Abstract
1-Methyl-4-phenylpyridinium (MPP(+))-induced neurotoxicity has previously been attributed to either caspase-dependent apoptosis or caspase-independent cell death. In the current study, we found that MPP(+) induces a unique, non-apoptotic nuclear morphology coupled with a caspase-independent but calpain-dependent mechanism of cell death in primary cultures of rat cerebellar granule neurons (CGNs). Using a terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) assay in CGNs exposed to MPP(+), we observed that these neurons are essentially devoid of caspase-dependent DNA fragments indicative of apoptosis. Moreover, proteolysis of a well recognized caspase-3 substrate, poly (ADP ribose) polymerase (PARP), was not observed in CGNs exposed to MPP(+). In contrast, calpain-dependent proteolysis of fodrin and pro-caspases-9 and −3 occurred in this model coupled with inhibition of caspase-3/−7 activities. Notably, several key members of the Bcl-2 protein family appear to be prominent calpain targets in MPP(+)-treated CGNs. Bid and Bax were proteolyzed to truncated forms thought to have greater pro-death activity at mitochondria. Moreover, the pro-survival Bcl-2 protein was degraded to a form predicted to be inactive at mitochondria. Cyclin E was also cleaved by calpain to an active low MW fragment capable of facilitating cell cycle re-entry. Finally, MPP(+)-induced neurotoxicity in CGNs was significantly attenuated by a cocktail of calpain and caspase inhibitors in combination with the antioxidant glutathione. Collectively, these results demonstrate that caspases do not play a central role in CGN toxicity induced by exposure to MPP(+), whereas calpain cleavage of key protein targets, coupled with oxidative stress, plays a critical role in MPP(+)-induced neurotoxicity. Our findings underscore the complexity of MPP(+)-induced neurotoxicity and suggest that calpain may play a fundamental role in causing neuronal death downstream of mitochondrial oxidative stress and dysfunction.
Keywords: Cerebellar granule neurons, MPP(+), Calpain, Oxidative stress, Neurotoxicity, Bcl-2
Introduction
1-Methyl-4-phenylpyridinium (MPP(+)) is an inhibitor of mitochondrial complex I and has been widely used to study protease involvement and oxidative stress in relation to neurodegenerative disease (González-Polo et al. 2004; Jourdi et al. 2009; Leist et al. 1998; Yang et al. 2009). A large variety of in vitro models support a complex interplay between the calpain and caspase families of cysteine proteases during MPP(+)-induced neurotoxicity, contributing to several distinct modes of neuronal death being proposed for this toxin. Moreover, factors including the concentration of MPP(+) and the duration of toxin exposure may lead to differential regulation of calpain and caspase activities in neurons exposed to MPP(+) (Choi et al. 2001; Du et al. 1997). All of these effects contribute to the ultimate mode of neuronal death induced by MPP(+) (i.e., apoptosis, necrosis, or other less well-defined pathways to cell death) (Kroemer et al. 2009).
Caspase-dependent neuronal death through the mitochondrial apoptotic pathway has been demonstrated as a prominent mechanism underlying neurotoxicity and neurodegenerative disease (Ekshyyan and Aw 2004; Okouchi et al. 2007). In a host of studies elucidating the cellular signaling mechanisms involved in MPP(+)-induced neurotoxicity including SH-SY5Y human neuroblastoma cells (Cheng et al. 2009; Wang and Xu 2005), dopaminergic N-27 cells (Anantharam et al. 2007), and PC12 cells (Bo et al. 2005; Kim et al. 2009), caspase-dependent mechanisms have been shown to be the primary mode of neuronal death (Table 1). On the other hand, contrasting studies involving dopaminergic MN9D (Choi et al. 2001; Chu et al. 2005), neuron-like NT2 human teratocarcinoma cells (Domingues et al. 2008), and PC12 cells (Wales et al. 2008) were found to exhibit alternate modes of cell death which were calpain-dependent and/or caspase-independent (Table 1). Therefore, the specific mechanism of cell death induced by MPP(+) neurotoxicity appears to be largely dependent on the cell system used.
Table 1.
Comparison of modes of cell death in dopaminergic cell lines and primary neuronal cultures exposed to MPP(+)
| Literature cited | Cell culture system | MPP(+) dose range | Duration of MPP(+) treatment (hours) | Primary mode of cell death |
|---|---|---|---|---|
| Anantharam et al. 2007 | N-27 | 300 μM | 45 min–24 h | Apoptosis |
| Bo J. et al. 2005 | PC12 | 400 μM | 6–24 | Apoptosis |
| Cheng et al. 2009 | SH-SY5Y | 1000 μM | 24–48 | Apoptosis |
| Choi et al. 2008 | MN9D | 50 μM | 48 | Necrosis (calpain-dependent) |
| Chu et al. 2005 | MN9D | 30 μM | 24–72 | Non-apoptotic (caspase-independent) |
| Domingues et al. 2008 | NT2 | 1000 μM | 24 | Non-apoptotic (calpain-dependent) |
| Han et al. 2003 | E14 rat embryo ventral mesencephalon | 3 μM | 48 | Non-apoptotic (caspase-independent) |
| Jourdi et al. 2009 | 9–11 day postnatal rat hippocampal and mesencephalic sections | Hippocampal (250 μM); mesencephalic (20 μM) | 24 | Non-apoptotic (caspase-independent) |
| Kim et al. 2009 | PC12 | 5000 μM | 24 | Apoptosis |
| Wales et al. 2008 | PC12 | 500–5000 μM | 24 | Calpain-dependent programmed cell death |
| Wang and Xu 2005 | SH-SY5Y | 100–500 μM | 24 | Apoptosis |
| This study | Rat CGNs | 150 μM | 24 | Non-apoptotic (calpain-dependent) |
Furthermore, cross-communication between the calpain and caspase proteolytic systems is complex and has been characterized in conflicting manners. For example, in different studies, calpains have been shown to either activate or inactivate caspases. Blomgren et al. (2001) demonstrated that the executioner caspase-3 of the apoptotic pathway could be directly activated by m-calpain. On the other hand, McGinnis et al. (1999) argued that caspases are actively repressed by members of the calpain family. Therefore, as Neumar et al. (2003) suggested, the timing of calpain activation in concert with the magnitude of cysteine protease activation may be critical factors in determining the ultimate mechanism of neuronal cell death.
