Translational research (TR) signifies a multidisciplinary approach to expedite availability and development of clinically relevant affordable products and new strategies for improving the existing healthcare system. It encompasses new discoveries, innovations, technologies and medical devices, on one hand, and policy-based research, on the other, so as to address the medical problem/issues more effectively1. Scientific communities of various countries define TR as per their own local needs and socio-economic scenario. The broad vision and goal of TR is to connect different stakeholders and thus promote 'bench-to-bedside and bedside-to-community' research with the principal aim of addressing a clinical problem more effectively. The mission of TR is to hasten the processes involved in new discovery and innovations with the ultimate benefit to the community2,3. Accordingly, the primary aim is to facilitate the mission and vision of TR by bringing all interdisciplinary stakeholders on a common platform and introducing new products/technologies into the market and medical advancements in a cost-efficient manner for social benefits4.
All funding agencies and regulatory bodies worldwide are focusing on three main agendas of TR: (i) identifying basic research information or new innovation having TR potential, (ii) expedite grant approvals within the ethical prevue and patents in judicial boundaries, and (iii) speedy availability of these medical therapies or technologies to the community. As a result, health policies are being reformed at the government level, while workforce training and development are being initiated at various levels and awareness programmes at academia and industry levels5. However, the legal and governance framework needs greater refinements to expedite the need of healthcare system.
Multidisciplinary stakeholders including appropriate faculty, institutions, industry regulators and policymakers, both at the national and international levels, are needed to achieve more translational leads and innovations. Currently, revolutionary advancements in biomedical research using gene- and cell-based therapy, nanotechnology, proteomics, genomics, artificial intelligence and robotic technologies are redefining the research strategies. Therefore, there is a need to redefine traditional research strategies from fundamental basic research to clinical applications to lead new technologies and therapeutics5.
Understanding early phase of TR means that other groups can reproduce the research component, with validation by a third party, while the Intellectual Property Rights (IPR) remains with the innovator. Effective TR approaches are needed to develop process of proof of concept, to file patent and to validate prototype. Further, the research leads may reach the market more faster thorough technology transfer and commercialization. Hence, an integrated approach is required to support innovation from basic research to the delivery of potential public healthcare products6,7.
This article is an effort to identify the checkpoints and bottlenecks of TR and possible perspectives to redefine concrete strategies that protect and improve human health. As Indian scientists are well on the way to adopt translational biotechnologies including translational engineering, it is time to harmonize TR in biomedical sciences and redesign TR strategies with new innovations, therapeutic modalities and clinical research (CR) programmes for public health policies.
International scenario - translational research initiatives
To deal with the challenges of TR, various guidelines/models/regulatory amendments are being formulated to ease the innovative methods and technologies and thus fast track their outreach to the community. National Institutes of Health (NIH), United States, is following disease-oriented drug-targeted strategies for TR8. The Centre for Drug Evaluation and Research, United States-Food and Drug Administration (US-FDA) has established a separate cell, namely 'Office of Translational Sciences', to catalyze and provide thrust to the basic researchers for technology transfer, drug regulatory review process, and all other facilities to expedite TR9. US-FDA is also facilitating accelerated approvals for drug-targeted therapies, especially for cancer and for breakthrough therapies10. The UK has also developed one of the remarkable Translational Medicine (TM) systems in the European Union. The Medical Research Council targets fundamental discoveries from basic research and works with multidisciplinary experts. The SPARK programme of British Council, an initiative for partnership between university and industry experts, has been established to provide a cost-effective model to generate proof of concept using industrial standards11,12. Two types of biomedical research centres (BRCs) have been developed in the UK, which are playing critical roles for advancement of TR, i.e. Comprehensive BRCs and Specialist BRCs13. Another initiative 'Translation Together' is to link the gap of new innovations between basic fundamental discoveries related to human disease and the delivery of new therapeutic options to the patients. The efforts are collectively been initiated among LifeArc in the UK, the European Infrastructure for TM in the European Union, The Centre for Drug Research and Development in Canada, Therapeutic Innovation, Australia, and the NIH's National Center for Advancing Translational Sciences in the US14. For Indian research setup, the SPARK model would be the most suitable model. This model includes more than 100 experts drawn from different scientific fields, social areas and various industrial subject matter experts (SMEs) work together as per the project basis. They focus more on fundamental research for drug discovery and development process.
