Abstract
Background:
Reduced cholesterol levels are associated with increased organ failure and mortality in sepsis. Cholesterol levels may vary by infection type (gram negative vs positive), possibly reflecting differences in cholesterol-mediated bacterial clearance.
Methods:
This was a secondary analysis of a combined data set of 2 prospective cohort studies of adult patients meeting Sepsis-3 criteria. Infection types were classified as gram negative, gram positive, or culture negative. We investigated quantitative (levels) and qualitative (dysfunctional high-density lipoprotein [HDL]) cholesterol differences. We used multivariable logistic regression to control for disease severity.
Results:
Among 171 patients with sepsis, infections were gram negative in 67, gram positive in 46, and culture negative in 47. Both gram-negative and gram-positive infections occurred in 11 patients. Total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and HDL cholesterol (HDL-C) levels were lower for culture-positive sepsis at enrollment (TC, P < .001; LDL-C, P < .001; HDL-C, P = .011) and persisted after controlling for disease severity. Similarly, cholesterol levels were lower among culture-positive patients at 48 hours (TC, P = .012; LDL-C, P = .029; HDL-C, P = .002). Triglyceride (TG) levels were lower at enrollment (P =.033) but not at 48 hours (P = .212). There were no differences in dysfunctional HDL. Among bacteremic patients, cholesterol levels were lower at enrollment (TC, P = .010; LDL-C, P = .010; HDL-C, P .001; TG, P = .005) and at 48 hours (LDL-C, P = .027; HDL-C, P < .001; TG, P = .020), except for 48 hour TC (P = .051). In the bacteremia subgroup, enrollment TC and LDL-C were lower for gram-negative versus gram-positive infections (TC, P = .039; LDL-C, P = .023).
Conclusion:
Cholesterol levels are significantly lower among patients with culture-positive sepsis and bacteremia.
Keywords: organ failure, gram negative, gram positive, culture positive, lipids
Introduction
Preclinical data suggest cholesterol has protective functions in patients with sepsis. Both high-density and low-density lipoprotein cholesterol (HDL-C, LDL-C) can bind and clear bacterial toxins and transport cholesterol to the adrenal glands for steroid synthesis.1,2 Reduced lipoprotein cholesterol levels are associated with mortality and early organ failure in patients with sepsis.3–6 Functional lipoprotein changes also occur. High-density lipoprotein cholesterol (HDL-C) has anti-inflammatory properties that become altered, rendering it dysfunctional and pro-inflammatory (dysfunctional HDL [Dys-HDL]).1,3,7–11 Increased dysfunctional lipoprotein levels in early sepsis predict organ failure.3,10
Both HDL-C and LDL-C facilitate bacterial toxin clearance and may serve as a pathogen “lipid sink.”12 Lipoproteins bind and neutralize both gram-positive and gram-negative endotoxins; however, this may be particularly important in gram-negative sepsis.1,2,13,14 Although the mechanisms have not been precisely elucidated, both LDL-C and HDL-C are dynamically regulated in the septic state (Figure 1). Animal models suggest lipoprotein-mediated bacterial recognition and clearance has a higher affinity for gram-negative endotoxin, whether this occurs in human sepsis is less clear.2,13–15
Figure 1.
At a glance—Lipoprotein-mediated mechanisms of bacterial toxin clearance and defense against sepsis.
Sepsis-induced lipoprotein dysregulation occurs frequently, and lipid metabolic pathways may serve as targets for novel therapies.16–18 However, results of previous interventional trials have failed to show a mortality benefit. This may be due to the heterogeneity of the sepsis population or insufficiently powered studies in the setting of lower than expected mortality rates.16,17 However, there may be a cohort that is more responsive to these therapies. Some data suggest that the degree of cholesterol dysregulation may vary by bacterial infection type.2,13–15,19 Thus, there may be patient or pathogen factors that predict responsiveness to lipid-targeted treatments. The long-term goal of this work is to better understand the role of cholesterol in bacterial pathogen clearance and to inform future novel sepsis therapies.
The objective of this study was to determine differences in lipoprotein cholesterol levels among gram-negative, gram-positive, and culture-negative sepsis. We also sought to report qualitative (functional and oxidative) differences in HDL-C by bacterial infection type. We hypothesized that total cholesterol, HDL-C, and LDL-C levels would be significantly lower in gram-negative sepsis compared with gram-positive and culture-negative sepsis.
Methods
Patient Selection
This was a secondary analysis of a combined data set of 2 prospective studies both investigating the association between Dys-HDL and organ failure severity in sepsis.3,10,11 The study period spanned from February 2015 through June 2018. Consecutive, adult (>18 years) patients meeting criteria were prospectively enrolled within 24 hours of presenting to the UF Health—Jacksonville Emergency Department. Enrollment criteria for both studies included suspected or confirmed infection being treated with an evidence-based sepsis bundle and Sequential Organ Failure Assessment (SOFA) score ≥ 2. Exclusion criteria were (1) pregnancy, (2) incarceration, (3) inability to obtain consent, (4) genetic disorders of lipid metabolism, (5) alterative or confounding diagnosis causing shock, (6) cardiopulmonary resuscitation prior to enrollment, or (7) patients deemed futile care or having advanced directives limiting resuscitative efforts. Data from both prospective studies were combined and analyzed. Adult patients were included if they had (1) suspected infection (2) a primary admission diagnosis of sepsis, and (3) SOFA score 2. Potential patients were excluded if they were < 18 years of age, pregnant, incarcerated, or had a familial disorder of lipid metabolism. Patients were screened and enrolled 7 days a week by an in-house research team.
