcipants (n = 736) with metabolomic measures.
beta coefficient from a LASSO regression that initially included all the metabolites among (n = 453) participants without diabetes.
beta coefficient from a LASSO regression that initially included all the metabolites among (n = 329) participants who had Mediterranean diet score of 5 or above.
metabolites also identified in linear regression analyses after FDR correction (Table 2). Results are only presented with FDR adjusted p-value <0.05.
Sensitivity analyses
When the LASSO regression was repeated on the subset of participants without diabetes, four of the metabolites identified in full-cohort LASSO remained associated with good cognition: the amino acid 4-methoxyphenol sulfate (βnodiabetes: 0.073), EPA (20: 5n3) (βnodiabetes: 0.051), 12,13-DiHOME (βnodiabetes: 0.088), and β-cryptoxanthin (βnodiabetes: 0.195). Of the metabolites identified in full sample LASSO as related to poor cognition, eight were retained in analyses restricted to those without diabetes: N-acetylisoleucine (βnodiabetes: −0.287), glycosyl-N-(2-hydroxynervonoyl)-sphingosine (d18 : 1/24: 1(2OH)) (βnodiabetes: −0.051), tyramine O-sulfate (βnodiabetes: −0.053), linoleoyl-arachidonoyl-glycerol (18:2/20: 4) (βnodiabetes: −0.168), sucrose (βnodiabetes: −0.070), 1-oleoyl-2-arachidonoyl-GPE (18 : 1/20: 4) (βnodiabetes: −0.092), acisoga (βnodiabetes: −0.372), and N-acetylleucine (βnodiabetes: −0.122) were also retained in the full analyses. In the AUROC analysis among those without diabetes, with a 50% validation/training split, the AUC of the model was 0.734 (95% CI: 0.614, 0.854).
Likewise, in analyses restricted to participants who scored 4 or above on the Mediterranean diet score, positive associations with 4-methoxyphenol sulfate, N-stearoylserine, EPA (20 : 5n3), 1-linoleoyl-2-linolenoyl-GPC(18 : 2/18 : 3), tryptophan, and methylmalonate (MMA) remained, as did inverse associations with N-acetylisoleucine, sphingomyelin (d18 : 2/16 : 0, d18: 1/16:1), (N(1)+N(8))-acetylspermidine, glycosyl-N-(2-hydroxynervonoyl)-sphingosine (d18 : 1/24 : 1(2OH)), sucrose, glycerol 3-phosphate, and 1-oleoyl-2-arachidonoyl-GPE (18 : 1/20 : 4)
Figure 2 shows the mean standardized levels, in the full sample, of the metabolites consistently associated with good or poor cognition, those metabolites were retained in all three LASSO analyses: LASSO analysis in the full sample, in those without diabetes, and in those with good adherence to Mediterranean diet (also highlighted in bold in Table 3).
Fig. 2.