Translational research initiatives in India
To combat with global conundrum of TR, certain initiatives have been taken by various scientific and biomedical funding agencies of the Government of India (GoI). Some initiatives on TR by the Indian organizations are narrated below.
The Department of Biotechnology (DBT), Ministry of Science and Technology (MoST), GoI, took an initiative to establish an autonomous institution named Translational Health Science and Technology Institute in 2010. Initially, the aim of this institution was to promote fundamental research with multidisciplinary approach. The focus was on translating the identified leads to scientific and technological prototypes, and to promote innovations and patents by transforming the scientific leads for improvement in healthcare system. The ultimate objective is to develop new strategies for diagnosis and management of diseases. These programmes will help in creation of an inter-institutional ecosystem in the Biotech Science Cluster, focussed on the development of an academia-biotech-industry collaboration within major innovation hubs15.
The Biotechnology Industry Research Assistance Council (BIRAC) is a not-for-profit Public Sector enterprise, set up by the DBT, MoST, GoI. The initiative of BIRAC is mainly focussed to encourage and empower the emerging biotech industries in the Indian continent. The modus operandi is still being developed for funding; focusing more on targeted funding, IP management, technology transfer and partnership schemes that help bring innovation excellence to Indian biotech firms and strengthens them to get global recognition16. BIRAC has provided financial and mentoring support to more than 500 companies and nearly 500 young entrepreneurs or start-ups. BIRAC has initiated partnerships with several national and global network including more than 15 implementing agencies. In addition, nearly 700 national experts from academia, industry, public and private research laboratories, donor agencies and other government departments have been working hand in hand to contribute towards building the innovation ecosystem of India16.
Standford India Biodesign (SIB) programme started in 2007 and continued till 2014. SIB is an innovation programme implemented by DBT, MoST, All India Institute of Medical Sciences (AIIMS), New Delhi, and Indian Institute of Technology, Delhi (IITD), in collaboration with Queensland University of Technology, Australia, and Hiroshima University, Japan. For implementation of the programme, the DBT has engaged Biotechnology Consortium of India Limited to manage the techno-legal activities of this programme. The mandate of this programme is to train next generation of medical technology innovators in India. The focus is on invention/innovation and early-stage development of affordable, accessible and available medical technologies for Indian population17. During the last decade since inception, this interdisciplinary programme has plotted some landmark achievements; it has trained more than 100 medical technology innovators (doctors, engineers, designers, juvenile entrepreneurs); more than 50 prototypes have been developed so far which have been further refined and validated internationally and tested [both preclinical and clinical trials (CTs)]; more than 50 medical devices developed by young innovators from IITD and faculty of AIIMS got patented; 15 technologies have been transferred and nine medical technology have been sync with the Start-up India programme17.
Apart from basic biomedical research, the Central Drug Standard Control Organization (CDSCO), India, is also trying to reform rules for approval of investigational new drugs, innovations, new interventions, CTs and new medical devices as per the current requirements18. The multinational companies are introducing their medical devices by tailoring their product portfolio as per the Indian market needs to reduce out-of-pocket expenditure for patients18.
Observations of translational research leads: Redefining funding policies
India, being a signatory of United Nations Mission for Sustainable Development Goals till 20304, is actively participating to improve health research in the country on macro and micro levels. Under the Ministry of Health and Family Welfare, the Department of Health Research (DHR) has initiated many programmes including Standard Treatment Workflow (STW) and Health Technology Assessment (HTA) in India as a part of TR to evaluate new cost-effective health technologies and medical devices which can be included in the National Health Programmes4. The Indian Council of Medical Research (ICMR), New Delhi, initiated TR programme under DHR in 2008.