Many immortalized dopaminergic cell lines utilized to study MPP(+)-induced neurotoxicity are characterized by high dose range dependency of MPP(+), on the order of 250–1,000 μM or even higher concentrations to cause neuronal death (see Table 1). In contrast, CGNs—a primary neuronal system—are susceptible to relatively lower doses of MPP(+) from 100 to 150 μM (González-Polo et al. 2001). CGNs share with the dopaminergic cell culture models the ability to transport MPP(+) across the plasma membrane. CGNs achieve MPP(+) uptake through a characterized organic cation transporter 3 (OCT 3) (Shang et al. 2003) or a cationic amino acid transporter (CAT) (González-Polo et al. 2001). Thus, although CGNs lack a bonafide dopamine transporter, their capacity to accumulate MPP(+) makes them a unique primary neuronal system for investigating the mechanism of cell death induced by this neurotoxin. Moreover, the high homogeneity of CGN cultures (>95% granule neurons) makes them amenable to standard biochemical techniques such as western blotting.
The objectives of this study were two-fold. First, we sought to compare the mechanism of MPP(+)-induced neurotoxicity to a well-characterized model of caspase-dependent, classical apoptosis in CGNs. Removal of an extracellular, high-potassium depolarization stimulus in CGNs was previously shown to activate a caspase-dependent, mitochondrial apoptotic cascade (D’Mello et al. 1993; Linseman et al. 2002). This model of neuronal apoptosis was utilized in parallel with MPP(+) to compare and contrast the effects of calpains and caspases on critical cellular substrates, nuclear morphology, and mitochondrial viability in primary CGNs. Second, we aimed to identify key calpain substrates which are cleaved in CGNs during MPP(+)-induced neurotoxicity. The identification of these target proteins should contribute to our understanding of the specific cell death pathways that are activated in neurons exposed to MPP(+) or other similar neurotoxins.
Materials and Methods
Materials
1-Methyl-4-phenylpyridium iodide (MPP(+)), 4,6-diamidino-2-phenylindole (DAPI), and Hoechst Dye number 33258 were purchased from Sigma (St. Louis, MO). The calpain inhibitors, ALLN and ALLM, and glutathione monoethyl ester (GSH) were purchased from Calbiochem (San Diego, CA). The pan-caspase family inhibitor Boc-D (OMe)-FMK was from Alexis Biochemicals (San Diego, CA). The ApopTag© Red apoptosis detection kit (TUNEL) was obtained from Chemicon International (Temecula, CA). CellQuanti-MTT™ Assay Kit was from BioAssay Systems (Hayward, CA). Caspase Glo 3/7 Assay for detection of active caspases was purchased from Promega (Madison, WI). The mouse monoclonal antibody to fodrin was obtained from Chemicon (Temecula, CA). Rabbit polyclonal antibodies to Bax (P-19), Bcl-2 (N-19), Bid (FL-195), Caspase-3 (H-277), Cyclin E (M-20), and PARP (H-250) were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). The mouse monoclonal and rat polyclonal antibodies to pro-caspase-9 and β-actin, respectively, were from Cell Signaling Biotechnology (Beverly, MA). Horseradish peroxidase-conjugated secondary antibodies and reagents for chemiluminescence detection were purchased from Amersham Biosciences (Piscataway, NJ).
Cerebellar Granule Neuron (CGN) Culture
CGNs were isolated from 7-day-old Sprague-Dawley rat pups as described previously (Linseman et al. 2002) and plated in basal modified Eagle’s medium containing 10% fetal bovine serum, 25 mM KCl, 2 mM L-glutamine, and 100 units/ml penicillin/100 μg/ml streptomycin (Invitrogen, CA) at a density of 4.0 × 106 cells/well on poly-L-lysine coated, 35 mm diameter plastic dishes. Twenty-four hours after plating, cytosine arabinoside (10 μM) was added to the culture medium to inhibit glial growth. Experiments were performed 6–8 days post-plating and were ~95% pure for CGNs.
Quantification of Nuclear Morphology
CGNs were treated with various pro-death stimuli and incubated for 24 h. Post incubation, cells were fixed with 4% paraformaldehyde and nuclei stained with Hoechst dye (8 μg/ml). Apoptotic nuclei (Apo) were identified by intense chromatin condensation and/or chromatin fragmentation, usually into one to three large fragments. Nuclei were categorized as undergoing non-apoptotic chromatin aggregation (ChAgg) upon the appearance of a prominent raspberry-like morphology consisting of many small chromatin aggregates. Viable cells were approximately 10 μM in diameter and lacked any visible signs of nuclear damage. Typically, ~300 cells were counted from each 35 mm well by randomly counting three fields in a 63× objective.
TUNEL Assay for Detection of Apoptotic Nuclei
CGNs were plated on polyethyleneimine-coated glass coverslips and treated as described in “Results” section. Cells were incubated for 24 h. After incubation, cells were washed with phosphate-buffered saline (PBS), pH 7.4, and fixed with 1% paraformaldehyde (PF) for 30 min. Following the fixation, cells were washed and permeabilized with ethanol and acetic acid (2:1 v/v) for 5 min at −20°C. After permeabilization, cells were washed and ApoTag Equlibrium Buffer (EB) was added for a 1-min incubation. EB was removed and cells were incubated in 55 μl/coverslip ApoTag© Terminal Deoxy-nucleotidyl Transferase (TdT) Enzyme at 37°C for 1 h in a humidified chamber. The reaction was then stopped by the addition of 1 ml ApoTag© Stop/Wash Buffer. Media were removed followed by 10 min incubation in 0.5 ml/coverslip of new Stop/Wash buffer at room temperature (RT). Stop/Wash buffer was aspirated and coverslips were washed three times with PBS. Following this wash, cells were incubated with Digoxigenin antibody in a humidified chamber for 30 min at RT. The antibody solution was removed and the coverslips were washed three times with PBS followed by mounting onto glass slides with 0.1% p-phenylenediamine in 75% glycerol in PBS to prevent quenching. Images were captured using an oil immersion objective (63×) on a Zeiss Axioplan 2 fluorescence microscope equipped with a Cooke Sensicam CCD camera and Slidebook Image analysis software (Intelligent Imaging Innovations, Inc., Denver, CO).
CGN Lysate Preparation and Immunoblotting
Following treatment as indicated in “Results” section, CGNs were lysed and immunoblotted according to a previously published method (Loucks et al. 2006). Blots shown are representative of a minimum of three independent experiments.
Caspase 3/7 Activity Assay
Cells were gently washed with 1 ml PBS and lysed in lysis reagent obtained from Promega over a 15-min incubation period. Cells were then scraped, transferred into a 1.6-ml microcentrifuge tube, and spun down at 10,000 rpm for 2 min. A 100-μl aliquot of the supernatant (lysate) was incubated with 100 μl of Casp-Glo reagent for 1 h at RT. Luminescence displaying active caspase 3/7 was measured in a TD-20/20 Luminometer. Results are representative of three independent experiments.
