Abstract
Aims:
Women with ischemia but no obstructive coronary artery disease (INOCA) often have coronary microvascular dysfunction (CMD). Left ventricular (LV) circumferential strain (CS) is often lower in INOCA compared to healthy controls; however, it remains unclear whether CS differs between INOCA women with and without CMD. We hypothesized that CS would be lower in women with CMD, consistent with CMD-induced LV mechanical dysfunction.
Methods and results:
Cardiac magnetic resonance (cMR) images were examined from women enrolled in the Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction Project. CS by feature tracking in INOCA women with CMD, defined as myocardial perfusion reserve index (MPRI) <1.84 during adenosine-stress perfusion cMR, was compared with CS in women without CMD. In a subset who had invasive coronary function testing (CFT), the relationship between CS and CFT metrics, LV ejection fraction (LVEF) and cardiovascular risk factors was investigated. Among 317 women with INOCA, 174 (55%) had CMD measured by MPRI. CS was greater in women with CMD compared to those without CMD (23.2 ± 2.5% vs. 22.1 ± 3.0%, respectively, P = 0.001). In the subset with CFT (n = 153), greater CS was associated with increased likelihood of reduced vasodilator capacity (OR = 1.33, 95%CI = 1.02–1.72, p = 0.03) and discriminated abnormal vs. normal coronary vascular function compared to CAD risk factors, LVEF and LV concentricity (AUC: 0.82 [0.73–0.96 95%CI] vs. 0.65 [0.60–0.71 95%CI], respectively, P = 0.007).
Conclusion:
The data indicate that LV circumferential strain is related to and predicts CMD, although in a direction contrary with our hypothesis, which may represent an early sign of LV mechanical dysfunction in CMD.
Keywords: Circumferential strain, Coronary microvascular dysfunction, Coronary microvascular disease, Mechanical dysfunction, Subclinical dysfunction
1. Introduction
Ischemia with no obstructive coronary artery disease (INOCA) is an increasingly recognized pathological phenotype, particularly in women, and associated with increased risk of adverse outcomes [1,2], including heart failure with preserved ejection fraction (HFpEF) [3]. The Women’s Ischemia Syndrome Evaluation (WISE) has also observed that the majority of women with INOCA present with coronary microvascular dysfunction (CMD) by invasive coronary function testing (CFT) [4,5], or non-invasive myocardial perfusion reserve index (MPRI) by cardiac magnetic resonance (cMR) imaging [6]. Despite increased understanding, the mechanistic role of CMD in progression to HFpEF in the INOCA cohort remains unclear, and was therefore the focus of this investigation.
Patients with HFpEF often have impaired left ventricular (LV) diastolic function with reduced longitudinal and circumferential diastolic strain rate [7,8]. Our group has observed similar findings in women with CMD, demonstrating impaired LV relaxation and reduced systolic strain [9]. However, it remains unclear whether CMD is a contributing mechanism driving these differences, as prior observations have only been compared with healthy reference subjects [9]. Given that both LV systolic and diastolic function are highly energy dependent processes [10], we hypothesize that ischemia, secondary to CMD in INOCA women, is associated with LV mechanical dysfunction, and that this will be reflected in longitudinal and circumferential strain and strain rates. To test this hypothesis, we selected subjects enrolled in the Women’s Ischemic Syndrome Evaluation - Coronary Vascular Dysfunction (WISE-CVD) Project [11], which enrolled symptomatic women who underwent clinically indicated coronary angiography; the majority of whom underwent rest-stress cMR imaging and many also underwent CFT.
2. Methods
Detailed descriptions of the WISE-CVD (NCT00832702) Project rationale and methodology have been previously published [6,11]. All subjects were assessed at either Cedars-Sinai Medical Center, Los Angeles, or the University of Florida, Gainesville. The study protocol was approved by the institutional review committee at each study site, and all women provided written informed consent. All subjects completed standardized demographic/health history questionnaires, including the Seattle Angina Questionnaire (SAQ) [12] and Duke Activity Status Index (DASI) [13]. Reference subjects were age-matched women without symptoms of ischemic heart disease or coronary artery disease (CAD) risk factors who had no evidence of cardiovascular disease during a maximal Bruce-protocol exercise treadmill stress test.
To test the hypothesis that women with CMD-related ischemia have LV mechanical dysfunction, 374 women with INOCA from the NHLBI-sponsored WISE-CVD database enrolled between January 2009 and August 2015, who underwent rest/stress cMR, were considered for analysis. Women with INOCA were grouped according to the presence or absence of CMD using the previously defined threshold of MPRI <1.84 [6]. In a subset of INOCA subjects (n = 153) who also underwent invasive coronary function testing (CFT), and in whom all cMR data was available, the association between longitudinal and circumferential strain and strain rates and CFT was evaluated by multivariable logistic regression. Reference control subjects underwent rest/stress cMR and were considered <1% likelihood of having ischemic heart disease.