Run on to identify potential translational leads emerging from research done in yester years, the ICMR constituted expert committee to screen the intramural completed project reports to identify any potential lead for further research. During these programmes, diagnostic kits for various diseases and vaccines were developed. Till date, 23 technologies have been transferred to the industry, of which four have been commercialized and 19 are in pipeline for commercialization (https://main.icmr.nic.in/technology). Later, in 2017, similar screening was initiated for extramural projects to identify translational potential, if any in the completed project reports of all technical divisions of the Council, viz. Non-communicable Diseases, Epidemiology and Communicable Diseases, Reproductive Biology and Child Health, Nutrition, Bioinformatics and Basic Medical Sciences.
The project reports from basic medical sciences were exploratory, observational and experimental. Although these were reasonably good research projects, the translation component was missing in them. A few of these were comparative studies involving both basic and clinical sciences. These were well started up to T0 level (discovery stage, in silico, in vitro) and T1 level (animal studies) of translation. Although the data emerging from these reports were informative, most lacked proof of concept and a large majority required proper study design. The observed outcome could not reach up to Phase III in clinical randomized control trial; hence, no proof of concept or potential translational value could be observed. Only a few projects reached at T2 level (i.e. development of product/patent/proof of concept/prototype development/validation level). A more in-depth planning could have helped to achieve the goals particularly from wet laboratory (experimental studies) or any clinical laboratory set-ups.
Some encouraging findings in various forms of successful translation were observed in the preview of funding agency. Around 3.2 per cent reports reflected translation in various forms of basic research and clinical findings, being used successfully by other researchers, doctors or public on various portals (development of various registries). Some findings have been submitted to the Ministry of Health (State and national levels) for policy and guidelines formulations.
Challenges in translational research from basic research to product development
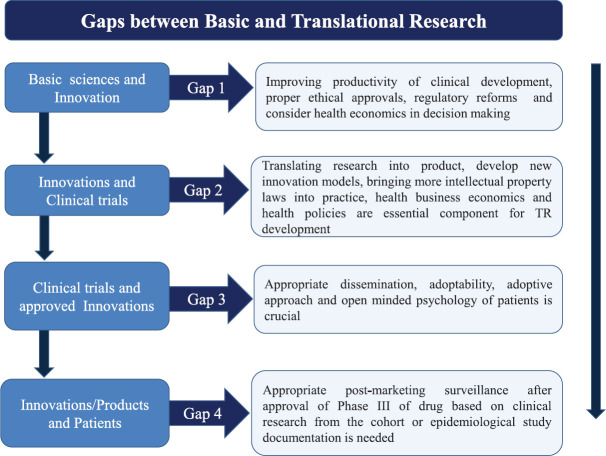
The TR is not just a linear process; it can be interconnected with translational bridges between disciplines. While there are many hurdles at every stage of development, the challenges in translating include: (i) basic non-CR to new innovation/product and diagnostic or prognostic biomarker assays, (ii) new drug development and interventions, (iii) CR to develop new therapeutic modalities, (iv) evidence-based open-minded community studies to add and develop healthcare policies/programmes, and (v) formulating new guidelines for disease prevention by policymakers. The major bottlenecks identified are slow regulatory approvals, slow patent updates and expensive patents on high revenue products, less community support, untrained workforce, inadequate research funding support and increasing competitions with other companies (indigenous as well as foreign importers)19,20. The major gaps from basic research to CR and innovations to reach market for healthcare system are discussed below5,6,7,19,20 (Fig. 1).
Fig. 1.
Gaps between basic and translational research. Source: Refs 5,6,7,19,20.
