Abstract
Background:
Post-herpetic neuralgia (PHN) is usually a constant or intermittent burning, stabbing, or sharp shooting pain with hyperalgesia or allodynia, persisting beyond the healing of herpetic skin lesions. This review was carried out in concordance to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. We used PICOS (Population, Intervention, Control, and Outcome Study) design for inclusion of potential studies into this review. Online literature available in PubMed, Cochrane, and Embase was searched for studies from January 1995 till March 2020, which evaluated interventional treatments in PHN by an independent reviewer, using the relevant medical subject heading (MeSH) terms. We analyzed the following outcome parameters with regard to each intervention—pain status at predefined fixed intervals after the intervention, quality of sleep using any of the reported questionnaires, analgesic consumption, functional evaluation, and quality of life assessment after the intervention.
Conclusion:
Interventional pain management options provide effective and long-lasting pain relief to patients not responding to medical management. The choice of intervention will depend on the region involved, cost, and invasiveness. Simple procedures such as intercostal nerve blocks/neurolysis, stellate ganglion blocks, paravertebral neurolysis, epidural steroid injections, and dorsal root ganglion–radiofrequency ablation are effective interventions, and if they fail, spinal cord stimulators could be effective in the hands of experienced pain physicians.
Keywords: Post herpetic neuralgia, Interventional pain management, Stellate ganglion block, Epidural steroid block, Intercostal RFA
INTRODUCTION
Post-herpetic neuralgia (PHN) is usually a constant or intermittent burning, stabbing, or sharp shooting pain with hyperalgesia or allodynia, persisting beyond the healing of herpetic skin lesions (more than 4 weeks after the rash onset). Another proposed definition of herpes-related pain is subacute herpetic neuralgia 30–90 days from Herpes Zoster (HZ), and PHN if the pain persists beyond 3 months.[1] The lifetime prevalence of HZ is between 20%–30%, rising to 50% by the age of 80 years.[2] If eruptions last more than 3 months, it is taken as the criteria to define PHN; studies report that 5%–15% of HZ cases convert to PHN.[3,4]
The strongest risk factors for PHN among zoster patients are old age, immunocompromised state, and/or recently diagnosed lymphoma/leukemia. Other risk factors include autoimmune conditions (rheumatoid arthritis, systemic lupus erythematosus, and inflammatory bowel disease), chronic obstructive pulmonary disease, depression, diabetes mellitus, asthma, lower socioeconomic status, smoking, and nontruncal zoster.
Like any chronic pain state, PHN also warrants a multimodal multispecialty team approach for its cure. The patient is offered first-line medical management most often by the dermatologist or the primary care physician. Medical management in the form of antiepileptic drugs (gabapentin or pregabalin), tricyclic antidepressants (amitriptyline), selective norepinephrine reuptake inhibitors (duloxetine), and local application of lignocaine 5%, and 8% capsaicin patches are the usual therapy, but still a large population is left unrelieved, and have severe neuropathic pain to the tune of allodynia.[5] It should be noted that spontaneous remission of intractable PHN is rare. The patients with severe persistent PHN recalcitrant to medical treatment or developing intolerable adverse effects to medications should be offered interventional pain management (IPM) options. But the role of IPM techniques for PHN is still not a widely accepted modality among most specialties due to lack of awareness and hesitation in referring or administering IPM themselves due to lack of training. The second hurdle is to choose the best among large armamentarium of interventional pain procedures in a particular patient.
This systemic review focuses on the relevant pathophysiology of PHN with emphasis on the targets amenable for modulation by IPM techniques, to fill up the lacunae about existing modalities of IPM in PHN.
MATERIALS AND METHODS
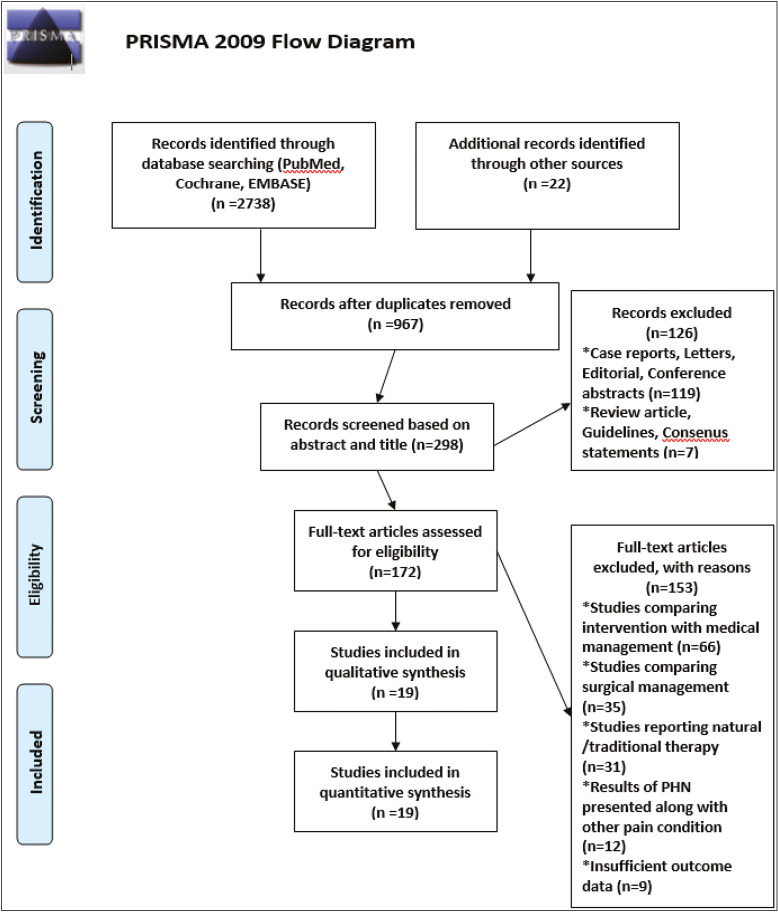
This review was carried out in concordance to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [Figure 1]. We used PICOS (Population, Intervention, Control, and Outcome Study) design [Table 1] for the inclusion of potential studies into this review. Randomized controlled studies, observational studies, and case series investigating various interventions for PHN were included in this review. Studies comparing efficacy of intervention versus conventional medical therapy were excluded.
