Abstract
Aim:
The aim of this study was to compare between equine and human bone blocks in the osteogenic differentiation of cultured human periodontal ligament stem cells (hPDLSCs) at 14 and 21 days of culture, using confocal laser microscopy and scanning electron microscopy.
Materials and Methods:
In vitro cultures of commercially obtained hPDLSCs were seeded onto equine and human bone blocks. At 14 days and 21 days of culture, confocal laser microscope images were obtained to assess cellular differentiation and adhesion, and scanning electron microscope images were obtained to validate the osteogenic differentiation by showing the morphological characteristics of the new bone cells.
Results:
Both equine and human bone blocks showed positive staining for newly formed bone cells through the confocal laser microscope analysis, however, a higher signal intensity was expressed at 21 days of culture. These findings indicate the biocompatibility of hPDLSC with both types of bone blocks, cellular differentiation, and adhesion. Scanning electron microscopy images validated the osteogenic differentiation by showing the common characteristics of bone cells as flattened, polygonal morphology with multiple extending cytoplasmic processes.
Conclusion:
Both equine and human bone blocks were able to confirm the osteogenic capability of seeded human PDLSC. There was no significant difference between equine and human bone blocks on the human PDLSC differentiation. Superior osteogenic differentiation of cultured hPDLSCs was evident at 21 days in comparison to 14 days.
Keywords: Allograft, bone blocks, equine, osteogenic differentiation, periodontal ligament stem cells, stem cells, tissue engineering, xenograft
Introduction
One of the main challenges that are encountered in rehabilitating edentulous area is insufficient bone height or width. Several techniques for alveolar ridge augmentation have been introduced, however, with continual search for the most predictable regenerative outcome and least complications, the concept of tissue engineering was introduced. For successful tissue engineering, two important factors should be considered. First, engineering features related to the biomechanical properties of the scaffold, the architectural geometry, and the space maintenance properties. Second, biological features related to the biological functions of the engineered matrix, including cell recruitment, permission of neovascularization, and delivery of the requisite growth factors for tissue regeneration.[1] Regarding the engineering features, several materials have been used as carrier to support cellular migration and space maintenance including bone grafts.[2] Different types of bone grafts including autografts, allografts, and xenografts are available according to its source of origin. Earlier studies have showed that autogenous bone is the “gold standard” for bone augmentation procedures, however, shortcomings such as donor site morbidity, bone resorption, and high operative and hospital costs have urged the exploration of alternative materials, such as allografts, xenografts, and bone-mimetic synthetic materials. Allograft is a source of bone substitute from the same species that has been introduced mainly due to the complications associated with harvesting autogenous bone. Unlike the autografts, it lacks the osteogenic cells and consequently acts only as an osteoinductive and osteoconductive material.[3] Xenograft is a source of bone substitute from another species such as coral, bovine, and equine. During the processing of xenografts, organic components such as cells and proteinaceous materials are removed to produce an inert absorbable bone scaffold, which assists in revascularization, osteoblast migration, and new bone formation, and so it mainly acts as an osteoconductive material only.[3,4] One type of xenografts which have recently gained much popularity is the equine bone graft. It is preferred for its low transmissibility of disease, as reports have showed that horses, rabbits, and dogs are the only mammalians known to be resistant to infections of prion diseases from other species.[5] Studies have also showed that its demineralized matrix is high in different types of growth factors that include insulin-like growth factor (IGF)-I, IGF-II, transforming growth factor-beta, platelet-derived growth factor, and bone morphogenetic proteins, all of which make it a great choice to simulate bone formation.[6]
Regarding the biological features of tissue engineering, mesenchymal stem cells (MSCs) are considered proper candidates due to their extensive expansion rate and its potential to differentiate into different types of cells. Of these MSCs, human periodontal ligament stem cells (hPDLSCs) are now considered as a more appropriate cell type for developing novel periodontal tissue engineering strategies, as it displays high expression of runt-related transcription factor 2 and alkaline phosphatase which are closely associated with the differentiation into hard tissue-forming cells, and it also has the ability to differentiate to clonogenic colonies that are self-renewable and have the ability to produce a range of dental and nondental tissues, including cementoblast-like cells, adipocytes, and collagen-forming cells.[7]
The seeding of cells onto scaffolds or carriers should provide a structure for cell adhesion, proliferation, and differentiation at the grafting site. Several documented attempts have been made to seed the hPDLSC on different carriers, which have shown a significant osteogenic differentiation of the seeded hPDLSC.[8] Knowing that there are different types of scaffolds, the choice of the best construct remains a major issue to imitate the extracellular matrix architecture and biological functions; a scaffolds are not only required to provide mechanical support but also to carry inductive molecules to supply signals for the newly formed bone.
