Abstract
Among the emerging economies Brazil, Russia, India, China and South Africa (together known as the BRICS countries) share collectively approximately 40 per cent of the global population and contribute to 25 per cent of the world gross domestic products. All these countries are facing the formidable challenge of rising incidence of breast cancer and significant number of premature deaths from the disease. A multidimensional approach involving prevention, early detection and improved treatment is required to counteract the growing burden of breast cancer. A growing trend in the prevalence of major preventable risk factors of breast cancer such as obesity, western dietary habits, lack of physical activity, consumption of alcohol and smoking is contributing significantly to the rising burden of the disease in BRICS nations. Specific interventions are needed at the individual and population levels to mitigate these risk factors, preferably within the broader framework of non-communicable disease control programme. Population-based quality assured mammography-based screening of the 50-69 yr old women can reduce breast cancer mortality at least by 20 per cent. However, none of the BRICS countries have been able to implement population-based organized screening programme. Large scale opportunistic screening with mammography targeting predominantly the younger women is causing harms to the women and wasting precious healthcare resources. There are recent national recommendations to screen women with mammography in Brazil and Russia and with clinical breast examination in China (along with ultrasound) and India. Given the challenges of implementing systematic screening of the population, the BRICS countries should prioritize the early diagnosis approach and invest in educating the women about the breast cancer symptoms, training the frontline health providers to clinically detect breast cancers and appropriately refer for diagnostic confirmation, and creating improved access to good quality diagnostic and treatment facilities for breast cancer. The early diagnosis approach has been proved to achieve downstaging and improve survival at a fraction of the resources needed for population screening. The countries also need to focus on improving the services and capacity for multidisciplinary treatment of breast cancer, histopathology and immunohistochemistry, safe administration of chemotherapy and palliative care.
Keywords: BRICS, breast cancer, CBE, early detection, mammography, prevention, screening
Introduction
A study of global economic trends over the next half-century has predicted that Brazil, Russia, India, China and South Africa (grouped as BRICS countries) would play an increasingly important role in the global economy, contributing collectively to nearly 25 per cent of the world Gross Domestic Product (GDP)1. The combined GDP of BRICS countries tripled from 7.9 per cent of global GDP in 1990 to 22.3 per cent in 20152. These five countries undergoing rapid economic transition are highly populous and share more than 40 per cent of the world population. The mammoth-sized population of these countries can heavily influence the disease and healthcare burden across the globe. In spite of the rapid economic growth, huge inequity in the reach and quality of healthcare delivery has been reported within each country. Progress towards achieving universal health coverage has been hindered by their insufficient public spending in health, resulting in limited access to quality healthcare by the ordinary citizens3. The growing burden of non-communicable diseases (NCDs) and consequential loss of high number of years of life and disability-adjusted life years will derail the recent initiatives for health system reforms in these countries4. Breast cancer being the most commonly diagnosed cancer among women globally as well as in the BRICS countries needs to be addressed on a priority basis keeping in mind the changing risk factors and the unique health system challenges of these five major emerging economies. Tackling the formidable challenge of rising breast cancer burden requires a multidimensional approach encompassing prevention, early detection and improved care of the patients affected by the disease. The purpose of this review article focusing on the BRICS countries is to highlight the modifiable risk factors for breast cancer that can be prevented through individual- and population-level interventions. Such interventions complemented by the adoption of resource-appropriate and pragmatic early detection strategies can reduce the morbidity and mortality from the disease.
Burden of breast cancer in BRICS countries
Breast cancer is the leading cancer among women and the number one cause of cancer deaths worldwide. According to the estimates by the Global Burden of Diseases Study, 2.38 million women were detected to have breast cancer and 0.52 million women died of the cancer across the globe in 20155. The International Agency for Research on Cancer (IARC) estimated that the BRICS countries contributed collectively to 33.6 per cent of global new cases of breast cancer and 36.9 per cent of global deaths from the disease in 20186. The incidence in the BRICS countries (ranging from 24.7/100,000 in India to 62.9/100,000 in Brazil) is still significantly lower than the reported incidence from the developed countries (Table I). Like several other developing countries, the BRICS countries are also observing a rising trend in breast cancer incidence due to population growth, ageing population and changing risk factors. The increase is not limited to the urban women or women belonging to the higher socio-economic status. For example, the population-based cancer registries in India reported almost similar annual percentage increase in age-adjusted incidence rates of breast cancer in 2012-14 in rural areas (Barshi - 1.87%), non-metropolitan cities (Bhopal - 2.00%) and metropolitan cities (Mumbai - 1.42% and Delhi - 1.44%)7.
Table I.
Estimated breast cancer burden in the BRICS (Brazil, Russia, India, China, South Africa) countries in 2018
| BRICS country | Number of new breast cancer cases detected in 2018 | Breast cancer incidence rate (/100,000) | Number of breast cancer deaths in 2018 | Breast cancer mortality rate (/100,000) |
|---|---|---|---|---|
| Brazil | 85,620 | 62.9 | 18,442 | 13.0 |
| Russian Federation | 71,426 | 53.6 | 23,181 | 15.1 |
| India | 162,468 | 24.7 | 87,090 | 13.4 |
| China | 367,900 | 36.1 | 97,972 | 8.8 |
| South Africa | 14,097 | 49.0 | 4,690 | 16.3 |
Source: Ref. 6
Survival of breast cancer has significantly increased in the 'Western' world over the past few decades due to improved health-seeking behaviour of the women, better access to diagnostic facilities, well-organized population-based screening and stage-appropriate treatment for cancers. In Australia, the five-year relative survival from breast cancer improved from 74 per cent in 1985-1989 to 91 per cent in 2011-20158. Other than China, the BRICS countries have not been able to achieve such a significant improvement in the survival rates (Fig. 1). None of the BRICS countries were among the top 25 countries with the highest age-standardized five-year net breast cancer survival estimated in women diagnosed during 2010-20149. The most critical reason for this disparity is the late stage at diagnosis. While in the developed countries 60-80 per cent of the breast cancers are detected at Stage I or Stage II, 60-70 per cent of the patients in the BRICS countries present at more advanced stages (Stage III or Stage IV)10,11. A wide variation in breast cancer survival between regions has been reported from India, China and Russia highlighting the inequity in accessing the diagnostic and treatment services within these vast countries.
Fig. 1.
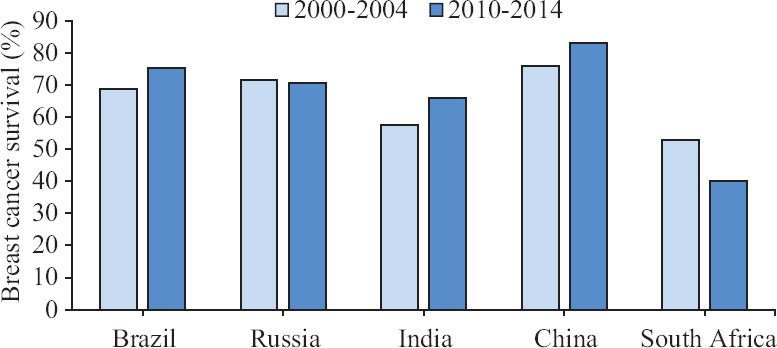
Age-standardized five-year net survival (%) of adults (15-99 yr) diagnosed with breast cancer in 2000-2004 and 2010-2014. Source: Ref. 9.