Figure 1.
PRISMA flow diagram
Table 1.
PICOS framework
| Population | Adult patients with PHN |
| Interventions | IPM for PHN* |
| Controls | Comparison with other intervention (nonsurgical) |
| Outcomes | Primary objective: |
| • Pain at fixed time points after the intervention | |
| Secondary objectives: | |
| • Quality of sleep using any of the reported questionnaires | |
| • Analgesic consumption after the intervention | |
| • Functional evaluation and quality of life assessment after the intervention overall adverse events, if any | |
| Study design | Randomized controlled trials |
| Observational studies | |
| Case series |
*Intervention studies include any intrathecal MP, ESIs (interlaminar or transforaminal), paravertebral blocks/neurolysis, intercostal nerve chemical neurolysis, intercostal nerve and DRG-pulsed RFA, stellate ganglion blocks, and spinal cord stimulation
Literature search strategy
Online literature was searched for studies that evaluated interventional treatments in PHN, by an independent reviewer using the medical subject heading (MeSH) terms “post herpetic neuralgia,” “post herpetic pain,” “intrathecal methylprednisolone,” “epidural steroid,” “paravertebral block,” “paravertebral neurolysis,” “stellate ganglion block,” “Intercostal nerve chemical neurolysis,” “Intercostal Nerve RFA,” “DRG Pulsed RFA,” and “spinal cord stimulation.” The search was limited to human studies published in English language in PubMed, Embase, and Cochrane searched from January 1995 till March 15, 2020. Bibliographies and references of selected publications on interventional treatments in PHN were further manually screened. The full text of each article was studied once the abstract was analyzed by the searching reviewer and found appropriate. The decision to include a study into the final analysis was based on an independent assessment performed by another reviewer. The authors conducted the literature search themselves. A librarian or search specialist was not involved in the literature search. The initial electronic search using MeSH terms we used is completely repeatable, and PubMed search was emphasized the most.
Studies were excluded if they were conducted in healthy volunteers or in patients with a diagnosis other than PHN; only evaluated pharmacologic, surgical, or noninterventional treatments; study methods were not adequately described with regard to study design, intervention, and outcomes; study results reported for PHN were combined with those for other pain conditions; and/or if there were insufficient data on study results.
Data extraction
Data were extracted from the full-text article of each included study in to a Microsoft Excel spreadsheet (Microsoft, Microsoft Corporation, Seattle, Washington), using a standardized data extraction form. From each study, the following data were extracted—year and country of publication, study design, patient demographic profile, intervention used for treatment of PHN, outcome studied after intervention, and any complications due to the intervention. We analyzed the following outcome parameters with regard to each intervention—pain status at predefined fixed intervals after the intervention, quality of sleep using any of the reported questionnaires, analgesic consumption, functional evaluation, and quality of life assessment after the intervention. The included studies were assessed for bias by analyzing the method of randomization used, concealed treatment allocation, blinded data collection and analysis, blinded adjudication of study endpoints, and completeness of data, by two independent reviewers. However, a statistical analysis for possible publication bias using funnel plot and Egger’s test were not done. We had a well-framed PICOS framework before conducting the review. We had not registered in the International Prospective Register of Systematic Reviews (PROSPERO) or any other databases. Only a qualitative analysis of data was done from the various eligible studies. Data extraction for quantitative synthesis was not done.
Studies included
For each intervention, systematic reviews and meta-analysis were considered first, followed by randomized controlled trials (RCTs), observational studies, and then case series and case reports, if no better evidence was available.
OBSERVATIONS
RCTs were found only for interventions of intrathecal methylprednisolone (MP), intercostal/dorsal root ganglion–radiofrequency ablation (DRG RFA), and stellate ganglion RFA. English-language selection bias could not excluded. RCTs were manually screened for random sequence generation and allocation concealment (selection bias). Performance and detection bias was assessed based on the blinding of participants or personnel and outcome assessment. Attrition bias was attributed on incomplete reporting of outcomes. However, a pooled analysis of bias using RevMan software was not done.
Intrathecal injection of MP with local anesthetics
As the initiating event in pathophysiology of PHN is an inflammatory process, a possible role of MP was under consideration. RCTs conducted on the role of intrathecal MP performed in consecutive years 1999 and 2000 by Kotani et al.[6] showed global pain relief in majority of patients when followed up for 2 years. They showed an inflammatory marker interleukin-8 (IL-8), likely to be causative of PHN, decreased markedly after intrathecal-MP. It is interesting to note that a study conducted in 2013 by Rijsdijk et al.[7,8] on intrathecal MP showed increased pain at 8 weeks, and the IL-8 levels also increased as compared to control group. The trial was stopped because of safety concerns and futility. Various studies on the role of intrathecal MP in PHN is summarised in Table 2.[6,7,9]
Table 2.
