Abstract
The severe acute respiratory syndrome (SARS)-coronavirus- 2 (CoV-2) outbreak in Wuhan, China has now spread to many countries across the world including the India with an increasing death toll. On March 11, 2020, the new clinical condition COVID-19 (Corona-Virus-Disease-19) was declared a pandemic by the World Health Organization (WHO). Owing to its infectivity, high risk of transmission, and limited handling of dead bodies, published data on the course of diseases has been limited. Most patients with COVID-19 have a mild disease course and remain as asymptomatic carrier; however, few patients of older age and with co-morbidites develop severe disease leading on to fatality. If due to COVID-19 infection death occurs, an autopsy is unlikely. However in unnatural deaths the legal duty impels the proper performance of a full autopsy, to find out the cause and manner of death. The detailed autopsy examination along with histo-pathological findings in the organs of asymptomatic patient of COVID-19 and its comparison with microscopic findings in Aluminium Phosphide poisoning are discussed below. This will summarizes the research status for COVID-19 deaths, which will be important for evaluation of cause of death, prevention, control and clinical strategies of COVID-19.
Keywords: Aluminium phosphide, asymptomatic carrier, autopsy, COVID-19, histo-pathological
Introduction
The emergence of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the respiratory disease coronavirus disease 2019 (COVID-19), caused a global pandemic that has emerged as an unprecedented worldwide public health emergency.[1] However, with the global outbreak, the diseases ranged from minimal symptoms to severe pneumonia. Asymptomatic infections refer to the positive detection of nucleic acid of SARS-CoV-2 in patient samples by reverse transcriptase-polymerase chain reaction (RT-PCR) but have no typical clinical symptoms or signs, and no apparent abnormalities in images, including lung computed tomography (CT).[2]
The guidelines from the Government of India mention that autopsy should be avoided in confirmed COVID-19 cases-related deaths.[3] However, section 174 of the code of criminal procedure, in India, authorizes the investigating police officer to hold an inquest into suspicious, and unnatural deaths, and forensic experts are called upon for conducting autopsy to accurately diagnose the cause of death.[4] On conducting the medicolegal autopsy the gross and histopathological findings of an asymptomatic COVID-19 patient who consumed aluminum phosphide[5] were compared with, the histopathology of aluminum phosphide poisoning with no previous history of SARS-CoV-2.
Case Report and Observation
An apparent healthy, afebrile, nonsmoker 31-year-old female individual presented to the emergency department at 11:45 PM, with alleged history of consumption of poisonous ssubstance, at about 11:30 PM at her residence. She was managed as case of poisoning, routine blood investigation showed hemoglobin (Hb)-9.7 gm/dl, total leukocyte count 20.000 cells/cu. mm, sodium (Na+)-136 mmol/L, potassium (K+)-3.08 mmol/L, random blood glucose 291 mg/dl, serum urea-31 mg/dl, pO2 35.2 mmHg, pCO2 33.0 mmHg, and pH 7.133, however, with best treatment modalities, she was declared dead at 12:55AM, the following morning. Being a COVID-19 pandemic, a nasopharyngeal swab test[2] was conducted before shifting the body to mortuary. She was labeled as COVID-19 because of a positive test result by RT-PCR for SARS-CoV-2. She had no history of contact or travel history to the high-risk zone of COVID-19. Autopsy was done in infection isolation autopsy rooms, by health personnel using personal protective equipment in accordance, with guidelines of the Indian Government.[3]
On autopsy, body was wrapped in a double airproof plastic zipped bag, she was average built, 161 cm in length, rigor mortis was present on the whole body, and post mortem staining was involving the back area, except the areas of contact with the surface. No external injuries were found. Internally, on the opening stomach, the mucosa was found to be congested and grayish material adherent to it with garlic odor. The solid organs comprising liver, spleen, and kidneys, were found to be congested. On gross examination, lungs are enlarged, heavy, boggy, edematous [Figure 1a] and showed a mosaic-like [Figure 1b], pattern of pale fields and slightly dark purple discoloration [Figure 1c], with marked fluid retention which oozed out on compression [Figure 1d], as compared to the aluminium phosphide poisoning lung. The right lung weighed 870 g and the left lung weighed 760 g and the combined lung weight was 1630 g. The whole of the laryngeal-tracheal lumen was filled with frothy fluid. All major parenchymal organs including both lungs were dissected out and were submerged in buffered 10% formalin solution and sampled for histopathological examination, and in supersaturated salt solution for chemical examination for final cause of death.