The BRICS countries face a considerable economic consequence of high breast cancer-related premature mortality, with $2 billion productivity loss reported in 2012 alone12.
Risk factors of breast cancer and preventive interventions
The risk factors for breast cancer are classified as modifiable and non-modifiable. Certain preventive measures at individual and population levels can be planned to mitigate at least some of the modifiable risk factors.
Age is the most important non-modifiable risk factor. Female life expectancy in BRICS countries increased steadily over the past decade to exceed 70 yr and became at par with the global level in 20142. The population ageing has naturally resulted in a spurt in post-menopausal breast cancers. Higher proportion of breast cancers is detected at younger age in these countries due to a proportionately large number of young women in the population, and this fact is often erroneously used as a justification to initiate screening at a younger age13. There is no evidence that the incidence of breast cancer in younger women is higher in the BRICS countries compared to the Western countries, as is obvious in the comparison of the age-specific incidence between the different countries (Fig. 2)14.
Fig. 2.
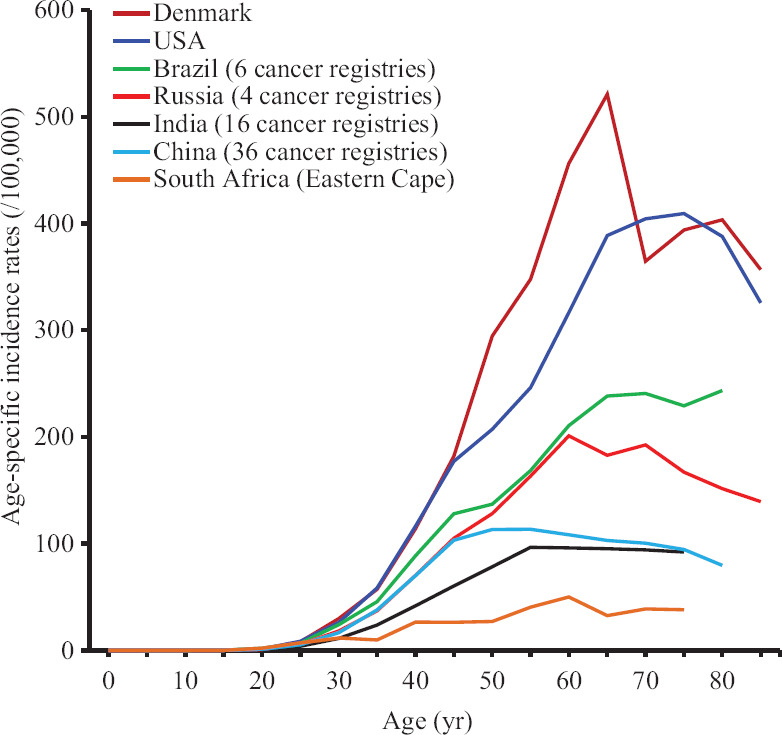
Age-specific incidence rates of breast cancer in the BRICS countries in comparison with USA and Denmark. Online analysis tool at Cancer Incidence in Five Continents, Vol. XI (electronic version) used.
Breast cancer risk increases significantly with the family history, another non-modifiable risk factor. Women with a history of breast cancer in one first-degree relative have nearly two times higher risk of breast cancer compared to those without any family history. The risk increases by three to four times when two or more first-degree relatives are affected15. Mutations of breast cancer susceptibility genes 1 and 2 (BRCA1 and BRCA2) are reported in approximately 5-10 per cent of all breast cancer patients. Women with a BRCA1 mutation have nearly six times higher risk of breast cancer compared to the women without such mutation16. The estimated risk is about three times for BRCA2 mutation. BRCA1/BRCA2 mutations are also associated with increased risk of ovarian, fallopian tube and primary peritoneal cancers17. Those women with significantly high family history or personal history of breast and the above-mentioned cancers should have genetic counselling by appropriately trained counsellor and be tested for potentially harmful (not all mutations are clinically significant) BRCA1/BRCA2 mutations, only if indicated. The management of women with such mutations requires multidisciplinary approach by a specialized team.
Women at high risk of breast cancer (assessed by different risk prediction models) may be considered for risk-reducing medications; however, such medications should not be initiated before 35 yr of age. Several randomized controlled trials (RCTs) evaluated the preventive effect of selective oestrogen receptor modulators (tamoxifen and raloxifene) and aromatase inhibitors (anastrozole and exemestane) used for five years. A meta-analysis of these RCTs observed that 7-9 fewer breast cancers were detected per 1000 users of tamoxifen or raloxifene and 16 less breast cancers detected per 1000 users of aromatase inhibitor, compared to the controls18,19. The reduction was observed only for the oestrogen receptor-positive (which have better prognosis) breast cancers without any impact on breast cancer-specific mortality. The drugs have significant side effects (increased thromboembolic events with tamoxifen and raloxifene, increased endometrial cancer with tamoxifen, musculoskeletal and vasomotor symptoms with raloxifene and aromatase inhibitors). The NICE (National Institute for Health and Care Excellence) Guidelines of the UK (published in 2013 and last updated in 2019) recommend tamoxifen for five years in pre-menopausal women at high risk of breast cancer unless they are at an increased risk for thromboembolism or endometrial cancer20. The same guidelines advise anastrozole for post-menopausal women for five years, the only contraindication being significant osteoporosis. Prescription of such preventive medications requires proper risk estimation using one of the validated tools, appropriate counselling of the women to ensure high adherence to the medicines over a prolonged period, and regular health check-ups.
Breast cancer is strongly associated with several lifestyle factors, some of which are modifiable. Being a resident of urban areas or areas with high socio-economic development itself is associated with a high incidence of breast cancer. A meta-analysis of several studies demonstrated a significantly increased risk of 17 and 25 per cent associated with higher income and higher composite socio-economic status (measured by education, income and poverty), respectively21. Certain non-modifiable reproductive health factors related to economic affluence like earlier age at menarche and delayed menopause are reported in women from the BRICS nations22,23. Each one-year delay in menarche is associated with a reduction of breast cancer risk by approximately 5.0 per cent24. Women achieving natural menopause at 55 yr have nearly twice the risk of breast cancer compared to women achieving menopause by 45 yr of age25.
Nulliparous women have up to two-fold increased risk of breast cancer compared to parous women. Each birth is associated with an average of seven per cent long-term reduction in the relative risk of breast cancer26. With better education, improved access to contraception and enhanced women's right to self-determination, the first childbirth is being delayed in the BRICS countries. Women are having less number of children, and the overall breastfeeding duration is becoming short. The Nurses' Health Study has reported that delaying the first childbirth by each year is associated with a significant three per cent increase in the risk of oestrogen receptor-positive breast cancers27. Breastfeeding has a small yet significant protective effect against breast cancer with a dose-response relationship28. The promotion of breastfeeding through better population awareness and creating a faciliatory environment for breastfeeding at workplaces is an important risk mitigation measure.
