Abstract
Background
Prolonged viral RNA detection in respiratory samples from patients with COVID-19 has been described, but the clinical relevance remains unclear. We studied the dynamics of SARS-CoV-2 on a group and individual level in intubated ICU patients.
Methods
In a cohort of 86 patients, we analysed SARS-CoV-2 RT-PCR results on nasopharyngeal and sputum samples (obtained as part of clinical care twice a week) according to time after intubation. Subsequently, we performed survival analyses.
Results
870 samples were tested by RT-PCR. Overall viral load was highest in the first week (median nasopharynx 3.5, IQR 1.5–4.3; median sputum 4.3, IQR 3.3–5.6) and decreased over time. In 20% of patients a relapsing pattern was observed. Nasopharyngeal and sputum PCR status on day 14 was not significantly associated with survival up to day 60 in this small cohort.
Conclusion
In general SARS-CoV-2 RNA levels in respiratory samples in patients with severe COVID-19 decrease after the first week after intubation, but individual SARS-CoV-2 RNA levels can show a relapsing pattern. Larger studies are needed to address the association of clearance of SARS-CoV-2 RNA from respiratory samples with survival, because we observed a trend towards better survival in patients with early clearance from sputum.
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by infection with SARS-CoV-2, has a broad clinical spectrum of disease, ranging from asymptomatic infection to severe respiratory disease requiring intensive care unit (ICU) admission and mechanical ventilation.
For diagnosis of COVID-19, guidelines recommend SARS-CoV-2 nucleic acid amplification testing (NAAT) on respiratory samples (nasopharyngeal/oropharyngeal samples and sputum) [1,2]. Although respiratory samples have the greatest yield, SARS-CoV-2 can also be detected in non-respiratory samples, like stool samples [3,4].
A pooled analysis of 28 studies reporting on SARS-CoV-2 RNA shedding showed a median duration of shedding of 18.4 days in respiratory samples from mainly hospitalized patients [5]. Prolonged shedding has been described in patients with mild and severe disease, including RNA detection in respiratory samples for more than 3 months after onset of symptoms [5,6]. Severe disease has been shown to be associated with prolonged shedding [[7], [8], [9]]. In addition multiple cases have been reported of patients testing positive after being discharged from isolation. To date the question remains whether this is caused by intermittent low load RNA shedding or reinfection with SARS-CoV-2 [10]. Whether prolonged RNA detection in respiratory samples is associated with a poor prognosis within the group of patients with severe COVID-19 is unclear.
Previous studies have reported on the SARS-CoV-2 RNA load over time in various sequential samples (upper/lower respiratory samples or faeces) in COVID-19 patients in general [[11], [12], [13], [14]] or in hospitalized COVID-19 patients [7,15,16]. Most of these studies describe an increase in viral load in the first week of the infection, followed by a decline in viral load [7,13,15]. For the most severely ill COVID-19 patients (i.e. admitted to the ICU requiring invasive mechanical ventilation) the correlation between nasopharyngeal and sputum load, the evolution of these loads through time, the presence of specific patterns in individual viral dynamics and their prognostic value remains to be elucidated. Furthermore, it is of interest whether these viral dynamics resemble other respiratory infections like influenza or SARS-CoV.
In this study, we aim to describe nasopharyngeal and sputum SARS-CoV-2 RNA dynamics on a group level and on an individual level in a large cohort of patients with severe COVID-19 admitted to the ICU and requiring invasive mechanical ventilation. Further we aim to investigate whether prolonged shedding is related to mortality.
2. Methods
2.1. Patients and samples
COVID-19 patients admitted to the Intensive Care Unit (ICU) in the University Medical Center Groningen (UMCG), The Netherlands between March 24 th and May 25th 2020 and requiring invasive mechanical ventilation were included in this study. Patients were excluded if no SARS-CoV-2 RT-PCR results were available.
The following clinical data were collected from the electronic patient files: age, sex, BMI, relevant comorbidity, date of onset symptoms, start and end date of mechanical ventilation, the need of extra corporeal life support, hydroxychloroquine treatment (optional treatment according to national guidelines at the time of inclusion, although no longer at time of publication [17]), date of hospital discharge, date of transfer from or to another hospital and date of death. Comorbidity was categorized in 10 categories: diabetes mellitus, hypertension, hypercholesterolemia, cardiovascular disease, chronic pulmonary disease, malignancy, renal insufficiency, thromboembolic disease, auto-immune disorders or solid organ transplantation. Respiratory sample SARS-CoV-2 RT-PCR results and CRP levels of these patients were extracted from the laboratory information system. ICU capacity during the inclusion period was sufficient for the relatively small number of patients from our own region and for transfer of patients from other regions in The Netherlands (the majority of patients included). Most patients were transferred due to ICU capacity problems and a few patients transferred because extra corporeal life support was considered. The first diagnostic samples of transferred patients were tested elsewhere and these initial Ct values were therefore not available for most patients.