Outcomes, Study Measures, and Data Collection
Demographic information, comorbidities, vital signs, organ dysfunction severity, and treatment data were prospectively collected at enrollment and at 48 hours. After completion of the study, all cases were manually adjudicated for primary sepsis diagnosis, source of infection, culture results, and infection type. Two authors (L.P.B., F.W.G.) performed 10% overlapping reviews to adjudicate for sepsis and infection type with percent agreements of 94% and 88%, respectively.
Infection Type
For the primary outcome, infection type was determined by reviewing culture results. Blood, urine, respiratory, and wound cultures were evaluated. Blood cultures were considered positive if a pathogenic bacterial species was isolated from at least one blood culture, except for coagulase-negative staphylococcus, which was considered a contaminant unless isolated from 2 blood samples. Urine culture was considered positive if an organism grew out in excess of 105 colony forming units/mL urine. In the setting of a suspected respiratory source of infection, respiratory cultures were considered positive if an organism grew out of an acceptable specimen (greater than 25 leukocytes and fewer than 10 epithelial cells per low power field). Culture data were classified as gram negative, gram positive, or culture negative. Patients with sepsis primarily from fungal organisms or atypical bacteria were excluded from the analysis.
Cholesterol Levels and HDL Function
Serum samples were collected within 24 hours of sepsis recognition (enrollment) and again 48 hours later. Serum total cholesterol, HDL-C, and triglyceride levels were directly measured from serum samples by the UF Health Jacksonville clinical laboratory. Low-density lipoprotein cholesterol was calculated using the Friedewald formula from total cholesterol, HDL-C, and triglyceride, unless triglycerides were > 400 mg/dL, in which case LDL-C was directly measured.20 Dysfunctional HDL was quantified using a cell-free assay and expressed as HDL Inflammatory Index (HII), as previously described.3,10 Briefly, the ability of sample HDL-C to protect LDL-C from oxidation was quantified by a decline in fluorescence from standard values obtained for LDL-C alone; subsequently this value was set at 1.0 for the sample. If sample HDL-C combined with LDL-C resulted in a decline in fluorescence, the HII was less than 1.0, and classified as anti-inflammatory. If sample HDL-C combined with LDL-C resulted in increased fluorescence, corresponding to an HII of 1.0 or greater, HDL-C was classified as pro-inflammatory or Dys-HDL.
Data Analysis
Categorical variables were summarized using counts and percentages and analyzed using χ2 or Fisher exact tests. Continuous variables were summarized using means and standard deviations or medians and interquartile ranges (IQR), based on normality. Analysis of variance or 2-tailed Student’s t test was used for normally distributed data and Wilcoxon rank sum or Kruskal-Wallis tests for nonparametric analyses.
Patients with more than one type of positive cultures (ie, blood and urine) who had discordant gram stain results between culture types were considered to have mixed gram-positive and gram-negative cultures. The cases with mixed cultures were excluded from comparisons between gram-positive, gram-negative, and culture-negative sepsis. Similarly, in the bacteremia subgroup, only patients with one gram stain phenotype were included in comparisons between gram-negative and gram-positive bacteremia. These patients were excluded as the individual effect of each infection type on cholesterol levels could not be clearly discerned. However, the patients with mixed gram-positive and gram-negative cultures were included in culture-positive versus culture-negative analyses as well as bacteremia versus nonbacteremia comparisons. In these latter comparisons, they were included in the culture-positive or bacteremia groups, respectively.
As organ failure severity is related to decreased cholesterol levels in sepsis, significant univariate differences were further evaluated to assess whether observed differences were confounded by differences in organ failure severity. We used multivariable logistic regression to adjust for organ failure severity, quantified as SOFA score, at enrollment or at 48 hours. Additionally, we used multivariable logistic regression to create a predictive model for the association between lipoprotein cholesterol levels and culture-positive sepsis, adjusting for other covariates. The model was created using backward elimination with a P value of .05 considered significant. Candidate predictors included age, race, gender, comorbidities (ie, diabetes, end-stage renal disease, human immunodeficiency virus infection status), organ failure severity, lactate levels, statin therapy, and features of sepsis management (fluid resuscitation, time to antibiotics, time to vasopressor initiation, mechanical ventilation dependence). The final number of candidate predictors was limited to 1 per 10 events per variable to prevent overfitting. Statistical analysis was performed using the Stata statistical software (version 15.1).
Results
Among 171 patients were enrolled, 90 from cohort one and 81 from cohort two; 164 patients had all serum samples available for analysis. Three patients were missing values for total cholesterol and triglyceride levels, and 7 patients were missing LDL-C levels. Thirteen patients did not have laboratory test results or organ severity scores available for 48-hour analysis, predominately due to death. The mean age was 62 years; 96 (56%) were male, 85 (50%) were black, and 84 (49%) were white. The median SOFA scores were 6 (IQR 4–10) at enrollment and 4 (IQR 1–7) at 48 hours. Nearly half (49%) of the patients required vasopressors for cardiovascular failure (n = 84). The overall 28-day mortality rate was 27%, and 42% when confined to those patients requiring vasopressors.
Among all patients, 124 had culture-positive (blood, urine, sputum, or wound) sepsis and 47 had culture-negative sepsis. Of patients with positive cultures, 67 had only gram-negative bacterial cultures, 46 had only gram-positive bacterial cultures, and 11 patients had mixed gram-positive and gram-negative cultures. There were 78 patients who had positive blood cultures, 56 who had positive urine cultures, 14 who had positive wound cultures, and 33 who had positive sputum cultures. Fifty-three patients had more than one type (blood, urine, etc) of positive culture result. In all but 7 of these patients, the gram types isolated were the same across cultures, predominately due to the same gram type growth in multiple cultures. Among bacteremic patients, 29 were gram positive only, 46 were gram negative only, and 3 grew out both gram-positive and gram-negative organisms.