Calpain Incubation in Isolated Mitochondria
The protocol for isolation of rat brain mitochondria was given to us courtesy of Dr. M. Patel (Department of Pharmaceutical Sciences, University of Colorado Health Sciences Center). It is described in Liang and Patel (2004) with modifications as described in Loucks et al. (2009).
MTT Cell Viability Assay
CGNs were incubated in control conditions, 25 mM KCl with 10% Fetal Bovine Serum (FBS), with either MPP(+) alone or MPP(+) co-incubated with 2 mM glutathione (GSH), 10 μM ALLN, and 20 μM Boc-D (OMe)-FMK (BOC). After 16 h of incubation, 187.5 μl of CellQuanti-MTT™ Reagent with Assay Buffer (15 μl/80 μl cell culture) was added directly to the culture medium and incubated for 4 h at 37°C/10% CO2. Following incubation, 1,250 μl/well Solubilization Solution was added directly to the media (100 μl/80 μl cell culture) and mixed on an orbital shaker for 1 h at RT. Next, the contents of each well were scraped and pipetted into individual 15 ml conical tubes and centrifuged for 2 min at 13,000 rpm. A 500-μl aliquot was removed from each sample, and the absorbance was read at a wavelength of 570 and 650 nm. The average of the data was plotted relative to the control mean absorbance for each independent experiment (n = 5).
Statistical Analyses
Data represent the means ± SEM for the number (n) of independent experiments that were performed. One-way analysis of variance tests followed by post-hoc Dunnett’s or Tukey’s tests were done to evaluate statistical significance between the data sets. A P value of <0.05 was considered statistically significant. Images are representative of at least three experiments.
Results
CGNs Exposed to the Complex I Inhibitor, MPP(+), Exhibit a Unique TUNEL-Negative, Caspaseindependent Nuclear Morphology
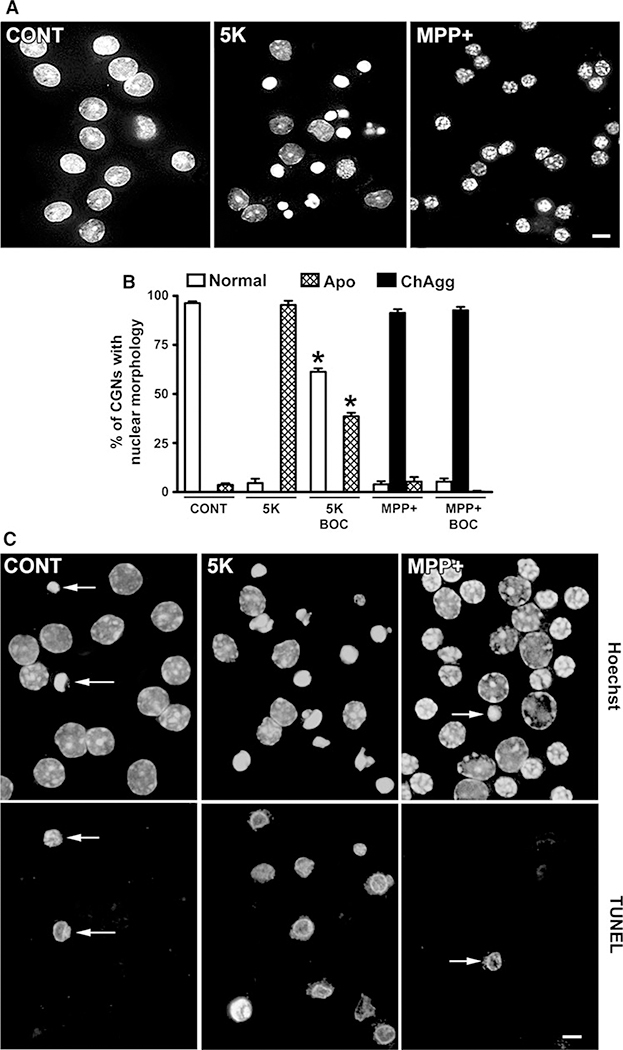
Under control conditions, CGNs require a high potassium-regulated depolarization stimulus for survival and display large, intact nuclei (D’Mello et al. 1993) (Fig. 1a, left panel). Removal of the depolarization stimulus (5 K) is a classical apoptotic stimulus in CGNs ultimately resulting in cytochrome c release from mitochondria and activation of the intrinsic or mitochondrial apoptotic pathway (Linseman et al. 2002, 2004). After a 24-h incubation in 5 K conditions, CGNs experience caspase activation leading to nuclear DNA condensation, fragmentation at nucleosomal linkage regions, and the formation of apoptotic bodies (Martin et al. 1994; Rubin et al. 1994), as indicated by Hoechst staining (Fig. 1a, middle panel). In contrast, CGNs incubated for 24 h in medium containing depolarizing potassium but also exposed to 150 μM MPP(+) display a unique “raspberry-like” nuclear morphology characterized by marked chromatin aggregation (Fig. 1a, right panel).
Fig. 1.
MPP(+) induces a unique nuclear morphology in CGNs that is caspase-independent and TUNEL-negative. a CGNs were incubated for 24 h in control medium containing 25 mM KCl and 10% FBS (CONT) or apoptotic medium containing 5 mM KCl and lacking FBS (5 K). Alternatively, CGNs were exposed to MPP(+) (150 μM) in CONT medium. Following incubation, cells were fixed and nuclei stained with Hoechst. Scalebar = 10 μm. b CGNs were incubated essentially as described in a, but for 48 h and ± the pan-caspase inhibitor, BOC (20 μM). Nuclear morphology was quantified as either “normal”, “classically apoptotic” (Apo), or “chromatin aggregation” (ChAgg). Data represent the means ± SEM (n = 3); *P < 0.01 versus 5 K alone. c CGNs were treated exactly as described in a. Following incubation, TUNEL-positive cells were labeled with rhodamine (lower panels). Nuclei were stained with Hoechst (upper panels). Arrows indicate TUNEL-positive CGNs. In the 5 K condition, essentially all of the apoptotic cells were TUNEL-positive. Scale bar = 10 μm
First, we examined the role of caspases to elucidate the mechanisms responsible for the distinct nuclear morphology caused by MPP(+)-induced neurotoxicity. Contrasting studies have suggested a central role for caspase activation in MPP(+)-induced neurotoxicity (Gonzalez-Polo et al. 2001, 2003a, b, 2004) while other studies have demonstrated a caspase-independent mode of cell death (Choi et al. 2001; Chu et al. 2005). To address caspase involvement, we used CGNs incubated for 48 h in 5 K conditions ± the pan-caspase inhibitor, BOC, as a positive control for caspase-dependent apoptosis (Fig. 1b). In 5 K conditions, apoptotic nuclei composed ~90% of the overall CGN nuclear morphology observed. The addition of BOC significantly decreased the appearance of this classically apoptotic morphology with a corresponding increase in the percentage of healthy nuclei. In MPP(+)-treated CGNs, we predominantly observed the unique nuclear morphology characterized by extensive chromatin aggregation (Fig. 1b). In marked contrast to the 5 K condition, CGNs exposed to MPP(+) and co-incubated with BOC demonstrated no apparent reduction in the level of chromatin aggregation compared to CGNs exposed to MPP(+) alone.