The most important modifiable risk factors for breast cancer are obesity and lack of physical exercise. Obesity has reached an epidemic proportion in Brazil, Russia and South Africa (much higher than the global average) and is showing a rising trend in China and India as a consequence of the adoption of western dietary patterns and less physical activities (Table II)29. Obesity increases the risk of post-menopausal (but not pre-menopausal) breast cancer. Waist-to-hip ratio of ≥0.95 compared to ratio ≤0.84 was found strongly associated with the risk of breast cancer in both rural and urban populations30. The World Cancer Research Fund has estimated that the risk of post-menopausal breast cancer increases by six per cent per 5 kg of adult weight gain31. The lack of physical activity was the most important preventable risk factor for breast cancer deaths in Brazil in 2015, contributing to 12 per cent of the breast cancer deaths in the country; other risk factors (alcohol, overweight and high-calorie diet) combined accounted for 6.5 per cent of all breast cancer deaths32. Physically active individuals have 14 per cent reduced risk of breast cancer along with significantly reduced risk of colon cancer, diabetes and ischaemic heart disease33.
Table II.
Prevalence of different risk factors for non-communicable diseases and breast cancer in the female populations of the BRICS countries in 2016
| Risk factor | World average (%) | Brazil (%) | Russia (%) | India (%) | China (%) | South Africa (%) |
|---|---|---|---|---|---|---|
| Obesity (BMI ≥30 kg/m2) prevalence in adult females (18+ yr) (%) | 13# | 26 | 31 | 5 | 7 | 39 |
| Prevalence of physical inactivity in adult females (18+ yr) (%) | 28# | 53 | 19 | 43 | 12 | 48 |
| Total consumption of pure alcohol per female aged 15+ yr in the year (l) | 6.4# | 2 | 6 | 2 | 3 | 3 |
| Prevalence of current tobacco smoking in adult females (15+ yr) (%) | 6 | 10 | 20 | 2 | 2 | 8 |
#For both males and females. BMI, body mass index. Source: Ref. 29
IARC identified sufficient evidence favouring a linear dose-response relationship between the consumption of alcohol and the risk of breast cancer irrespective of the type of alcohol consumed34. The Million Women's Study reported that the risk of breast cancer increased by a significant 12 per cent for every 10 g/day increase in alcohol consumption35. Tobacco smoking, especially smoking at an early age and several decades of smoking are associated with a high breast cancer risk25. High and rising prevalence of alcohol consumption and smoking have been reported in the BRICS countries (Table II).
All the BRICS countries have adopted certain strategies to counter the growing threats from the NCDs risk factors in their national NCD action plans, though the implementation of such strategies is sub-optimal. As signatories to the WHO's Framework Convention on Tobacco Control36, the BRICS countries should enforce better basic ingredients of the framework convention - advertising bans, smoke-free spaces, graphic warnings on the packages and increased taxation on tobacco products. Alcohol consumption can be reduced by increasing the excise taxes on the products, introducing legally binding regulations on alcohol advertising, alcohol sponsorship or sales promotion, and enforcing legally required health warning labels on alcohol advertisement and containers. The government should initiate focused campaigns on television and other media on the ill effects of tobacco and alcohol use and the benefits of quitting by the existing users. An age-restriction policy to regulate cigarette or alcohol purchase by children needs to be enforced.
Community-based awareness programmes, including media campaigns, are necessary to educate the population about the ill effects of consuming the sugary drinks and fast foods and the benefits of regular exercise to keep the bodyweight under control. The schools must provide nutritional guidance to the students and their parents because the food preferences and eating behaviour established in childhood are difficult to modify later. The government can regulate the size of a single serving of sugary drinks and junk foods, limit access (e.g. not allowing vending machines in and around schools) and raise taxes on them. There is evidence that the consumption of sugary drink can be reduced by 10-12 per cent by raising the tax by 10 per cent on them37. The schools should promote games and sports and create adequate provisions for such activities. Better urban planning and creating facilities for exercise, jogging and cycling are highly effective in promoting physical activities. The civil society organizations have a very crucial role to play in creating the public awareness. The frontline healthcare providers including the primary care physicians should be educated to disseminate the health promotional messages and appropriate educational materials (e.g. posters and flipcharts) should be made available to them.
The current users of combined oral contraceptive pills (OCP) have slightly higher risk compared to the non-users (7% increased risk for every five years of use)38. The risk depends on the duration of use and attenuates after the stoppage of OCP. The risk of breast cancer among the current users of hormone replacement therapy (HRT) in post-menopausal women was reported to be 33 per cent higher in the current users compared to the non-users39. However, no increased risk of breast cancer has been found among oestrogen-only HRT users40. The clinicians should assess the woman's breast cancer risk prior to prescribing OCP and HRT and appropriately counsel them.
Breast density detected on mammography is a non-modifiable risk factor largely determined by age (high in younger women) and genetic predisposition. Compared to the women with average breast density, the women with moderately and maximum dense breasts have 1.53 times and 2.34 times increased risk of breast cancer, respectively, even after adjusting for age, BMI, parity and use of HRT41. Asian women have higher breast density compared to their age-matched counterparts from other races42. This has a practical implication in breast cancer early detection as the high breast density can obscure a small lesion or micro-calcification on mammography and thus make the test less sensitive.
Screening for breast cancer
Screening for breast cancer involves a systematic examination of asymptomatic women with average risk to detect breast cancers at in situ or early invasive stages when treatment can achieve cure of the disease, and more conservative treatment can improve the quality of life. Screening is a large-scale population-level intervention, highly complex, resource-intensive and ultimately aims to reduce mortality in the entire target population. The best evidence on the effectiveness of different screening tests and strategies should ideally be gathered from the RCTs that demonstrate significant mortality reduction in the screened population. Till date, only the trials evaluating mammography to screen women between 50 and 69 yr of age have conclusively demonstrated a reduction in mortality. The relative risks of breast cancer deaths among the mammography screened women compared to controls observed by different meta-analyses of the breast cancer randomized trials are listed in Table III43,44,45,46,47,48. An expert group convened by IARC in 2015 reviewed the evidence from the RCTs as well as the well-designed large cohort studies and concluded that mammography screening reduced the risk of death by 23 per cent among the women invited to screening and by 40 per cent among women actually undergoing the test49. Some reduction in the risk of dying from breast cancer was observed from mammography screening among 45-50 and 70-74 yr old women as well. Screening women below 45 yr of age with mammography causes more harms than benefits25.
Table III.