The aim of this study was to compare between equine and human bone blocks as scaffolds on the osteogenic potentials of hPDLSCs using confocal laser microscopy to examine cell adhesion, intercellular continuity, cytoskeletal development, and potential damage, and further with the use of scanning electron microscopy to assess the cellular morphology of the newly formed bone cells, in a timely fashion that corresponds to the osteogenic differentiation stages at 14 and 21 days of culture.
Materials and Methods
Ethical guidelines
This study was conducted in accordance with protocols of King Saud University.
Approval of the Ethical Committee was obtained from College of Dentistry Research Center and the Institutional Review Board.
Work protocol of the study was conducted using facility and support of “molecular and cell biology” laboratory at the College of Dentistry, King Saud University, Riyadh, Saudi Arabia.
Scaffold material
Allograft scaffold material used is allograft wedge system (Integra LifeSciences, Plainsboro, NJ, USA). It is a processed human allograft tissue composed of compressed cancellous bone providing strength, rigidity, and volume enhancement for rapid healing and revascularization of the graft. The xenograft scaffold material is OsteoBiol SP-block Norm (Tecnoss Dental, Coazze, TO, Italy), an equine bone block graft that combines the mineral properties of cancellous particulate with the space maintenance of collagen providing a convenient approach to filling periodontal and maxillofacial defects. All block samples were unified in a dimension of 5 mm × 5 mm × 5 mm.
Cell culture
hPDLSC was supplied from ScienCell Company, isolated from human periodontal tissue, cryopreserved, and delivered frozen in vial containing >5 × 10^5 cells in 1 ml volume. hPDLSCs were cultured at passage 4 in regular growth media and placed in a T-75 flask, cells were allowed to grow, and the media was changed every other day until it reached a confluency of 85% within 14 days of culture.
After that, cell counting was done using a hemocytometer to ensure that cell proliferation and growth has reached the desired confluency. Subsequently, PDLSCs were seeded with an amount of 250,000 of cells on each block in accordance with the following four groups:
Group 1: (A-14 days) where PDLSCs were cultured with an allograft and assessed at 14 days of culture, Group 2: (X-14 days) where PDLSCs were cultured with an xenograft block and assessed at 14 days of culture, Group 3: (A-21 days) where PDLSCs were cultured with an allograft and assessed at 21 days of culture, and Group 4: (X-21 days) where PDLSCs were cultured with an xenograft block and assessed at 21 days of culture.
All samples were cultured in a 48-well plate, in regular growth culture media, and it was changed every other day until the day of assessment. Subsequently, samples were imaged through confocal laser microscope as a whole block, after that the blocks were sectioned into thin sections to be assessed by the scanning electron microscope (SEM).