As an additional measure of caspase involvement, we employed a TUNEL assay. Typically, control CGNs displayed very few TUNEL-positive cells (Fig. 1c, left panels), whereas TUNEL-positive cells were the majority of the population in 5 K-treated CGNs (Fig. 1c, middle panels). In contrast, CGNs incubated with MPP(+) displayed only an occasional TUNEL-positive cell and moreover, the unique nuclear morphology induced by MPP(+) was uniformly TUNEL-negative (Fig. 1c, right panels). These data support a key role for caspase-independent CGN death following exposure to MPP(+).
MPP(+)-Treated CGNs Display Calpain-dependent, Caspase-Independent Proteolysis of Known Substrates
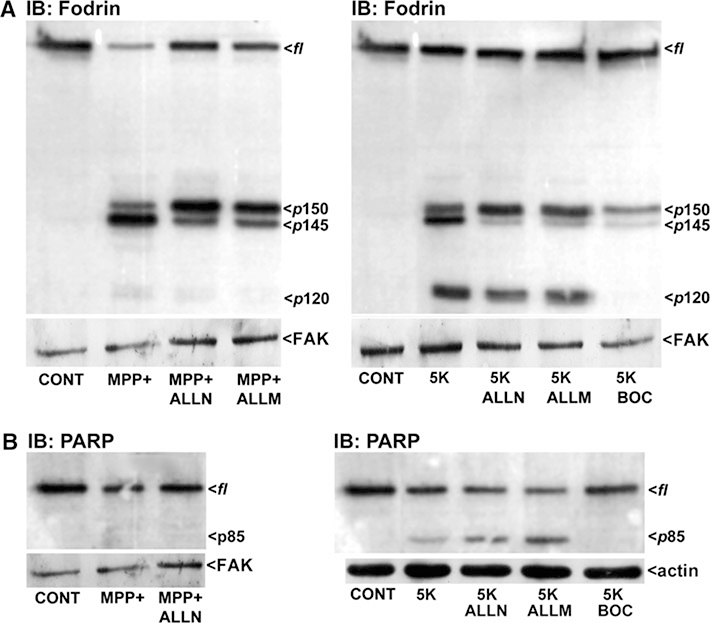
To compare the activities of calpains, Ca2+-dependent cysteine proteases, and caspases, cysteine-dependent aspartate-specific proteases, in MPP(+)-induced neurotoxicity, we assessed non-erythroid α-spectrin (fodrin) cleavage. CGNs were treated with MPP(+) ± the calpain inhibitors, ALLN or ALLM, to analyze calpain-dependent proteolysis. CGNs incubated for 24 h with MPP(+) displayed p150 and p145 kDa cleavage products of fodrin and a marked decrease in full-length (fl) fodrin (Fig. 2a, left blot). CGNs co-incubated with MPP(+) and the calpain inhibitors, ALLN or ALLM, showed a reduction in the p145 cleavage product and a corresponding increase in full-length (fl) fodrin. Wang et al. (Nath et al. 1996) have shown that the p145 kDa cleavage product of fodrin is a calpain-dependent fragment, explaining the partial blockage of fodrin cleavage in MPP(+)-treated CGNs with calpain inhibitors. This partial blockage of p145 formation may indicate the spatial and/or temporal regulation of calpain. Various members of the calpain family may be expressed or activated at different times or within different cellular compartments following MPP(+) exposure. Moreover, different calpains may be sensitive to distinct inhibitors or show limited-to-no susceptibility to ALLN or ALLM, for example. Therefore, ALLN and ALLM likely do not act as pan-calpain inhibitors and thus, retention of some of the p145 cleavage fragment is observed. Interestingly, 5 K also produced the p145 cleavage product and BOC decreased the appearance of this band under 5 K conditions, suggesting that caspases may actually act upstream of calpains in 5 K-treated CGNs. Wang et al. (Zhang et al. 2009) have recently shown that the p150 fodrin fragment may actually contain both calpain and caspase-generated products making this a less useful indicator of which specific protease is involved.
Fig. 2.
MPP(+) stimulates calpain-dependent proteolysis in the absence of significant caspase activation. CGNs were incubated for 24 h in either control medium containing 25 mM KCl and 10% FBS (CONT), apoptotic medium containing 5 mM KCl without FBS (5 K), or in CONT medium containing MPP(+) (150 μM). Some cultures were co-incubated with either the calpain inhibitors, ALLN or ALLM (2.5 μM), or the pan-caspase inhibitor, BOC (20 μM). Following incubation, cells were lysed, proteins resolved by SDS-PAGE, and membranes immunoblotted (IB) with antibodies to either Fodrin (a) or PARP (b). Western blots for focal adhesion kinase (FAK) and actin are shown as corresponding loading controls. fl, full-length; p150 and p145, calpain breakdown products of fodrin; p120, caspase-generated fragment of fodrin; p85, caspase-generated fragment of PARP
In contrast to the relatively nonspecific nature of the p150 cleavage fragment, the p120 kDa cleavage fragment of fodrin is definitively a caspase-generated fragment (Nath et al. 1996; Zhang et al. 2009). CGNs exposed to 5 K showed a striking increase in the p120 cleavage product. Following normalization to the loading control (FAK), the p120 band induced by 5 K was not decreased by either ALLN or ALLM. However, p120 was completely prevented by BOC consistent with this being a caspase-generated fragment of fodrin (Fig. 2a, right blot). In contrast to 5 K, MPP(+)-treated CGNs displayed barely detectable levels of this p120 caspase-generated fragment (Fig. 2a, left blot).
Further analyzing calpain and caspase involvement, we assessed the proteolysis of PARP which has a known caspase-dependent 85 kDa cleavage product (O’Brien et al. 2001; Wesierska-Gadek et al. 2004). In CGNs exposed to 5 K conditions, the p85 cleavage fragment is generated and is completely blocked by co-incubation with BOC. However, 5 K-treated CGNs co-incubated with calpain inhibitors do not show any reduction in the p85 cleavage product (Fig. 2b, right blot). In contrast, proteolysis of PARP to its p85 fragment is not observed in MPP(+)-treated CGNs (Fig. 2b, left blot).