2.1. Coronary function testing (CFT)
CFT was performed using a standardized protocol [14]. Briefly, a Doppler guide wire was placed in the proximal left anterior descending coronary artery for assessment of coronary blood flow (CBF) and coronary diameter by quantitative angiography at rest, and in response to intracoronary adenosine (18 and 36 μg), acetylcholine (0.182 and 18.2 μg/mL), and nitroglycerin (200 μg); with standardized definitions of normal versus abnormal [14]. Four CFT measures were assessed: 1) abnormal macrovascular endothelial function defined as a change in epicardial coronary artery diameter ≤ 0% in response to a maximum dose of acetylcholine; 2) abnormal coronary flow reserve (CFR), defined as CFR < 2.5 in response to adenosine; 3) abnormal microvascular endothelial dysfunction, defined as an increase in CBF ≤50% in response to acetylcholine; 4) abnormal non-endothelial function defined as a change in epicardial coronary artery diameter ≤ 20% in response to nitroglycerin.
2.2. Cardiac magnetic resonance (cMR) imaging
cMR was performed using the same model 1.5-Tesla scanners and protocol at both centers (Avanto, Siemens Healthcare, Erlangen, Germany), with electrocardiogram gating and phased-array surface coil (CP Body Array Flex; Siemens Healthcare), and subjects in the supine position [6]. First-pass perfusion images were acquired in basal, mid and distal short-axis image planes under resting conditions and in response to intra-venous adenosine (140 μg/kg/min over ~4 mins; Adenoscan, Astellas Pharma US, Inc., Northbrook, IL) or regadenoson (0.4 mg) infusion, with use of a gadolinium-based contrast agent (0.05 mmol/kg at 4 mL/s) also administered intravenously as previously described [6]. Splenic switch off was confirmed in all patients who received adenosine. All data were analyzed with the operator blinded to the clinical status of each participant. Dedicated CAAS MRV software (Pie Medical Imaging, B.V., Netherlands) with semi-automated cMR segmentation was used for calculation of the MPRI, LV mass, LV volume, volumetric filling profiles, and LV ejection fraction (LVEF) [6]. LV concentricity was defined by the ratio of LV mass to LV end diastolic volume. Short-axis cine images, together with the 2- and 4-chamber long-axis cine images, were selected for assessment of circumferential and longitudinal strain and strain rates, measured by feature tracking (CVI42v v5.3.0; Circle Cardiovascular Imaging Inc., Calgary, AB, Canada) [9].
2.3. Statistical analysis
Statistical analyses of group cross-sectional comparisons were performed using SPSS (version 25, IBM SPSS Statistics, Armonk, NY) and R Studio (version 3.5.3, R Studio Inc., Boston, MA). Normal distribution was confirmed in all dependent variables using Shapiro-Wilk tests. Group differences between CMD and non-CMD women were compared using unpaired Student t-tests. Categorical variables were summarized using percentages (counts) and compared using the Chi-squared test, while continuous variables are described as mean ± standard deviation. A multivariable model was used to evaluate the probability of any abnormal CFT given clinical and cMR-derived variables using logistic regression. Reference control women were included in the multivariable analysis of the association between cMR parameters and CFT and assumed to have normal CFT. For multivariable model selection, 60% of the observations were randomly chosen to create a training data set with the rest reserved as a testing dataset in which our model could be validated. The model was based on clinical relevance of the variables in affecting vasoreactivity and the performance of the multivariable model was tested for its ability to predict CFT in the testing data set. Prediction of CFT by the model was expressed as the area under the receiver operating characteristic curve computed with 100 bootstrap replicates. Performance of the model which included MPRI as well as circumferential and longitudinal strain variables was compared to prediction of abnormal CFT by a model that only included presence of 1 or more CAD risk factors (i.e. hypertension, hyperlipidemia, diabetes, family history of premature atherosclerotic disease, cigarette smoking and peripheral arterial disease), LVEF and LV concentricity. Trends in MPRI and circumferential strain among patients stratified by the number of CFT abnormalities was evaluated by Jonckheere-Terpstra test. A two-tailed P-value <0.05 was considered statistically significant and 95% confidence intervals are reported.
3. Results
3.1. CMD and LV function
Following exclusion for incomplete MPRI data (n = 25) and insufficient feature tracking quality (n = 32), cMR data from 317 women with INOCA were considered for comparisons, of which 174 (55%) met the criteria for CMD (MPRI <1.84; Supplemental Fig. 1).
Demographics for group comparisons are summarized in Table 1. Body mass index (BMI) was similar between groups, and there were no differences in disease history, while the CMD group tended to be prescribed a statin more often (P = 0.057). Treatment satisfaction from the SAQ score was higher in the CMD group (P = 0.002), while all other indices from the SAQ and DASI questionnaires were not different.