Relative risk of breast cancer deaths in women screened with mammography compared to the unscreened women or women screened with alternative screening tests (e.g. clinical breast examination) in the meta-analyses of randomized clinical trials evaluating the efficacy of mammography screening
| Meta-analysis reference (yr) | Age at entry (yr) | RR of breast cancer deaths | 95% CI |
|---|---|---|---|
| IARC (2002)43 (excluded trials with CBE screening) | 40-49 | 0.81 | 0.65-1.01 |
| 50-69 | 0.75 | 0.67-0.85 | |
| Nelson et al44 | 39-49 | 0.85 | 0.75-0.96 |
| 50-59 | 0.86 | 0.75-0.99 | |
| 60-69 | 0.68 | 0.54-0.87 | |
| 70-74 | 1.12 | 0.73-1.72 | |
| Tonelli et al45 | 40-49 | 0.85 | 0.75-0.96 |
| 50-69 | 0.79 | 0.68-0.90 | |
| 70-74 | 0.68 | 0.45-1.01 | |
| Magnus et al46 | 39-49 | 0.83 | 0.72-0.97 |
| Gøtzsche and Jørgensen47 | 39-49 | 0.84 | 0.73-0.96 |
| ≥50 | 0.77 | 0.69-0.86 | |
| Marmot et al48 | 40-74 | 0.80 | 0.73-0.89 |
CBE, clinical breast examination; CI, confidence interval; RR, relative risk; IARC, International Agency for Research on Cancer
A well-recognized harm of mammography screening is over-diagnosis, which is defined as the detection of a less aggressive early cancer that would not have been clinically evident in the woman's lifetime and was detected only because of screening. Over-diagnosis obviously leads to unnecessary treatment and resultant harms. Over-diagnosis is usually estimated by comparing the number of cancers detected in the screened arm compared to the unscreened arm of a clinical trial after adjusting for the contemporaneous rising trend in breast cancer incidence and the lead time (early detection of the prevalent cancers through screening will cause an early increase in the cancer detection rate, which will come down below the detection rate in the unscreened arm if the screened cohort is followed up over adequate number of years). Although the estimates vary widely across the trials, it is now accepted that the rate of over-diagnosis in a quality assured mammography programme is between 10 and 20 per cent50,51,52. Based on the benefits demonstrated by the RCTs, most of the developed countries have introduced mammography screening every 2-3 yr at least for the women in the age group of 50-69 yr. Considering the complexities and high resource demand of mammography screening, the WHO recommended that such programmes should be implemented through an organized population-based approach only in the high resourced countries or in limited resourced countries with relatively strong health systems53. To be considered as an organized population-based programme, mammography screening at a national or sub-national level must fulfil the following criteria54:
(i) There should be a documented policy to provide quality-assured screening, diagnostic and treatment services to all eligible women with equity. The policy should also specify the structure of management, organization of services and maintaining coordination between all stakeholders.
(ii) A written protocol specifying the target population, methods of identifying and inviting the eligible women, screening and further evaluation protocol, referral mechanism etc. should be followed by all levels of service providers and programme managers.
(iii) Adequate workforce, infrastructure and financial resources should exist to sustain mammography screening of the entire target population every 2-3 yr and further investigate the women tested positive (at least 5% of total screened).
(iv) Sufficient resources should be available to procure and install mammography machines, engage a sufficient number of trained radiographers, radiologists and physicists, maintain regular supply of consumables and have appropriate maintenance and servicing facilities. Countries with reasonably effective mammography-based screening programmes have 15-30 mammography machines per one million inhabitants55.
(v) The presence of an information system linking the population registers to the screening database will allow identification of the eligible women and invite them to ensure high screening coverage (at least 70%).
(vi) The programme should have a robust system of quality assurance based on regular collection of performance data through an effective health information system. The performance should be evaluated using a set of validated indicators and standards and appropriate actions should be taken if any deficiencies are detected. A good proportion (~30%) of the total screening programme budget should be allocated to the implementation of quality assurance.
Unfortunately, none of the BRICS countries have been able to implement well-organized breast cancer screening programmes, though a large number of mammograms are performed in the opportunistic setting without any quality assurance with a relatively low coverage. Moreover, the guidelines on breast cancer screening vary widely among BRICS countries (Table IV)56,57,58,59,60,61. In Brazil, the National Cancer Institute Guidelines on breast cancer screening recommend mammography every two years for 50-69 yr old women. Although women aged 40-49 yr are not the focus of the programme, according to Brazilian Federal legislation mammography screening should be guaranteed annually to all women from 40 yr of age onwards through the Brazilian Public Health System56,57. The Brazilian National Health Survey in 2013 reported that 60 per cent of the women between 50 and 69 yr had a mammogram in the past two years with a high coverage in the more affluent South and Southeast regions62. However, analysis of the data from the national breast cancer screening registry showed that half of the screening mammograms were performed outside the recommended age of 50-69 yr63.
Table IV.
National recommendations for breast cancer early detection in BRICS countries
| BRICS country | Breast cancer early detection/screening recommendation (National Cancer Control Plan) |
|---|---|
| Brazil56,57 | Brazilian NCI guidelines propose mammography every two years for women aged 50-69 yr and mammography for high risk women starting at the age of 35 yr. |
| Federal Law guarantees mammography screening annually to all women from 40 yr of age onwards. | |
| Russian Federation58 | Mammography every two years for women aged 40-75 yr. |
| India59 | Mammography is not recommended in the national breast cancer screening guidelines. |
| Women aged 30 yr and above are screened with CBE. | |
| China60 | Mammography is not recommended in the national breast cancer screening guidelines. |
| Women aged 35-64 yr are screened with CBE. | |
| South Africa61 | Mammography every year for women aged 40-54 yr. |
| Mammography every two years for women aged 55 yr and older; or if they choose, continue with an annual mammogram. |
CBE, clinical breast examination; NCI, National Cancer Institute
Mammography-based breast cancer screening is recommended in South Africa starting at the age of 40 yr. A population-based survey in South Africa in 2012 reported less than 20 per cent women above the age of 39 yr ever having mammography with a huge inequity based on wealth, place of residence, education and racial characteristics61. The Ministry of Health in Russia issued an order in 2012 to initiate nation-wide opportunistic breast cancer screening in the country64. There were several changes to the order, and the latest guidelines issued in 2019 recommend mammography every two years at the age of 40-75 yr. However, the quality control of this programme is not implemented, and the regional uptake is not clear58. Mammography is not recommended in the national breast cancer screening guidelines in India and China, though a lot of opportunistic tests are performed in both these countries. The overall opportunistic mammograms screening rate was 21.7 per cent in 2010 in China65.
Clinical breast examination (CBE) has been evaluated as an alternative to mammography. CBE involves a systematic visual examination followed by palpation of the breast and the axilla. CBE is simple, requires no special equipment or consumables, can be performed at primary care settings and non-clinician providers can be trained to perform the test. The sensitivity of CBE (approximately 50%) is much lower compared to mammography (above 80% in women aged 50-69 yr). The positive predictive value of the test in a screening setting is quite low (1%) implying that a large number of screen-positive women would require unnecessary diagnostic investigations. Two large randomized studies to compare CBE with no screening have been conducted in India and neither could demonstrate mortality reduction after three rounds of CBE66,67 . IARC initiated a cluster-randomized trial in southern India in 2006, in which healthy women aged 30-69 yr (n=115,652) were randomly allocated either to be screened with CBE every three years or to receive education about the early symptoms of breast cancer but no screening67. The trial demonstrated that significantly lower proportion of breast cancer cases were detected in the advanced stages, which improved the survival of the women. However, the RCT failed to show any significant reduction of breast cancer-specific mortality in the trial arm.
Even though the evidence favouring CBE screening from RCTs is limited, an economic modelling has demonstrated that yearly CBE among 40-60 yr old women can be comparable to mammography screening in reducing mortality but at a substantially lower cost in the emerging economies like the BRICS68. China and India decided to incorporate the test in the national breast cancer screening programmes. The Chinese Ministry of Health launched the Chinese National Breast Cancer Screening Programme in 2008 covering 29 provinces and screened more than 1.22 million Chinese women aged between 35 and 64 yr in urban (n=398,184) and rural (n=828,530) China. The CBE-positive women in the urban areas underwent mammography with or without ultrasound, while those in the rural areas underwent ultrasound (mammography, if necessary only) for diagnosis confirmation60,69.