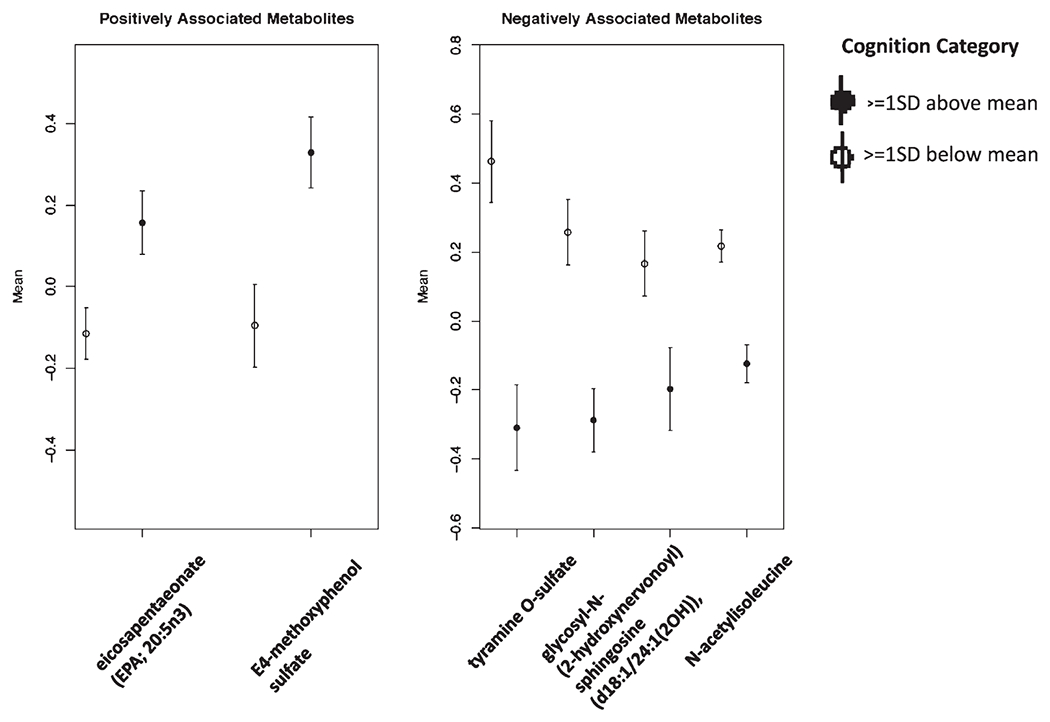
Mean standardized levels of cognition-associated metabolites identified via LASSO, comparing participants with the lowest (≤1SD below the mean) versus the highest (≥1SD above the mean) of cognitive function (n = 736).
In secondary analyses restricted to participants who were not carriers of the APOE ε4 allele, only glucose was inversely associated with cognitive function in FDR-corrected multivariable linear regression (β = −0.18 (95% CI: −0.267, −0.093); p = 0.036), and no metabolites were positively associated with cognitive function. Among participants who were not carriers of the APOE ε4 allele, LASSO resulted in 12 positive associations, including with 4-methoxyphenol sulfate, EPA (20 : 5n3), 12,13-DiHOME, and β-cryptoxanthin, and 15 negative associations, including N-acetylisoleucine, glycosyl-N-(2-hydroxynervonoyl)-sphingosine (d18 : 1/24 : 1(2OH)), tyramine O-sulfate, linoleoyl-arachidonoyl-glycerol, sucrose, 1-oleoyl-2-arachidonoyl-GPE (18 : 1/20 : 4), and asocoga, which were also identified in main-cohort analyses. In sensitivity analyses restricted to participants 60 years or older at baseline, in FDR-corrected linear regression analyses, we confirmed negative associations with N-acetylisoleucine (β = −0.144 (95% CI: −0.212, −0.075); p = 0.028) and mannose (β = −0.167 (95% CI: −0.253, −0.082); p = 0.043).
In sensitivity analyses restricted to participants with complete data on all model covariates (N = 578) only N-acetylisoleucine was significantly associated with cognitive function (β = −0.108; 95% CI: −0.183, −0.032); p(FDR) = 0.0338) after FDR correction. All the other metabolites significantly associated with cognition in our main analyses, were also associated with cognition without FDR correction in these sensitivity analyses. These sensitivity analyses are presented in Supplementary Table 2.
DISCUSSION
In this group of Puerto Rican older adults, multivariable linear regression with FDR adjustment and LASSO identified metabolites that were significantly associated with cognitive function. β-cryptoxanthin was consistently associated with better cognition in our cohort across all analyses. β-cryptoxanthin was significantly associated with cognitive function in multivariable-models adjusted for covariates, and was retained in LASSO models as predictive of good cognition, in the full sample, as well as in analyses restricted to participants without diabetes. β-cryptoxanthin is a pre-vitamin A carotenoid and antioxidant found in fruits and vegetables, including oranges, tangerines, and persimmons [32, 33]. Carotenoid intakes and concentrations have been associated with better cognitive function in prior studies [34, 35]. Marginal deficiency of vitamin A has been associated with worse cognition in AD patients, and vitamin A supplementation has been shown to improve cognitive function [36].