Table 1:
Characteristics of women with CMD and non-CMD for baseline comparison.
| Variable | Non-CMD (n = 143) | CMD (n = 174) | P-value |
|---|---|---|---|
| Demographics | |||
| Age (years) | 53 ± 10 | 57 ± 11 | 0.38 |
| Height (cm) | 163 ± 6 | 163 ± 7 | 0.58 |
| Weight (kg) | 72.6 ± 17.7 | 72.6 ± 19.4 | 0.84 |
| BSA (m2) | 1.81 ± 0.21 | 1.82 ± 0.21 | 0.60 |
| Caucasian % (n) | 73 (105) | 79 (137) | 0.23 |
| Resting Hemodynamics | |||
| Heart Rate (bpm) | 67 ± 12 | 69 ± 12 | 0.21 |
| Systolic BP (mmHg) | 130 ± 21 | 134 ± 22 | 0.14 |
| Diastolic BP (mmHg) | 60 ± 12 | 65 ± 15 | 0.01 |
| Health Status | |||
| DASI | 9.1 ± 5.7 | 8.2 ± 5.7 | 0.17 |
| SAQ - Exertional Capacity | 68 ± 24 | 67 ± 24 | 0.71 |
| SAQ - Anginal Stability | 50 ± 26 | 47 ± 27 | 0.24 |
| SAQ - Anginal Frequency | 63 ± 26 | 65 ± 26 | 0.48 |
| SAQ - Disease Perception | 64 ± 28 | 73 ± 23 | 0.002 |
| SAQ - Treatment Satisfaction | 48 ± 23 | 50 ± 25 | 0.52 |
| Medications | |||
| Hormone Replacement Therapy % (n) | 47 (67) | 49 (86) | 0.67 |
| ACE-I % (n) | 20 (29) | 17 (29) | 0.48 |
| ARB % (n) | 7 (10) | 6 (11) | 0.85 |
| β-Blocker % (n) | 31 (45) | 32 (55) | 0.80 |
| Ca2+ Channel Blocker % (n) | 17 (25) | 22 (39) | 0.21 |
| Diuretics % (n) | 10 (15) | 13 (23) | 0.41 |
| Vasodilator % (n) | 4 (6) | 3 (5) | 0.58 |
| Full-Dose Aspirin % (n) | 13 (19) | 8 (14) | 0.15 |
| Low-Dose Aspirin % (n) | 57 (82) | 62 (108) | 0.33 |
| Statin % (n) | 36 (52) | 47 (82) | 0.06 |
| Nitrates % (n) | 34 (48) | 29 (49) | 0.39 |
Non-CMD – no coronary microvascular dysfunction; CMD – coronary microvascular dysfunction; BSA – body surface area; BP – blood pressure; DASI – Duke Activity Status Index; SAQ – Seattle Angina Questionnaire; ACE-I – angiotensin-converting enzyme inhibitor; ARB – angiotensin receptor blocker; Ca2+ − calcium. Bold text highlights P values < 0.05.
Compared to women without CMD, women with CMD had a similar end-diastolic volume (P = 0.27) and a lower end-systolic volume (P = 0.009), contributing to an increased LVEF (P = 0.017; Table 2). Likewise, women with CMD had increased absolute circumferential strain (P = 0.001; Table 2) and absolute circumferential systolic strain rate (P = 0.039; Table 2). All other measures of systolic and diastolic deformation were similar between groups.
Table 2:
Left ventricular structure and function for baseline comparison.
| Variable | Non-CMD (n = 143) | CMD (n = 174) | P-value |
|---|---|---|---|
| Volumes and mass | |||
| EDV (mL) | 126 ± 25 | 119 ± 23 | 0.27 |
| EDVi (mL/m2) | 70.0 ± 13.0 | 66.2 ±11.6 | 0.15 |
| ESV (mL) | 42 ± 15 | 38 ± 12 | 0.009 |
| ESVi (mL/m2) | 23.5 ± 8.3 | 20.8 ± 6.6 | 0.006 |
| SV (mL) | 83 ± 17 | 82 ± 16 | 0.69 |
| Stroke Index (mL/m2) | 46.3 ± 8.6 | 45.1 ± 8.0 | 0.41 |
| Cardiac Output (L/min) | 5.53 ± 1.23 | 5.55 ± 1.34 | 0.23 |
| Cardiac Index (L/min/m2) | 3.06 ± 0.58 | 3.05 ± 0.64 | 0.35 |
| Ejection Fraction (%) | 67 ± 7 | 69 ± 7 | 0.017 |
| LV Mass (g) | 92.5 ± 16.1 | 91.8 ± 17.5 | 0.60 |
| LV Mass Index (g/m2) | 51.4 ± 7.2 | 50.5 ± 7.0 | 0.18 |
| Peak Filling Rate (mL/min) | 361 ± 93 | 346 ± 99 | 0.56 |
| Time to Peak Filling Rate (ms) | 204 ± 85 | 206 ± 82 | 0.92 |
| Perfusion | |||
| MPRI | 2.28 ± 0.35 | 1.49 ± 0.25 | By Design |
| Epi-to-Endo Gradient | 0.40 ± 0.28 | 0.20 ± 0.21 | 0.001 |
| LV Mechanics | |||
| Longitudinal Strain (%) | −20.6 ± 3.1 | −20.7 ± 3.4 | 0.33 |
| Longitudinal Systolic SR (/s) | −1.00 ± 0.21 | −0.99 ± 0.59 | 0.19 |
| Early Longitudinal Diastolic SR (/s) | 1.13 ± 0.32 | 1.09 ± 0.31 | 0.96 |
| Late Longitudinal Diastolic SR (/s) | 0.69 ± 0.26 | 0.73 ± 0.24 | 0.41 |
| Circumferential Strain (%) | −22.1 ± 3.0 | −23.2 ± 2.5 | 0.001 |
| Circumferential Systolic SR (/s) | −1.08 ± 0.20 | −1.12 ± 0.20 | 0.039 |
| Early Circumferential Diastolic SR (/s) | 1.38 ± 0.37 | 1.38 ± 0.35 | 0.90 |
| Late Circumferential Diastolic SR (/s) | 0.56 ± 0.21 | 0.61 ± 0.21 | 0.60 |
Non-CMD – no coronary microvascular dysfunction; CMD – coronary microvascular dysfunction; EDV – end-diastolic volume; EDVi – end-diastolic volume index; ESV – end-systolic volume; ESVi – end-systolic volume index; SV – stroke volume; LV – left ventricular; MPRI – myocardial perfusion reserve index; Epi-to-Endo -epicardial-to-endocardial; SR – strain rate.