Analysis
Confocal laser scanning microscope
To stain the samples, the culture media was discarded, and cells were fixed with 4% paraformaldehyde in 0.1 m sodium PBS. Afterward, permeabilization was done through adding 0.5% Triton X-100 for 10 min and then blocking with skimmed milk for 30 min. Primary monoclonal antibody to vinculin (Acris, Rockville, MD, USA) was added and incubated overnight at 4°C, then material was discarded, and samples were washed with phosphate buffer solution (PBS) three times. That was followed by incubation with the secondary antibody, Alexa Fluor 488 goat anti-mouse immunoglobulin 1 (Molecular Probes, Invitrogen, Eugene, OR, USA) for 1 h at room temperature to stain the focal adhesions of vinculin, then incubated with Alexa Flour 594 phalloidin (Molecular Probes, Invitrogen, Eugene, OR, USA) also for 1 h at room temperature to mark the actin cytoskeleton. After that, samples were washed three times with PBS and imaged with Nikon C2 Confocal Microscope system connected to inverted Nikon TI Eclipse microscope consuming an argon laser beam with excitation lines at 488 nm and a helium–neon source. The microscope setting was set to visualize the focal adhesion of vinculin in red color while the actin cytoskeleton in green color.
Scanning electron microscopy scanning electron microscope
Preparation of the samples for SEM analysis started by washing away any proteinaceous fluid. After that, samples were fixed in 2.5% glutaraldehyde for 48 h in room temperature, dried in increasing ethanol concentrations, and then critical point dried to ensure that the samples are free of any volatile solvents. They were then mounted on aluminum stubs and gold coated to visualize the shape of the cells, the most important thing to consider during gold sputter coating is to start with a short burst of coating lasting only one or a few seconds at a low coating current, and allow the sample surface to cool again before continuing with the main coating operation.
Results
Confocal laser microscopy
At 14 days of culture, confocal images showed positive representations of new bone cells, where the green signals for the cytoskeleton outlined the scaffolds' margin. However, at 21 days of culture, the images displayed higher signal intensity, where the signals are showing more yellow color that signifies the overlapping of high intensities of both green and red signals. This indicates more spreading of the new bone cells and increased osteogenic differentiation compared to what is found at 14 days of culture [Figure 1].
Figure 1.
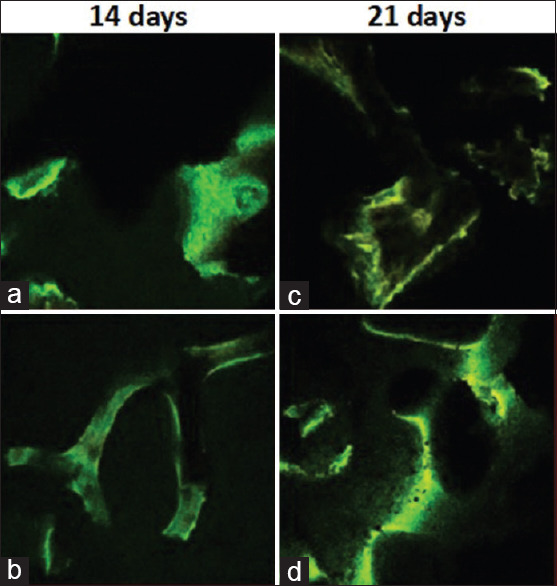
Confocal laser microscopy images. (a) 14 days of cultured human periodontal ligament stem cell on: Allograft bone block. (b): 14 days of cultured human periodontal ligament stem cell on: Xenograft bone block. (c): 21 days of cultured human periodontal ligament stem cell on: Allograft bone block. (d): 21 days of cultured human periodontal ligament stem cell on: Xenograft bone block
Scanning electron microscopy
At first, imaging of both types of scaffolds was done prior to culture, to show its topographical morphology. The images reveal the porous architecture of the scaffolds; it shows that the pore sizes are smaller in the allograft sample compared to the xenograft samples. In relation to the pore size, the allograft sample pores were smaller but covering a large surface area making them more in quantity, while the xenograft samples had larger pores covering smaller surface area, which make the xenograft samples less porous than the allograft samples. Results show that cultured hPDLSC had grown within the scaffolds as illustrated by the cell colonies that are formed and adhered to the scaffolds. These newly formed cells are showing flat, spread-out, and polygonal appearance with extending cytoplasmic processes indicating their osteoblastic characteristics. These processes were closely linked with the pores of the scaffold indicating the positive anchorage of the cells on the scaffold. At day 21 of culture, the newly formed cells were more coalesced with higher expression of extending cytoplasmic processes that increased the cell-to-cell contact and cellular bridging covering most of the scaffolds' surface area in comparison to the 14-day culture groups [Figure 2].