All samples were collected and tested as part of clinical care. For all ICU COVID-19 patients nasopharyngeal samples and sputum samples (in intubated patients) were routinely collected twice a week. The use of samples and data was approved by the review board of the UMCG (METc 2020/344). The objection register for use of data and biological materials was checked for all patients. Data were pseudonymised after collection from the electronic patient files and laboratory information system and the pseudonymisation key was stored separately.
Nasopharyngeal swabs were preserved in 3 ml of universal viral transport medium prior to testing. Sputum samples were transported to the laboratory in native conditions. In some viscous sputum samples 1 ml of viral transport medium was added. For RNA extraction 190 μl or 200 μl of sample was used (depending on the platform used). All samples were tested within 24 h and stored at 4 °C prior to testing.
2.2. RNA extraction and real-time PCR
Total nucleic acid was extracted on the NucliSENS® EasyMAG® (Biomerieux, France) according to the instructions of the manufacturer. RT-PCR was performed by using an in-house assay targeting the E-gene of SARS-CoV-2 on the Applied Biosystems 7500 Real-time PCR system (Applied Biosystems, USA) or with the GeneFinder™ COVID-19 Plus RealAmp Kit on the ELITe InGenius® RT-PCR system (amplification of RdRp gene, E gene and N gene) (EliTech, France). Ct-values of the N gene were used in the analysis for samples tested on the InGenius system. We estimated two calibration curves (one for the in-house assay and one for the GeneFinder™ assay) of Ct values versus the load measured by digital droplet PCR using data from a quality control panel, and used these curves for conversion of our study sample Ct values to log copies/ml. The lower limits of detection based on the quality control panel were 1.7 log copies/ml (in-house assay) and 2.0 log copies/ml (GeneFinder™ assay, N-gene). Any lower values in our study data should be interpreted as positive outside range of quantification.
To investigate whether unexpected increases in viral load were attributable to variations in sample quality human housekeeping gene RNAse P (included as an endogenous internal control in the GeneFinder COVID-19 Plus RealAmp assay) was used as a comparison gene if the amount of sample was sufficient for an additional PCR.
3. Data analysis
3.1. Patient characteristics
Patient characteristics were described as numbers with percentages (categorical variables) or median with interquartile range (continuous variables). To calculate the duration of admission, the date of discharge home or to a physical rehabilitation hospital was used.
3.2. Duplicate samples on one day
Differences in SARS-CoV-2 load between duplicate samples on a single day were calculated as a measure of replicability (see Supplementary Table 1). For all further analyses, the mean of the duplicate samples on one day was used.
3.3. Analysis of SARS-CoV-2 RNA dynamics on a group level
Categories of 1 week were made to plot the viral load in nasopharynx and sputum over time. If more than one sample of a patient was tested within this time period of 1 week, a value of a random sample within this week was taken for the analysis.
3.4. Analysis of individual SARS-CoV-2 RNA dynamics
An increase in SARS-CoV-2 viral load was scored in samples when 2 log copies/ml increase compared to the previous samples was observed and the Ct value was ≤33 (corresponding to a load of 2.3 log copies/ml in the in-house PCR). For the purpose of calculation of these differences, negative samples were set to −1 log copies/ml. To study the association between CRP and increase in viral load, an increase of 50 mg/l in the interval from 2 days before to 3 days after a SARS-CoV-2 peak was used.
3.5. Survival analysis
We compared survival of patients with at least 1 negative SARS-CoV-2 PCR on a nasopharyngeal swab by day 14 after intubation to survival of patients without a negative nasopharyngeal swab by day 14 after intubation. Similarly, we compared survival of patients with at least 1 negative SARS-CoV-2 PCR on a sputum sample by day 14 after intubation to survival of patients without a negative a sputum sample by day 14 after intubation. For this analysis, we included only those patients still on mechanical ventilation in the UMCG on day 14 after intubation. The 14 day time point was chosen because the cohort of patients on mechanical ventilation in the UMCG ICU was relatively large at day 14 (i.e. most patients were transferred from elsewhere by this time and few patients were already detubated) and a substantial minority of patients had negative PCR results by this time. Follow-up ended 60 days after intubation, or on June 15th 2020 if earlier. Patients who were transferred to another hospital were censored on the day of transfer. Patients who were discharged home or to a physical rehabilitation hospital were presumed to be alive on day 60. Survival curves were estimated using Kaplan-Meier's methodology. To assess the differences between the estimated survival curves the log-rank test was used.
All analyses were performed in StataSE 14 and/or GraphPad Prism 7.0.
4. Results
4.1. Patients and samples
Eighty-six patients with proven COVID-19 admitted to the ICU were included in the study. Patient characteristics are listed in Table 1 . Most patients were transferred from a different region in The Netherlands (n = 84, 98%) and intubated before or on the day of transport (n = 79, 94% of those transferred), with a median duration of mechanical ventilation before transfer of 2 days (IQR 0–5). In 63 (73%) patients one or more relevant comorbidities were present, of whom 41 (48% of all patients) had diabetes mellitus, hypertension and/or hypercholesterolemia. The median Body Mass Index of the patients was 27 (IQR 25–31). For 66 patients, the first day of illness was known. Intubation occurred at a median of 9 days (IQR 7–14) after onset of symptoms. None of these patients were treated for COVID-19 with dexamethasone, because this was not recommended at time of inclusion (but some patients may have received corticosteroids for other indications).