Baseline characteristics comparing patients with gram-positive, gram-negative, and culture-negative sepsis are summarized in Table 1. Disease severity was significantly lower at enrollment as estimated by SOFA score in the culture-negative group, though this difference was no longer significant at 48 hours. The incidence of vasopressor-dependent septic shock also differed significantly between gram-negative, gram-positive, and culture-negative patients with sepsis. Time to antibiotics and volume of intravenous crystalloid did not differ significantly between groups. Patient with gram-positive sepsis required mechanical ventilation more often than those with gram-negative or culture-negative sepsis (Table 2). There were no differences in statin therapy between groups (Table 1). Additionally, in this population of patients with acute sepsis, statin therapy did not significantly impact cholesterol levels (Supplemental Table 1).
Table 1.
Baseline Characteristics.a
| Overall, N = 171b | Gram negative, n = 67 | Gram positive, n = 46 | Culture negative, n = 47 | P Value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years)c | 62(14) | 62 (15) | 65 (13) | 59 (12) | .1152d |
| Sex: male | 96 (56.1) | 36 (53.7) | 26 (56.5) | 27 (57.5) | .916e |
| Female | 75 (43.9) | 31 (46.3) | 20 (43.5) | 20 (42.6) | |
| Race: black | 85 (49.7) | 35 (52.2) | 19 (41.3) | 26 (55.3) | .490e |
| White | 84 (49.1) | 32 (47.8) | 26 (56.5) | 20 (42.6) | |
| Other | 2(1.2) | 0 | 1 (2.17) | 1 (2.13) | |
| Initial vital signs | |||||
| Systolic blood pressure (mm Hg)c | 116 (33) | 111 (30) | 120 (38) | 113 (31) | .3645d |
| Heart rate (beats/min) c | 108 (26) | 107 (27) | 107 (24) | 106 (28) | .9885d |
| Respiratory rate (breaths/min)f | 20 (18–26) | 20 (18–26) | 20 (18–26) | 20 (18–22) | .6885g |
| Temperature (°F)f | 99 (98–101) | 99 (99–102) | 98 (98–101) | 99 (98–101) | .0446g |
| SpO2 (%)f | 97 (94–99) | 97 (94–99) | 96 (93–98) | 97 (93–99) | .6091g |
| Comorbidities & indwelling line status | |||||
| Statin use | 67 (39.4) | 25 (37.3) | 20 (43.5) | 19 (40.4) | .838e |
| Chronic obstructive pulmonary disease | 31 (18.1) | 11 (16.4) | 9 (19.6) | 9 (19.2) | .811e |
| Diabetes mellitus | 75 (43.9) | 26 (38.8) | 18 (41.3) | 23 (48.9) | .549e |
| Human immunodeficiency virus status | 8(4.7) | 5 (7.5) | 1 (2.2) | 2 (4.3) | .539h |
| End-stage renal disease | 20 (11.7) | 11 (16.4) | 4 (8.7) | 5 (10.6) | .471h |
| Active cancer | 17 (9.9) | 8 (11.9) | 5 (10.9) | 3 (6.4) | .674h |
| Organ transplant | 3(1.8) | 2 (3.0) | 0 (0) | 1 (2.1) | .784h |
| Indwelling vascular Catheter | 7(4.1) | 4 (6.0) | 2 (4.4) | 1 (2.1) | .803h |
Abbreviations: ANOVA, analysis of variance; IQR, interquartile range; SpO2, peripheral capillary oxygen saturation.
Data are counts and (percentages), unless otherwise specified.
Eleven patients with mixed growth cultures excluded from between group analyses (N = 160 for comparisons).
Mean (standard deviation).
ANOVA.
χ2.
Median (IQR).
Kruskal-Wallis.
Fisher exact.
Table 2.
Sepsis Resuscitation and Organ Failure Severity.a
| Overall,b N = l7l | Gram negative, n = 67 | Gram positive, n = 46 | Culture negative, n = 47 | P value | |
|---|---|---|---|---|---|
| Initial SOFA score (first 24 hours) | 6 (4–10) | 6 (4–10) | 8 (5–10) | 4 (3–7) | .0001c |
| 48-hour SOFA score | 4 (1–7) | 4.5 (2–7) | 5 (1– 8) | 2 (1–6) | .0848c |
| Lactate (mmol/L) | 3 (2.2–4.6) | 3.0 (2.4–4.8) | 3.15 (2.1–4.6) | 2.6 (2.0–4.0) | .1952c |
| Mechanical ventilationd | 69 (40.6) | 22 (33.3) | 26 (56.5) | 15 (31.9) | .021e |
| Time from ED triage to antibiotics (minutes) | 113 (77–175) | 127 (84–187) | 102.5 (75–144) | 108 (75–167) | .2452c |
| Fluids in first 6 hours (mL) | 3000 (2000–4000) | 3000 (2000–4200) | 3000 (2000–4000) | 2750 (1000–4000) | .4556c |
| Fluids in first 24 hours (mL) | 4000 (3000–6000) | 5000 (3000–7000) | 4625 (2350–6050) | 4000 (3000–5750) | .4168c |
| Septic shockd | 84 (49.4) | 37 (56.1) | 28 (60.9) | 15 (31.9) | .010e |
| Mortalityd | 46 (27.1) | 18 (26.9) | 15 (33.3) | 11 (23.4) | .557e |
| Septic shock mortalityd | 35 (42.2) | 14 (37.8) | 12 (44.4) | 7 (46.7) | .794e |
Abbreviations: ED, emergency department; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment Score.