Designs of studies using intrathecal methylprednisolone injection to treat PHN
| Study | Study design | Inclusion criteria | Groups | N | Route | Dose | Outcome | Funding |
|---|---|---|---|---|---|---|---|---|
| Kikuchi et al.[9] | RCT | Intractable PHN (pain > 1 year) | IT MP, epidural MP | 14, 15 | IT, epidural | IT: 3 mL of 2% lidocaine and 60-mg MP; Epidural: 5 mL of 2% lidocaine and 60-mg MP (QW*4) | ≥50% global pain relief: IT 92.3% vs. epidural 16.7% (P < 0.01). | The work was performed in the Department of Anesthesiology, University of Hirosaki, and supported in part by grants-in-aid for Scientific Research No. 08457399 (Department of Education, Japan). |
| Persistent reductions in pain, lancinating pain, and allodynia for 24 weeks in IT group (P < 0.005). | ||||||||
| Kotani et al.[6] | RCT, blinded | Intractable PHN (pain > 1 year) | MP-lidocaine, lidocaine, and no treatment | 89, 91, 90 | IT | 3 mL of 3% lidocaine, 60 mg of MP (QW*4) | ≥50% global pain relief. Greater improvement in the severity of burning and lancinating pain, allodynia, and areas of maximal pain and allodynia in the MP-lidocaine group for 2 years (P < 0.001). | Supported by a grant-in-aid for Scientific Research No. 08457399 from the Ministry of Education, Tokyo, Japan. |
| Rijsdijk et al.[7] | RCT | Intractable PHN (pain > 6 months), VAS score ≥ 4 | MP-lidocaine and lidocaine alone | 6, 4 | IT | MP 60 mg, lidocaine 60 mg, or lidocaine 60 mg only (QW*4) | VAS scores for global pain and lancinating pain decreased significantly in lidocaine group. Analgesic use unchanged. *The trial was stopped because of safety concerns and futility of IT MP | None |
IT = intrathecal, MP = methylprednisolone, NS = normal saline, QOW*4 = once every 2 weeks for four times
Intercostal neurolysis for PHN
The intercostal nerves were chosen as the target of treatment of PHN as peripheral nerve sensitization is important to central nerve sensitization in neuropathic pain. Peripheral nerve electricity modulation can reduce allodynia for a long time.[10] Tactile brush stimulation in the peripheral allodynia areas has been shown to reduce pain by more than 30% and last for several days. But there is a paucity of literature for intercostal nerve ablation or neurolysis in managing PHN. Isolated case reports of paraplegia after use of intercostal neurolysis in patients of cancer with thoracic wall pain have been reported. Postulated mechanism for paraplegia could be from phenol diffusing along either the spinal nerves or the paravertebral venous plexus into the subarachnoid space.[11] Pneumothorax has been reported in 14 of 161 patients undergoing intercostal nerve block in a study by Shanti et al.;[12] various studies[13] on intercostal neurolysis for PHN are summarized in Table 3.
Table 3.
Studies using intercostal nerve chemical neurolysis and epidural steroid injections
| Authors | Study design | Inclusion criteria | Route | N | Dose | Outcome |
|---|---|---|---|---|---|---|
| Jayaraman and Parthasarathy[13] | Observational study | PHN > 3 months | Intercostal 50% alcohol under USG guidance | 6 | 8 mL of the mixture of 4 mL of 0.5% bupivacaine with 4 mL of 100% alcohol | VAS scores came down to near 1 or 2 in all the cases. The reduction in pain scores persisted for 3 months. |
| Ghanavatian et al.[16] | Retrospective analysis | PHN | Epidural steroid injection 41 interlaminar and 1 transforaminal | 42 | Not mentioned | 24 of 42 patients had a moderate-to-good pain relief 2 weeks after ESI, 19 patients (79%) had persistent relief after 12 weeks. |
| PHN duration <11 months was predictive of moderate-to-good pain relief at 12 weeks’ post-ESI, with a positive predictive value of 55.2%. |
Epidural steroid injection
It is not clear whether interlaminar to transforaminal epidural steroid injection (ESI) can make any difference as far as duration of pain relief is concerned. In transforaminal approach,[14] drug is deposited close to the site of inflammation of the targeted DRG and spinal nerve, thereby possibly providing the greatest potential for benefit with limited systemic impact.[15] Various studies[16] on interlaminar/transforaminal ESI for PHN are summarized in Table 3.
Pulsed RFA of intercostal nerves and DRG
The thoracic nerves (T1-T12) are the most common region affected in PHN with an incidence of up to 50%. The most common targets of pulsed radiofrequency (PRF) treatment are the segmental DRG responsible for the pain. PRF is a novel therapeutic strategy that has recently been used by pain practitioners as a non/minimally neuroablative technique, where short bursts of high-frequency current are applied to nervous tissue. PRF is known to be effective in short- or long-term pain relief of cervical or lumbar postoperative pain and PHN.[17,18,19,20,21,22] Various studies[17,23,24,25,26] on PRF of DRG for PHN are summarized in Table 4.
Table 4.