Calpain Degrades Pro-caspases-9 and −3 in MPP(+)-Treated CGNs
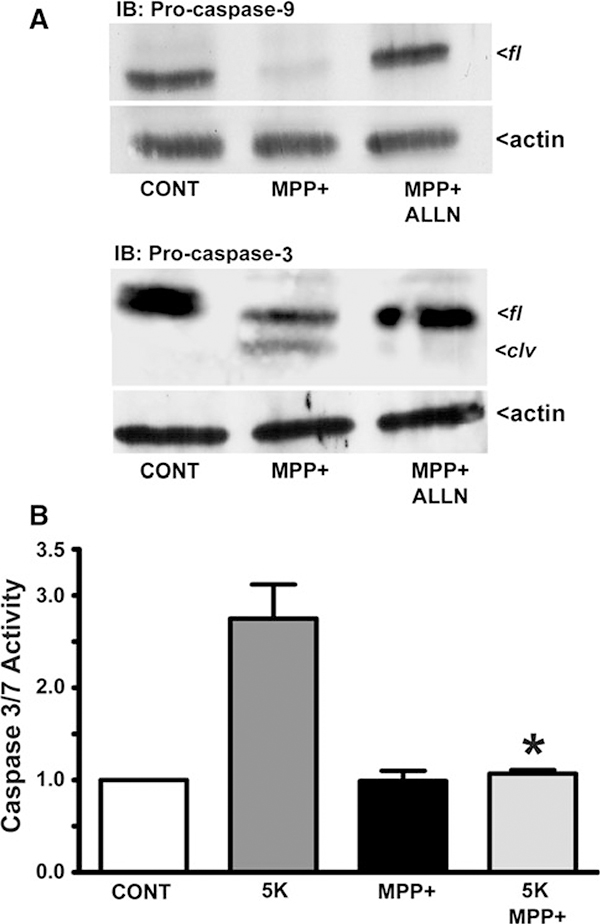
Next, we assessed the propensity of calpains to negatively regulate caspase activity in CGNs exposed to MPP(+). Previous studies indicate that calpains can selectively regulate caspases through inactivating proteolysis (Bizat et al. 2003; Chua et al. 2000; McGinnis et al. 1999). We examined the cleavage of endogenous pro-caspases-9 and −3 in CGNs incubated for 24 h with MPP(+). Complete degradation of pro-caspase-9 occurred which was prevented by co-incubation with ALLN (Fig. 3a, upper blot). Similarly, MPP(+) induced the degradation of full-length (fl) pro-caspase-3 to a lower molecular weight cleavage (clv) product, an effect that was similarly blocked by co-incubation with ALLN (Fig. 3a, lower blot).
Fig. 3.
MPP(+) inactivates pro-caspases-9 and −3 in CGNs via calpain-dependent degradation. a CGNs were incubated for 24 h in either control medium containing 25 mM KCl and 10% FBS (CONT), CONT medium including MPP(+) (150 μM), or CONT medium containing MPP(+) (150 μM) and the calpain inhibitor ALLN (2.5 μM). Following incubation, cells were lysed, proteins separated by SDS-PAGE and membranes immunoblotted (IB) with antibodies to either pro-caspase-9 (upper panel) or pro-caspase-3 (lower panel). Western blots for actin are shown as corresponding loading controls. fl, full-length; clv, calpain-generated cleavage product of pro-caspase-3. b Caspase 3/7 activity was quantified after a 4 h incubation period for CGNs subjected to either CONT medium or apoptotic medium with 5 mM KCl and lacking FBS (5 K), in either the absence of presence of MPP(+) (150 μM). The results are expressed as the fold change in caspase activity relative to the CONT which was set at a value of 1.0. Data represent the means ± SEM (n = 3); * P < 0.01 versus 5 K alone
To evaluate the potential consequences of pro-caspase degradation triggered by MPP(+)-induced neurotoxicity, we utilized a caspase 3/7 assay to detect the activity of these executioner caspases. CGNs were incubated for 4 h in either control medium, 5 K, MPP(+), or 5 K simultaneously incubated with MPP(+). Caspase-3 and −7 activities were then measured by luminescence as described in “Materials and Methods” section. Active caspase activity was highest in 5 K-treated CGNs, displaying approximately a three-fold increase over control (Fig. 3b). Neurons exposed to MPP(+) displayed no increase over controls in active caspase activity. Surprisingly, CGNs incubated in 5 K conditions and exposed simultaneously to MPP(+) also showed no increase in caspase activity over controls (Fig. 3b; *P < 0.01, 5 K + MPP(+) vs. 5 K alone). Collectively, these data suggest that MPP(+)-induced neurotoxicity in CGNs is caspase-independent likely as a result of calpain-specific degradation and inactivation of caspases.
Bcl-2 Family Members, Bid, Bax, and Bcl-2 are Putative Calpain Substrates in CGNs Exposed to MPP(+)
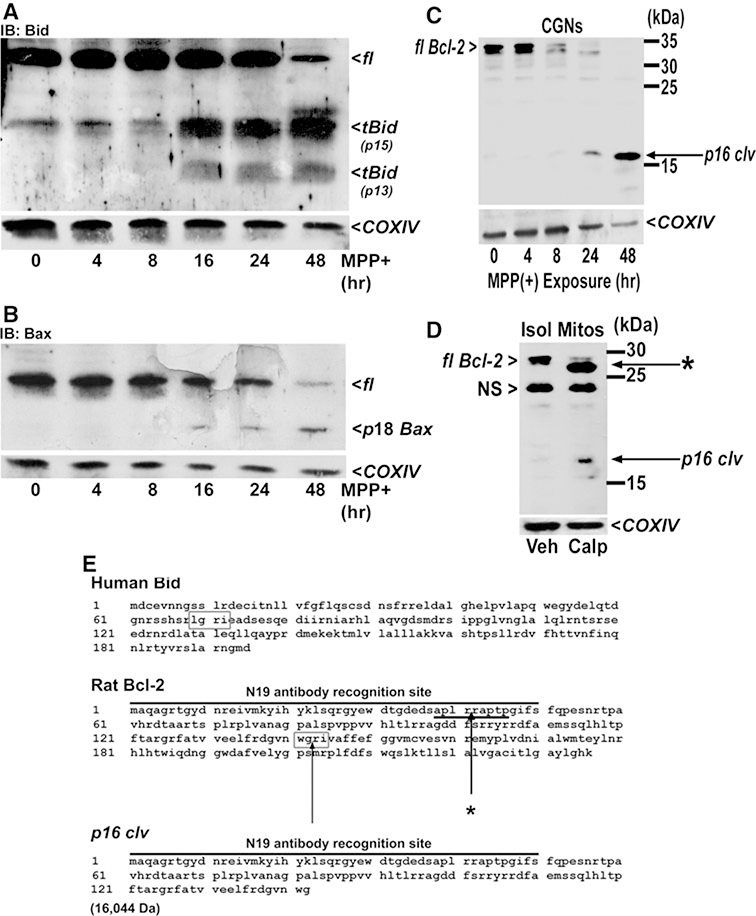
The calpain-dependence of MPP(+)-induced neurotoxicity in CGNs warranted study of Bcl-2 family members for proteolysis. Bcl-2 plays pivotal roles in regulating both oxidative stress and mitochondrial apoptosis by sequestering Bcl-2 homology-3 domain (BH3)-only proteins which regulate Bax-dependent cytochrome c release (Cheng et al. 2001). Bcl-2 regulates Bid, a BH3-only protein which has been shown to be proteolyzed in a calpain-dependent manner (Chen et al. 2001; Mandic et al. 2002). Proteolysis of Bid results in a truncated form of Bid (tBid) with the potential to induce Bax oligomerization at the mitochondrial outer membrane forming a cytochrome c pore (reviewed by Willis and Adams 2005). Moreover, Bid appears to be a necessary protein for signaling the Bax-dependent generation of oxygen radicals at the mitochondria (Ding et al. 2004). In addition to Bid, an active 18 kDa, calpain-dependent cleavage product of Bax has been previously identified, and this fragment has the potential to further enhance mitochondrial oxidative stress (Cao et al. 2003; Choi et al. 2001; Gao and Dou 2000; Wood and Newcomb 2000). In a 48-h timecourse, CGNs exposed to MPP(+) displayed marked Bid (tBid) and Bax (p18) truncation predominantly beginning at 16 h after treatment (Fig. 4a, b). These results indicate that Bid and Bax are probable calpain substrates in MPP(+)-treated CGNs.