Data are median and (IQR), unless otherwise specified.
Eleven patients with mixed growth cultures excluded from between group analyses.
Kruskal-Wallis.
Count and (%).
χ2.
Quantitative Cholesterol Metrics
Among patients with culture-positive sepsis compared to culture-negative sepsis, levels of total cholesterol, LDL-C, and HDL-C were significantly lower at enrollment. Similarly at 48 hours, levels of total cholesterol, LDL-C, and HDL-C were also different (Table 3). Triglyceride levels were significantly lower among culture-positive patients at enrollment, but not at 48 hours.
Table 3.
Quantitative and Qualitative Cholesterol Differences by Culture Positivity.a
| Overall, N = 171 | Culture positive, n = 124 | Culture negative, n = 47 | P value | |
|---|---|---|---|---|
| Initial total cholesterol (mg/dL) | 102.20 (33.53) | 96.67 (30.87) | 116.85 (30.19) | .0004b |
| Initial LDL (mg/dL) | 49.90 (26.40) | 44.95 (23.68) | 62.59 (28.96) | .0001b |
| Initial HDL (mg/dL) | 27.06 (18.53) | 24.85 (18.76) | 32.89 (16.75) | .0109b |
| Initial triglycerides (mg/dL) | 125.45 (66.57) | 132.21 (71.70) | 107.67 (46.79) | .0329b |
| 48-hour total cholesterol (mg/dL) | 104.05 (32.43) | 100.03 (30.35) | 114.48 (35.57) | .0116b |
| 48-hour LDL (mg/dL) | 52.34 (24.29) | 49.67 (23.90) | 59.16 (24.22) | .0294b |
| 48-hour HDL (mg/dL) | 19.14 (15.81) | 16.76 (14.94) | 25.30 (16.51) | .0021b |
| 48-hour triglycerides (mg/dL) | 160.68 (90.90) | 166.30 (93.96) | 146.11 (81.65) | .2119b |
| T0 HIIc | 2.22 (1.48–3.48) | 2.34 (1.49–3.60) | 1.82 (1.35–3.13) | .2587d |
| T48 HIIc | 2.03 (1.34–2.98) | 2.11 (1.41–3.14) | 1.68 (1.21–2.42) | .1872d |
Abbreviations: HDL, high-density lipoprotein; HII, HDL Inflammatory Index; LDL, low-density lipoprotein; IQR, interquartile range.
Data are mean and (standard deviations), unless otherwise specified.
Median (IQR).
t test.
Wilcoxon rank sum.
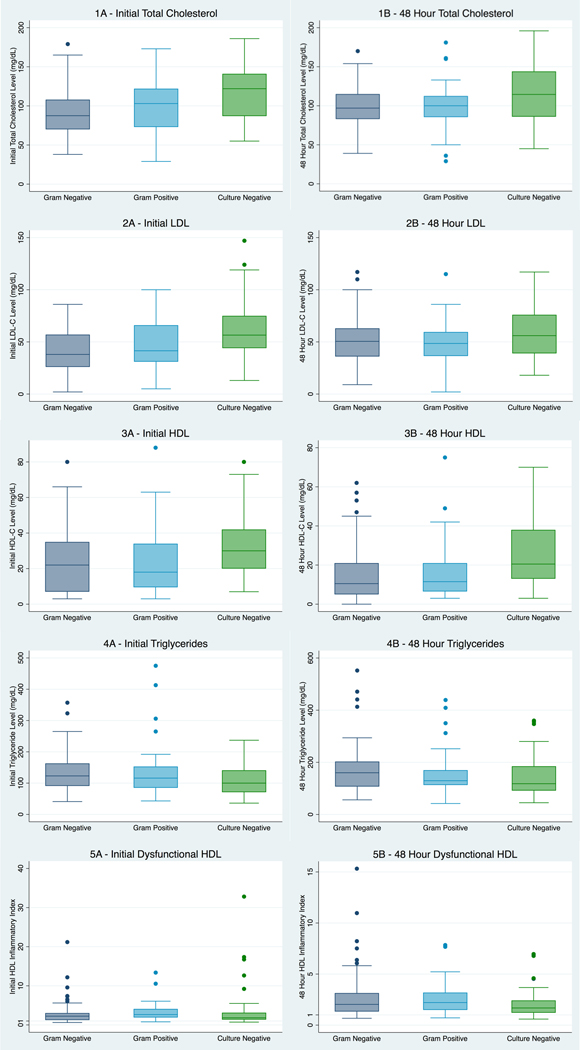
When further divided into gram-negative, gram-positive, and culture-negative sepsis, total cholesterol, LDL-C, and HDL-C levels were significantly different between groups at enrollment and at 48 hours (Figure 2; Supplemental Table 2). In general, levels increased in a stepwise pattern, with the lowest cholesterol levels observed in the gram-negative group, slightly higher levels in the gram-positive group, and the highest levels in the culture-negative group. However, in pairwise comparisons, the significant differences in cholesterol levels were driven largely by the culture-negative group (Supplemental Table 3). Cholesterol levels were significantly lower among patients with gram-negative sepsis compared to culture-negative sepsis and gram-positive sepsis compared to culture-negative sepsis, but no significant differences were found between gram-negative and gram-positive sepsis (Supplemental Table 3).
Figure 2.
Quantitative and qualitative cholesterol differences by infection type.