Studies using pulsed-radiofrequency ablation of intercostal nerves and dorsal root ganglion
| Study design | Inclusion criteria | Groups | N | Route of administration | Dose | Outcome | Funding | |
|---|---|---|---|---|---|---|---|---|
| Kim et al.[17] | Observational | PHN refractory to conservative treatment | 49 | Pulsed DRG fluoroscopic guidance | PRF was performed three times adjacent to the DRG of corresponding levels under the fluoroscopic guidance. | Excellent pain relief (about 55%) at 4 weeks after PRF lesioning and maintained at subsequent 12-week follow-up. | ||
| Ke et al.[23] | Double-blinded RCT | Refractory PHN > 6 months, VAS score > 3 | PRF or sham group | 48, 48 | Fluoroscopy-guided, intercostal nerve-pulsed RFA | 42°C, 120s, twice for the same level every weekly for 3 weeks | Greater improvement of VAS for 6 months (P < 0.0001) and SF-36 score (P < 0.05), and lesser analgesic dosage (P < 0.001) in PRF group. | There was no external funding. Conflict of interest: none. |
| Saxena et al.[24] | Double-blinded RCT | PHN, VAS score ≥ 3, T3-T11 | PRF plus PO pregabalin or PO pregabalin | 30, 30 | Fluoroscopy-guided in three consecutive intercostal spaces | 42°C, 180s, QOW | Higher efficacy rate in PRF group at 1 week, persisted for 4 weeks (P < 0.001). Greater improvements in VAS, NRS sleep, and GPE scores at 2 weeks in PRF group. | The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. |
| Wang et al.[25] | Single-blinded RCT | PHN > 3 months, VAS score > 5, thoracic to lumbar | PRF plus PO medication or PO medication only | 30, 30 | Ultrasound-guided, lesion in one segment and adjacent DRG | 42°C, 180s, twice | Greater pain relief and lower SF-MPQ scores in PRF group (P = 0.001). | No mention of financial disclosure on site |
| Kim et al.[26] | Retrospective study | Zoster group vs. PHN group | 29 , 29 | Fluoroscopic-guided pulsed DRG RFA | It was followed for 3 months after doing pulsed RFA. | The degree of pain reduction was significantly higher in the early PRF group. Moreover, more patients discontinued their medication in the early PRF group. |
DRG = dorsal root ganglion, GPE = global perceived effect, NRS = numeric rating scale, PO = orally, PSQI score = Pittsburgh Sleep Quality Index, QW*3 = weekly for 3 times, QOW = once every 2 weeks, SF-MPQ = Short-Form McGill Pain Questionnaire
Paravertebral steroid injection and chemical neurolysis
Paravertebral block, a common alternative to epidural injection, is more beneficial for patients in whom pain is unilateral and involves a limited number of spinal segments. Literature on use of neurolytic paravertebral block in managing intractable cancer pain is in abundance but scarce in patients with PHN.[27,28,29] Phenol or alcohol may be used for neurolysis. If diagnostic paravertebral block provides good short duration pain relief, chemical paravertebral neurolysis can be used to achieve long-tern pain relief. Various studies[30] on paravertebral interventions for PHN are summarized in Table 5.
Table 5.
Studies using paravertebral steroid injection/chemical neurolysis; stellate ganglion blocks; and RFA for PHN
| Study | Interventional technique | Study design | Inclusion group | Method | Observations | Affiliation or disclosure |
|---|---|---|---|---|---|---|
| Naja et al.[30] | Paravertebral block | Case report | Mainly T1 extending to T4 | Single injection at T1-T2 levels paravertebral catheter placement at T3 level. Bupivacaine 0.5% (19 mL) and clonidine 150 μg/mL (1 mL); repeated injection of same solution QOD for 3 weeks. | Before: VAS 7–8/10 with mood changes and sleep disturbance even while using ACT, NSAIDs, tramadol, carbamazepine 1200 mg/day, amitriptyline 30 mg/day, and gabapentin 3600 mg/day. After: pain free during an 8-month follow-up period. | Nil |
| Ding et al.[33] | SGB and SG-pulsed RFA followed for 6 months duration | RCT total 84, 48 each in two groups | PHN on the face or upper limbs more than 1 month duration | CT-guided SGB vs. SG-pulsed RFA, only once | VAS decreased in both groups (P < 0.05). In the SG-B group, VAS increased after 1 month, whereas in the SG-P group, VAS gradually decreased at later follow-up time points. VAS decreased more significantly in the SG-P group (P < 0.05). The total effective rates of the SG-B and SG-P groups were 64.3 and 83.3%, respectively. | This study was supported by the Natural Science Foundation of Liaoning Province (No. 20170541032) and Shenyang Young and Middle-aged Science and Technology Innovation Talent Support Plan (No. RC170045). |
| Milligan and Nash[34] | SGB | Observational study, 34 had PHN more 1 year duration and 8 had PHN < 1 year duration | PHN of thoracic, cervical, or trigeminal distribution. | 65 SGB done on 42 patients. | Landmark-based SGB: anterior paratracheal approach to the ganglion was used, and 10 mL 0.25% bupivacaine injected. | 12 (29%) showed no improvement, 11 (26%) were improved, and 19 (45%) had a good result. Patients with PHN < 1-year duration had better results. |
| One patient received nine blocks. |
ACT = acetaminophen, QOD = once every 2 days
Stellate ganglion blocks and RFA
Role of sympathetic nervous system in pathophysiology of chronic pain is well known. There is abnormal activation of adrenergic receptors in primary afferent neutrons and direct interaction between primary afferent and efferent sympathetic nerves due to collateral sprouting after a nerve injury or tissue trauma.[31] Some data suggest a link between sympathetic activity and pain in PHN, as patients with PHN show increased pain and worsening allodynia after local administration of adrenergic agonists.[32] Thus, administration of sympathetic nerve blocks may theoretically interrupt the sympathetic–sensory interactions, giving pain relief. Studies[33,34] related to stellate ganglion block (SGB) in PHN are listed in Table 5.
Role of spinal cord stimulator
Spinal cord stimulator works on the principle of “Gate control theory” given by Wall and Melzack. Stimulating large diameter A-α and A-β neurons, inhibits the pain signal transmission carried by C-fibers. This suggests that electrical spinal cord stimulation could reasonably modulate pain. Spinal cord stimulation may also affect the levels of γ-aminobutyric acid and adenosine in the dorsal horn and may consequently reduce neuropathic pain.[35] The first human trial of electrical spinal cord stimulation as a neuromodulatory method for treating pain was conducted in 1967.[36] Currently, this modality is applied for analgesia for complex regional pain syndrome, brachial plexus injury, refractory chronic limb ischemia–related pains, and failed back surgery syndrome.