Figure 2.
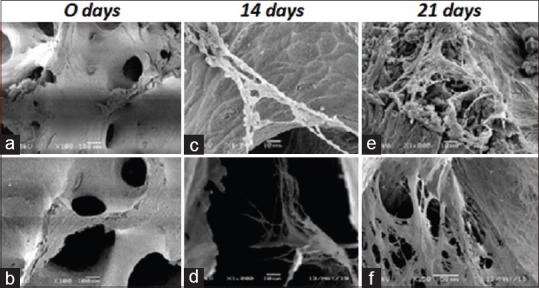
Scanning electron microscopy scanning electron microscope images. (a): Allograft bone block. (b): Xenograft bone block. (c): 14 days of cultured human periodontal ligament stem cell on: Allograft bone block. (d): 14 days of cultured human periodontal ligament stem cell on: Xenograft bone block. (e): 21 days of cultured human periodontal ligament stem cell on: Allograft bone block. (f): 21 days of cultured human periodontal ligament stem cell on: Xenograft bone block
Discussion
The challenge to find the best components for bone tissue engineering has evolved to explore different types of cells, scaffolds, and growth factors. Massive experimental attempts have been made on different types of bone graft materials to find the most appropriate scaffolds; most studies have agreed that for a scaffold to support bone regeneration, it should be biocompatible and capable to support cell growth and differentiation.[9] The aim of this study was to compare between equine and human bone blocks on cultured osteogenic differentiation of hPDLSCs at 14 and 21 days of culture, in regular growth media, using confocal laser microscopy and scanning electron microscopy. The confocal laser microscopy was used to assess the structural integrity of the new bone cells and their adhesion properties by observing the expression of actin and focal adhesion of vinculin, which are critical proteins for cell attachment and sustainability. Our confocal microscope images of the samples demonstrated positive growth and differentiation of the seeded PDLSCs, indicating the biological plausibility of both types of scaffold material and its compatibility with seeded PDLSCs. Reports on the effect of bovine and equine scaffolds on expanded bone marrow stem cells have showed that both scaffolds induced a significant increase in alkaline phosphatase, osteopontin, and calcium deposits signifying the biocompatibility and osteogenic potential of equine scaffolds, which comes in support to our findings.[10] The osteogenic differentiation of seeded PDLSCs was demonstrated in the confocal images by the green fluorescent signals outlining the scaffold margins, which represents the focal adhesions of vinculin. These focal adhesions are membrane-associated complexes that serve as nucleation sites for actin filaments that link between the cell exterior and the actin cytoskeleton inside.[11] The dynamic changes upon the osteogenesis of MSCs exhibit membrane–cytoskeleton interactions that are mediated by transmembrane glycoproteins at the focal contacts; these focal adhesions were displayed in higher density in the osteoblasts compared to the undifferentiated MSCs. This fact makes the membrane–cytoskeleton interactions in the osteoblast much stronger.[12] Our study findings have also shown that with time progression, the osteogenic differentiation of PDLSCs was more pronounced as the intensity of the green fluorescent signals increased from 14 days of culture to 21 days of culture. That could be explained by the decreasing density of the actin cytoskeleton during the osteogenic differentiation into mature osteoblast, which makes the intensity of the green florescent signals to be more pronounced. The structural changes in the membrane cytoskeleton are related to the mechanical characteristics of the differentiated cells, as osteoblasts are subjected to different mechanical strains it should have a strong structure to withstand these loads. Hence, upon osteogenesis, the actin cytoskeleton starts to decrease in density after 7 days, and the focal adhesion along with other protein linkers will start to be reorganized around 14 days, and as time progresses, the density will increase as well, resulting in a strong membrane–cytoskeleton interactions characteristic for the mature osteoblast.