Bacteremia Subgroup
Levels of total cholesterol, LDL-C, HDL-C, and triglycerides were all significantly lower at enrollment among patients with bacteremia compared to those with negative blood cultures. This was also true at 48 hours, except for 48-hour total cholesterol (Table 4). Enrollment total cholesterol and LDL-C levels were significantly lower among patients with gram-negative versus gram-positive bacteremia. Other cholesterol levels were similar between patients with gram-negative and gram-positive bacteremia (Table 4).
Table 4.
Quantitative Differences in Cholesterol Levels Among Bacteremic Patients.a
| Quantitative differences among bacteremic vs nonbacteremic patients | ||||
| Overall, N = 171 | Bacteremia, n = 78 | No bacteremia, n = 93 | P value, (t test) | |
| Initial total cholesterol (mg/dL) | 102.20 (33.53) | 94.88 (27.73) | 108.24 (36.72) | .0098 |
| Initial LDL (mg/dL) | 49.90 (26.40) | 44.03 (22.20) | 54.60 (28.59) | .0104 |
| Initial HDL (mg/dL) | 27.06 (18.53) | 21.87 (16.52) | 31.42 (19.08) | .0007 |
| Initial triglycerides (mg/dL) | 125.45 (66.57) | 141.37 (78.30) | 112.47 (52.14) | .0049 |
| 48-hour total cholesterol (mg/dL) | 104.05 (32.43) | 98.48 (31.17) | 108.60 (32.91) | .0508 |
| 48-hour LDL (mg/dL) | 52.34 (24.29) | 47.42 (23.77) | 56.17 (24.13) | .0265 |
| 48-hour HDL (mg/dL) | 19.14 (15.81) | 13.34 (11.63) | 23.87 (17.20) | <.0001 |
| 48-hour triglycerides (mg/dL) | 160.68 (90.90) | 179.30 (106.41) | 145.48 (73.13) | .0195 |
| Quantitative differences among bacteremic patients by infection type | ||||
| Overall, n = 75b | Gram negative, n = 46 | Gram positive, n = 29 | P value (t test) | |
| Initial total cholesterol (mg/dL) | 94.29 (27.64) | 89.04 (25.39) | 102.71 (29.45) | .0390 |
| Initial LDL (mg/dL) | 42.86 (21.38) | 38.27 (20.39) | 50.15 (21.24) | .0226 |
| Initial HDL (mg/dL) | 22.40 (16.60) | 22.91 (16.85) | 21.59 (16.45) | .7385 |
| Initial triglycerides (mg/dL) | 141.14 (79.29) | 136.02 (68.62) | 149.18 (94.45) | .4964 |
| 48-hour total cholesterol (mg/dL) | 97.33 (29.93) | 96.50 (28.67) | 98.63 (32.31) | .7754 |
| 48-hour LDL (mg/dL) | 46.08 (22.33) | 46.29 (21.91) | 45.78 (23.33) | .9283 |
| 48-hour HDL (mg/dL) | 13.49 (11.75) | 12.38 (11.36) | 15.22 (12.36) | .3307 |
| 48-hour triglycerides (mg/dL) | 178.86 (106.57) | 189.21 (114.70) | 162.74 (92.29) | .3175 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Data are mean and (standard deviations).
Three mixed blood cultures excluded.
Adjusting for Organ Failure Severity
As lower cholesterol levels have been associated with increased disease severity,4–6 we used logistic regression to control for SOFA score to evaluate whether observed differences were independent of organ failure severity. Significant differences in enrollment total cholesterol, LDL-C, and HDL-C persisted between culture-positive and culture-negative sepsis, with similar results in the bacteremic cohort (Supplemental Table 4A). Similarly, differences between enrollment total cholesterol and LDL-C among patients with gram-negative and gram-positive bacteremia also remained significant. In the same manner, we used logistic regression to evaluate whether 48-hour cholesterol differences persisted after accounting for severity of illness. Fewer of the differences observed in 48-hour cholesterol levels remained statistically significant after controlling for 48-hour organ failure (Supplemental Table 4B).
Logistic Regression Model
A logistic regression model was developed to evaluate the association between culture-positive sepsis and LDL-C. Significant independent predictors of culture-positive sepsis included age (odds ratio [OR]: 1.03; 95% CI: 1.001–1.061; P = .043), initial SOFA score (OR: 1.22; 95% CI: 1.075–1.380; P = .002), and enrollment LDL-C (OR: 0.975; 95% CI: 0.960–0.990; P = .001). Human immunodeficiency virus infection status was also included in the model as a nonsignificant predictor (OR: 2.820; 95% CI: 0.405–19.646; P = .295). The model showed no evidence overfitting, Hosmer-Lemeshow test P = .19 (using the standard 10 groups). Enrollment LDL-C was a significant predictor of culture-positive sepsis, after adjustment for other variables. Each 1 mg/dL decrease in enrollment LDL-C was associated with a 2.5% increased odds of having culture positive sepsis.
Qualitative Cholesterol Metrics
There were no significant differences between Dys-HDL (expressed as HII) at enrollment or at 48 hours among patients with culture-positive and culture-negative sepsis (Table 3). Similarly, there were no differences in Dys-HDL between gram-negative, gram-positive, and culture-negative sepsis (Figure 2; Supplemental Table 2). In the bacteremia subgroup, Dys-HDL did not differ significantly between bacteremic and nonbacteremic patients, nor patients with gram-negative versus gram-positive bacteremia (Supplemental Table 5).