Studies suggest that patients with pain and allodynia caused by central sensitization and those with preserved neuronal and dorsal column function would respond well to spinal cord stimulation. By contrast, patients with marked sensory loss and those experiencing constant pain without allodynia would not benefit from spinal cord stimulation, as deafferentation and degeneration of the dorsal column might be the dominant mechanism.[37] It is therefore important to select patients who are mechanistically more likely to respond to spinal cord stimulation to achieve better pain relief. The various studies[38,39,40,41,42,43] are summarized in Table 6.
Table 6.
Studies in which PHN was treated with spinal cord stimulation
| Study | Study design | N | Localization | Setting of stimulation | Outcome |
|---|---|---|---|---|---|
| Iseki et al.[38] | Case series (subacute herpes zoster, 2 months) | 2 | T3-T4 (responsive to CEB, barbiturate, and ketamine; refractory to lidocaine and morphine) →spinal cord to central nerve level | Pulse width: 210ms; frequency: 15 Hz; current: 3 V (lead placement: tip at T1, end at T3) | Before: VAS scores 8/10, gabapentin 600 mg/day, amitriptyline 10 mg/day with drowsiness. After 1-week treatment: VAS 1–2/10 (discharge with gabapentin 300 mg/day and amitriptyline 10 mg/day; gabapentin 300 mg/day 1 year later). |
| Harke et al.[39] | Case series (patients with preserved sensory function) | Range from C2-S1 | Pulse width: 90–450ms, frequency: 50–130 Hz, current: 1–6 V | Before: VAS scores, 7–10/10. After: Long-term responders: VAS, 1/10 until 29-month follow-up (23 of 28), normalized PDI scores, completely removed/significantly reduced analgesic, corticosteroid, and anticonvulsant use. Short-term responders: average VAS 1 → 7 after 8 months (5 of 28). | |
| Moriyama[40] | Case series (refractory even to CEB) | 14 | Range from T3-T10 | Initial: pulse width, 210 μs; frequency, 50 Hz; current, 3.8–8.2 V. Adjustment: pulse width, 450 μs; frequency, 20–80 Hz; current, 3.8–8.2 V. | Before: VAS scores 60–100 (mean = 89). After CEB: VAS 34–100 (mean = 68). After SCS: VAS 0–48 (mean = 14), opioid cessation. Adverse effects: hypotension (3 of 14), ischuria (7 of 14). |
| Baek et al.[41] | Case series | 11 | Range from C5-L2 (those with permanent SCS: C5-L1) | Those with > 50% reduction in pain receive permanent SCS (4 of 11) | Before: VAS score 8/10. After permanent SCS: VAS 1.5–2.9/10. |
| Yanamoto and Murakawa[42] | Case series | 33 | (cervical: 5; thoracic: 28) | — | Before: average VAS score of 6.8. After: average VAS 3.8 (63.3% persisted > 6 months). |
| Liu et al.[43] | Case series | 6 | Range from T6-T12 | DREZotomy after confirmed SCS for 1 week. SCS settings: 50–150 Hz, 2.8–5.4 V, 150–500 μs. | Before: average VAS score 8.4. After: average VAS 2.4 (persists for 1 year). |
CEB = continuous epidural blocks, DREZotomy = dorsal root entry zone lesion, PDI = pain disability index, SCS = spinal cord stimulation
DISCUSSION
The pathophysiology of PHN is not fully understood yet. A subacute or chronic inflammatory process involving spinal cord may play a role in pathogenesis of PHN. Peripheral sensitization develops because of inflammatory mediators, such as substance-P, histamine, and cytokines that reduce the stimulus threshold of nociceptors. Levels of IL-8 are increased initially but as the duration of PHN increase the levels go down, suggesting that gradually the inflammation resolves spontaneously and other mechanisms start to play a role. The swelling accompanying the inflammation can compress the sensory ganglion in the intervertebral foramen, resulting in ischemia and neuronal tissue damage. As the chronicity increase, deafferentation develops primary afferent neural body and axon degeneration, spinal cord atrophy, scarring of the DRG, and loss of epidermal innervation.[44] These changes contribute to increased N-methyl-D-aspartate (NMDA) receptor–dependent excitability of spinal DRG. Central sensitization is related to an increasingly stronger response from nerve cells in the occipital horn to continuous stimulation by nociceptive C-fibers.
IPM can provide excellent pain relief and improve the quality of life of patients with recalcitrant PHN. At present, there is a paucity of good quality RCTs for each intervention as PHN cases are sporadic, hence performing an RCT is also sometimes difficult unless the dermatologist/pain physicians are aware about interventional treatments, and they regularly start referring such patients to interventional pain specialists.
Intrathecal MP use was found useful in initial studies by Kotani et al.[6] but the results were not reproducible in further studies and there were serious safety concerns. Even in the past, intrathecal MP use was largely abandoned after Nelson and Landau reported serious neurologic complications, including adhesive arachnoiditis and meningitis;[45,46] we infer that intrathecal MP lacked evidence to support its use in PHN.
Intercostal neurolysis seems to be a safe and cost-effective method for long-term pain relief in PHN. Intercostal nerve chemical neurolysis has been carried out for numerous painful conditions successfully, such as intercostal neuralgia,[47] intractable cancer-associated chest wall pain,[48] and postsurgical thoracic wall pain with excellent results, but studies on PHN are scarce. Only one observational study performed on six patients with PHN showed excellent pain relief till 3 months of follow-up.[13] The incidence of procedure-related risks such as pneumothorax and chemical neuritis is very low with this intervention.