India has a comprehensive NCD control programme and the guidelines recommend breast cancer screening of women aged 30 yr and above with CBE to be performed by the nurses and midwives at the primary health centers59. The recommended interval of five years is long for breast cancer screening. The programme is purely opportunistic and is being implemented only in a few districts covering an insignificant proportion of the vast target population. The Government of the southern state of Tamil Nadu implemented its own NCD control programme through a World Bank-supported project (Tamil Nadu Health Systems Project) launched in 2005 as a pilot70. The community health workers and the female members of the rural self-help groups are trained to create awareness in the community and mobilize women to undergo screening. Dedicated NCD nurses have been trained to perform CBE and other screening tests. The screening is offered in 1753 primary health centres, 267 government hospitals and 100 municipal dispensaries in the State71. During 2012-2018, nearly 23 million women were screened, and the CBE positivity was one per cent.
Conventional hand-held breast ultrasound (HHUS) has been evaluated as a screening tool, either alone or as an adjunct to mammography. It was hypothesized that ultrasound would work better or at least as good as mammography in Asian women with dense breasts. The Japan Strategic Anti-cancer Randomized Trial (J-START) was initiated in 2007 and randomized 72,998 women aged 40-49 yr to undergo screening either with mammography along with ultrasound (study group) or mammography alone (control group)72. An interim analysis in 2016 revealed that sensitivity was significantly higher in the study group (91.1 vs. 77.0%), but specificity was significantly lower (87.7 vs. 91.4%). The detection rate of breast cancer in the study group was higher than the control group (0.50 vs. 0.34%) with more cancers being detected at Stage 0 or Stage 1 in the study arm compared to the control (52.0%). The study was not able to demonstrate any difference between the two trial arms to reduce breast cancer mortality, and the harm-benefit balance was unclear given the large number of women having a false-positive diagnosis on ultrasound and requiring further interventions. A meta-analysis has demonstrated that in women with dense breasts ultrasound detects additional four breast cancer cases per 1000 mammography-negative women but nearly doubles the referral rate to further assessment73. Breast ultrasound is being extensively used to screen women in the national programme in China (along with CBE)74.
HHUS is very much operator-dependent, necessitates the presence of a radiologist and requires a considerable amount of time to assess both breasts. By contrast, high-frequency automated breast three-dimensional ultrasound (ABUS) is less operator dependent, requires less time, can be performed by trained nurses or technicians and the results are more reproducible. Studies have shown that in asymptomatic women with normal mammography but dense breasts ABUS is capable of detecting approximately two breast cancers per 1000 women tested75. A multicentric hospital-based cross-sectional study carried out in China in 2016 compared HHUS, ABUS and mammography in 1974 CBE-positive women aged 30-69 years76. The results suggest that ABUS has the comparable clinical performance to HHUS for breast cancer detection, and both traditional ultrasound and ABUS have better performance than mammography, especially among women with high-density breasts. Except China, none of the BRICS countries use ultrasound extensively to screen women for breast cancer. A multicentric study to compare ABUS, HHUS and mammography techniques for breast cancer screening targeting 63,000 Chinese women was launched in China in 201874. Advising women to systematically examine their own breast every month as a routine (breast self-examination) is not effective in improving breast cancer survival or reducing mortality and is not recommended as a screening test25.
Early diagnosis of breast cancer
Early diagnosis is a strategy to detect breast cancer early and is an alternative to systematic screening. Implementation of early diagnosis requires altering the health-seeking behaviour of the women by educating the community about the early symptoms of breast cancer and the necessity of its early detection. In addition, training the frontline healthcare providers to recognize the breast cancer symptoms and signs and promptly refer the women with suspected disease, reducing the barriers for the referred women to access good quality diagnostic facilities and ensuring treatment of the women with cancer without delay are the important components of the early diagnosis approach. While screening is quite complex and resource-intensive as it focuses on the entire apparently normal population at risk, early diagnosis approach is directed to much less number of women with symptoms only and is logistically much simpler and affordable for all settings77. Although early diagnosis may not be as efficient as systematic screening to detect the breast cancers at early stage, yet there is adequate evidence that breast cancer survival as well as quality of life of the patients can be significantly improved through such a simple strategy. The health staffs in 18 district hospitals and 154 rural clinics were trained to detect breast cancers early as part of the Early Cancer Surveillance Programme in Sarawak Province in Malaysia starting in 199478. Simultaneously, the community was sensitized through pamphlets, posters and counselling by health workers. Within four years, the proportion of breast cancers detected in Stage III and Stage IV was significantly reduced from 60 to 35 per cent. A cohort study initiated in 2013 sent awareness materials on breast cancer annually to 22,500 women aged 30-69 yr who were beneficiaries of an occupational health scheme in Mumbai, India. The women with symptoms were provided prompt diagnostic services and treatment through a specialized breast clinic79. Within three years, the proportion of early-stage breast cancer increased from 74 to 81 per cent, the proportion of patients eligible for breast-conserving surgery increased from 39 to 51 per cent and the proportion of patients requiring adjuvant chemotherapy decreased from 84 to 56 per cent79.
The BRICS countries may consider revising their breast screening policies; in the best scenario, they should move for an organized, population-based, mammograph-based screening, as this is the recommendation by the WHO53 considering the best cost-benefit. If this is not feasible, they need to prioritize the early diagnosis of breast cancer. This requires a concerted and innovative approach to ensure that women are aware of the common symptoms of breast cancer and appreciate the need to consult a health provider if any such symptoms exist. The primary healthcare providers (nurses, general practitioners and primary care physicians) should be trained to perform a good quality clinical examination of the symptomatic women. A systematic CBE by an appropriately trained provider has high negative predictive value and can efficiently rule out the presence of disease. The women suspected to have abnormalities on clinical examination should have access to diagnostic imaging (mammography and/or ultrasound) followed by diagnosis confirmation with either core needle biopsy (CNB) or fine-needle aspiration cytology (FNAC). CNB has advantages over FNAC in providing tissue for immunohistochemistry and diagnosing more efficiently the atypical ductal hyperplasia and in situ carcinomas80. However, FNAC is rapid, logistically simpler and more acceptable to the patients with sensitivity and specificity marginally lower than those of CNB, especially in the women with palpable lumps80. Setting up breast ultrasound and guided FNAC facilities at secondary level of care can significantly improve early diagnosis of breast cancers in the BRICS countries.
Conclusions
The progressive privatization of healthcare resulting in higher inequity, fragmented public health services and high out of pocket expenditures is a common feature of the BRICS countires. In spite of major progresses in health reforms, many of the key health indicators show sub-optimal performance in these countries81. The formidable challenge posed by the NCDs is still somewhat underappreciated in the BRICS countries, and they need to be more responsive to the WHO Global Action Plan to prevent and control NCDs, including a 25 per cent relative reduction in premature mortality from NCDs82. Achieving the voluntary targets for NCD control (reducing smoking and alcohol consumption, reduced prevalence of obesity and physical inactivity, etc.) will have a significant effect on breast cancer burden. Recognizing the fact that the implementation of quality assured population-based screening and achieving a target of 70 per cent coverage (the coverage recommended by the WHO to achieve any significant impact of screening) would be a herculean task for each of these populous countries, the BRICS countries should consider reviewing their approach and policies towards breast cancer screening. It is important to monitor the existing screening activities in the countries where there is a national recommendation (mammography-based screening in Brazil and Russia, and CBE-based screening in China and India). A proper evaluation of the programmes will not only allow improvement of quality of services but also will generate valuable evidence on the effectiveness of screening in the countries 'in transition'. Breast cancer is curable at an early stage. Better surgical care with a multidisciplinary approach, availability of good quality anaesthesia facilities, improving the histopathology and basic immunohistochemistry capacities, ensuring the availability of tamoxifen for the estrogen receptor positive tumours and cytotoxic chemotherapy for more biologically aggressive cancers will have a huge impact on breast cancer survival83. The policymakers need to be more committed and invest rationally to save the lives of the thousands of women succumbing to a curable cancer at present.