3.2. Associations between strain and strain rate and coronary function testing
Characteristics of women stratified by abnormal CFT, and 19 healthy reference women, are summarized in Supplemental Table 1. Due to clinical feasibility and safety issues, one of the INOCA women only had one pathway evaluated at the time of CFT. Unadjusted values for the cMR-derived parameters are summarized in Supplemental Table 2 stratified by normal and abnormal CFT. MPRI was lower in women with any CFT abnormalities compared to those with normal CFT. There was a trend towards progressive reduction in MPRI with increasing number of CFT abnormalities (Supplemental Fig. 2A).
Circumferential strain was not associated with increasing number of abnormal CFT pathways (P = 0.33, Supplemental Fig. 2B); however, in a univariable logistic regression, greater circumferential strain was associated with increased odds of abnormal CFT (Supplemental Table 3). Furthermore, the multivariable model that best described the association between cMR variables and abnormal CFT, included age, MPRI, and circumferential strain (Supplemental Table 4). In this multivariable model, greater circumferential strain was associated with increased likelihood of abnormal CFT after adjusting for other clinical and cMR variables.
The multivariable model derived from the training cohort was further validated in a second subset of INOCA and reference control women which formed the testing cohort. The AUC of the ROC curve for identifying women with CMD measured by the presence of at least 1 CFT abnormality in the testing cohort with a model that only included >1 CAD risk factor, LVEF and LV concentricity was 0.65, (95% CI: 0.60–0.71; Fig. 1). Addition of MPRI, circumferential strain, longitudinal strain and the respective strain rates to the model increased the AUC to 0.82 (95% CI: 0.73–0.96, P = 0.007). Circumferential strain and MPRI were important predictors of any abnormal CFT in the multivariable model.
Fig. 1.
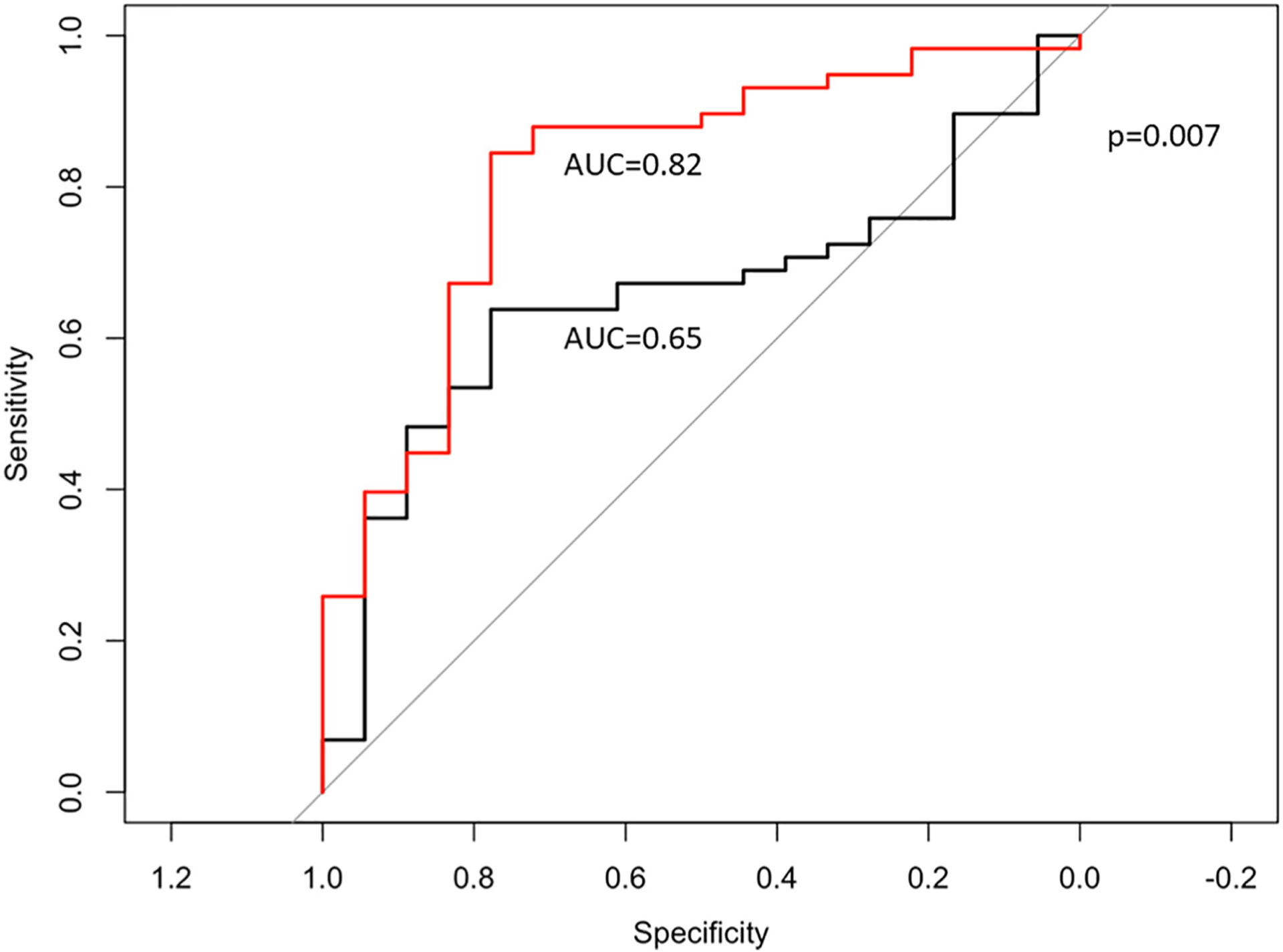
Receiver-operating characteristic curves show an improved ability to predict coronary microvascular dysfunction as defined by ≥1 abnormal pathway on CFT using a model that included LVEF LV concentricity, MPRI, circumferential strain, longitudinal strain, circumferential strain rates, longitudinal strain rates and presence of >1 CAD risk factor (red line) compared to a model that only included LVEF, LV concentricity and > 1 CAD risk factor alone (black line; 0.82 vs. 0.65, P = 0.007). CFT - coronary reactivity testing; CAD - coronary artery disease; LVEF – left ventricular ejection fraction; MPRI - myocardial perfusion reserve index.
4. Discussion
Our study sought to determine if CMD in women with INOCA is associated with LV mechanical dysfunction. The data herein suggest that LV circumferential strain may be related to CMD, although in a direction contrary with our original hypothesis, with greater LV circumferential strain associated with CMD.