Table 1.
Patient characteristics.
| Age (years), median (IQR) | 62 (55–69) |
| Male sex, n (%) | 63 (73) |
| Transfer from another hospital | |
| Transferred, n (%) | 84 (98) |
| Intubated on/before day of transfer, n(%) | 79 (94) |
| Duration of mechanical ventilation upon transfer (days), median (IQR) | 2 (0–5) |
| Duration of symptoms before intubation (days)a, median (IQR) | 9 (7–14) |
| Comorbidity, n(%) | |
| Diabetes mellitus | 25 (29) |
| Hypertension | 27 (31) |
| Dyslipidemia | 6 (7) |
| Cardiovascular disease | 15 (17) |
| Chronic pulmonary disease | 10 (12) |
| Malignancy | 11 (13) |
| Renal insufficiency | 5 (6) |
| DVT and/or pulmonary embolism | 2 (2) |
| Autoimmune disease | 5 (6) |
| Solid organ transplantation | 2 (2) |
| BMI, median (IQR) | 27 (25–31) |
| Hydroxychloroquine treatment, n (%) | 57 (66) |
| Extra corporeal life support, n (%) | 5 (6) |
Information available for 66 patients.
In our cohort, 19 patients died (22%). Death occurred between 5 and 50 days after intubation (median 21 days). Patients who survived (n = 67) received mechanical ventilation for a median of 17 days (range 7- ≥60 days). Out of 5 patients who received extra corporeal life support (ECLS), 2 patients died. Two patients were transferred to another hospital and 59 patients were discharged home or to a rehabilitation hospital before end of follow-up. At 60 days after intubation 6 patients were still in our hospital.
4.2. Viral RNA shedding in nasopharyngeal and sputum samples
From these 86 patients a total of 870 samples (485 nasopharyngeal samples and 385 sputum samples taken mostly simultaneously) were tested by RT-PCR. The median number of nasopharyngeal samples per patient was 5 (range 1–13), the median number of sputum samples per patient was 4 (range 0–14).
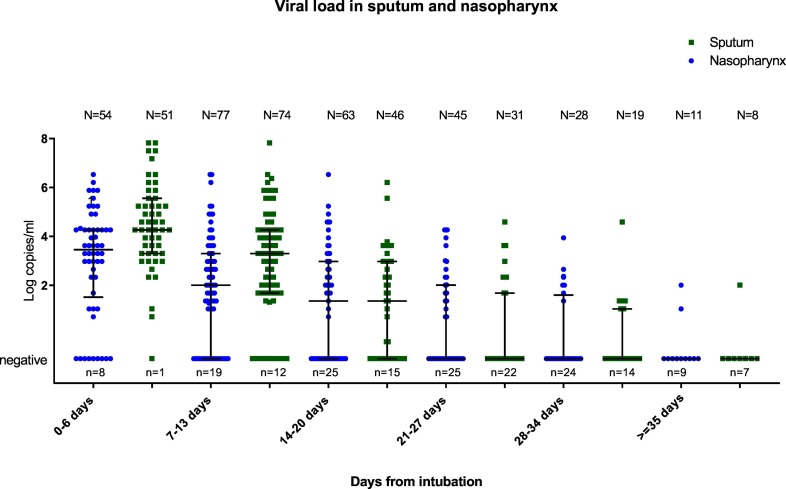
In the first week after intubation, nasopharyngeal samples of 54 patients were tested for SARS-CoV-2 RNA, of which 46 were positive for SARS-CoV-2 RNA, with a median viral load of 3.5 Log copies/ml (IQR 1.5–4.3). Sputum from 51 patients was obtained during the first week after intubation, in all but one SARS-CoV-2 RNA was detected with a median viral load of 4.3 Log copies/ml (IQR 3.3–5.6). In the first two weeks of admission the viral load was higher in sputum samples than in nasopharyngeal samples (median second week sputum 3.3 (IQR 1.7–4.3), nasopharynx 2.0 (IQR negative – 3.3)). As shown in Fig. 1 overall viral load (sputum and nasopharynx) was highest in the first days after intubation and gradually decreased.
Fig. 1.
SARS-CoV-2 viral load in nasopharyngeal and sputum samples.
Y-axis indicating the viral load. X-axis indicating the time from intubation. The bars represent median and IQR. The total number of samples in a category is indicated by N = … The number of negative samples in a category is indicated by n = …. Samples with <2 log copies/ml should be interpreted as positive but not in quantitative range of the PCR.