[12] This could interpret the increasing intensity of the green florescent signals at 21 days of culture compared to 14 days of culture. Positive osteogenic differentiation of PDLSCs has been shown on confocal images as accumulated green florescent signals showing an increased expression of multiple anchoring vinculin junctions and focal adhesions indicating an increased infiltration of osteogenic cells[13,14] and that explains the increased green florescent signals in our samples at 21 days. According to the present findings, positive osteogenic differentiation of PDLSCs was expressed as the cells displayed a predominant flattened, polygonal morphology with extending filopodia and fiber-like processes that connects between the newly formed cells and the scaffold forming intercellular bridging and anchorage and that was observed on the images of the scanning electron microscopy that was used to identify the morphological characteristics of the newly formed cells. Cellular morphology has long been used as an important indicator to characterize the presence of new cells and to assess cell quality. Several reports have showed that cell geometry is highly correlated with the differentiation into osteogenic lineages.[15] The results of two other studies have confirmed the evidence of the osteogenic differentiation of seeded bone marrow stem cells and hPDLSCs through SEM images, showing close contact with the scaffold through extended cytoplasmic processes and filopodia indicating cellular anchorage.[16,17] In addition, in the present study, the SEM images have also showed positive osteogenic differentiation at 14 days of culture, however, superior osteogenic differentiation was displayed more at on 21 days of culture. Other studies showed that evidence of osteogenic differentiation at 14 days of culture corresponds to the early stage of cellular adhesion which is considered the most important stage as the ability of the cells to attach and adhere is crucial indicator for continual cell proliferation and spread, and with progressive differentiation around 21–28 days of culture, the cells seem to spread out with extension of many projections that cover the majority of the surface area of the scaffold, along with production of a fibrillar extracellular matrix that enhances the cell–material interactions.[18,19] And the same was found in the present study which confirms the superior osteogenic differentiation at 21 days.
Conclusion
Both equine and human bone blocks were able to confirm the osteogenic capability of seeded human PDLSC. There was no significant difference between equine and human bone blocks on the human PDLSC differentiation. However, superior osteogenic differentiation of cultured hPDLSCs at 21 days in comparison to 14 days was found.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bartold PM, McCulloch CA, Narayanan AS, Pitaru S. Tissue engineering: A new paradigm for periodontal regeneration based on molecular and cell biology. Periodontol 2000. 2000;24:253–69. doi: 10.1034/j.1600-0757.2000.2240113.x. [DOI] [PubMed] [Google Scholar]
- 2.Pagni G, Kaigler D, Rasperini G, Avila-Ortiz G, Bartel R, Giannobile WV. Bone repair cells for craniofacial regeneration. Adv Drug Deliv Rev. 2012;64:1310–9. doi: 10.1016/j.addr.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore WR, Graves SE, Bain GI. Synthetic bone graft substitutes. ANZ J Surg. 2001;71:354–61. [PubMed] [Google Scholar]
- 4.Scaglione M, Fabbri L, Dell'Omo D, Gambini F, Guido G. Long bone nonunions treated with autologous concentrated bone marrow-derived cells combined with dried bone allograft. Musculoskelet Surg. 2014;98:101–6. doi: 10.1007/s12306-013-0271-2. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J. The structural stability of wild-type horse prion protein. J Biomol Struct Dyn. 2011;29:369–77. doi: 10.1080/07391102.2011.10507391. [DOI] [PubMed] [Google Scholar]