Transforaminal and interlaminal ESI work on the principle of steroid application close to DRG and spinal nerves, thereby reducing the inflammation at these targets. Most studies have shown the success of ESI when they are given before 11 months of onset of rash probably because as the duration of PHN increases the inflammation subsides on its own and other aspects of pathophysiology such as axon degeneration, spinal cord atrophy, and DRG scarring starts to play the major role. Case reports have been published describing the application of ESI in HZ and PHN.[16]
Thoracic paravertebral block and neurolysis can be one of the good interventions but till date literature on its use in PHN is lacking. With single paravertebral block, we can target multiple intercostal nerves, DRG, and the thoracic sympathetic chain, and thus its works on different targets. Whether doing it fluoroscopic guided or sonography guided is an individual choice, but under fluoroscopic guidance, dye spread is visualized and spread of neurolytic agent can be seen as well. Major risk is pneumothorax as pleura is closer, and some isolated case reports have also mentioned paraplegia due to spread of neurolytic agent into the intrathecal space.
SGBs with local aesthetics or its pulsed RFA have been used successfully for PHN of face, cervical, and upper limb distribution, but effectiveness is more and long term if duration of PHN is less than 1 year.[33,34,49]
Spinal cord stimulation is used to treat unendurable PHN both in the subacute and chronic stages. For subacute PHN, temporary stimulation provided for 7–10 days to a median of 2.5 months yielded immediate and persistent pain relief for >1 year.[38,39] For patients with chronic PHN, permanent device placement is always conducted following a successful temporary trial. The adverse effects related to spinal cord stimulation include hypotension (21%) and ischuria (33%).[40]
Strength of evidence of each interventional procedure is summarized in Table 7.
Table 7.
Strength of evidence
| Intervention | Strength of evidence |
|---|---|
| 1. Intrathecal methylprednisolone | Very low |
| 2. Intercostal nerve chemical neurolysis | Low |
| 3. Epidural steroid injection | Low |
| 4. Pulsed RFA for intercostal nerve and dorsal root ganglion | Moderate |
| 5. Paravertebral steroid injection and chemical neurolysis | Low |
| 6. Stellate ganglion blocks and RFA | Moderate |
| 7. Spinal cord stimulation | Low |
Definitions of grades of evidence:
High = further research is unlikely to change our confidence in the estimate of effect, moderate = further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate, low = further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate, very low = any estimate of effect is very uncertain
Our systematic review has some limitations. Some of the interventions, such as Botox therapy or acupuncture or trigger point injections, were not included in our analysis. There is a paucity of literature for some of the key interventions carried out in PHN. For some of the interventions, there are only case reports or case series so the strength of evidence is not strong. For some of the interventions for which RCTs are carried out, the results were not reproducible, reducing the strength of evidence for them. The numbers of participants in the available studies are small.
CONCLUSION
PHN remains a potentially debilitating and undertreated form of neuropathic pain. With the advent of IPM options, one can provide effective and long-lasting pain relief to patients not responding to medical management. The choice of intervention will depend on the region involved, cost, and invasiveness. Procedures such as intercostal nerve blocks/neurolysis, SGBs, paravertebral neurolysis, ESIs, and DRG RFA are effective interventions; however, if they fail, spinal cord stimulators could be effective in the hands of experience pain physicians.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002;18:350–4. doi: 10.1097/00002508-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Johnson RW, Alvarez-Pasquin MJ, Bijl M, Franco E, Gaillat J, Clara JG, et al. Herpes zoster epidemiology, management, and disease and economic burden in Europe: a multidisciplinary perspective. Ther Adv Vaccines. 2015;3:109–20. doi: 10.1177/2051013615599151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gialloreti LE, Merito M, Pezzotti P, Naldi L, Gatti A, Beillat M, et al. Epidemiology and economic burden of herpes zoster and post-herpetic neuralgia in Italy: a retrospective, population-based study. BMC Infect Dis. 2010;10:230. doi: 10.1186/1471-2334-10-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weitzman D, Shavit O, Stein M, Cohen R, Chodick G, Shalev V. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013;67:463–9. doi: 10.1016/j.jinf.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Shrestha M, Chen A. Modalities in managing postherpetic neuralgia. Korean J Pain. 2018;31:235–43. doi: 10.3344/kjp.2018.31.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotani N, Kushikata T, Hashimoto H, Kimura F, Muraoka M, Yodono M, et al. Intrathecal methylprednisolone for intractable postherpetic neuralgia. N Engl J Med. 2000;343:1514–9. doi: 10.1056/NEJM200011233432102. [DOI] [PubMed] [Google Scholar]