Of the patients who died of COVID-19, 14 out of 19 (74%) were SARS-CoV-2-RNA positive in the last nasopharyngeal sample, with a median viral load of 2.6 (IQR 1.7–3.6) log copies/ml. At time of detubation, 32 out of 66 patients (48%) still had detectable virus in the last nasopharyngeal swab, with a median viral load of 3.0 (IQR 2.3–3.6) log copies/ml. SARS-CoV-2 remained detectable in nasopharyngeal swabs for extremely long periods of more than 35 days following intubation in 5 patients (in at least 1 sample), with a load of ≥2.3 log copies/mL (Ct-value ≤33) in 3 of these patients.
4.3. Viral dynamics in serial samples and C-reactive protein
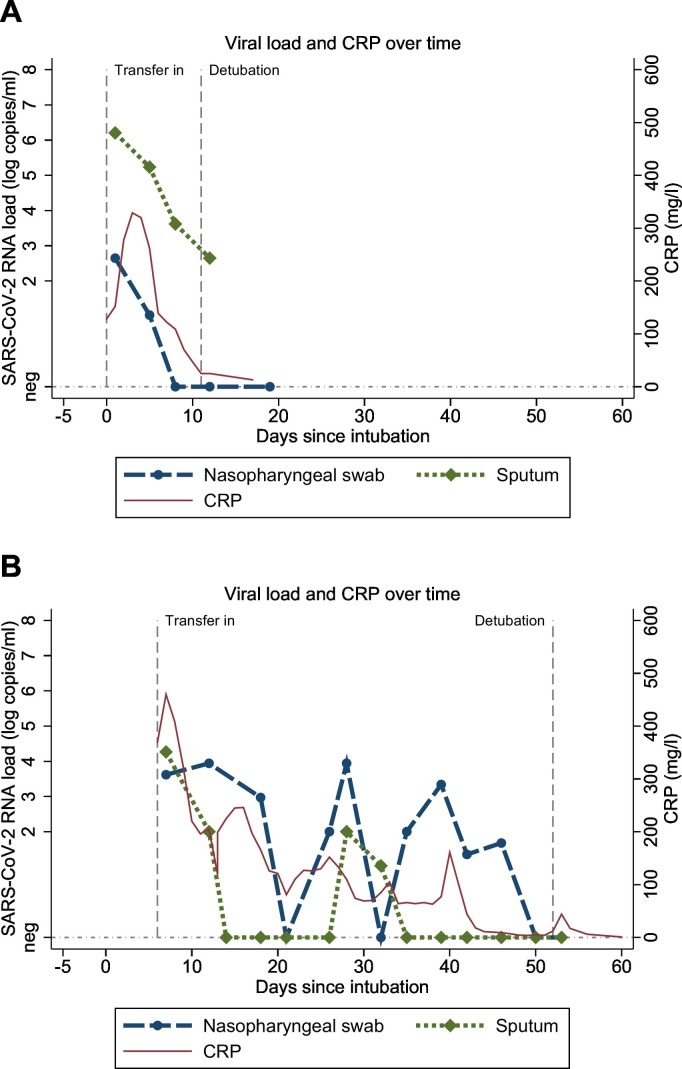
Next we examined the dynamics of viral load through time in all individual patients. In most patients viral loads were high on admission to the intensive care unit and decreased over time (typical example shown in Fig. 2A). However, in a considerable proportion of patients a relapsing pattern was observed (example shown in Fig. 2B). Seventeen out of 86 patients (20%) had a relevant increase in nasopharyngeal SARS-CoV-2 RNA load (defined as an increase of >2 log copies/mL in 1 or 2 increments, with a minimal “peak” load of 2.3 log copies/mL). Four out of 86 patients (5%) had a relevant increase in sputum SARS-CoV-2 viral load. For 6 patients with an increase in nasopharyngeal SARS-CoV-2 RNA load, the CT-value of housekeeping gene RNAse P was available for both the peak sample and the pre-peak sample. The difference in RNAse P CT-value between the peak sample and the pre-peak sample ranged from −2.6 (i.e. more human DNA in the peak sample) to +1.7 (i.e. less human DNA in the peak sample). Therefore the increases in viral load are not clearly caused by differences in sample quality. In 2/17 patients the increase in SARS-CoV-2 RNA nasopharyngeal load was accompanied by a simultaneous CRP increase (more than 50 mg/l in any interval between 2 days before and 3 days after the peak in viral load). Furthermore, in 2 patients a positive nasopharyngeal PCR (>2.3 log copies/ml) was observed following ≥2 consecutive negative nasopharyngeal PCRs.
Fig. 2.
Time course graph of SARS-CoV-2 viral load and CRP. a. Typical example of a patient with a gradually decreasing viral load pattern. b. Typical example of a patients with a relapsing pattern. X-axis time from intubation. Y-axis left SARS-CoV-2 viral load (log copies/ml). Y-axis right CRP (mg/L).