Fig. 4.
The Bcl-2 family proteins, Bid, Bax, and Bcl-2 are putative calpain substrates in MPP(+)-treated CGNs. CGNs were incubated for up to 48 h in medium containing 25 mM KCl and 10% FBS and including MPP(+) (150 μM). Following incubation, cells were lysed, proteins resolved by SDS-PAGE, and membranes immunoblotted (IB) with antibodies to either, Bid (a), Bax (b), or Bcl-2 (c). d In a separate experiment, mitochondria were isolated from rat brain and were incubated in vitro with either vehicle or recombinant calpain I. Mitochondrial lysates were subsequently western blotted for Bcl-2. e Possible sites on rat Bcl-2 yielding putative calpain-generated fragments are indicated. Western blots for COXIV are shown in figures a–d as corresponding loading controls. fl, full-length; p16 clv, 16 kDa fragment of Bcl-2 generated by active calpain I; *, N-terminal truncated fragment of Bcl-2 generated by active calpain I; p18 Bax, calpain-generated fragment of Bax; NS non-specific band; tBid (p15), truncated Bid of ~15 kDa; tBid (p13), truncated Bid of ~13 kDa
As opposed to the pro-apoptotic and pro-oxidant effects of Bax and Bid at mitochondria, anti-apoptotic Bcl-2 has recently been shown to regulate mitochondrial susceptibility to oxidative stress through direct interaction with the intracellular antioxidant glutathione (GSH) (Zimmermann et al. 2007). In a 48-h timecourse of MPP(+ exposure, CGN lysates immunoblotted for Bcl-2 demonstrated the appearance of a 16-kDa cleavage product (p16 clv) after 24 h and a corresponding marked reduction of full-length Bcl-2 (fl Bcl-2) (Fig. 4c). The appearance of this 16 kDa fragment agrees with the presence of a putative cleavage site between G142 and R143 (WGRI) which is analogous to a known calpain cleavage site on human Bid between G69 and R70 (LGRI) (Fig. 4e). The comparable rat Bid sequence is HGRI (Itoh et al. 2003). Cleavage at G142/R143 of rat Bcl-2 would result in a predicted 16,044 Da (~ 16 kDa) fragment containing the N19 antibody recognition site (Fig. 4e). Upon incubating isolated rat brain mitochondria (Isol Mitos) with active calpain I in vitro, the calpain-incubated fraction exhibited the appearance of a 16-kDa Bcl-2 fragment while the fl Bcl-2 was nearly completely degraded (Fig. 4d). An additional fragment (*) was present in the calpain-incubated fraction possibly due to cleavage of Bcl-2 between R41 and R42 (Fig. 4e), a site consistent with Friedrich and colleagues’ predictions of consensus calpain cleavage sites (Tompa et al. 2004). The absence of this additional Bcl-2 fragment (denoted by (*) in Fig. 4d) in MPP(+)-treated CGNs (see Fig. 4c) suggests that calpain I may not be the principal calpain isoform activated by MPP(+) in these neurons. Alternatively, this putative calpain cleavage site may be masked in the endogenous Bcl-2 protein found in CGNs. Our findings indicate a likely role for calpain-dependent proteolysis of Bcl-2 family members in MPP(+)-induced neurotoxicity.
Significant Protection of MPP(+)-Treated CGNs is Observed Through a Combination of Cysteine Protease Inhibition and Addition of the Critical Antioxidant Glutathione
Accumulation of MPP(+) in CGNs results in a coordinated activation of calpains and production of ROS at mitochondria resulting in neuronal death. Since calpains directly cleave and inactivate caspases in CGNs exposed to MPP(+) (see Fig. 3), calpain inhibition might be expected to de-repress caspases under these conditions. Therefore, we predicted that inhibitors of both calpains and caspases may need to be used in combination to significantly reduce MPP(+) neurotoxicity. Moreover, Bid truncation (Chen et al. 2001; Mandic et al. 2002) and Bax oligomerization in the outer mitochondrial membrane result in augmentation of ROS production at this organelle (Starkov et al. 2002; Wei et al. 2001; Wood and Newcomb 2000). In addition, we recently showed a key role for Bcl-2 in regulating the mitochondrial GSH antioxidant pool and the potential for BH3-only proteins to inhibit this antioxidant function of Bcl-2 (Zimmermann et al. 2007). Given that Bid, Bax, and Bcl-2 are proteolyzed in MPP(+)-treated CGNs, we hypothesized that exogenous GSH may also be necessary to attenuate MPP(+) neurotoxicity.