EPA (20 : 5n3) was consistently associated with better cognitive function in LASSO analyses of the full sample, and in the sub-samples restricted to 1) participants diabetes, and 2) those with good adherence to Mediterranean diet. However, it was not significantly associated with cognitive function in FDR corrected linear models. EPA dietary supplements [37], as well as blood concentration of this metabolite, have been associated with better cognitive function [38], increased brain volume, and slower brain aging [39–41]. Fish consumption, a major dietary source of omega-3 fatty acids such as EPA, has also been linked with better cognitive outcomes [42].
Several of the metabolites we identified as beneficial for cognitive function (β-cryptoxanthin, EPA) have been previously identified as markers of a healthy dietary pattern in US postmenopausal women [30]. This supports an important role for healthy diet in cognitive health. The associations reported here were adjusted for Mediterranean diet score, and this did not meaningfully alter the associations between most of the metabolites with cognitive function. Likewise, analyses restricted to participants who had a Mediterranean diet score of 4 or above, confirmed many of the results of our main analyses.
The metabolites identified in our study strongly implicate diabetes, metabolic syndrome, and insulin resistance as key factors linked to cognition. Our study cohort is a disadvantaged minority population with very high rates of metabolic syndrome [43], diabetes and obesity. Metabolic syndrome has been associated with an increased risk of dementia in several studies [44, 45]. Type II diabetes and impaired fasting glucose has been linked with increased risk of cognitive impairment [46] and dementia, particularly in older women [47], who make up the majority of this study.
Many of the metabolites identified in this study as significantly associated with cognitive function reflect the high prevalence of diabetes, poor glycemic control, obesity, and metabolic syndrome in our population and underscore the potential role of these factors in dementia. Full-sample FDR adjusted linear regression analyses identified the carbohydrate 1,5-anydroglucitol (1,5-AG), and the lipids 1-stearoyl-GPI, as associated with better cognition. 1,5-anydroglucitol (1,5-AG), the metabolite most strongly associated with cognitive function, is a short-term marker of glycemic control [48,49]. Circulating 1,5-anydroglucitol (1,5-AG) has been shown to drop in response to rising glucose concentration [49]. Correspondingly, in our study, 1,5-anydroglucitol (1,5-AG) concentration was strongly inversely correlated with glucose (Fig. 1), and with other carbohydrates, such as mannose, mannitol/sorbitol, and ribitol. 1-stearoyl-GPI is a phospholipid that has been associated with measures of insulin resistance and insulin secretion [50]. Of these metabolites, only β-cryptoxanthin remained significantly associated with cognition among participants without diabetes (Table 2).
N-acetylisoleucine was the metabolite most consistently associated with poor cognition in our cohort, both in FDR adjusted linear models and in LASSO, as well as in participants without diabetes (LASSO only). N-acetylisoleucine is an amino acid derivative of isoleucine and is part of the Leucine, Isoleucine, and Valine metabolism (Branched Chain Amino Acids/BCAA) pathway. Other metabolites in the BCAA pathway, including N-acetylleucine, 3-methylglutaconate (an intermediate in the leucine degradation pathway), and beta-hydroxyisovalerate (a biproduct of the leucine degradation pathway), were also negatively associated with cognitive function. BCAA play important roles in muscle metabolism as building blocks for proteins, as well as in insulin metabolism. In mice, leucine supplementation has been linked with improved glucose homeostasis, insulin metabolism, and satiety [51]. Prior studies have reported reduced BCAA in patients with dementia and AD [19], and inverse correlation between valine concentration and cognition [52].