4.4. Duration of viral RNA detection and survival
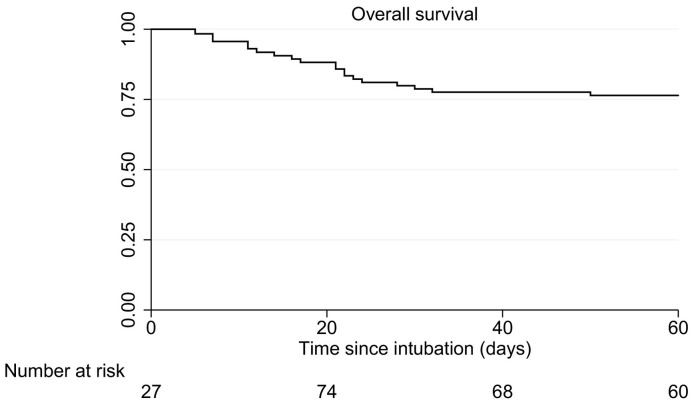
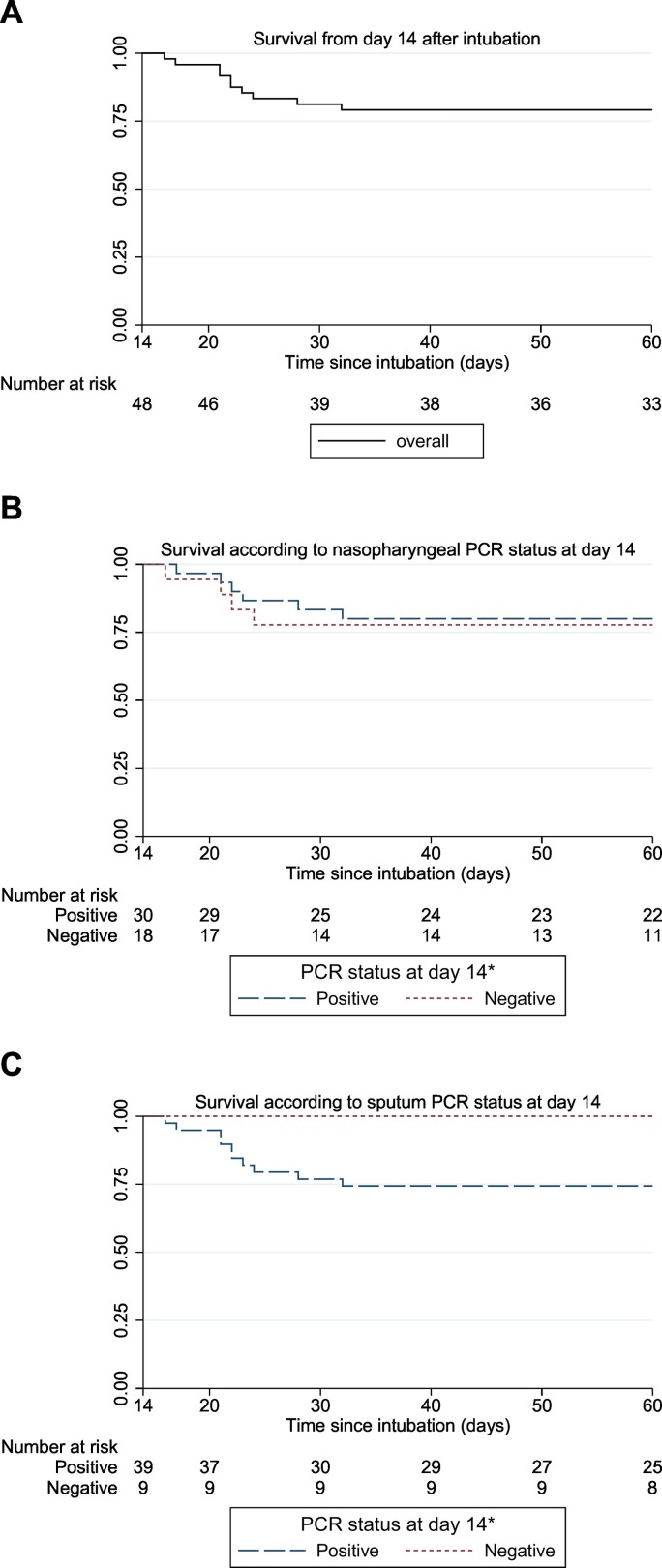
Overall 60 day survival as estimated by Kaplan-Meier in this cohort of patients with severe COVID-19 was 76% (Fig. 3 ). Most deaths occurred within the first 3 weeks after intubation (14%). To assess whether prolonged SARS-CoV-2 detection is associated with a poor prognosis, survival of patients with viral RNA detection at least 14 days was compared with patients with at least 1 negative sample within 14 days (restricted to patients still on mechanical ventilation on day 14). Overall 60 days survival for the patients still intubated after 14 days is shown in Fig. 4A. Fourteen days after intubation 30 patients had positive nasopharyngeal swabs and 18 patients had 1 or more negative nasopharyngeal swab. Fig. 4B shows the survival curve according to nasopharyngeal PCR status. Survival from 14 days to 60 days was 80% in the group of patients with positive nasopharyngeal swabs only up to day 14, while survival was 78% in the patients with at least 1 negative swab at 14 days after intubation (p = 0.81). Fourteen days after intubation 39 patients had positive sputum samples and 9 patients had 1 or more negative test results. Fig. 4C shows the survival curve from 14 days to 60 days according to sputum PCR status. Survival was 74% in the group of patients with positive sputum samples only up to day 14, while survival was 100% in the patients with at least 1 negative sputum sample at 14 days after intubation (p = 0.10).