Discussion
Our results show that culture-positive sepsis was associated with significantly lower enrollment total cholesterol, LDL-C, and HDL-C as compared to culture-negative sepsis. The strongest differences were found between the bacteremic and nonbacteremic patients, and these findings persisted when controlling for severity of illness. Further, gram-negative bacteremic patients had significantly lower cholesterol levels than gram-positive bacteremic patients. Our findings contribute to a growing body of work demonstrating that lipid metabolism is dynamically regulated and dysfunctional in sepsis.3,4,10,11,21 To our knowledge, this is the first study evaluating both quantitative and qualitative cholesterol differences among infection types in sepsis. These data provide circumstantial support for the hypothesis that cholesterol might act as a “lipid sink” in sepsis.12 Additionally, our results suggest that sepsis-mediated cholesterol differences may vary based on infection type.
Cholesterol is compelling as an early sepsis indicator because it may also be a therapeutic target. The test is rapidly available in clinical practice with test results frequently available within 1 hour. Early sepsis source-control strategies, such as early antibiotic initiation, have had the most reproducibly positive results.22,23 This implies that interventions that enhance source-control may be promising therapeutic targets. In addition to impacting sepsis-induced inflammation, cholesterol facilitates pathogen sequestration and clearance, as such, lipid-targeted therapies may work synergistically with other sepsis source-control strategies. Numerous studies have shown reduced cholesterol levels are associated with organ failure and mortality.3–6 Whether mitigating these drops confers a survival benefit is uncertain. Existing interventional studies have failed to show a difference in outcomes, but this may partially be due to the heterogeneity of the sepsis syndrome.16,17 Eritoran, a synthetic lipid A antagonist that blocks gram-negative lipopolysaccharide endotoxin binding, failed to show a mortality difference compared to placebo in a randomized control trial of patients with severe sepsis.16 Similarly, Dellinger and colleagues investigated whether a phospholipid emulsion could improve outcomes in patients with gram-negative severe sepsis and found no difference in mortality or organ failure among patients who received phospholipid emulsion compared to placebo.17 Our findings support the hypothesis that a subset of patients with sepsis may preferentially respond to a lipid-targeted sepsis therapies. These findings are more hypothesis generating than proving, but they may indicate an infection phenotype more amenable to benefit and suggest a mechanism in need of further exploration.
Existing clinical studies on sepsis-related lipid dysregulation have largely focused on host factors that regulate cholesterol metabolism (ie, cholesterol ester transfer protein, proprotein convertase subtilisin/kexin type 9).18,21,24–26 Less is known about pathogen factors that may mediate variations in lipid kinetics. Our data support preclinical evidence that suggests a varying degree of cholesterol dysregulation based on infection type.2,13–15 Our findings indicate that variations in lipid metabolism between gram-positive and gram-negative infections occur in some, but not all types of sepsis. When accounting for all culture data, we found similar cholesterol levels among patients with gram-negative and gram-positive sepsis. When limited to patients with bacteremia, we found cholesterol levels were different among patients with gram-negative and gram-positive infections. These results are consistent with those of Zou and colleagues, who found HDL-C levels were lower on enrollment among patients with gram-negative infections.19 However, they did not control for organ failure severity, a potential confounder as gram-negative infections have been associated with a larger inflammatory response and a greater severity of illness.27–30 Our study further extends our understanding of lipid metabolism in sepsis because we accounted for organ severity when analyzing cholesterol levels between groups.
Another potential application of our findings is in the prediction of culture-positive sepsis or bacteremia. Patients with culture-positive sepsis have increased disease severity and worse outcomes compared to those with culture-negative sepsis.31–33 Unfortunately the gold standard for diagnosis, culture and antibiotic sensitivity data, can take 24 to 48 hours to return. Prompt recognition of patients at risk for rapid illness progression is critical because patients who are admitted to the floor and subsequently upgraded to the intensive care have worse outcomes.34–37 Thus an early indication of increased likelihood of culture-positive sepsis could be valuable to the provider. Our evidence suggests that cholesterol levels, combined with standard clinical and microbiology data, may provide additional information of increased likelihood of certain types of infection prior to culture results, though these data remain hypothesis generating.
Notably, inferences based on our findings require an understanding of which patients are captured in the culture-negative sepsis group. Much like sepsis in general, culture-negative sepsis is a heterogenous group. We know that culture data is lacking in sensitivity.38,39 Among many proposed explanations, one is that this group may have a lower bacterial burden than its culture-positive counterparts.31,38,40 A lower bacterial load could mean less pathogen-bound lipid clearance, lower cholesterol turnover, and more stable levels. Additionally, it is also possible that this population included some cases of viral and nonbacterial sepsis.
Our study has several strengths, including the use of prospective data, controlling for disease severity, and both quantitative and qualitative testing. Our study considered all culture data in addition to blood culture data. We did not limit our analysis to culture-positive patients as a provider doesn’t know which patients will ultimately have positive cultures at the time they are ordered. We felt including only those patients with positive cultures would have represented a selection bias as it would have exclude a large number of patients who were not only identified as septic at the time of enrollment but also adjudicated for sepsis. Including all patients identified as septic at the time of enrollment, prior to culture results, is more reflective of physician practice.
Limitations
This was a single-center study and results may not be generalizable to other institutions. Our study included only 171 patients, 124 of whom were culture positive. The study may have been underpowered to identify differences between gram-negative and gram-positive sepsis. We categorized patients as culture positive and culture-negative based on pathogen identification, although the sensitivity and specificity of microbio-logical culture data is limited.38,39 We excluded cases of nonbacterial sepsis, however, it is possible that cases of undetected viral and parasitic sepsis were classified as culture negative.