Figure 1.
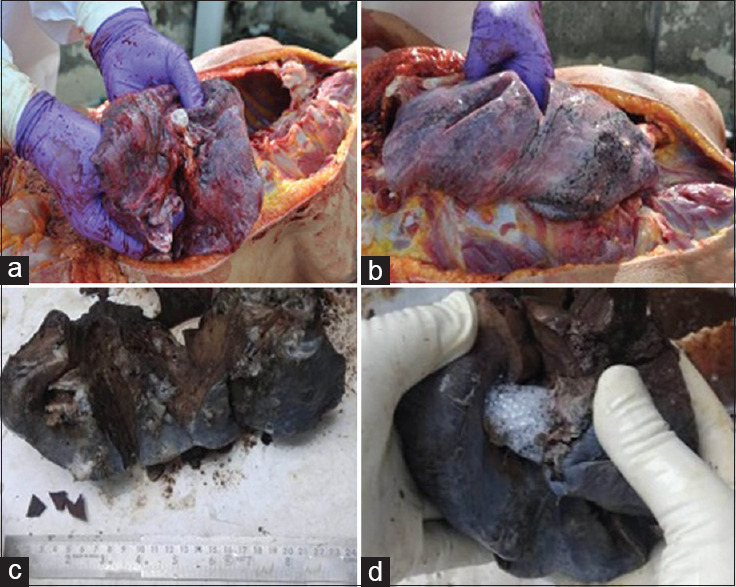
showing Gross examination of heavy edematous lungs 1b: showing Lung with mosaic pattern, and oozing of fluid, 1c Showing dark coloration on cut section of lungs and 1d: showing oozing of fluid from lung on compression
In addition, pieces of the lungs, liver, spleen, kidney, brain, peritoneal fluid, vitreous humor, blood, and tissues from the aerodigestive tract, were collected for virological study for detection of SARS-CoV-2 RNA by RTPCR. The viral RNA was isolated from various specimens by spin column technique (Q1Amp Viral RNA extraction kit). The highly pure RNA so obtained was subjected to amplification by RTPCR (biorad CFX-96). The NIV Pune (AB1 Thermofischer) screening and confirmatory assay were used for the amplification targeting E-gene, ORFI gene, and RNA dependent RNA polymerase (RdRP) gene.[6] CT value ≤36 was considered significant, and results are displayed below mentioned Table 1.
Table 1.
Results of real-time reverse-transcriptase-polymerase-chain-reaction testing for the 2019 novel coronavirus (severe acute respiratory syndrome coronavirus 2)
| Specimen | E gene (Ct Value) | Rdrp (Ct value) |
|---|---|---|
| Vitreous fluid | Negative | |
| Peritoneal fluid | Negative | |
| Blood | Negative | |
| Lungs | Positive (Ct - 30.19) | 32.22 |
| Spleen | Negative | |
| Kidney | Positive (Ct - 36.91) | 34.21 |
| Brain | Negative | |
| Liver | Positive (Ct - 36.51) | 32.42 |
| Tonsil | Positive (Ct - 34.67) | 30.23 |
| Tongue | Positive (Ct - 34.07) | 30.19 |
| Pharyngeal wall | Positive (Ct - 35.79) | 29.72 |
| Heart | Negative | |
| Esophagus | Positive (Ct - 33.00) | 28.17 |
| Submandibular salivary gland | Negative |
Rdrp: RNA dependent RNA polymerase
Lower cycle threshold (Ct) values indicate higher viral loads.
Routine histological stains were performed on formalin-fixed samples following standard protocols, and the findings are summarized in, below mentioned Table 2.
Table 2.