Multiple lines of evidence document that women with INOCA often have CMD [4,5], and are at increased risk of developing HFpEF [15]. One attractive hypothesis is that CMD contributes to heart failure progression in INOCA women. We have previously shown that women with CMD often have elevated LV filling pressures [16], impaired LV relaxation [17,18], abnormal myocardial substrate utilization [17] and subclinical LV systolic dysfunction [9] compared to reference subjects. Emerging evidence also suggests that women with CMD have elevated inflammatory markers [19–21], focal scar [22], and diffuse fibrosis [23]; which are characteristics observed in HFpEF [24–26]. These observations provide support for the hypothesis that CMD may contribute to HFpEF initiation and/or progression. However, until now, these comparisons have not been specifically evaluated between CMD and non-CMD women within the broader INOCA classification. Our results extend prior observations related to LV mechanical and CMD, by examining a cohort of INOCA women with and without CMD rigorously measured by experienced core laboratories.
That circumferential strain was greater in women with CMD versus women without CMD was unexpected and contrary to our original hypothesis. This observation is however consistent with a growing body of literature showing dissociations between longitudinal and circumferential deformation, with preserved LVEF [27–33]. For example, circumferential strain was greater in patients with hypertension-induced LV hypertrophy [34,35] and hypertrophic cardiomyopathy [36]; proposed by many as evidence of subclinical LV dysfunction [33,37–39]. While the mechanisms remain incompletely understood, one potential explanation is that subendocardial dysfunction may promote epicardial helical muscle fiber dominance, resulting in greater circumferential strain [37]. In support of this interpretation, our observations indicate that women with CMD had reduced epicardial-to-endocardial perfusion gradient versus women without CMD. Our observation that there was no graded response of circumferential strain based on CFT abnormalities suggests that circumferential strain may be associated with the presence or absence of CMD and independent of the number of CFT abnormalities. Caution is indeed warranted when interpreting this later observation however, as the sample size of participants with more than two abnormal CFT pathways was admittedly low.
We were also surprised to find no significant differences in LV diastolic function between women with and without CMD. While this may suggest that CMD may not be driving previously documented differences in diastolic function between women with INOCA and reference controls, several alternative explanations must also be considered: First, the cine images used to evaluate tissue deformation in the present study were performed under resting conditions. It therefore remains unclear if LV diastolic dysfunction would be unmasked in the CMD group if placed under physiological stress. Second, early LV diastolic relaxation is influenced by the extent and rigor of the preceding systolic contraction [40,41]. It is therefore possible that differences in LV diastolic function per se are being masked by the heightened systolic deformation in the CMD group observed herein. Finally, it is possible that the group with CMD had elevated LV filling pressures compared to those without CMD, which would similarly mask the extent of LV diastolic dysfunction, given the dynamic coupling between left atrial driving pressures and ventricular relaxation that governs the intraventricular pressure gradient and early LV diastolic filling [42,43]. We have indeed previously reported an increased incidence of elevated LV filling pressures in women with CMD [16]. Unfortunately, LV filling pressure was not measured in the entire cohort of patients studied herein, precluding the normalization of early diastolic strain rate for LV end-diastolic pressure.