- 7.Rijsdijk M, van Wijck AJ, Meulenhoff PC, Kavelaars A, van der Tweel I, Kalkman CJ. No beneficial effect of intrathecal methylprednisolone acetate in postherpetic neuralgia patients. Eur J Pain. 2013;17:714–23. doi: 10.1002/j.1532-2149.2012.00233.x. [DOI] [PubMed] [Google Scholar]
- 8.Rijsdijk M, Svensson CI, Wijck AJ, Kalkman CJ, Yaksh TL. Analgesic properties of intrathecal glucocorticoids in three well established preclinical pain models. Scand J Pain. 2016;10:90–102. doi: 10.1016/j.sjpain.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Kikuchi A, Kotani N, Sato T, Takamura K, Sakai I, Matsuki A. Comparative therapeutic evaluation of intrathecal versus epidural methylprednisolone for long-term analgesia in patients with intractable postherpetic neuralgia. Reg Anesth Pain Med. 1999;24:287–93. doi: 10.1016/s1098-7339(99)90101-3. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson HJ, Schouenborg J. Differential inhibitory effect on human nociceptive skin senses induced by local stimulation of thin cutaneous fibers. Pain. 1999;80:103–12. doi: 10.1016/s0304-3959(98)00205-x. [DOI] [PubMed] [Google Scholar]
- 11.Kim BH, No MY, Han SJ, Park CH, Kim JH. Paraplegia following intercostal nerve neurolysis with alcohol and thoracic epidural injection in lung cancer patient. Korean J Pain. 2015;28:148–52. doi: 10.3344/kjp.2015.28.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanti CM, Carlin AM, Tyburski JG. Incidence of pneumothorax from intercostal nerve block for analgesia in rib fractures. J Trauma. 2001;51:536–9. doi: 10.1097/00005373-200109000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Jayaraman G, Parthasarathy S. Evaluation of efficacy of ultrasound guided, alcohol based, neurolytic intercostal blocks in post herpetic neuralgia—an observational study. Eur J Biomed Pharma Sci. 2018;5:313–5. [Google Scholar]
- 14.Zheng P, Schneider BJ, Kennedy DJ, McCormick ZL. Safe injectate choice, visualization, and delivery for lumbar transforaminal epidural steroid injections: evolving literature and considerations. Curr Phys Med Rehabil Rep. 2019;7:414–21. [Google Scholar]
- 15.Opstelten W, van Wijck AJ, Moons KG, Essen GA, Stolker RJ, Kalkman CJ, et al. Treatment of patients with herpes zoster by epidural injection of steroids and local anaesthetics: less pain after 1 month, but no effect on long-term postherpetic neuralgia—a randomised trial. Ned TijdschrGeneeskd. 2006;150:2649–55. [PubMed] [Google Scholar]
- 16.Ghanavatian S, Wie CS, Low RS, Butterfield RJ, Zhang N, Dhaliwal GS, et al. Parameters associated with efficacy of epidural steroid injections in the management of postherpetic neuralgia: the Mayo Clinic experience. J Pain Res. 2019;12:1279–86. doi: 10.2147/JPR.S190646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YH, Lee CJ, Lee SC, Huh J, Nahm FS, Kim HZ, et al. Effect of pulsed radiofrequency for postherpetic neuralgia. Acta Anaesthesiol Scand. 2008;52:1140–3. doi: 10.1111/j.1399-6576.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- 18.Choi GS, Ahn SH, Cho YW, Lee DG. Long-term effect of pulsed radiofrequency on chronic cervical radicular pain refractory to repeated transforaminal epidural steroid injections. Pain Med. 2012;13:368–75. doi: 10.1111/j.1526-4637.2011.01313.x. [DOI] [PubMed] [Google Scholar]
- 19.Munglani R. The longer term effect of pulsed radiofrequency for neuropathic pain. Pain. 1999;80:437–9. doi: 10.1016/s0304-3959(98)00183-3. [DOI] [PubMed] [Google Scholar]
- 20.Guo L, Kubat NJ, Nelson TR, Isenberg RA. Meta-analysis of clinical efficacy of pulsed radio frequency energy treatment. Ann Surg. 2012;255:457–67. doi: 10.1097/SLA.0b013e3182447b5d. [DOI] [PubMed] [Google Scholar]
- 21.Tamimi MA, McCeney MH, Krutsch J. A case series of pulsed radiofrequency treatment of myofascial trigger points and scar neuromas. Pain Med. 2009;10:1140–3. doi: 10.1111/j.1526-4637.2009.00646.x. [DOI] [PubMed] [Google Scholar]
- 22.Cahana A, Van Zundert J, Macrea L, van Kleef M, Sluijter M. Pulsed radiofrequency: current clinical and biological literature available. Pain Med. 2006;7:411–23. doi: 10.1111/j.1526-4637.2006.00148.x. [DOI] [PubMed] [Google Scholar]
- 23.Ke M, Yinghui F, Yi J, Xeuhua H, Xiaoming L, Zhijun C, et al. Efficacy of pulsed radiofrequency in the treatment of thoracic postherpetic neuralgia from the angulus costae: a randomized, double-blinded, controlled trial. Pain Physician. 2013;16:15–25. [PubMed] [Google Scholar]
- 24.Saxena AK, Lakshman K, Sharma T, Gupta N, Banerjee BD, Singal A. Modulation of serum BDNF levels in postherpetic neuralgia following pulsed radiofrequency of intercostal nerve and pregabalin. Pain Manag. 2016;6:217–27. doi: 10.2217/pmt.16.3. [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Zhang K, Han S, Yu L. Painvision® apparatus for assessment of efficacy of pulsed radiofrequency combined with pharmacological therapy in the treatment of postherpetic neuralgia and correlations with measurements. Biomed Res Int. 2017;2017:5670219. doi: 10.1155/2017/5670219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim K, Jo D, Kim E. Pulsed radiofrequency to the dorsal root ganglion in acute herpes zoster and postherpetic neuralgia. Pain Physician. 2017;20:E411–8. [PubMed] [Google Scholar]