Histopathological findings
| Site of tissue | Microscopic findings |
|---|---|
| Lungs | Intraalveolar edema, congestion of alveolar septal capillaries and larger vessels, septal perivascular lymphocytic infiltrate and peribronchiolar lymphocytic infiltrate, focal interalveolar exudates of macrophages admixed with lymphocytes scattered small epithelioid cell granulomas with foreign body and Langerhans types of giant cells and peripheral cuffing by lymphocytes in predominantly perivascular distribution, however, ZN stain for AFB was negative [Figure 2] |
| Liver | Congestion, mild chronic inflammatory infiltrate in some portal tract, and occasional lymphocytic aggregate adjacent to central vein [Figure 3a and b] |
| Spleen | Congestion [Figure 3c] |
| Brain | No pathological changes observed |
| Kidney | Congestion, occasional tiny focus of chronic inflammatory cells aggregate [Figure 3d] |
| Heart | No pathological changes observed |
| Tracheal tissues | Sparse submucosa and perivascular lymphocytic infiltrate mucosal epithelium shows partial autolysis [Figure 4a and b] |
| Salivary gland | The salivary gland shows focal sparse perivascular lymphocytic infiltrate [Figure 5a] |
| Epiglottis | Sparse submucosal lymphocytic infiltrate [Figure 5b and c] |
| Upper Oesophagus | Shows focal sparse perivascular lymphocytic infiltrate [Figure 5d] |
| Tongue | Follicular lymphoid hyperplasia, on its posterior aspect [Figure 4c] |
| Pharyngeal tissues | Edema, sparse sub mucosal lymphocytic infiltrate, and few submucosal lymphoid follicles [Figure 4d] |
| Tonsils | Reactive follicular hyperplasia |
Figure 2.
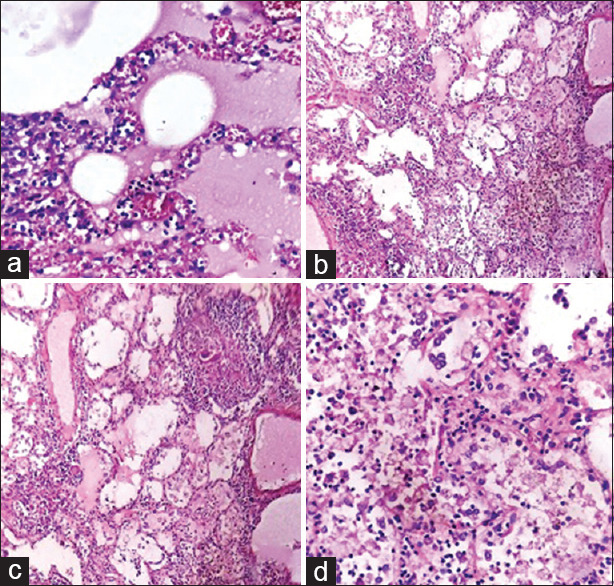
(a) Lung: Alveolar edema with septal lymphocytic infiltrate (H and E, ×400). (b) Lung: Intraalveolar exudate of macrophages with lymphocytes (H and E, ×100). (c) Lung: Epithelioid cell granuloma with peripheral lymphocytes (H and E, ×100). (d) Lung: Hyperplastic type II pneumocytes (in the upper part of picture ) and intraalveolar exudate of macrophages (H and E, ×100)
Figure 3.
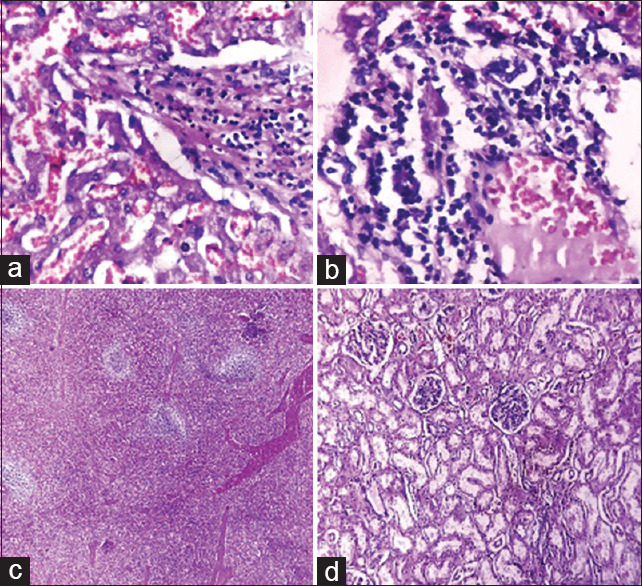
(a) Liver: Congestion of sinusoids (H and E, ×400). (b) Liver: lymphocytic infiltrate in portal tract (H and E, ×400). (c) Spleen: Congestion and white pulp depletion. (H and E, ×40. (d) Kidney: Congestion and autolytic changes (H and E, ×100)
Figure 4.