Other epidemiological studies have consistently reported that elevated BCAA are associated with higher risk of type 2 diabetes [53, 54] and insulin resistance [55], and that they change post-treatment [56]. Mice fed a diet enriched in BCAA showed higher tau neuropathology and performed worse in cognitive tests [57]. Furthermore, many of the metabolites negatively associated with cognition in our main analyses were carbohydrates, including glucose, and these associations did not remain in analyses restricted to participants without diabetes (ex: lactate, glucose, mannose, ribitol). Our study thus supports a potential role for BCAA metabolism in cognitive function. It also implicates diabetes, metabolic syndrome, and insulin resistance as key factors linked to cognition and suggests potential metabolites that may be responsible for these associations.
Whether the metabolites significantly related to cognitive function in our study, such as those in the BCAA pathway, 1,5-anydroglucitol (1,5-AG), and the carbohydrates glucose, mannose, ribitol serve as markers of type II diabetes, metabolic syndrome, and obesity or whether they are causally involved in pathways between these conditions and poor cognitive function/dementia should be explored in further studies.
Our analysis relied on a subset of BPRHS participants with metabolomic profiling. These participants were similar to those who did not have this (Table 1). The study lacked a validation cohort and was cross sectional in nature; thus, it will be important to see these results validated in other cohorts, as well as in future longitudinal studies. Nevertheless, the metabolites identified in this study support prior associations observed in other cohorts, lending external validity to our findings.
Due to the cross-sectional nature of this study, factors related to the establishment of cognitive reserve may have impacted the results. Our population is socioeconomically disadvantaged and has low levels of education, and generally reports occupations with low levels of cognitive complexity (personal communication) and thus potentially low opportunities for building cognitive reserve. We have attempted to address this concern by adjusting our analyses for known covariates such as education, diet, smoking and others, as well as conducting stratified analyses by these variables. We also conducted sensitivity analyses among participants without diabetes, among older participants (65+) and those known to be carriers of the APOE ε4 genotype, to identify metabolites most relevant in older age and among participants with a genetic predisposition to AD. Power to detect association was limited in these latter analyses, due to the small sample sizes, limiting our ability to identify metabolites significantly associated with cognition in these subgroups. Because of substantial missing data on some covariates, particularly APOE, and the Mediterranean diet score, we used the missing indicator method to address missingness in covariates in order to maximize the sample size in our analyses. This method makes assumptions about similarity of participants with missing data and their relationships with exposure and outcome which may not hold in our study. We thus conducted complete-case sensitivity analyses, which had a substantially lower number of participants and thus less power to identify metabolite-cognition relationships, but generally agreed with our main findings.
In summary, in this study of Boston area Puerto Rican older adults, we identified multiple metabolites associated with cognitive function. β-cryptoxanthin was consistently associated with better cognition, and mannose with worse cognition in our cohort. Several of the metabolites identified in our study implicate BCAA metabolism, insulin and glucose regulation, metabolic syndrome, and diabetes in relation to cognitive function. Future longitudinal studies in diverse populations are needed to confirm and refine this associations.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health grants P01 AG023394, P50HL105185, and R01 AG055948. The National Institutes of Health grant K01 DK107804 contributed to the metabolomic profiling of the samples. Natalia Palacios receives funding from the National Institutes of Health grant R01 NS097723. Rachel S Kelly is supported by K01 HL146980 from the NHLBI.
Footnotes
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-200040.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0040r2).