Conclusions
Cholesterol levels are significantly lower among patients with culture-positive sepsis and bacteremia. Among bacteremic patients, total and LDL cholesterol were significantly lower among patients with gram-negative bacteremia. These data suggest sepsis-induced cholesterol dysregulation may vary based on etiology of sepsis and may indicate a subset of patients more likely to respond to lipid-targeted therapies. Further studies are needed to evaluate these associations.
Supplementary Material
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The parent studies were supported by an NIGMS K23 (1K23GM115690-01A1 [Faheem W. Guirgis]) and a Society of Critical Care Medicine Vision Grant (Faheem W. Guirgis). The authors disclose the following salary support: NCATS 1KL2TROO1429 (Lauren Page Black); Minneapolis Medical Research Foundation (Michael A. Puskarich); Department of Defense W81XWH1810089; W81XWH-15-1-0403 (Rosemarie Fernandez); NIGMS 1K23GM115690-01A1 (Faheem W. Guirgis).
Footnotes
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Fernandez reports payment for speaking engagement from Physio-Control, Inc.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Catapano AL, Pirillo A, Bonacina F, Norata GD. HDL in innateand adaptive immunity. Cardiovasc Res. 2014;103(3):327–383. doi: 10.1093/cvr/cvu150 [DOI] [PubMed] [Google Scholar]
- 2.Morin EE, Guo L, Schwendeman A, Li XA. HDL in sepsis—risk factor and therapeutic approach. Front Pharmacol. 2015;6:244. doi: 10.3389/fphar.2015.00244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guirgis FW, Dodani S, Leeuwenburgh C, et al. HDL inflammatory index correlates with and predicts severity of organ failure in patients with sepsis and septic shock. PLoS One. 2018;13(9): e0203813. doi: 10.1371/journal.pone.0203813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guirgis FW, Donnelly JP, Dodani S, et al. Cholesterol levels and long-term rates of community-acquired sepsis. Crit Care. 2016; 20(1):408. doi: 10.1186/s13054-016-1579-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chien J, Jerng J, Yu C, Yang P. Low serum level of high-density lipoprotein cholesterol is a poor prognostic factor for severe sepsis. Crit Care Med. 2005;33(8):1688–1693. doi: 10.1097/01.CCM.0000171183.79525.6B [DOI] [PubMed] [Google Scholar]
- 6.Chien Y, Chen C, Hsu C, Chen K, Yu C. Decreased serum level of lipoprotein cholesterol is a poor prognostic factor for patients with severe community-acquired pneumonia that required intensive care unit admission. J Crit Care. 2015;30(3):506–510. doi: 10.1016/j.jcrc.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 7.Charles-Schoeman C, Watanabe J, Lee YY, et al. Abnormal function of high-density lipoprotein is associated with poor disease control and an altered protein cargo in rheumatoid. Arthritis Rheum. 2009;60(10):2870–2879. doi: 10.1002/art.24802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-banna N, Lehmann C. Oxidized LDL and LOX-1 in experimental sepsis. Mediators Inflamm. 2013;2013:761789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madahian S, Navab K, Pourtabatabaei N, et al. Inflammation, high density lipoprotein and endothelium. Curr Med Chem. 2014; 21(25):2902–2909. doi: 10.2174/0929867321666140414105530 [DOI] [PubMed] [Google Scholar]
- 10.Guirgis FW, Dodani S, Moldawer L, et al. Exploring the predictive ability of dysfunctional high-density lipoprotein for adverse outcomes in emergency department patients with sepsis: a preliminary investigation. Shock. 2017;48(5):539–544. doi: 10.1097/SHK.0000000000000887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guirgis FW, Leeuwenburgh C, Grijalva V, et al. HDL cholesterol efflux is impaired in older patients with early sepsis: a subanalysis of a prospective pilot study. Shock. 2018;50(1):66–70. doi: 10.1097/SHK.0000000000001030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones TK, Wong HR, Meyer NJ. HDL cholesterol: a “pathogen lipid sink” for sepsis? Am J Respir Crit Care Med. 2019;199(7): 812–814. doi: 10.1164/rccm.201811-2084ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khovidhunkit W, Kim M, Memon RA, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host 1. J Lipid Res. 2004;45(7): 1169–1196. doi: 10.1194/jlr.R300019-JLR200 [DOI] [PubMed] [Google Scholar]
- 14.Eckardstein AV. HDL in infectious disease and sepsis. In: Eckardstein AV, Kardassis D, eds. Handbook of Experimental Pharmacology. 224th ed. Springer; 2015:483–508. doi: 10.1007/978-3-319-09665-0 [DOI] [PubMed] [Google Scholar]
- 15.Grunfeld C, Marshall M, Shigenaga JK, Moser AH, Tobias P, Feingold KR. Lipoproteins inhibit macrophage activation by lipoteichoic acid. J Lipid Res. 1999;40(2):245–252. [PubMed] [Google Scholar]
- 16.Opal SM, Laterre P-F, Francois B, et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis. JAMA. 2013;309(11):1154–1162. doi: 10.1001/jama.2013.2194 [DOI] [PubMed] [Google Scholar]
- 17.Dellinger RP, Tomayko JF, Angus DC, et al. Efficacy and safety of a phospholipid emulsion (GR270773) in gram-negative severe sepsis: results of a phase II multicenter, randomized, placebo-controlled, dose-finding clinical trial. Crit Care Med. 2009; 37(11):2929–2938. doi: 10.1097/CCM.0b013e3181b0266c [DOI] [PubMed] [Google Scholar]