- 6.Pacaccio DJ, Stern SF. Demineralized bone matrix: Basic science and clinical applications. Clin Podiatr Med Surg. 2005;22:599–606, vii. doi: 10.1016/j.cpm.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Han J, Menicanin D, Marino V, Ge S, Mrozik K, Gronthos S, et al. Assessment of the regenerative potential of allogeneic periodontal ligament stem cells in a rodent periodontal defect model. J Periodontal Res. 2014;49:333–45. doi: 10.1111/jre.12111. [DOI] [PubMed] [Google Scholar]
- 8.Tour G, Wendel M, Moll G, Tcacencu I. Bone repair using periodontal ligament progenitor cell-seeded constructs. J Dent Res. 2012;91:789–94. doi: 10.1177/0022034512452430. [DOI] [PubMed] [Google Scholar]
- 9.Chou AM, Sae-Lim V, Hutmacher DW, Lim TM. Tissue engineering of a periodontal ligament-alveolar bone graft construct. Int J Oral Maxillofac Implants. 2006;21:526–34. [PubMed] [Google Scholar]
- 10.El-Sabban ME, El-Khoury H, Hamdan-Khalil R, Sindet-Pedersen S, Bazarbachi A. Xenogenic bone matrix extracts induce osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. Regen Med. 2007;2:383–90. doi: 10.2217/17460751.2.4.383. [DOI] [PubMed] [Google Scholar]
- 11.Burridge K, Nuckolls G, Otey C, Pavalko F, Simon K, Turner C. Actin-membrane interaction in focal adhesions. Cell Differ Dev. 1990;32:337–42. doi: 10.1016/0922-3371(90)90048-2. [DOI] [PubMed] [Google Scholar]
- 12.Titushkin I, Cho M. Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophys J. 2007;93:3693–702. doi: 10.1529/biophysj.107.107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Born AK, Rottmar M, Lischer S, Pleskova M, Bruinink A, Maniura-Weber K. Correlating cell architecture with osteogenesis: First steps towards live single cell monitoring. Eur Cell Mater. 2009;18:49–62. doi: 10.22203/ecm.v018a05. [DOI] [PubMed] [Google Scholar]
- 14.Manescu A, Giuliani A, Mohammadi S, Tromba G, Mazzoni S, Diomede F, et al. Osteogenic potential of dualblocks cultured with human periodontal ligament stem cells: In vitro and synchrotron microtomography study. J Periodontal Res. 2016;51:112–24. doi: 10.1111/jre.12289. [DOI] [PubMed] [Google Scholar]
- 15.Matsuoka F, Takeuchi I, Agata H, Kagami H, Shiono H, Kiyota Y, et al. Morphology-based prediction of osteogenic differentiation potential of human mesenchymal stem cells. PLoS One. 2013;8:e55082. doi: 10.1371/journal.pone.0055082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trubiani O, Orsini G, Zini N, Di Iorio D, Piccirilli M, Piattelli A, et al. Regenerative potential of human periodontal ligament derived stem cells on three-dimensional biomaterials: A morphological report. J Biomed Mater Res A. 2008;87:986–93. doi: 10.1002/jbm.a.31837. [DOI] [PubMed] [Google Scholar]
- 17.Annaz B, Hing KA, Kayser M, Buckland T, Di Silvio L. Porosity variation in hydroxyapatite and osteoblast morphology: A scanning electron microscopy study. J Microsc. 2004;215:100–10. doi: 10.1111/j.0022-2720.2004.01354.x. [DOI] [PubMed] [Google Scholar]
- 18.Barrias CC, Ribeiro CC, Lamghari M, Miranda CS, Barbosa MA. Proliferation, activity, and osteogenic differentiation of bone marrow stromal cells cultured on calcium titanium phosphate microspheres. J Biomed Mater Res A. 2005;72:57–66. doi: 10.1002/jbm.a.30217. [DOI] [PubMed] [Google Scholar]
- 19.Hatakeyama W, Taira M, Chosa N, Kihara H, Ishisaki A, Kondo H. Effects of apatite particle size in two apatite/collagen composites on the osteogenic differentiation profile of osteoblastic cells. Int J Mol Med. 2013;32:1255–61. doi: 10.3892/ijmm.2013.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]