REFERENCES
- [1].Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362, 329–344. [DOI] [PubMed] [Google Scholar]
- [2].Morris JC, Schindler SE, McCue LM, Moulder KL, Benzinger TLS, Cruchaga C, Fagan AM, Grant E, Gordon BA, Holtzman DM, Xiong C (2019) Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol 76, 264–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gurland BJ, Wilder DE, Lantigua R, Stern Y, Chen J, Killeffer EH, Mayeux R (1999) Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry 14, 481–493. [PubMed] [Google Scholar]
- [4].Espino DV, Lichtenstein MJ, Palmer RF, Hazuda HP (2001) Ethnic differences in mini-mental state examination (MMSE) scores: Where you live makes a difference. J Am Geriatr Soc 49, 538–548. [DOI] [PubMed] [Google Scholar]
- [5].Grigsby J, Kaye K, Shetterly SM, Baxter J, Morgenstern NE, Hamman RF (2002) Prevalence of disorders of executive cognitive functioning among the elderly: Findings from the San Luis Valley Health and Aging Study. Neuroepidemiology 21, 213–220. [DOI] [PubMed] [Google Scholar]
- [6].Mulgrew CL, Morgenstern N, Shetterly SM, Baxter J, Baron AE, Hamman RF (1999) Cognitive functioning and impairment among rural elderly Hispanics and non-Hispanic whites as assessed by the Mini-Mental State Examination. J Gerontol B Psychol Sci Soc Sci 54, P223–230. [DOI] [PubMed] [Google Scholar]
- [7].Gonzalez HM, Tarraf W, Gouskova N, Gallo LC, Penedo FJ, Davis SM, Lipton RB, Arguelles W, Choca JP, Catellier DJ, Mosley TH (2015) Neurocognitive function among middle-aged and older Hispanic/Latinos: Results from the Hispanic Community Health Study/Study of Latinos. Arch Clin Neuropsychol 30, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].(2015) Cognitive aging: Progress in understanding and opportunities for action. Mil Med 180, 1111–1113. [DOI] [PubMed] [Google Scholar]
- [9].Rangel-Huerta OD, Pastor-Villaescusa B, Gil A (2019) Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics 15, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liggi S, Griffin JL (2017) Metabolomics applied to diabetes-lessons from human population studies. Int J Biochem Cell Biol 93, 136–147. [DOI] [PubMed] [Google Scholar]
- [11].Cheng S, Shah SH, Corwin EJ, Fiehn O, Fitzgerald RL, Gerszten RE, Illig T, Rhee EP, Srinivas PR, Wang TJ, Jain M (2017) Potential impact and study considerations of metabolomics in cardiovascular health and disease: A scientific statement from the American Heart Association. Circ Cardiovasc Genet 10, e000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shao Y, Le W (2019) Recent advances and perspectives of metabolomics-based investigations in Parkinson’s disease. Mol Neurodegener 14, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bjornevik K, Zhang Z, O’Reilly EJ, Berry JD, Clish CB, Deik A, Jeanfavre S, Kato I, Kelly RS, Kolonel LN, Liang L, Marchand LL, McCullough ML, Paganoni S, Pierce KA, Schwarzschild MA, Shadyab AH, Wactawski-Wende J, Wang DD, Wang Y, Manson JE, Ascherio A (2019) Prediagnostic plasma metabolomics and the risk of amyotrophic lateral sclerosis. Neurology 92, e2089–e2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kelly RS, Giorgio RT, Chawes BL, Palacios NI, Gray KJ, Mirzakhani H, Wu A, Blighe K, Weiss ST, Lasky-Su J (2017) Applications of metabolomics in the study and management of preeclampsia; a review of the literature. Metabolomics 13, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kelly RS, Dahlin A, McGeachie MJ, Qiu W, Sordillo J, Wan ES, Wu AC, Lasky-Su J (2017) Asthma metabolomics and the potential for integrative omics in research and the clinic. Chest 151, 262–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bressler J, Yu B, Mosley