- 27.Antila H, Kirvelä O. Neurolytic thoracic paravertebral block in cancer pain. A clinical report. Acta Anaesthesiol Scand. 1998;42:581–5. doi: 10.1111/j.1399-6576.1998.tb05170.x. [DOI] [PubMed] [Google Scholar]
- 28.Malik T. Ultrasound-guided paravertebral neurolytic block: a report of two cases. Pain Pract. 2014;14:346–9. doi: 10.1111/papr.12072. [DOI] [PubMed] [Google Scholar]
- 29.Richardson J, Sabanathan S. Thoracic paravertebral analgesia. Acta Anaesthesiol Scand. 1995;39:1005–15. doi: 10.1111/j.1399-6576.1995.tb04219.x. [DOI] [PubMed] [Google Scholar]
- 30.Naja ZM, Maaliki H, Al-Tannir MA, El-Rajab M, Ziade F, Zeidan A. Repetitive paravertebral nerve block using a catheter technique for pain relief in post-herpetic neuralgia. Br J Anaesth. 2006;96:381–3. doi: 10.1093/bja/ael007. [DOI] [PubMed] [Google Scholar]
- 31.Wu CL, Marsh A, Dworkin RH. The role of sympathetic nerve blocks in herpes zoster and postherpetic neuralgia. Pain. 2000;87:121–9. doi: 10.1016/S0304-3959(00)00230-X. [DOI] [PubMed] [Google Scholar]
- 32.Choi B, Rowbotham MC. Effect of adrenergic receptor activation on post-herpetic neuralgia pain and sensory disturbances. Pain. 1997;69:55–63. doi: 10.1016/s0304-3959(96)03245-9. [DOI] [PubMed] [Google Scholar]
- 33.Ding Y, Yao P, Li H, Han Z, Wang S, Hong T, et al. CT-guided stellate ganglion pulsed radiofrequency stimulation for facial and upper limb postherpetic neuralgia. Front Neurosci. 2019;13:170. doi: 10.3389/fnins.2019.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milligan NS, Nash TP. Treatment of post-herpetic neuralgia. A review of 77 consecutive cases. Pain. 1985;23:381–6. doi: 10.1016/0304-3959(85)90008-9. [DOI] [PubMed] [Google Scholar]
- 35.Oakley JC, Prager JP. Spinal cord stimulation: mechanisms of action. Spine (Phila Pa 1976) 2002;27:2574–83. doi: 10.1097/00007632-200211150-00034. [DOI] [PubMed] [Google Scholar]
- 36.Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967;46:489–91. [PubMed] [Google Scholar]
- 37.Dones I, Levi V. Spinal cord stimulation for neuropathic pain: current trends and future applications. Brain Sci. 2018;8:138. doi: 10.3390/brainsci8080138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iseki M, Morita Y, Nakamura Y, Ifuku M, Komatsu S. Efficacy of limited-duration spinal cord stimulation for subacute postherpetic neuralgia. Ann Acad Med Singapore. 2009;38:1004–6. [PubMed] [Google Scholar]
- 39.Harke H, Gretenkort P, Ladleif HU, Koester P, Rahman S. Spinal cord stimulation in postherpetic neuralgia and in acute herpes zoster pain. Anesth Analg. 2002;94:694–700; table of contents. doi: 10.1097/00000539-200203000-00040. [DOI] [PubMed] [Google Scholar]
- 40.Moriyama K. Effect of temporary spinal cord stimulation on postherpetic neuralgia in the thoracic nerve area. Neuro-modulation. 2009;12:39–43. doi: 10.1111/j.1525-1403.2009.00186.x. [DOI] [PubMed] [Google Scholar]
- 41.Baek IY, Park JY, Kim HJ, Yoon JU, Byoen GJ, Kim KH. Spinal cord stimulation in the treatment of postherpetic neuralgia in patients with chronic kidney disease: a case series and review of the literature. Korean J Pain. 2011;24:154–7. doi: 10.3344/kjp.2011.24.3.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanamoto F, Murakawa K. The effects of temporary spinal cord stimulation (or spinal nerve root stimulation) on the management of early postherpetic neuralgia from one to six months of its onset. Neuromodulation. 2012;15:151–4. doi: 10.1111/j.1525-1403.2012.00438.x. [DOI] [PubMed] [Google Scholar]
- 43.Liu MX, Zhong J, Zhu J, Xia L, Dou NN. Treatment of postherpetic neuralgia using DREZotomy guided by spinal cord stimulation. Stereotact Funct Neurosurg. 2015;93:178–81. doi: 10.1159/000375174. [DOI] [PubMed] [Google Scholar]
- 44.Head H, Campbell AW, Kennedy PG. The pathology of herpes zoster and its bearing on sensory localisation. Rev Med Virol. 1997;7:131–43. doi: 10.1002/(sici)1099-1654(199709)7:3<131::aid-rmv198>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 45.Nelson DA, Landau WM. Intraspinal steroids: history, efficacy, accidentality, and controversy with review of United States Food and Drug Administration reports. J Neurol Neurosurg Psychiatry. 2001;70:433–43. doi: 10.1136/jnnp.70.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlatter J, Nguyen D, Zamy M, Kabiche S, Fontan JE, Cisternino S. Safety of intrathecal route: focus to methylprednisolone acetate (depo-medrol) use. Eur Spine J. 2019;28:21–30. doi: 10.1007/s00586-017-5387-x. [DOI] [PubMed] [Google Scholar]
- 47.Kang J, Liu Y, Niu L, Wang M, Meng C, Zhou H. Anesthesia upstream of the alcoholic lesion point alleviates the pain of alcohol neurolysis for intercostal neuralgia: a prospective randomized clinical trial. Clinics (Sao Paulo) 2020;75:e1296. doi: 10.6061/clinics/2020/e1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matchett G. Intercostal nerve block and neurolysis for intractable cancer pain. J Pain Palliat Care Pharmacother. 2016;30:114–7. doi: 10.3109/15360288.2016.1167804. [DOI] [PubMed] [Google Scholar]
- 49.Malec-Milewska M, Sękowska A, Kolęda I, Horosz B, Guć M, Jastrzębski J. Sympathetic nerve blocks for the management of postherpetic neuralgia—19 years of pain clinic experience. Anaesthesiol Intensive Ther. 2014;46:255–61. doi: 10.5603/AIT.a2014.0039. [DOI] [PubMed] [Google Scholar]