That circumferential strain predicted abnormal coronary reactivity in the broader INOCA population may be of potential clinical importance. cMR offers the advantage of being noninvasive and free of radiation, thus serving as a safe technique to study myocardial structure and function. We have previously shown that MPRI derived from cMR can distinguish individuals with abnormal CFT results [6]. Here we show that adding circumferential strain to the model significantly improves our ability to predict abnormal CFT.
4.1. Experimental considerations
Although the number of women studied was relatively small, our rigorously determined core laboratory cMR and CFT measures that include rest and vasodilator stress myocardial perfusion, myocardial strain and invasively determined CMD in INOCA women are strengths. Moreover, only a subset of women with INOCA included in this investigation underwent invasive CFT; however CFT is a rigorous reference standard for CMD determination. We interpret low MPRI in the present investigation to reflect impaired vasodilator reserve; however, abnormal MPRI can be influenced by higher resting myocardial perfusion. Future investigations should focus on quantitative flow assessment, especially as newer cMR approaches become more widely available. cMR T1 and T2 mapping were not available at the time of imaging, limiting our ability to determine whether alterations in myocardial tissue characteristics contributed to our results. Although we observed an association between myocardial strain and abnormal coronary function, our study was not designed to evaluate the mechanistic relationship between vasoreactivity and changes in myocardial deformation. Moreover, a specific “cut-off” value predicting abnormal coronary function remains equivocal. Instead, we interpret the data to reflect sub-clinical changes along a continuous spectrum of mechanical function. In this way, left ventricular function is a product of the very environment for which it operates.
5. Conclusion
Taken together, the data herein are hypothesis generating, and support the need for further prospective studies into the evolution of CMD and LV mechanics.
Supplementary Material
Funding
This work was supported by contracts from the National Institutes for Health, nos. 7R56HL131871, N01-HV-68161, HL33610, HL56921; UM1 HL087366, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U01 64829, U01 HL649141, U01 HL649241, T32 HL69751, K23HL105787, K23HL127262, K23HL125941, 1R03 AG032631 MO1-RR00425, UL1TR 001427, UL1TR000124 and UL1TR000064, grants from the Gustavus and Louis Pfeiffer Research Foundation; The Women’s Guild of Cedars-Sinai Medical Center; The Ladies Hospital Aid Society of Western Pennsylvania; QMED, Inc.; the Edythe L. Broad Women’s Heart Research Fellowship; the Barbra Streisand Women’s Cardiovascular Research and Education Program; the American Heart Association, nos. 16SDG2 7260115, 18POST34080330, 18PRE33960358; the Gatorade Trust and the McJunkin Family Foundation through funds distributed by the University of Florida; and PCORnet-OneFlorida Clinical Research Consortium CDRN-1501-26692.
Disclosures
C.N.B.M receives funding from Abbott Diagnostics, Sanofi (paid through CSMC) and serves as Board of Director for iRhythm. C. P. receives research grants from GE Healthcare, Merck, Sanofi, CLS Behring, Biocardia, McJunkin Family Foundation, Brigham & Women’s Hospital, Gatorade Trust through the University of Florida Department of Medicine, Athersys Inc., AMI MultiStem, and Mesoblast, Inc.; has received consultant fees/honoraria from Verily Life Sciences LLC Project Baseline OSMB (Google), Ironwood, XyloCor, Slack Inc., Imbria Pharmaceuticals, Milestone Pharmaceuticals Inc., Ventrix, Inc., AstraZeneca Pharmaceuticals, and Sanofi-Aventis. Dr. Mehta has received research grants from Gilead and General Electric.
Footnotes
Declaration of Competing Interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcard.2020.11.006.