Footnotes
Financial support & sponsorship: None.
Disclaimer: Authors identified as personnel of the International Agency for Research on Cancer (IARC)/WHO are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the IARC/WHO.
Conflicts of Interest: None.
References
- 1.Singh SP, Memory D. The BRICS and the new world order: A beginner's guide. Brazil: Centre for International Trade, Economics and Environment, South African Institute of International Affairs; 2013. [accessed on October 7, 2019]. Available from: https://saiia.org.za/research/the-brics-and-the-new-world-order-a-beginnersguide/ [Google Scholar]
- 2.World Health Organization. BRICS. Health and WHO. Country presence profile. Geneva: WHO; 2017. [Google Scholar]
- 3.Marten R, McIntyre D, Travassos C, Shishkin S, Longde W, Reddy S, et al. An assessment of progress towards universal health coverage in Brazil, Russia, India, China, and South Africa (BRICS) Lancet. 2014;384:2164–71. doi: 10.1016/S0140-6736(14)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jakovljevic MB, Milovanovic O. Growing burden of non-communicable diseases in the emerging health markets: The case of BRICS. Front Public Health. 2015;3:65. doi: 10.3389/fpubh.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted Life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global Burden of disease study. JAMA Oncol. 2017;3:524–48. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global cancer observatory: Cancer today. Lyon, France: International Agency for Research on Cancer; 2018. [accessed on October 17, 2019]. Available from: https://gco.iarc.fr/today . [Google Scholar]
- 7.National Cancer Registry Programme, National Centre for Disease Informatics and Research, Indian Council of Medical Research. Three-year report of population based cancer registries 2012-2014. incidence, distribution, trends in incidence rates and projections of burden of cancer (report of 27 PBCRs in India) [accessed on October 17, 2019]. Available from: http://ncdirindia.org/All_Reports/PBCR_REPORT_2012_2014/ALL_CONTENT/PDF_Printed_Version/Preliminary_Pages_Printed.pdf .
- 8.Cancer Australia. Breast Cancer in Australia Statistics. [accessed on October 17, 2019]. Available from: https://breast-cancer.canceraustralia.gov.au/statistics .
- 9.Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–75. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Justo N, Wilking N, Jönsson B, Luciani S, Cazap E. A review of breast cancer care and outcomes in Latin America. Oncologist. 2013;18:248–56. doi: 10.1634/theoncologist.2012-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, et al. Breast cancer in China. Lancet Oncol. 2014;15:E279–89. doi: 10.1016/S1470-2045(13)70567-9. [DOI] [PubMed] [Google Scholar]
- 12.Pearce A, Sharp L, Hanly P, Barchuk A, Bray F, de Camargo Cancela M, et al. Productivity losses due to premature mortality from cancer in Brazil, Russia, India, China, and South Africa (BRICS): A population-based comparison. Cancer Epidemiol. 2018;53:27–34. doi: 10.1016/j.canep.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Chopra B, Kaur V, Singh K, Verma M, Singh S, Singh A. Age shift: Breast cancer is occurring in younger age groups: Is it true? Clin Cancer Investig J. 2014;3:526–9. [Google Scholar]
- 14.Bray F, Colombet M, Mery L, Piñeros M, Znaor A, Zanetti R, Ferlay J. Cancer incidence in five continents (electronic version), Vol. XI. Lyon, France: International Agency for Research on Cancer; 2017. [accessed on October 9, 2019]. Available from: http://ci5.iarc.fr/CI5-XI/Default.aspx . [Google Scholar]
- 15.Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: Collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet. 2001;358:1389–99. doi: 10.1016/S0140-6736(01)06524-2. [DOI] [PubMed] [Google Scholar]
- 16.Kurian AW, Hughes E, Handorf EA, Gutin A, Allen B, Hartman A, et al. Breast and ovarian cancer penetrance estimates derived from germline multiple-gene sequencing results in women. Precis Oncol. 2017;1:1–2. doi: 10.1200/PO.16.00066. [DOI] [PubMed] [Google Scholar]
- 17.Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: A combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–30. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson HD, Fu R, Zakher B, McDonagh M, Pappas M, Stillman L. Medication use for the risk reduction of primary breast cancer in women: A systematic review for the U.S preventive services task force. Rockville (MD): Agency for Healthcare Research and Quality (US); 2019. [PubMed] [Google Scholar]
- 19.Nelson HD, Fu R, Zakher B, Pappas M, McDonagh M. Medication use for the risk reduction of primary breast cancer in women: Updated evidence report and systematic review for the US preventive services task force. JAMA. 2019;322:868–86. doi: 10.1001/jama.2019.5780. [DOI] [PubMed] [Google Scholar]
- 20.National Institute for Health and Care Excellence. Familial breast cancer: Classification, care Familial breast cancer: Classification, care and managing breast cancer and related and managing breast cancer and related risks in people with a family history of risks in people with a family history of breast cancer. [accessed on October 17, 2019]. Available from: https://www.nice.org.uk/guidance/cg164/resources/familial-breast-cancerclassification-care-and-managing-breast-cancer-and-relatedrisks-in-people-with-a-family-history-of-breast-cancer-pdf-35109691767493 . [PubMed]
- 21.Akinyemiju TF, Genkinger JM, Farhat M, Wilson A, Gary-Webb TL, Tehranifar P. Residential environment and breast cancer incidence and mortality: A systematic review and meta-analysis. BMC Cancer. 2015;15:191. doi: 10.1186/s12885-015-1098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forman MR, Mangini LD, Thelus-Jean R, Hayward MD. Life-course origins of the ages at menarche and menopause. Adolesc Health Med Ther. 2013;4:1–21. doi: 10.2147/AHMT.S15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozlov AI, Vershubsky G. Secular trends in average height and age at menarche of ethnic Russians and Komi-Permyaks of the Permsky Krai, Russia. Anthropol Anz. 2015;72:27–42. doi: 10.1127/anthranz/2014/0427. [DOI] [PubMed] [Google Scholar]
- 24.Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: Individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13:1141–51. doi: 10.1016/S1470-2045(12)70425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.International Agency for Research on Cancer Breast cancer screening. IARC handbooks of cancer prevention, Vol 15. Lyon, France: IARC; 2016. [Google Scholar]
- 26.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: Collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002;360:187–95. doi: 10.1016/S0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]
- 27.Sisti JS, Collins LC, Beck AH, Tamimi RM, Rosner BA, Eliassen AH. Reproductive risk factors in relation to molecular subtypes of breast cancer: Results from the Nurses' Health Studies. Int J Cancer. 2016;138:2346–56. doi: 10.1002/ijc.29968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Cancer Research Fund. London, UK: WCRF; 2017. Continuous update project systematic literature review: The associations between food, nutrition and physical activity and the risk of breast cancer. [Google Scholar]