TH, Knopman DS, Gottesman RF, Alonso A, Sharrett AR, Wruck LM, Boerwinkle E (2017) Metabolomics and cognition in African American adults in midlife: The atherosclerosis risk in communities study. Transl Psychiatry 7, e1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Oresic M, Hyotylainen T, Herukka SK, Sysi-Aho M, Mattila I, Seppanan-Laakso T, Julkunen V, Gopalacharyulu PV, Hallikainen M, Koikkalainen J, Kivipelto M, Helisalmi S, Lotjonen J, Soininen H (2011) Metabolome in progression to Alzheimer’s disease. Transl Psychiatry 1, e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].van der Lee SJ, Teunissen CE, Pool R, Shipley MJ, Teumer A, Chouraki V, Melo van Lent D, Tynkkynen J, Fischer K, Hernesniemi J, Haller T, Singh-Manoux A, Verhoeven A, Willemsen G, de Leeuw FA, Wagner H, van Dongen J, Hertel J, Budde K, Willems van Dijk K, Weinhold L, Ikram MA, Pietzner M, Perola M, Wagner M, Friedrich N, Slagboom PE, Scheltens P, Yang Q, Gertzen RE, Egert S, Li S, Hankemeier T, van Beijsterveldt CEM, Vasan RS, Maier W, Peeters CFW, Jorgen Grabe H, Ramirez A, Seshadri S, Metspalu A, Kivimaki M, Salomaa V, Demirkan A, Boomsma DI, van der Flier WM, Amin N, van Duijn CM (2018) Circulating metabolites and general cognitive ability and dementia: Evidence from 11 cohort studies. Alzheimers Dement 14, 707–722. [DOI] [PubMed] [Google Scholar]
- [19].Tynkkynen J, Chouraki V, van der Lee SJ, Hernesniemi J, Yang Q, Li S, Beiser A, Larson MG, Saaksjarvi K, Shipley MJ, Singh-Manoux A, Gerszten RE, Wang TJ, Havulinna AS, Wurtz P, Fischer K, Demirkan A, Ikram MA, Amin N, Lehtimaki T, Kahonen M, Perola M, Metspalu A, Kangas AJ, Soininen P, Ala-Korpela M, Vasan RS, Kivimaki M, van Duijn CM, Seshadri S, Salomaa V (2018) Association of branched-chain amino acids and other circulating metabolites with risk of incident dementia and Alzheimer’s disease: A prospective study in eight cohorts. Alzheimers Dement 14, 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tucker KL (2005) Stress and nutrition in relation to excess development of chronic disease in Puerto Rican adults living in the Northeastern USA. J Med Invest 52 Suppl, 252–258. [DOI] [PubMed] [Google Scholar]
- [21].Gao X, Scott T, Falcon LM, Wilde PE, Tucker KL (2009) Food insecurity and cognitive function in Puerto Rican adults. Am J Clin Nutr 89, 1197–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [23].Artiola Frotuny L, Hermosillo Romo D, Heaton RK, Pardee RE III, editors. (2000) Manual de normas y procedimientos para la bateria neuropsicologica en espanol. [Manual of norms and procedures for the Spanish neuropsychological battery.] Swets & Zeitlinger, Tucson, AZ. [Google Scholar]
- [24].Wolf-Klein GP, Silverstone FA, Levy AP, Brod MS (1989) Screening for Alzheimer’s disease by clock drawing. J Am Geriatr Soc 37, 730–734. [DOI] [PubMed] [Google Scholar]
- [25].Beery K (1989) The development test of visual-motor intergration manual. Modern Curriculum Press. [Google Scholar]
- [26].Groenwold RH, White IR, Donders AR, Carpenter JR, Altman DG, Moons KG (2012) Missing covariate data in clinical research: When and when not to use the missing indicator method for analysis. CMAJ 184, 1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Evans A, Bridgewater B, Liu Q, Mitchell M, Robinson R, Dai H, Stewart S, DeHaven C, Miller L (2004) High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics 4. doi: 10.4172/2153-0769.1000132. [DOI] [Google Scholar]