- 18.Walley KR. Role of lipoproteins and proprotein convertase subtilisin/kexin type 9 in endotoxin clearance in sepsis. Curr Opin Crit Care. 2016;22(5):464–469. doi: 10.1097/MCC.0000000000000351 [DOI] [PubMed] [Google Scholar]
- 19.Zou G, He J, Ren B, Xu F, Xu G, Zhang W. The delta high-density lipoprotein cholesterol ratio: a novel parameter for gram-negative sepsis. Springerplus. 2016;5(1):1044. doi: 10.1186/s40064-0162685-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18(6):499–502. [PubMed] [Google Scholar]
- 21.Walley KR, Francis GA, Opal SM, Stein EA, Russell JA, BoydJH. The central role of proprotein convertase subtilisin/kexin type 9 in septic pathogen lipid transport and clearance. Am J Respir Crit Care Med. 2015;192(11):1275–1286. doi: 10.1164/rccm.201505-0876CI [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9 [DOI] [PubMed] [Google Scholar]
- 23.Puskarich MA, Trzeciak S, Shapiro NI, et al. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med. 2012;39(9):2066–2071. doi: doi: 10.1097/CCM.0b013e31821e87ab [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyd JH, Fjell CD, Russell JA, Sirounis D, Cirstea MS, Walley KR. Increased plasma PCSK9 levels are associated with reduced endotoxin clearance and the development of acute organ failures during sepsis. J Innate Immun. 2016;8(2):211–220. doi: 10.1159/000442976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grion CMC, Cardoso LTQ, Perazolo TF, et al. Lipoproteins and CETP levels as risk factors for severe sepsis in hospitalized patients. Eur J Clin Invest. 2010;40(4):330–338. doi: 10.1111/j.1365-2362.2010.02269.x [DOI] [PubMed] [Google Scholar]
- 26.Trinder M, Genga KR, Kong HJ, et al. Cholesteryl ester transfer protein influences high-density lipoprotein levels and survival in sepsis. Am J Respir Crit Care Med. 2019;199(7):854–862. doi: 10.1164/rccm.201806-1157OC [DOI] [PubMed] [Google Scholar]
- 27.Abe R, Oda S, Sadahiro T, et al. Gram-negative bacteremia induces greater magnitude of inflammatory response than Gram-positive bacteremia. Crit Care. 2010;14(2):R27. doi: 10.1186/cc8898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjerre A, Brusletto B, Høiby EA, Kierulf P, Brandtzaeg P. Plasma interferon-gamma and interleukin-10 concentrations in systemic meningococcal disease compared with severe systemic Gram-positive septic shock. Crit Care Med. 2004;32(2):433–438. doi: 10.1097/01.CCM.0000104950.52577.97 [DOI] [PubMed] [Google Scholar]
- 29.Gao H, Evans TW, Finney SJ. Bench-to-bedside review: sepsis, severe sepsis and septic shock—does the nature of the infecting organism matter? Crit Care. 2008;12(3):213. doi: 10.1186/cc6862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feezor RJ, Oberholzer C, Baker H V, et al. Molecular characterization of the acute inflammatory response to infections with gram-negative versus gram-positive bacteria. Infect Immun. 2003;71(10):5803–5813. doi: 10.1128/IAI.71.10.5803-5813.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phua J, Ngerng W, See K, et al. Characteristics and outcomes of culture-negative versus culture-positive severe sepsis. Crit Care. 2013;17(5):R202. doi: 10.1186/cc12896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li S, Rong H, Guo Q, Chen Y, Zhang G, Yang J. Serum procalcitonin levels distinguish Gram-negative bacterial sepsis from Gram-positive bacterial and fungal sepsis. J Res Med Sci. 2016; 21(1):39. doi: 10.4103/1735-1995.183996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lang Y, Jiang Y, Gao M, et al. Interleukin-1 receptor 2: a new biomarker for sepsis diagnosis and gram-negative/gram-positive bacterial differentiation. Shock. 2017;47(1):119–124. doi: 10.1097/SHK.0000000000000714 [DOI] [PubMed] [Google Scholar]
- 34.Delgado MK, Liu V, Pines JM, Kipnis P, Gardner MN, Escobar GJ. Risk factors for unplanned transfer to intensive care within 24 hours of admission from the emergency department in an integrated healthcare system. J Hosp Med. 2013;8(1):13–19. doi: 10.1002/jhm.1979 [DOI] [PubMed] [Google Scholar]
- 35.Liu V, Kipnis P, Rizk NW, Escobar GJ. Adverse outcomes associated with delayed intensive care unit transfers in an integrated healthcare system. J Hosp Med. 2012;7(3):224–230. doi: 10.1002/jhm.964 [DOI] [PubMed] [Google Scholar]
- 36.Parkhe M, Myles PS, Leach DS, Maclean AV. Outcome of emergency department patients with delayed admission to an intensive care unit. Emerg Med. 2002;14(1):50–57. http://www.ncbi.nlm.nih.gov/pubmed/11993835 [DOI] [PubMed] [Google Scholar]
- 37.Wardi G, Wali AR, Villar J, et al. Unexpected intensive care transfer of admitted patients with severe sepsis. J Intensive Care. 2017;5(1):43. doi: 10.1186/s40560-017-0239-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lever A, Mackenzie I. Sepsis: definition, epidemiology, and diagnosis. BMJ. 2007;335(7625):879–883. doi: 10.1136/bmj.39346.495880.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bloos F, Sachse S, Kortgen A, et al. Evaluation of a polymerase chain reaction assay for pathogen detection in septic patients under routine condition: an observational study. PLoS One. 2012;7(9):e46003. doi: 10.1371/journal.pone.0046003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lisboa T, Waterer G, Rello J. We should be measuring genomic bacterial load and virulence factors. Crit Care Med. 2010;38(10): 656–662. doi: 10.1097/CCM.0b013e3181f2453a [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.