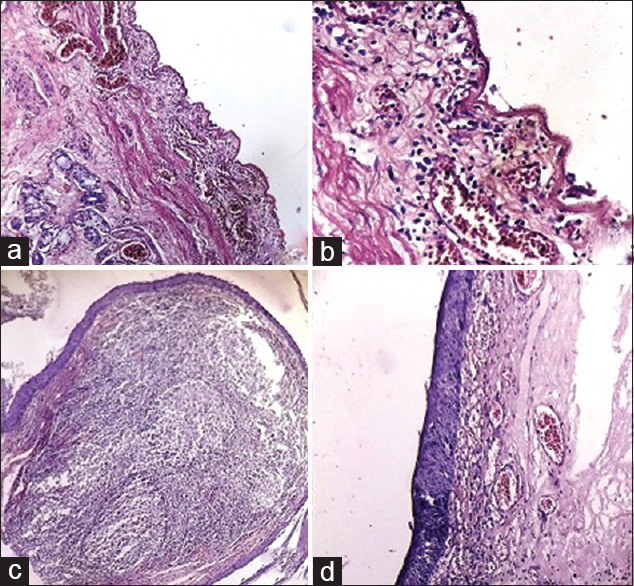
(a) Trachea: Congestion. Overlying epithelial cells lost due to autolytic changes. (H and E, ×100. (b) Trachea: Subepithelial lymphocytic infiltrate and congestion. Overlying epithelial cells lost due to autolysis (H and E, ×400). (c) Posterior tongue: lymphoid follicle (lingual tonsil) showing reactive hyperplasia (H and E, ×100). (d) Pharynx: Congestion, subepithelial lymphocytic infiltrate and edema (H and E, ×100)
Figure 5.
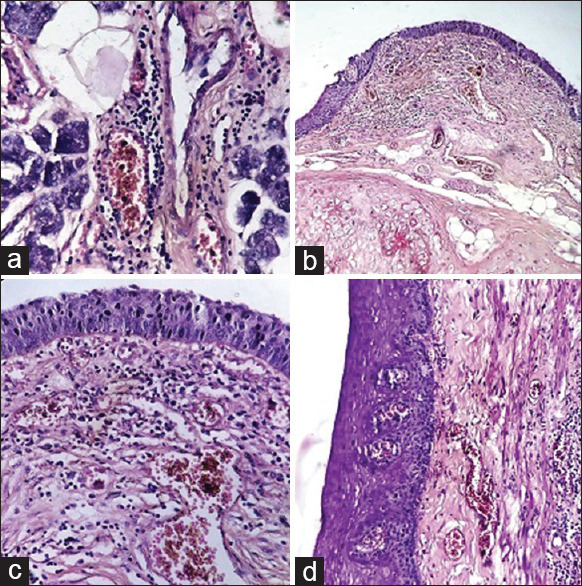
(a) Submandibular salivary gland: Perivascular lymphocytic infiltrate. (H and E, ×400). (b) Epiglottis: Subepithelial lymphocytic infiltrate and congestion (H and E, ×100). (c) Epiglottis: Subepithelial lymphocytic infiltrate and congestion (H and E, ×400). (d) Esophagus: Subepithelial congestion and lymphocytic infiltrate (right side of the picture) (H and E, ×200)
None of health professionals involved in the autopsy of this case developed COVID-19.
Discussion
Autopsies are considered the gold standard for establishing the cause of death, preexisting associated diseases, and role SARS-CoV-2 infections in the cause of death.[7] This case reports detection of SARSCOV-2 RNA in several organs and aerodigestive tissues from postmortem formalin-fixed sections in India. We detected SARS-CoV-2 RNA in a number of angiotensin-converting enzyme-2 (ACE2)[6] expressing tissues consisting of the lungs, liver, kidney, tissues from upper airways and upper esophagus, and lymphoid tissues from the base of the tongue, palatine tonsils, and the posterior pharyngeal wall, which is similar to the finding of studies done at autopsy[8] and was negative in the brain, spleen, heart, blood, peritoneal fluid, vitreous humor. The respiratory tract tissues showed Ct value ≤30 suggesting a high viral load. Less abundant viral RNA was noted in the kidney and liver (Ct = 36). In addition, our study also documents detection in lymphoid organs (lingual tonsil and palatine tonsil) and may lend credence to the theory that leukocytes could serve as a route for dissemination of the virus from airways to other organs.[7,8]
Microscopic examination of, lungs have been found to be the most consistently and severely affected.[7,8,9] As in our case the deceased was an asymptomatic carrier the findings of gross features of lungs in the form of increased lung weight, congestion, and edema were comparable to findings observed in other studies The histopathological findings showed the presence of septal lymphocytic infiltrate and intra alveolar exudates of macrophages and focal type 2 pneumocyte hyperplasia which is similar to observation which emphasized on edema, interstitial mononuclear inflammatory infiltrates (mainly lymphocytic), and multinucleated syncytial cells with viral cytopathic-like changes.[7,8,9] In aluminium phosphide poisoning features in form of severe edema and moderate hemorrhagic, collapsed alveoli and bronchioles, and alveolar dilatation are pronounced in the lungs[5] hence, the findings septal lymphocytic infiltrate and intra alveolar exudates of macrophages with lymphocytes perhaps represents findings due to COVID-19 infection in our case. In addition in our case we observed few epithelioid cell granulomas with cuffing by lymphocytes, it might be incidental finding due to hypersensitivity pneumonitis or it represents a novel additional histopathological finding of COVID-19 infection, (as special stains for mycobacteria or fungi were negative). Surprisingly, no observation of hyaline membrane was found, which would have been expected based on the main histopathological changes reported in COVID-19 lungs, this could be hypothesized that the patient was asymptomatic and might have developed histopathological changes, later on, had the patient survived.