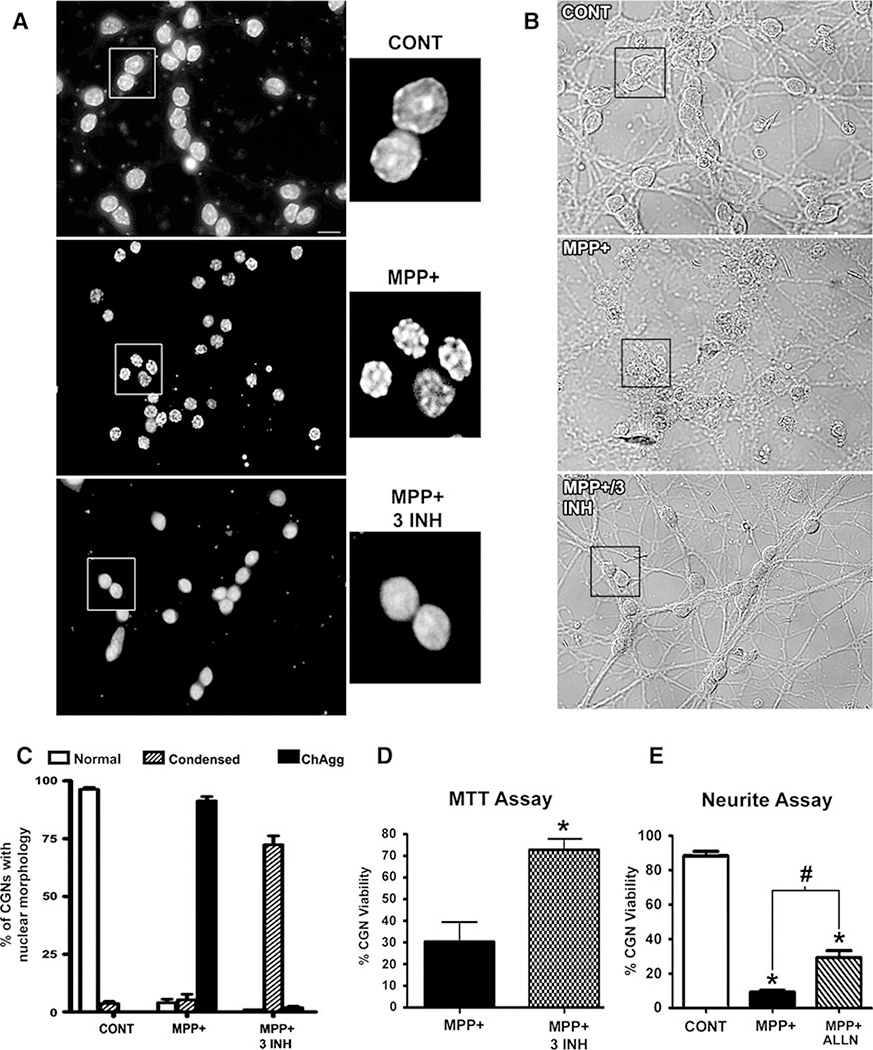
To test the above hypotheses, we evaluated the effects of a calpain inhibitor, a caspase inhibitor, and a cell-permeable ester of GSH, either alone or in combination, on CGN death induced by MPP(+). Hoechst staining of CGNs exposed to MPP(+) and co-incubated with an inhibitor/antioxidant cocktail including ALLN, BOC, and GSH, showed a distinct improvement in nuclear morphology characterized by a complete absence of chromatin aggregation normally induced by MPP(+) in these neurons (Fig. 5a, compare middle and lower panels). Although, the inhibitor/antioxidant cocktail essentially prevented MPP(+)-induced chromatin aggregation, the nuclei still remained somewhat condensed when compared to control CGNs (Fig. 5a,compare upper and lower panels). The inhibitor/antioxidant cocktail largely preserved CGN neuritic processes that were otherwise significantly fragmented in MPP(+)-treated CGNs (Fig. 5b). Quantitatively, we found that MPP(+)-treated CGNs co-incubated with any inhibitor alone, or a combination of any two inhibitors, did not show any significant difference in the amount of chromatin aggregation from that observed with MPP(+) alone (data not shown). In contrast, co-incubation of MPP(+)-treated CGNs with all three components resulted in a marked shift from chromatin aggregation (ChAgg) to a somewhat condensed, but non-fragmented and non-aggregated nuclear morphology (Fig. 5c). Co-incubation of MPP(+)-treated CGNs with all three inhibitors also significantly enhanced neuronal viability measured by an MTT assay (Fig. 5d). When viability was assayed by quantifying the percentage of CGNs with intact neuronal processes greater than two soma lengths, the addition of the calpain inhibitor, ALLN, was sufficient to induce a statistically significant, although modest, increase in viability relative to MPP(+) alone (Fig. 5e). These data demonstrate that a combined inhibition of calpains and caspases, in addition to supplementation with GSH, significantly decreases neurotoxicity induced by MPP(+) in CGNs.
Fig. 5.
MPP(+)-induced toxicity in CGNs is significantly attenuated by a drug cocktail including glutathione and inhibitors of caspases and calpains. a CGNs were incubated for 24 h in either control medium containing 25 mM KCl and 10%FBS (CONT, upper panel) or CONT medium containing 150 μM MPP(+) (middle panel). Additionally, the lower panel shows CGNs exposed to MPP(+) and co-incubated with a pan-caspase inhibitor, BOC (20 μM), a calpain inhibitor, ALLN (10 μM), and glutathione monoethyl ester (GSH; 2 mM) (MPP + 3 INH; i.e., an inhibitor cocktail). Following incubation, cells were fixed and nuclei stained with Hoechst. Areas demarcated by the boxes were enlarged 300% to show detailed nuclear morphology. Scale bar = 10 μm. b Corresponding brightfield images show gross cell morphology including neuritic processes. Orientation identical to (a). Note healthier neurites in the lower panel (MPP + 3INH) compared to the middle panel (MPP + alone). c CGNs were treated exactly as in a. Nuclei were quantified based on their morphology as either “normal” (as seen in CONT), “condensed” (as seen in MPP+ 3 INH), or “chromatin aggregation” (as seen in MPP+ alone) (ChAgg). Data represent mean ± SEM (n = 3). d CGNs were treated as described in (a) except for 16 h. Following incubation, percent cell viability (relative to CONT) of MPP(+)-treated CGNs and CGNs exposed to MPP+ 3INH was measured by the reduction of a tetrazolium salt, MTT (see Experimental Procedures). Percent viability of CONT cells was set at 100%. Data represent the mean ± SEM (n = 5); * P < 0.01 versus MPP(+) alone. E. CGNs were treated for 24 h with either CONT medium, MPP+ alone, or MPP+ in combination with ALLN (2.5 μM). Cells possessing neurites longer than two times the cell body were quantified as viable. Data represent the mean ± SEM (n = 5); * P < 0.01 versus CONT; # P < 0.05 versus MPP+ alone
MPP(+) Induces Calpain-Dependent Cleavage of Cyclin E to Active Low Molecular Weight Fragments
Cyclin E is a cell cycle protein which regulates cyclin-dependent kinase 2 at the G1/S checkpoint (Keyomarsi and Pardee 1993; Keyomarsi et al. 1995; Keyomarsi and Herliczek 1997; Harwell et al. 2000; Wang et al. 2003). Cyclin E can be cleaved to active low molecular weight fragments by calpain (Libertini et al. 2005; Wang et al. 2003). This activation of cyclin E induces cell cycle entry which may contribute to neurotoxicity and/or neurodegenerative diseases (Yang et al. 2001, 2003; Jordan-Sciutto et al. 2002).
