Figure 3.
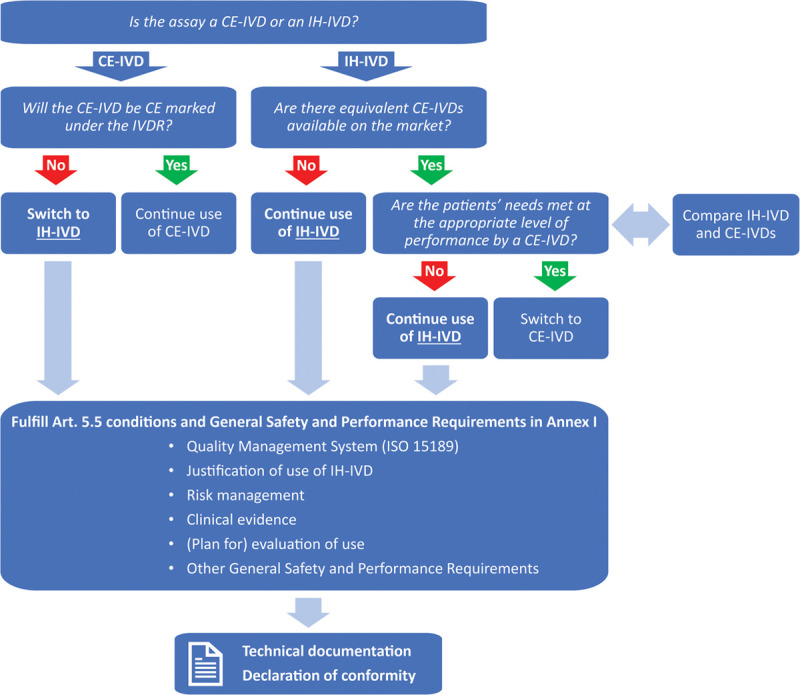
Assay inventory and IVDR compliance workflow. The assay inventory decision tree allows laboratories to plan their assay portfolio under the IVDR based on the tests that are currently implemented. Key questions are whether current CE-IVD tests will be CE marked under the IVDR (for the same intended purpose) and whether appropriate alternatives for in-house devices (IH-IVDs) are available (see also the discussion on IVDR Art. 5(5)d,e). Subsequently, for all IH-IVDs that are used after May 2022, the conditions and requirements for IH-IVDs as dictated by the IVDR should be fulfilled. The most important requirements are listed above and are discussed in more detail in the main text. Finally, fulfillment of the requirements should be documented in the technical documentation and publicly stated in the declaration of conformity. CE-IVDs = CE marked in vitro diagnostic medical devices; IH-IVDs = in-house in vitro diagnostic medical devices; IVDR = Regulation (EU) 2017/746 on in vitro diagnostic medical devices.