- [28].Kelly RS, Sordillo JE, Lasky-Su J, Dahlin A, Perng W, Rifas-Shiman SL, Weiss ST, Gold DR, Litonjua AA, Hivert MF, Oken E, Wu AC (2018) Plasma metabolite profiles in children with current asthma. Clin Exp Allergy 48, 1297–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mattei J, Bigornia SJ, Sotos-Prieto M, Scott T, Gao X, Tucker KL (2019) The Mediterranean diet and 2-year change in cognitive function by status of type 2 diabetes and glycemic control. Diabetes Care 42, 1372–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McCullough ML, Maliniak ML, Stevens VL, Carter BD, Hodge RA, Wang Y (2019) Metabolomic markers of healthy dietary patterns in US postmenopausal women. Am J Clin Nutr 109, 1439–1451. [DOI] [PubMed] [Google Scholar]
- [31].van den Brink AC, Brouwer-Brolsma EM, Berendsen AAM, van de Rest O (2019) The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diets are associated with less cognitive decline and a lower risk of Alzheimer’s Disease-a review. Adv Nutr 10, 1040–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Burri BJ (2015) Beta-cryptoxanthin as a source of vitamin A. J Sci Food Agric 95, 1786–1794. [DOI] [PubMed] [Google Scholar]
- [33].Burri BJ, La Frano MR, Zhu C (2016) Absorption, metabolism, and functions of beta-cryptoxanthin. Nutr Rev 74, 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Devore EE, Kang JH, Stampfer MJ, Grodstein F (2013) The association of antioxidants and cognition in the Nurses’ Health Study. Am J Epidemiol 177, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zuniga KE, Moran NE (2018) Low serum carotenoids are associated with self-reported cognitive dysfunction and inflammatory markers in breast cancer survivors. Nutrients 10, 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zeng J, Chen L, Wang Z, Chen Q, Fan Z, Jiang H, Wu Y, Ren L, Chen J, Li T, Song W (2017) Marginal vitamin A deficiency facilitates Alzheimer’s pathogenesis. Acta Neuropathol 133, 967–982. [DOI] [PubMed] [Google Scholar]
- [37].McGrattan AM, McEvoy CT, McGuinness B, McKinley MC, Woodside JV (2018) Effect of dietary interventions in mild cognitive impairment: A systematic review. Br J Nutr 120, 1388–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bigornia SJ, Scott TM, Harris WS, Tucker KL (2018) Prospective associations of erythrocyte composition and dietary intake of n-3 and n-6 PUFA with measures of cognitive function. Nutrients 10, 1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ammann EM, Pottala JV, Robinson JG, Espeland MA, Harris WS (2017) Erythrocyte omega-3 fatty acids are inversely associated with incident dementia: Secondary analyses of longitudinal data from the Women’s Health Initiative Memory Study (WHIMS). Prostaglandins Leukot Essent Fatty Acids 121, 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pottala JV, Yaffe K, Robinson JG, Espeland MA, Wallace R, Harris WS (2014) Higher RBC EPA + DHA corresponds with larger total brain and hippocampal volumes: WHIMSMRI study. Neurology 82, 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tan ZS, Harris WS, Beiser AS, Au R, Himali JJ, Debette S, Pikula A, Decarli C, Wolf PA, Vasan RS, Robins SJ, Seshadri S (2012) Red blood cell omega-3 fatty acid levels and markers of accelerated brain aging. Neurology 78, 658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang Y, Chen J, Qiu J, Li Y, Wang J, Jiao J (2016) Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: A dose-response meta-analysis of 21 cohort studies. Am J Clin Nutr 103, 330–340. [DOI] [PubMed] [Google Scholar]
- [43].Noel SE, Newby PK, Ordovas JM, Tucker KL (2010) Adherence to an (n-3) fatty acid/fish intake pattern is inversely associated with metabolic syndrome among Puerto Rican adults in the Greater Boston area. J Nutr 140, 1846–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB (2004) The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 292, 2237–2242. [DOI] [PubMed] [Google Scholar]