Fig. 3.
Overall 60-day survival.
Patients enter the set of patients at risk upon admission or transfer to the ICU of the UMCG, which in many cases is later than the day of intubation.
Fig. 4.
Association between PCR status at day 14 and survival up to day 60. a. Overall survival from day 14 in patients still intubated; b. Survival according to PCR status at day 14 in nasopharyngeal samples; c. Survival according to PCR status at day 14 in sputum samples. *Negative: patients with one or more negative samples up to day 14; positive: patients with only positive samples up to day 14.
5. Discussion
This study shows the viral dynamics of SARS-CoV-2 in a relatively large cohort of intensive care COVID-19 patients in the Netherlands. We found that on a group level 1. SARS-CoV-2 RNA loads were highest in the first week after intubation, decreasing thereafter and 2. during the first two weeks after intubation sputum SARS-CoV-2 RNA levels exceeded nasopharyngeal SARS-CoV-2 RNA load. On an individual level, we found diverse patterns of SARS-CoV-2 RNA dynamics in time, with some patients showing quickly and steadily decreasing levels and other patients showing peaks in load after an initial decrease, i.e. a relapsing pattern. In those patients still intubated after two weeks, clearance of SARS-CoV-2 RNA from the nasopharynx was not related to mortality, whereas clearance of SARS-CoV-2 RNA from sputum showed a trend towards lower mortality.
Most patients had high viral loads in the first week after intubation and these loads gradually decreased over time. A peak in viral load in the first week and gradual decrease of viral load over time has been described by several authors in mixed populations of patients with severe and mild disease [7,12,[14], [15], [16]]. Furthermore, we observed a higher load in sputum samples than in nasopharyngeal samples in the first 2 weeks after intubation, which also has been described previously [12,14,16,18].
In 20% of patients a relapsing pattern was observed. After an initial low and/or decreasing viral load, a sudden increase of ≥2 log copies/ml was observed. Although previous studies have described viral dynamics primarily on a group level and have not commented on the patterns of individual viral dynamics, upon inspection of published individual viral load graphs similar viral load increases can be observed [19,20]. The phenomenon of more than one peak in the viral load versus time curve is recognized for Influenza A, where it is thought to be the result of target cell depletion, temporal dynamics in interferon production by infected cells or developing adaptive immunity [21]. More recently it was shown that subgenomic RNA from double walled vesicles may be detected for prolonged periods of time following SARS-CoV-2 infections [22].
The significance of the increases in viral load after periods of decrease or low load which we describe in this study remains uncertain. More research is necessary to investigate if this is observed by others and if it is associated with adverse outcome and longer total virus shedding.
In many aspects, our study population was similar to previously described cohorts of ICU COVID-19 patients. Patients were admitted to the ICU between 4 and 22 days (mean 10 days) after onset of symptoms, which is similar to what has been reported before [23]. In 73% of the patients at least one of the recognized risk factors for severe COVID-19 infections was present, which is also similar to what has been reported before [24]. A distinctive aspect of our cohort of patients is the large proportion of patients transferred from another hospital. This may affect generalizability of our results, because patients who were intubated, but had a clinical condition suitable for transport were transferred to our hospital. The slightly better survival than previously described [25] may also be explained by the non-representative group of patients transferred to our hospital. How this may have affected results with regard to viral dynamics is difficult to predict.
Altered treatment recommendations may affect generalizability of our results to future patients. Most patients were treated with hydroxychloroquine, which may affect viral dynamics. However, several studies have shown that the use of hydroxychloroquine does not affect survival in COVID-19 patients [26]. Furthermore, the hydroxychloquine was discontinued upon admission to our ICU. Whereas hydroxychloroquine treatment is no longer recommended, current Dutch national guidelines recommend treatment of moderately to severely COVID-19 with dexamethasone. As this suppresses the inflammatory response, it may cause increased or prolonged viral shedding.
There are some technical limitations with regard to sampling and the RT-PCR procedure. Quality of both nasopharyngeal samples and sputum samples can vary, which means that an observed increase in viral load could be attributable to improved sampling compared with the previous sample. We further explored the effect of sampling variability through analysis of the CT-value of the human housekeeping gene RNAse P (expressed in human epithelial cells), which was available for only a limited number of relevant samples. This did not clearly indicate that the SARS-CoV-2 RNA peak samples were of better quality than the previous sample.
Due to routine sampling twice weekly during the time patients were intubated, there was no selective sampling based on clinical deterioration as long as patients were intubated. In detubated patients, nasopharyngeal samples were still submitted at regular intervals to guide infection prevention measures. However, in detubated patients sputum was only incidentally tested. This may have led to a sampling bias, because it is more likely that sputum was tested in patients with clinical deterioration.