References
- [1].Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, et al. , Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s ischemia syndrome evaluation study and the St James women take heart project, Arch. Intern. Med 169 (2009) 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, et al. , Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events, Eur. Heart J 33 (2012) 734–744. [DOI] [PubMed] [Google Scholar]
- [3].Nelson MD, Wei J, Bairey Merz CN, Coronary microvascular dysfunction and heart failure with preserved ejection fraction as female-pattern cardiovascular disease: the chicken or the egg? Eur. Heart J 39 (2018) 850–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, et al. , Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, lung and blood institute WISE (Women’s ischemia syndrome evaluation) study, J. Am. Coll. Cardiol 55 (2010) 2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, et al. , Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study, Am. Heart J 141 (2001) 735–741. [DOI] [PubMed] [Google Scholar]
- [6].Thomson LE, Wei J, Agarwal M, Haft-Baradaran A, Shufelt C, Mehta PK, et al. , Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation, Circ. Cardiovasc. Imaging (2015) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zile MR, Baicu CF, Gaasch WH, Diastolic heart failure–abnormalities in active relaxation and passive stiffness of the left ventricle, N. Engl. J. Med 350 (2004) 1953–1959. [DOI] [PubMed] [Google Scholar]
- [8].Kawaguchi M, Hay I, Fetics B, Kass DA, Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations, Circulation 107 (2003) 714–720. [DOI] [PubMed] [Google Scholar]
- [9].Nelson MD, Sharif B, Shaw JL, Cook-Wiens G, Wei J, Shufelt C, et al. , Myocardial tissue deformation is reduced in subjects with coronary microvascular dysfunction but not rescued by treatment with ranolazine, Clin. Cardiol 40 (2017) 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gorski PA, Ceholski DK, Hajjar RJ, Altered myocardial calcium cycling and energetics in heart failure–a rational approach for disease treatment, Cell Metab 21 (2015) 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Quesada O, AlBadri A, Wei J, Shufelt C, Mehta PK, Maughan J, et al. , Design, methodology and baseline characteristics of the Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction (WISE-CVD), Am. Heart J 220 (2019) 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, et al. , Development and evaluation of the Seattle angina questionnaire: a new functional status measure for coronary artery disease, J. Am. Coll. Cardiol 25 (1995) 333–341. [DOI] [PubMed] [Google Scholar]
- [13].Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, et al. , A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index), Am. J. Cardiol 64 (1989) 651–654. [DOI] [PubMed] [Google Scholar]
- [14].Wei J, Mehta PK, Johnson BD, Samuels B, Kar S, Anderson RD, et al. , Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study, JACC Cardiovasc. Interv 5 (2012) 646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bakir M, Nelson MD, Jones E, Li Q, Wei J, Sharif B, et al. , Heart failure hospitalization in women with signs and symptoms of ischemia: a report from the women’s ischemia syndrome evaluation study, Int. J. Cardiol 223 (2016) 936–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wei J, Mehta PK, Shufelt C, Yang Y, Gill E, Kahlon R, et al. , Diastolic dysfunction measured by cardiac magnetic resonance imaging in women with signs and symptoms of ischemia but no obstructive coronary artery disease, Int. J. Cardiol 220 (2016) 775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wei J, Nelson MD, Szczepaniak EW, Smith L, Mehta PK, Thomson LE, et al. , Myocardial steatosis as a possible mechanistic link between diastolic dysfunction and coronary microvascular dysfunction in women, Am. J. Physiol. Heart Circ. Physiol 310 (2016) H14–H19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nelson MD, Szczepaniak LS, Wei J, Haftabaradaren A, Bharadwaj M, Sharif B, et al. , Diastolic dysfunction in women with signs and symptoms of ischemia in the absence of obstructive coronary artery disease: a hypothesis-generating study, Circ. Cardiovasc. Imaging 7 (2014) 510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].AlBadri A, Lai K, Wei J, Landes S, Mehta PK, Li Q, et al. , Inflammatory biomarkers as predictors of heart failure in women without obstructive coronary artery disease: a report from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE), PLoS One 12 (2017), e0177684,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kip KE, Marroquin OC, Shaw LJ, Arant CB, Wessel TR, Olson MB, et al. , Global inflammation predicts cardiovascular risk in women: a report from the Women’s ischemia syndrome evaluation (WISE) study, Am. Heart J 150 (2005) 900–906. [DOI] [PubMed] [Google Scholar]
- [21].Marroquin OC, Kip KE, Mulukutla SR, Ridker PM, Pepine CJ, Tjandrawan T, et al. , Inflammation, endothelial cell activation, and coronary microvascular dysfunction in women with chest pain and no obstructive coronary artery disease, Am. Heart J 150 (2005) 109–115. [DOI] [PubMed] [Google Scholar]