- [45].Vanhanen M, Koivisto K, Moilanen L, Helkala EL, Hanninen T, Soininen H, Kervinen K, Kesaniemi YA, Laakso M, Kuusisto J (2006) Association of metabolic syndrome with Alzheimer disease: A population-based study. Neurology 67, 843–847. [DOI] [PubMed] [Google Scholar]
- [46].Roberts RO, Geda YE, Knopman DS, Christianson TJ, Pankratz VS, Boeve BF, Vella A, Rocca WA, Petersen RC (2008) Association of duration and severity of diabetes mellitus with mild cognitive impairment. Arch Neurol 65, 1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K (2004) Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology 63, 658–663. [DOI] [PubMed] [Google Scholar]
- [48].Buse JB, Freeman JL, Edelman SV, Jovanovic L, McGill JB (2003) Serum 1,5-anhydroglucitol (GlycoMark ): A short-term glycemic marker. Diabetes Technol Ther 5, 355–363. [DOI] [PubMed] [Google Scholar]
- [49].Dungan KM (2008) 1,5-anhydroglucitol (GlycoMark) as a marker of short-term glycemic control and glycemic excursions. Expert Rev Mol Diagn 8, 9–19. [DOI] [PubMed] [Google Scholar]
- [50].Palmer ND, Okut H, Hsu FC, Ng MCY, Chen YI, Goodarzi MO, Taylor KD, Norris JM, Lorenzo C, Rotter JI, Bergman RN, Langefeld CD, Wagenknecht LE, Bowden DW (2018) Metabolomics identifies distinctive metabolite signatures for measures of glucose homeostasis: The Insulin Resistance Atherosclerosis Family Study (IRAS-FS). J Clin Endocrinol Metab 103, 1877–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Macotela Y, Emanuelli B, Bang AM, Espinoza DO, Boucher J, Beebe K, Gall W, Kahn CR (2011) Dietary leucine–an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One 6, e21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Toledo JB, Arnold M, Kastenmuller G, Chang R, Baillie RA, Han X, Thambisetty M, Tenenbaum JD, Suhre K, Thompson JW, John-Williams LS, MahmoudianDehkordi S, Rotroff DM, Jack JR, Motsinger-Reif A, Risacher SL, Blach C, Lucas JE, Massaro T, Louie G, Zhu H, Dallmann G, Klavins K, Koal T, Kim S, Nho K, Shen L, Casanova R, Varma S, Legido-Quigley C, Moseley MA, Zhu K, Henrion MYR, van der Lee SJ, Harms AC, Demirkan A, Hankemeier T, van Duijn CM, Trojanowski JQ, Shaw LM, Saykin AJ, Weiner MW, Doraiswamy PM, Kaddurah-Daouk R (2017) Metabolic network failures in Alzheimer’s disease: A biochemical road map. Alzheimers Dement 13, 965–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O’Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE (2011) Metabolite profiles and the risk of developing diabetes. Nat Med 17, 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bloomgarden Z (2018) Diabetes and branched-chain amino acids: What is the link? J Diabetes 10, 350–352. [DOI] [PubMed] [Google Scholar]
- [55].Chen T, Ni Y, Ma X, Bao Y, Liu J, Huang F, Hu C, Xie G, Zhao A, Jia W, Jia W (2016) Branched-chain and aromatic amino acid profiles and diabetes risk in Chinese populations. Sci Rep 6, 20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Walford GA, Davis J, Warner AS, Ackerman RJ, Billings LK, Chamarthi B, Fanelli RR, Hernandez AM, Huang C, Khan SQ, Littleton KR, Lo J, McCarthy RM, Rhee EP, Deik A, Stolerman E, Taylor A, Hudson MS, Wang TJ, Altshuler D, Grant RW, Clish CB, Gerszten RE, Florez JC (2013) Branched chain and aromatic amino acids change acutely following two medical therapies for type 2 diabetes mellitus. Metabolism 62, 1772–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tournissac M, Vandal M, Tremblay C, Bourassa P, Van cassel S, Emond V, Gangloff A, Calon F (2018) Dietary intake of branched-chain amino acids in a mouse model of Alzheimer’s disease: Effects on survival, behavior, and neuropathology. Alzheimers Dement (N Y) 4, 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