COVID-19 associated hepatic injuries cannot be ignored as the ACE2 receptors[8,9] have also been detected in the hepatobiliary system, and can also directly affect the liver cells, and were characterized by congestion, lymphocytic infiltrate in some portal tracts, which was consistent to studies[7,8,9] which revealed mild inflammation in the hepatic lobular portal region. However, in kidney microscopic findings in the form of congestion were appreciable, as compared to tubular dilation, edema, and even balloon degeneration in aluminum phosphide poisoning.[5]
In the hematopoietic system, the spleen showed congestion and white pulp depletion along with features of autolysis which was similar.[8] Sub carinal lymph node showed normal follicular architecture, and features of autolysis,[8,9] In the oral cavity, microscopic sections from the base of the tongue and bilateral palatine tonsils showed reactive hyperplasia, which can be seen in viral infections and might be the possible pathogenetic mechanism of olfactory and gustatory disorders.[10] Esophagus also showed features of lymphocytic infiltration due to its tropism for the gastrointestinal tract.[8] Microscopic studies from the section of tracheal mucosa, epiglottis, and pharyngeal walls showed features of mild inflammatory changes, in the form of edema and subepithelial lymphocytic infiltrate as was also observed in study done in Washington.[8] In contrast changes in aluminium phosphide[5] poisoning are comprised of congestion and petechial hemorrhages of the mucosa.
Conclusion
A complete and meticulous, autopsy in COVID-19 patients is a useful tool to study, the exact pathophysiology of such new diseases, and can be undertaken with necessary precautions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Statement on the Second Meeting of the International Health Regulations. Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV) 2005. [Last accessed on 2020 Aug 01]. Available from: https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov)
- 2.WHO. Clinical Management of COVID-19 Interim Guidance 27 May 2020. [Last assessed on 2020 Sept 01]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance-publications .
- 3.COVID-19: Guidelines on Dead Body Management. EMR Division, Directorate General of Health Services, Ministry of Health & Family Welfare. Government of India. 2020. [Last assessed on 2020 Sep 01]. Available from: https://www.mohfw.gov.in/pdf/1584423700568_COVID19GuidelinesonDeadbodymanagement.pdf .
- 4.The Code of Criminal Procedure Act. Legislative department. Government of India. 1973. [Last assessed on 2020 Aug 11]. Available from: http://legislative.gov.in/actsofparliamentfromtheyear/code-criminal-procedure-act-1973 .
- 5.Liang Y, Tong F, Huang F, Liu Y, Zhu L, Le Grange JM, et al. Pathological changes induced by phosphine poisoning: A study on 8 children. Int J Legal Med. 2020;134:217–28. doi: 10.1007/s00414-019-02169-z. [DOI] [PubMed] [Google Scholar]
- 6.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keresztesi AA, Perde F, Ghita-Nanu A, Radu CC, Negrea M, Keresztesi G. Post-mortem diagnosis and autopsy findings in SARS-CoV-2 infection: Forensic case series. Diagnostics (Basel) 2020;10:1070. doi: 10.3390/diagnostics10121070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: A case series. Lancet. 2020;396:320–32. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elsoukkary SS, Mostyka M, Dillard A, Berman DR, Ma LX, Chadburn A, et al. Autopsy findings in 32 patients with COVID-19: A single-institution experience. Pathobiology. 2021;88:1–3. doi: 10.1159/000511325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mildto- moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–61. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