Gaps between basic translational research to clinical research (CR)
Immediate need is to redefine the linear drug development processes used in life-threatening diseases, viz. cancer, infectious diseases such as HIV and vaccines. The following considerations may lead us to achieve our goals: (i) innovative medical devices with established proof of concept in pilot study regulators need to be facilitated with faster approval process2. (ii) For these trials, the difference between exploratory and confirmatory CTs can be practiced rather than sticking to the traditional expensive and cumbersome Phase I-IV studies. This can be achieved by conducting CTs with the freedom of conducting ethically approved and clinically proved efficacy and safety, and/or proof of concept in a viable number of patients with appropriately adopted randomizations7. (iii) Confirmatory CTs need to be less expensive as compared to the classical ways of regulatory approvals of these trials. To achieve this, collaborative set-up should be encouraged to conduct CR in various platforms having expertise in specific components and data collection. There is also a need for early controlled access to patients once the confirmatory trial reaches interim conclusions or results7. (iv) Pharmacovigilance is required and pharmaco-economics need to be well analyzed before wide-access and long-term re-embracement policies21. (v) Identifying new gold standards for diagnostic and treatment approach for TR with proper and viable validations21. (vi) Validation of clinically proven and most feasible biomarkers with standardized procedures needs to be adopted before technology transfer22. (vii) Risk-benefit appraisals need to be improved with a relook into the cost-effective research set-up. (viii) Drug patent facilities of Indian Pharmaceutical Industries should fasten the process23. (ix) Quicker third party validation for life-threatening drugs needs to be dealt by an independent cell with fast-track approvals23.
Translational research gaps in institutional set-up: Road map
After screening more than 1000 project reports, the following points were emerged: (i) the study design needs to be much focussed to address outcome in terms of leads/translational value or transformation of any developed prototype. However, it should not be simply observational, experimental or exploratory study, (ii) statistical evaluations need to be appropriate as per the study design and it should be possible to draw valuable meaning from the outcome, (iii) infrastructure needs to be updated/upgraded from the older techniques used by principal investigators (PIs) to obtain faster and accurate results. Re-engineering of the research environment is needed, (iv) one of the important challenges is validation of research outcome which is not in regular practice by the researchers. Validation may be in-house within the same institution or same organization by different group (first party) or second party in National Accreditation Board for Testing and Calibration Laboratories. It was noted that the PIs were not aware about the process of validation of the data or prototype of their research outcome. Awareness programmes from funding agencies can help to deal this challenge, (v) the concept of commercialization and technology transfer needs to be inculcated in the PIs. For this, the funding agency can play an important role of effective catalyst, (vi) expenditure on obtaining patent for any lead from basic research and developed prototype must be borne by the funding agency to promote more patents applications, (vii) initiatives need to be taken by the PIs towards confirming basic research findings and develop leads into clinical efficacy and foster research collaboration with clinicians in hospitals and clinical institutions. Funding agency can also facilitate such a collaboration with own or other relevant institutions, (viii) while the PI is required to take initiatives for FDA filings for their research findings to commercialize or market their product, the funding agency must support and facilitate these initiatives, (ix) the PIs need to upgrade knowledge through educational and training opportunities provided by the funding agencies and/or develop industrial collaboration to scale-up the product at low costs, and (x) integrated research network needs to be developed within the institution or intra-institution set-ups. Serious thinking is needed to deal with the identified barriers for successful implementation of TR and TM, so that the affordable therapies can reach the community.