In 5 patients nasopharyngeal viral RNA detection of more than 35 days after intubation was observed. Huang et al. [16] have described viral shedding of >28 days after onset of symptoms in 69% of critically ill patients. Prolonged positivity and testing positive after initial negative test(s), may have implications for infection control. Van Kampen et al. [27] assessed the duration and determinants of infectious virus shedding in 129 patients with severe COVID-19. Viral loads above 7 log10 RNA copies/mL and absence of serum neutralizing antibodies were independently associated with isolation of infectious SARS-CoV-2 from the respiratory tract using viral culture. In our study we did not perform viral culture. Therefore, it is unknown 1. up to which point the prolonged detection of SARS-CoV-2 RNA indicates the presence of viable virus and 2. whether a relapsing pattern may indicate the presence of viable virus. The relapsing pattern suggests that a single PCR test with a high Ct value may not be sufficient for patients with severe COVID-19 to be taken out of isolation.
SARS-CoV-2 clearance from sputum before 14 days after intubation showed a trend towards better outcome, however not significantly due to small numbers of patients included in this analysis. Therefore, the association between survival and early clearance of SARS-CoV-2 needs to be investigated in larger prospective studies.
Because we did not have follow-up data of patients discharged home or to a rehabilitation hospital, patients discharged before day 60 after intubation were assumed to be alive at day 60. Although there is a small chance that we may have underestimated mortality, this will have resulted in less bias than censoring all discharged patients (i.e. presuming that their risk of dying is the same as that of patients still in hospital). Sputum samples were infrequently obtained once patients were detubated, and nasopharyngeal swabs no longer obtained once patients were discharged. This could potentially lead to misclassification in survival analysis according to PCR status, due to patients with a last positive sample before detubation or discharge remaining in the positive category. However, because we performed the analysis on patients still intubated at day 14, the risk of misclassification in our analysis is low.
In conclusion, we describe a relapsing viral load in a relatively large cohort of patients with severe COVID-19. We observed a higher viral load in sputum samples than in nasopharyngeal samples in the first two weeks after intubation and viral RNA was detected in nasopharyngeal samples for more than 35 days in 5 patients. Whether prolonged or relapsing viral detection also has implications for infection control remains to be elucidated. Routinely monitoring of sputum viral load in severe COVID-19 patients may be beneficial for development of infection control guidelines and prediction of prognosis.
Funding
None.
Declaration of Competing Interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcrc.2021.04.010.
Appendix A. Supplementary data
Supplementary material
References
- 1.WHO/2019-nCoV/Lab_Assessment_Tool/2020.2 . 2 October 2020. Assessment tool for laboratories implementing SARS-CoV-2 testing: interim guidance. [Google Scholar]
- 2.Corman, VM, Landt, O, Kaiser, M, Molenkamp, R, Meijer, A, Chu, DK, Bleicker, T, Brunink, S, Schneider, J, Schmidt, ML, Mulders, DG, Haagmans, BL, van der Veer, B, van den Brink, S, Wijsman, L, Goderski, G, Romette, JL, Ellis, J, Zambon, M, Peiris, M, Goossens, H, Reusken, C, Koopmans, MP, Drosten, C. 2020. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 25:Doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed]
- 3.Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. doi: S2468-1253(20)30083-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung K.S., Hung I.F.N., Chan P.P.Y., Lung K.C., Tso E., Liu R., et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [doi: S0016-5085(20)30448-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontana, LM, Villamagna, AH, Sikka, MK, McGregor, JC. 2020. Understanding viral shedding of severe acute respiratory coronavirus virus 2 (SARS-CoV-2): review of current literature. Infect Control Hosp Epidemiol 1-10. Doi: 10.1017/ice.2020.1273. [DOI] [PMC free article] [PubMed]
- 6.Kim, SM, Hwang, YJ, Kwak, Y. 2020. Prolonged SARS-CoV-2 detection and reversed RT-PCR results in mild or asymptomatic patients. Infect Dis (Lond) 1-7. Doi: 10.1080/23744235.2020.1820076. [DOI] [PubMed]
- 7.Zheng, S, Fan, J, Yu, F, Feng, B, Lou, B, Zou, Q, Xie, G, Lin, S, Wang, R, Yang, X, Chen, W, Wang, Q, Zhang, D, Liu, Y, Gong, R, Ma, Z, Lu, S, Xiao, Y, Gu, Y, Zhang, J, Yao, H, Xu, K, Lu, X, Wei, G, Zhou, J, Fang, Q, Cai, H, Qiu, Y, Sheng, J, Chen, Y, Liang, T. 2020. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 369:m1443. Doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed]