- [22].Wei J, Bakir M, Darounian N, Li Q, Landes S, Mehta PK, et al. , Myocardial scar is prevalent and associated with subclinical myocardial dysfunction in women with suspected ischemia but no obstructive coronary artery disease: from the Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction Study, Circulation 137 (2018) 874–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shaw JL, Nelson MD, Wei J, Motwani M, Landes S, Mehta PK, et al. , Inverse association of MRI-derived native myocardial T1 and perfusion reserve index in women with evidence of ischemia and no obstructive CAD: a pilot study, Int. J. Cardiol 270 (2018) 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].DeVore AD, McNulty S, Alenezi F, Ersboll M, Vader JM, Oh JK, et al. , Impaired left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: insights from the RELAX trial, Eur. J. Heart Fail 19 (7) (2017) 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tadic M, Pieske-Kraigher E, Cuspidi C, Genger M, Morris DA, Zhang K, et al. , Left ventricular strain and twisting in heart failure with preserved ejection fraction: an updated review, Heart Fail. Rev 22 (2017) 371–379. [DOI] [PubMed] [Google Scholar]
- [26].Mahmod M, Pal N, Holloway C, Ferreira V, Dass S, Francis J, et al. , 42 myocardial lipid accumulation and cardiac remodelling in heart failure with reduced and preserved ejection fraction, Heart 101 (2015) (A24–A5). [Google Scholar]
- [27].Cheng-Baron J, Chow K, Pagano JJ, Punithakumar K, Paterson DI, Oudit GY, et al. , Quantification of circumferential, longitudinal, and radial global fractional shortening using steady-state free precession cines: a comparison with tissue-tracking strain and application in Fabry disease, Magn. Reson. Med 73 (2015) 586–596. [DOI] [PubMed] [Google Scholar]
- [28].Kosmala W, Plaksej R, Strotmann JM, Weigel C, Herrmann S, Niemann M, et al. , Progression of left ventricular functional abnormalities in hypertensive patients with heart failure: an ultrasonic two-dimensional speckle tracking study, J. Am. Soc. Echocardiogr 21 (2008) 1309–1317. [DOI] [PubMed] [Google Scholar]
- [29].Eleid MF, Caracciolo G, Cho EJ, Scott RL, Steidley DE, Wilansky S, et al. , Natural history of left ventricular mechanics in transplanted hearts: relationships with clinical variables and genetic expression profiles of allograft rejection, JACC Cardiovasc. Imaging 3 (2010) 989–1000. [DOI] [PubMed] [Google Scholar]
- [30].Fallah-Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, et al. , The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy, J. Am. Coll. Cardiol 57 (2011) 2263–2270. [DOI] [PubMed] [Google Scholar]
- [31].Ho E, Brown A, Barrett P, Morgan RB, King G, Kennedy MJ, et al. , Subclinical anthracycline- and trastuzumab-induced cardiotoxicity in the long-term follow-up of asymptomatic breast cancer survivors: a speckle tracking echocardiographic study, Heart 96 (2010) 701–707. [DOI] [PubMed] [Google Scholar]
- [32].Stanton T, Leano R, Marwick TH, Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring, Circ Cardiovasc. Imaging 2 (2009) 356–364. [DOI] [PubMed] [Google Scholar]
- [33].Cikes M, Solomon SD, Beyond ejection fraction: an integrative approach for assessment of cardiac structure and function in heart failure, Eur. Heart J 37 (2016) 1642–1650. [DOI] [PubMed] [Google Scholar]
- [34].Imbalzano E, Zito C, Carerj S, Oreto G, Mandraffino G, Cusma-Piccione M, et al. , Left ventricular function in hypertension: new insight by speckle tracking echocardiography, Echocardiography 28 (2011) 649–657. [DOI] [PubMed] [Google Scholar]
- [35].Sun JP, Xu T, Yang Y, Yang XS, Shang Q, Li Y, et al. , Layer-specific quantification of myocardial deformation may disclose the subclinical systolic dysfunction and the mechanism of preserved ejection fraction in patients with hypertension, Int. J. Cardiol 219 (2016) 172–176. [DOI] [PubMed] [Google Scholar]
- [36].Urbano-Moral JA, Rowin EJ, Maron MS, Crean A, Pandian NG, Investigation of global and regional myocardial mechanics with 3-dimensional speckle tracking echocardiography and relations to hypertrophy and fibrosis in hypertrophic cardiomyopathy, Circ Cardiovasc. Imaging 7 (2014) 11–19. [DOI] [PubMed] [Google Scholar]
- [37].Vasanji Z, Sigal RJ, Eves ND, Isaac DL, Friedrich MG, Chow K, et al. , Increased left ventricular extracellular volume and enhanced twist function in type 1 diabetic individuals, J. Appl. Physiol. (1985) 123 (2017) 394–401. [DOI] [PubMed] [Google Scholar]
- [38].Nallur Shivu G, Abozguia K, Phan T, Ahmed I, Weaver R, Wagenmakers A, et al. , Increased left ventricular twist as an early manifestation of diabetic cardiomyopathy in patients with uncomplicated type 1 diabetes, Heart 95 (2009) 21. [Google Scholar]
- [39].Vigneault DM, Yang E, Jensen PJ, Tee MW, Farhad H, Chu L, et al. , Left ventricular strain is abnormal in preclinical and overt hypertrophic cardiomyopathy: cardiac MR feature tracking, Radiology 290 (2019) 640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Notomi Y, Martin-Miklovic MG, Oryszak SJ, Shiota T, Deserranno D, Popovic ZB, et al. , Enhanced ventricular untwisting during exercise: a mechanistic manifestation of elastic recoil described by Doppler tissue imaging, Circulation 113 (2006) 2524–2533. [DOI] [PubMed] [Google Scholar]
- [41].Notomi Y, Popovic ZB, Yamada H, Wallick DW, Martin MG, Oryszak SJ, et al. , Ventricular untwisting: a temporal link between left ventricular relaxation and suction, Am. J. Physiol. Heart Circ. Physiol 294 (2008) H505–H513. [DOI] [PubMed] [Google Scholar]
- [42].Hieda M, Parker J, Rajabi T, Fujimoto N, Bhella PS, Prasad A, et al. , Left ventricular volume-time relation in patients with heart Failure with preserved ejection fraction, Am. J. Cardiol 121 (5) (2017) 609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Opdahl A, Remme EW, Helle-Valle T, Edvardsen T, Smiseth OA, Myocardial relaxation, restoring forces, and early-diastolic load are independent determinants of left ventricular untwisting rate, Circulation 126 (2012) 1441–1451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