Perspectives: Policy consideration to accelerate translational research in India
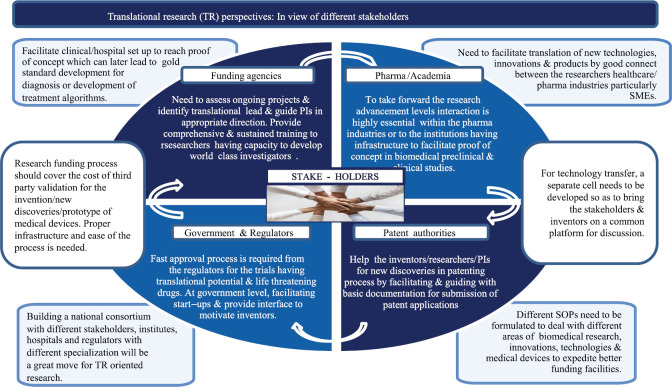
The biomedical funding organizations in India need to improve the fundamental or basic research infrastructure before approval of biomedical project. A strong coordination is required within the stakeholders to achieve the specific outcome in a predefined framework when planned for goal-oriented TR projects. In addition, new innovation models are required to work for discovery through validation which includes translational management team (TMT) to facilitate deliverables24. The TMT may facilitate validation of leads (i) from animal studies, CTs and biotech research by having collaboration/outsource with multidisciplinary team with Good Manufacturing Practices for animal testing or biotech innovations, (ii) Good Clinical Practices compliance in hospital set-ups or medical research institutions or CT management, and (iii) facilitate through validation consortium. For example, recently, many Pharma companies including Pfizer (on bio-therapeutic discoveries and development), Johnson and Johnson (on schizophrenia drug development), and AstraZeneca (on stem cell collaborations) have developed the innovation models and working on their developed model system24,25. In Figure 2, a few considerations are highlighted to accelerate TR in India7,12,20,21,22,23,24,25.
Fig. 2.
Translational research perspectives in India: In view of different stakeholders. PIs, principle investigators; SMEs, subject matter experts; SOPs, standard operating procedures. Source: Refs 7,12,20,21,22,23,24,25.
Conclusion
Biomedical as well as clinical research and development require a shift with an integrated approach from researchers and clinicians to initiate innovative ideas and discoveries which can fulfill the need driven requirements of the patients. The fundamental basic research needs to be evidence based, can be validated and target oriented which will facilitate identification of potential leads for translational discoveries. The funding agencies need initiatives to deal with the TR conundrum by redefining their policies, strategies and formulate the guidelines to support fundamental research by bridging the translational gaps. Regulatory reforms are essential to facilitate early approvals for ongoing new drug discoveries and CR. Restructuring and modifications in patent laws are required to hasten up the process of translational research which may facilitate the innovations and primary health technologies to hit market in affordable rates. There is a need to conceptualize TR programs with close collaboration within all stakeholders on an open exchange ongoing forum.
Acknowledgment:
Authors thank for language editing to Dr N.K. Mehra, Former Dean Research, All India Institute of Medical Sciences (AIIMS), New Delhi, currently holds the ICMR Dr C.G. Pandit National Chair at AIIMS, New Delhi.
Footnotes
Conflicts of Interest: None.
References
- 1.Ostergren JE, Hammer RR, Dingel MJ, Koenig BA, McCormick JB. Challenges in translational research: The views of addiction scientists. PLoS One. 2014;9:e93482. doi: 10.1371/journal.pone.0093482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahu S, Panja S. Current status and challenges of medical device innovations - Indian perspectives. J Intellect Prop Rights. 2017;99:329–35. [Google Scholar]
- 3.Wan J. Drug discovery and development in India. BioPharm Int. 2015;28:10–11. [Google Scholar]
- 4.Downey LE, Mehndiratta A, Grover A, Gauba V, Sheikh K, Prinja S, et al. Institutionalising health technology assessment: Establishing the medical technology assessment board in India. BMJ Glob Health. 2017;2:e000259. doi: 10.1136/bmjgh-2016-000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Homer-Vanniasinkam S, Tsui J. The continuing challenges of translational research: Clinician-scientists' perspective. Cardiol Res Pract. 2012;2012:246710. doi: 10.1155/2012/246710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim B. Insight for clinical technology transfer from international environmental and human rights law. J Intellect Prop Rights. 2018;23:7–21. [Google Scholar]
- 7.Mann DL, MD, Mochly-Rosen D. Translational medicine: Proceed at your own risk. Nat Rev Drug Discov. 2013;12:327–8. doi: 10.1038/nrd4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cimino JJ, Ayres EJ, Remennik L, Rath S, Freedman R, Beri A, et al. The National Institutes of Health's Biomedical Translational Research Information System (BTRIS): Design, contents, functionality and experience to date. J Biomed Inform. 2014;52:11–27. doi: 10.1016/j.jbi.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Food & Drug Administration. Office of Translational Sciences. [accessed on November 14, 2019]. Available from: https://www.fda.gov/aboutfda/ centersoffices/officeofmedicalproductsandtobacco/cder/ucm 153534.htm .