- 29.World Health Organization. Noncommunicable diseases and mental health.Noncommunicable diseases country profiles. Geneva: WHO; 2018. [Google Scholar]
- 30.Nagrani R, Mhatre S, Boffetta P, Rajaraman P, Badwe R, Gupta S, et al. Understanding rural-urban differences in risk factors for breast cancer in an Indian population. Cancer Causes Control. 2016;27:199–208. doi: 10.1007/s10552-015-0697-y. [DOI] [PubMed] [Google Scholar]
- 31.World Cancer Research Fund/American Institute for Cancer Research. Continuous update project expert report 2018.Diet, nutrition, physical activity and breast cancer. London, UK: WCRF/AICR; 2018. [Google Scholar]
- 32.Silva DAS, Tremblay MS, Souza MFM, Guerra MR, Mooney M, Naghavi M, et al. Mortality and years of life lost due to breast cancer attributable to physical inactivity in the Brazilian female population (1990-2015) Sci Rep. 2018;8:11141. doi: 10.1038/s41598-018-29467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: Systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ. 2016;354:i3857. doi: 10.1136/bmj.i3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. A review of human carcinogens: Personal habits and indoor combustions, Part E. Lyon, France: IARC; 2012. [PMC free article] [PubMed] [Google Scholar]
- 35.Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, et al. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101:296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- 36.Framework Convention Alliance. Parties to the WHO FCTC (ratifications and accessions) [accessed on October 8, 2020]. Available from: https://www.fctc.org/parties-ratifications-and-accessions-latest/
- 37.World Health Organization. e-Library of Evidence for Nutrition Actions (eLENA) Reducing consumption of sugarsweetened beverages to reduce the risk of unhealthy weight gain in adults. [accessed October 8, 2020]. Available from: https://www.who.int/elena/bbc/ssbs_adult_weight/en/
- 38.Zhu H, Lei X, Feng J, Wang Y. Oral contraceptive use and risk of breast cancer: A meta-analysis of prospective cohort studies. Eur J Contracept Reprod Health Care. 2012;17:402–14. doi: 10.3109/13625187.2012.715357. [DOI] [PubMed] [Google Scholar]
- 39.Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36:114–36. doi: 10.1093/epirev/mxt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chlebowski RT, Rohan TE, Manson JE, Aragaki AK, Kaunitz A, Stefanick ML, et al. Breast cancer after use of estrogen plus progestin and estrogen alone: Analyses of data from 2 women's health initiative randomized clinical trials. JAMA Oncol. 2015;1:296–305. doi: 10.1001/jamaoncol.2015.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pettersson A, Graff RE, Ursin G, Santos Silva ID, McCormack V, Baglietto L, et al. Mammographic density phenotypes and risk of breast cancer: A meta-analysis. J Natl Cancer Inst. 2014;106:pii: dju078. doi: 10.1093/jnci/dju078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.del Carmen MG, Halpern EF, Kopans DB, Moy B, Moore RH, Goss PE, et al. Mammographic breast density and race. AJR Am J Roentgenol. 2007;188:1147–50. doi: 10.2214/AJR.06.0619. [DOI] [PubMed] [Google Scholar]
- 43.International Agency for Research on Cancer. Breast cancer screening. IARC handbooks of cancer prevention, Vol. 7. Lyon, France: IARC Press; 2002. [Google Scholar]
- 44.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L, et al. Screening for breast cancer: An update for the US Preventive services task force. Ann Intern Med. 2009;151:727–37. doi: 10.1059/0003-4819-151-10-200911170-00009. W237-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tonelli M, Connor Gorber S, Joffres M, Dickinson J, Singh H, Lewin G, et al. Recommendations on screening for breast cancer in average-risk women aged 40-74 years. CMAJ. 2011;183:1991–2001. doi: 10.1503/cmaj.110334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magnus MC, Ping M, Shen MM, Bourgeois J, Magnus JH. Effectiveness of mammography screening in reducing breast cancer mortality in women aged 39-49 years: A meta-analysis. J Womens Health (Larchmt) 2011;20:845–52. doi: 10.1089/jwh.2010.2098. [DOI] [PubMed] [Google Scholar]
- 47.Gøtzsche PC, Jørgensen KJ. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2013;2013:CD001877. doi: 10.1002/14651858.CD001877.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer screening: An independent review. Br J Cancer. 2013;108:2205–40. doi: 10.1038/bjc.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lauby-Secretan B, Scoccianti C, Loomis D, Benbrahim-Tallaa L, Bouvard V, Bianchini F, et al. Breast-cancer screening - viewpoint of the IARC Working Group. N Engl J Med. 2015;372:2353–8. doi: 10.1056/NEJMsr1504363. [DOI] [PubMed] [Google Scholar]
- 50.Puliti D, Duffy SW, Miccinesi G, de Koning H, Lynge E, Zappa M, et al. Overdiagnosis in mammographic screening for breast cancer in Europe: A literature review. J Med Screen. 2012;19(Suppl 1):42–56. doi: 10.1258/jms.2012.012082. [DOI] [PubMed] [Google Scholar]
- 51.Zackrisson S, Andersson I, Janzon L, Manjer J, Garne JP. Rate of over-diagnosis of breast cancer 15 years after end of Malmö mammographic screening trial: Follow-up study. BMJ. 2006;332:689–92. doi: 10.1136/bmj.38764.572569.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Houssami N. Overdiagnosis of breast cancer in population screening: Does it make breast screening worthless? Cancer Biol Med. 2017;14:1–8. doi: 10.20892/j.issn.2095-3941.2016.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Health Organization. WHO position paper on mammography screening. Geneva: WHO; 2014. [PubMed] [Google Scholar]
- 54.Perry N, Broeders M, de Wolf C, Törnberg S, Holland R, von Karsa L. European guidelines for quality assurance in breast cancer screening and diagnosis. 4th ed. Luxembourg: Office for Official Publications of the European Communities; 2006. [accessed on October 30, 2019]. Available from: http://screening.iarc.fr/doc/ND7306954ENC_002.pdf . [DOI] [PubMed] [Google Scholar]
- 55. [accessed on October 15, 2019];OECDiLibrary. Mammography Machines (Indicator). OECDiLibrary. 2019 https://www.oecd-ilibrary.org/socialissues-migration-health/mammography-machines/indicator/english_685c9c5e-en . [Google Scholar]
- 56.Migowski A, Silva GAE, Dias MBK, Diz MDPE, Sant'Ana DR, Nadanovsky P. Guidelines for early detection of breast cancer in Brazil.II – New national recommendations, main evidence, and controversies. Cad Saude Publica. 2018;34:e00074817. doi: 10.1590/0102-311X00074817. [DOI] [PubMed] [Google Scholar]
- 57.Barcelos MRB, Nunes BP, Duro SMS, Tomasi E, Lima R, Chalupowski MN, et al. Utilization of breast cancer screening in Brazil: An external assessment of primary health care access and quality improvement program. Health Syst Reform. 2018;4:42–55. [Google Scholar]
- 58.The Ministry of Healthcare of the Russian Federation. The Order of the Ministry of Healthcare of the Russian Federation #124n from 13.03.2019’ on Implementing the Order of Prophylactic Medical Check-Up and Dispenserization of Certain Groups of Adult Population. 2019. [accessed on October 17, 2019]. Available from: https://cdnimg.rg.ru/pril/168/48/51/54495.pdf .