- 8.Xu, K, Chen, Y, Yuan, J, Yi, P, Ding, C, Wu, W, Li, Y, Ni, Q, Zou, R, Li, X, Xu, M, Zhang, Y, Zhao, H, Zhang, X, Yu, L, Su, J, Lang, G, Liu, J, Wu, X, Guo, Y, Tao, J, Shi, D, Yu, L, Cao, Q, Ruan, B, Liu, L, Wang, Z, Xu, Y, Liu, Y, Sheng, J, Li, L. 2020. Factors associated with prolonged viral RNA shedding in patients with coronavirus disease 2019 (COVID-19). Clin Infect Dis 71:799-806. Doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed]
- 9.Fang Z., Zhang Y., Hang C., Ai J., Li S., Zhang W. Comparisons of viral shedding time of SARS-CoV-2 of different samples in ICU and non-ICU patients. J Inf Secur. 2020;81:147–178. doi: 10.1016/j.jinf.2020.03.013. [doi: S0163-4453(20)30139-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Centre for Disease Prevention and Control . ECDC; 2020. Reinfection with SARS-CoV: considerations for public health response. [Google Scholar]
- 11.Zou, L, Ruan, F, Huang, M, Liang, L, Huang, H, Hong, Z, Yu, J, Kang, M, Song, Y, Xia, J, Guo, Q, Song, T, He, J, Yen, HL, Peiris, M, Wu, J. 2020. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 382:1177-1179. Doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed]
- 12.Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [doi: S1473-3099(20)30113-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The COVID-19 Investigation Team, Kujawski S.A., Wong K.K., et al. Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Nat Med. 2020;26:861–868. doi: 10.1038/s41591-020-0877-5. [DOI] [PubMed] [Google Scholar]
- 14.Yu, F, Yan, L, Wang, N, Yang, S, Wang, L, Tang, Y, Gao, G, Wang, S, Ma, C, Xie, R, Wang, F, Tan, C, Zhu, L, Guo, Y, Zhang, F. 2020. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis 71:793-798. Doi: 10.1093/cid/ciaa345. [DOI] [PMC free article] [PubMed]
- 15.Wolfel, R, Corman, VM, Guggemos, W, Seilmaier, M, Zange, S, Muller, MA, Niemeyer, D, Jones, TC, Vollmar, P, Rothe, C, Hoelscher, M, Bleicker, T, Brunink, S, Schneider, J, Ehmann, R, Zwirglmaier, K, Drosten, C, Wendtner, C. 2020. Virological assessment of hospitalized patients with COVID-2019. Nature. 581:465-469. Doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed]
- 16.Huang Yongbo, Chen Sibei, Yang Zifeng, Guan Wenda, et al. SARS-CoV-2 viral load in clinical samples from critically ill patients. Am J Respir Crit Care Med. 2020;201(11) doi: 10.1164/rccm.202003-0572LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stichting Werkgroep Antibioticabeleid . Vol. 2021. 2021. Medicamenteuze behandeling voor patiënten met COVID-19 (infectie met SARS–CoV-2) [Google Scholar]
- 18.Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J., et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323:1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X., et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020 doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 20.Kim J.Y., Ko J.H., Kim Y., Kim Y.J., Kim J.M., Chung Y.S., et al. Viral load kinetics of SARS-CoV-2 infection in first two patients in Korea. J Korean Med Sci. 2020 doi: 10.3346/jkms.2020.35.e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baccam P., Beauchemin C., Macken C.A., Hayden F.G., Perelson A.S. Kinetics of influenza A virus infection in humans. J Virol. 2006;80:7590–7599. doi: 10.1128/jvi.01623-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexandersen, S, Chamings, A, Bhatta, TR. 2020. SARS-CoV-2 genomic and subgenomic RNAs in diagnostic samples are not an indicator of active replication. Nat Commun 11:6059-7. Doi: 10.1038/s41467-020-19883-7. [DOI] [PMC free article] [PubMed]
- 23.Wang, D, Hu, B, Hu, C, Zhu, F, Liu, X, Zhang, J, Wang, B, Xiang, H, Cheng, Z, Xiong, Y, Zhao, Y, Li, Y, Wang, X, Peng, Z. 2020. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama. 323:1061-1069. Doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed]
- 24.Zhou Y., Yang Q., Chi J., Dong B., Lv W., Shen L., et al. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta-analysis. Int J Infect Dis. 2020;99:47–56. doi: 10.1016/j.ijid.2020.07.029. http://www.sciencedirect.com/science/article/pii/S1201971220305725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quah, P, Li, A, Phua, J. 2020. Mortality rates of patients with COVID-19 in the intensive care unit: a systematic review of the emerging literature. Crit Care 24:285-1. Doi: 10.1186/s13054-020-03006-1. [DOI] [PMC free article] [PubMed]
- 26.RECOVERY Collaborative Group. Horby P., Mafham M., Linsell L., Bell J.L., Staplin N., et al. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383(21):2030. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Kampen Jeroen J.A., van de Vijver David A.M.C., Fraaij Pieter L.A., Haagmans Bart L., Lamers Mart M., Okba Nisreen, et al. 2020. Shedding of infectious virus in hospitalized patients with coronavirus disease-2019 (COVID-19): Duration and key determinants. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material