- 10.Andrews A. Can FDA Drug approval keep pace with translational research? Econom Cancer Care. 2013;4:13466. [Google Scholar]
- 11.The SPARK model for translational research has proven to be a successful paradigm which can be replicated at other academic institutions. [accessed on November 23, 2019]. Available from: https://sparkmed.stanford.edu/
- 12.Kim ES, Omura PMC, Lo AW. Accelerating biomedical innovation: A case study of the SPARK program at Stanford University, School of Medicine. Drug Discov Today. 2017;22:1064–8. doi: 10.1016/j.drudis.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Soderquest K, Lord GM. Strategies for translational research in the United Kingdom. Sci Transl Med. 2010;2:53cm28. doi: 10.1126/scitranslmed.3001129. [DOI] [PubMed] [Google Scholar]
- 14.Top global translational science organizations to catalyze translational innovation through new initiative - Translation Together. [accessed on November 23, 2019]. Available from: http://www.translationtogether.org/press_release.pdf .
- 15.Translational Health Science and Technology Institute. [accessed on January 26, 2019]. Available from: http://thsti.res.in/index.php .
- 16.Biotechnology Industry Research Assistance Council Bio-Innovations. Propelling the Bio-economy. [accessed on January 26, 2019]. Available from: http://www.dbtindia.nic.in/birac/
- 17.Biodesign - A novel Medical Technology Innovation Programme. [accessed on January 26, 2020]. Available from: http://www.dbtindia.nic.in/biodesign-a-novel-medical-technology-innovationprogramme/
- 18.Central Drugs Standard Control Organization. [accessed on January 26, 2019]. Available from: https://cdsco.gov.in/opencms/opencms/en/Notifications/Circulars/
- 19.Parrish MC, Tan YJ, Grimes KV, Mochly-Rosen D. Surviving in the valley of death: Opportunities and challenges in translating academic drug discoveries. Annu Rev Pharmacol Toxicol. 2019;59:405–21. doi: 10.1146/annurev-pharmtox-010818-021625. [DOI] [PubMed] [Google Scholar]
- 20.Vaidya M, Garg S, Singh C, Mahajan M. Changing dimensions of drug patents of Indian pharmaceutical industry. J Intellect Prop Rights. 2018;23:111–8. [Google Scholar]
- 21.Kaitin KI. Translational research and the evolving landscape for biomedical innovation. J Investig Med. 2012;60:995–8. doi: 10.231/JIM.0b013e318268694f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu D. Translational research: Current status, challenges and future strategies. Am J Transl Res. 2011;3:422–33. [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma G, Kumar H. Exploring the possibilities of utility models patent regime for grassroots innovations in India. J Intellect Prop Rights. 2018;23:119–30. [Google Scholar]
- 24.Gehr S, Garner CC. Rescuing the lost in translation. Cell. 2016;165:765–70. doi: 10.1016/j.cell.2016.04.043. [DOI] [PubMed] [Google Scholar]
- 25.Gillman J, Pillinger M, Plottel CS, Galeano C, Maddalo S, Hochman JS, et al. Teaching translational research to medical students: The New York University School of Medicine's Master's of Science in clinical investigation dual-degree program. Clin Transl Sci. 2015;8:734–9. doi: 10.1111/cts.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]