- 59.National Programme for Prevention and Control of Cancer. Diabetes, Cardiovascular Diseases & Stroke. Operational Guidelines (Revised: 2013-17) [accessed on October 15, 2019]. Available from: https://mohfw.gov.in/sites/default/files/Operational%20Guidelines%20 of%20 NPCDCS%20%28 Revised%20-%202013-17%29.pdf .
- 60.Huang Y, Dai H, Song F, Li H, Yan Y, Yang Z, et al. Preliminary effectiveness of breast cancer screening among 1.22 million Chinese females and different cancer patterns between urban and rural women. Sci Rep. 2016;6:39459. doi: 10.1038/srep39459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Phaswana-Mafuya N, Peltzer K. Breast and cervical cancer screening prevalence and associated factors among women in the South African general population. Asian Pac J Cancer Prev. 2018;19:1465–70. doi: 10.22034/APJCP.2018.19.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Viacava F, Bellido JG. Health, access to services and sources of payment, according to household surveys. Cien Saude Colet. 2016;21:351–70. doi: 10.1590/1413-81232015212.19422015. [DOI] [PubMed] [Google Scholar]
- 63.Tomazelli JG, Migowski A, Ribeiro CM, Assis M, Abreu DM. Assessment of actions for breast cancer early detection in Brazil using process indicators: A descriptive study with Sismama data, 2010-2011. Epidemiol Serv Saude. 2017;26:61–70. doi: 10.5123/S1679-49742017000100007. [DOI] [PubMed] [Google Scholar]
- 64.Barchuk A, Bespalov A, Huhtala H, Chimed T, Laricheva I, Belyaev A, et al. Breast and cervical cancer incidence and mortality trends in Russia 1980-2013. Cancer Epidemiol. 2018;55:73–80. doi: 10.1016/j.canep.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 65.Wang B, He M, Wang L, Engelgau MM, Zhao W, Wang L. Breast cancer screening among adult women in China, 2010. Prev Chronic Dis. 2013;10:E183. doi: 10.5888/pcd10.130136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mittra I, Mishra GA, Singh S, Aranke S, Notani P, Badwe R, et al. A cluster randomized, controlled trial of breast and cervix cancer screening in Mumbai, India: Methodology and interim results after three rounds of screening. Int J Cancer. 2010;126:976–84. doi: 10.1002/ijc.24840. [DOI] [PubMed] [Google Scholar]
- 67.Sankaranarayanan R, Ramadas K, Thara S, Muwonge R, Prabhakar J, Augustine P, et al. Clinical breast examination: Preliminary results from a cluster randomized controlled trial in India. J Natl Cancer Inst. 2011;103:1476–80. doi: 10.1093/jnci/djr304. [DOI] [PubMed] [Google Scholar]
- 68.Okonkwo QL, Draisma G, der Kinderen A, Brown ML, de Koning HJ. Breast cancer screening policies in developing countries: A cost-effectiveness analysis for India. J Natl Cancer Inst. 2008;100:1290–300. doi: 10.1093/jnci/djn292. [DOI] [PubMed] [Google Scholar]
- 69.Shen S, Zhou Y, Xu Y, Zhang B, Duan X, Huang R, et al. A multi-centre randomised trial comparing ultrasound vs.mammography for screening breast cancer in high-risk Chinese women. Br J Cancer. 2015;112:998–1004. doi: 10.1038/bjc.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tamil Nadu Health System Project, Government of Tamil Nadu. 2015. [accessed on October 16, 2019]. Available from: http://www.tnhsp.org .
- 71.Selvavinayagam TS. Screening 35 million for hypertension and diabetes mellitus through public system: Experiences of Tamil Nadu, India. Int J Community Med Public Health. 2017;4:3882–7. [Google Scholar]
- 72.Ohuchi N, Suzuki A, Sobue T, Kawai M, Yamamoto S, Zheng YF, et al. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): A randomised controlled trial. Lancet. 2016;387:341–8. doi: 10.1016/S0140-6736(15)00774-6. [DOI] [PubMed] [Google Scholar]
- 73.Rebolj M, Assi V, Brentnall A, Parmar D, Duffy SW. Addition of ultrasound to mammography in the case of dense breast tissue: Systematic review and meta-analysis. Br J Cancer. 2018;118:1559–70. doi: 10.1038/s41416-018-0080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jia M, Lin X, Zhou X, Yan H, Chen Y, Liu P, et al. Diagnostic performance of automated breast ultrasound and handheld ultrasound in women with dense breasts. Breast Cancer Res Treat. 2020;181:589–97. doi: 10.1007/s10549-020-05625-2. [DOI] [PubMed] [Google Scholar]
- 75.Brem RF, Tabár L, Duffy SW, Inciardi MF, Guingrich JA, Hashimoto BE, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: The SomoInsight Study. Radiology. 2015;274:663–73. doi: 10.1148/radiol.14132832. [DOI] [PubMed] [Google Scholar]
- 76.Zhang X, Lin X, Tan Y, Zhu Y, Wang H, Feng R, et al. A multicenter hospital-based diagnosis study of automated breast ultrasound system in detecting breast cancer among Chinese women. Chin J Cancer Res. 2018;30:231–9. doi: 10.21147/j.issn.1000-9604.2018.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harford JB. Breast-cancer early detection in low-income and middle-income countries: Do what you can versus one size fits all. Lancet Oncol. 2011;12:306–12. doi: 10.1016/S1470-2045(10)70273-4. [DOI] [PubMed] [Google Scholar]
- 78.Devi BC, Tang TS, Corbex M. Reducing by half the percentage of late-stage presentation for breast and cervix cancer over 4 years: A pilot study of clinical downstaging in Sarawak, Malaysia. Ann Oncol. 2007;18:1172–6. doi: 10.1093/annonc/mdm105. [DOI] [PubMed] [Google Scholar]
- 79.Gadgil A, Sauvaget C, Roy N, Muwonge R, Kantharia S, Chakrabarty A, et al. Cancer early detection program based on awareness and clinical breast examination: Interim results from an urban community in Mumbai, India. Breast. 2017;31:85–9. doi: 10.1016/j.breast.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 80.Mitra S, Dey P. Fine-needle aspiration and core biopsy in the diagnosis of breast lesions: A comparison and review of the literature. Cytojournal. 2016;13:18. doi: 10.4103/1742-6413.189637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gómez EJ. Confronting health inequalities in the BRICS: Political Institutions, foreign policy aspirations and state-civil societal relationships. Glob Policy. 2016;7:500–9. [Google Scholar]
- 82.World Health Organization. Noncommunicable diseases and mental health. About 9 voluntary global targets. [accessed on October 15, 2019]. Available from: https://www.who.int/nmh/ncd-tools/definition-targets/en/
- 83.Clarke M. Meta-analyses of adjuvant therapies for women with early breast cancer: The Early Breast Cancer Trialists' Collaborative Group overview. Ann Oncol. 2006;17(Suppl 10):x59–62. doi: 10.1093/annonc/mdl238. [DOI] [PubMed] [Google Scholar]