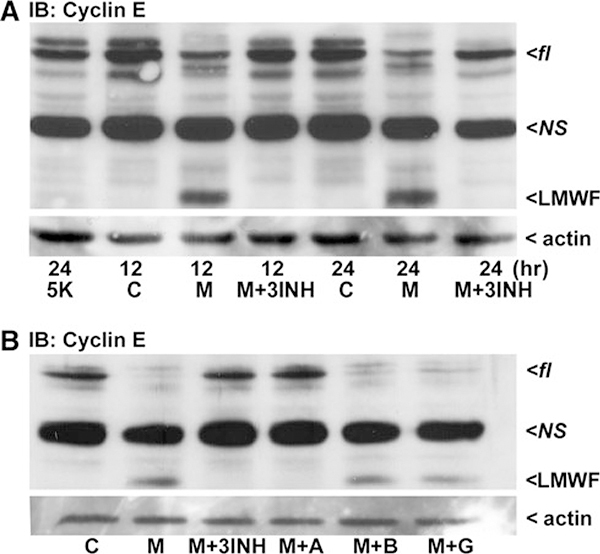
Moreover, recent studies have suggested that MPP(+) may trigger cell cycle re-entry in CGNs (Alvira et al. 2007). Therefore, we examined cyclin E processing and found control neurons lacked the appearance of an active low molecular weight fragment (LMWF) of cyclin E. On the other hand, CGNs exposed to MPP(+) produced a LMWF of cyclin E by 12 h which continued to be present at 24 h (Fig. 6a). The formation of this LMWF was completely blocked in CGNs co-incubated with the combination inhibitor/antioxidant cocktail used previously (Fig. 6a). In contrast, 5 K-treated CGNs did not display the LMWF of cyclin E indicating that the mechanism responsible for cyclin E processing was not activated by this apoptotic stimulus (Fig. 6a, first lane).
Fig. 6.
The cell cycle protein, Cyclin E, is processed by calpain to an active low molecular weight fragment in MPP(+)-treated CGNs. CGNs were incubated for either 12 or 24 h in control medium containing 25 mM KCl and 10% FBS (c), or control medium containing 150 μM MPP(+) (M). Alternatively, CGNs were co-incubated with MPP(+) and either a pan-caspase inhibitor, BOC (B; 20 μM), a calpain inhibitor, ALLN (A; 10 μM), glutathione (G; 2 mM), or all three inhibitors together (3INH). To compare to a classical apoptotic stimulus, cells were incubated for 24 h in medium containing 5 mM KCl minus FBS (5 K). Following incubation, cells were lysed, proteins resolved by SDS-PAGE, and membranes immunoblotted (IB) with antibodies to Cyclin E (a and b). Western blots for actin are shown as corresponding loading controls. fl, full-length; NS non-specific band, LMWF low-molecular weight fragment produced by calpain
Consistent with this finding, co-incubation of MPP(+)-treated CGNs with BOC alone had no effect on cyclin E processing (Fig. 6b), demonstrating that caspases were not required. Similarly, GSH had no effect on MPP(+)-induced cyclin E processing. However, ALLN completely prevented cyclin E proteolysis in MPP(+)-treated CGNs consistent with an essential role for calpain in neuronal death (Fig. 6b). These findings are in accordance with Libertini et al. (2005) and suggest that Complex I inhibition by MPP(+) may induce the cell cycle in CGNs through calpain-dependent proteolysis of cyclin E.
Discussion
Our results indicate a central role of calpain in the mechanism of CGN death induced by exposure to the complex I inhibitor and neurotoxin MPP(+). However, inhibition of calpain alone does not significantly attenuate MPP(+)-induced neurotoxicity. Instead, a combination of calpain and caspase inhibitors, coupled with the critical antioxidant GSH, is necessary to significantly ameliorate MPP(+)-induced toxicity in CGNs. These findings suggest that multiple pro-death pathways (e.g., mitochondrial oxidative stress) act in a coordinated manner with calpain to cause MPP(+)-induced neurotoxicity.
There are many examples demonstrating either caspase-dependent, classically apoptotic modes of neuronal death or non-apoptotic mechanisms of MPP(+)-induced neurotoxicity (Table 1). In the same dopaminergic cell line (PC12), the primary mechanism of cell death induced by MPP(+) may be either apoptotic or non-apoptotic (Kim et al. 2009; Wales et al. 2008). In a similar manner, CGN death induced by MPP(+) has either been shown to exhibit strong caspase dependence (Du et al. 1997), or alternatively displays a key role for calpain activation as well (Leist et al. 1998). In order to systematically evaluate the roles of calpains and caspases in CGNs, low potassium (5 K) conditions were used as a well-characterized model of caspase-dependent apoptosis which could be directly contrasted to MPP(+) toxicity in this primary neuronal system. By comparing these models directly in CGNs, we find that MPP(+)-induced toxicity shows little-to-no similarities to classical apoptosis such as that triggered by 5 K.
Our results demonstrate a prominent role for calpain and a mostly caspase-independent mechanism for MPP(+)-induced neurotoxicity in CGNs as evidenced by: (1) the unique nuclear morphology of MPP(+)-treated CGNs compared to 5 K (apoptosis), (2) the lack of caspase-dependent proteolytic fragments of fodrin and PARP, (3) the calpain-mediated degradation and inhibition of caspases, (4) calpain-dependent proteolysis of pro-death Bcl-2 family members Bid and Bax, (5) calpain-dependent proteolysis of pro-survival Bcl-2, and (6) calpain-sensitive proteolysis of cyclin E. Previous study demonstrated that fragments of fodrin at 120 kDa (Nath et al. 1996) and PARP at 85 kDa (O’Brien et al. 2001; Wesierska-Gadek et al. 2004) are caspase-dependent; however, these fragments were notably absent in MPP(+)-treated CGNs indicating a lack of caspase activation. The above findings are consistent with the results showing that pro-caspase-9 and −3 are degraded in a calpain-dependent manner in MPP(+)-treated CGNs which likely contributes to the observed lack of caspase activity. Many groups have reported that calpain-dependent cleavage can result in the inactivation of caspases (Bizat et al. 2003; Chua et al. 2000; McGinnis et al. 1999). Furthermore, we show that caspase-3/7 activity induced by culturing CGNs in 5 K medium is suppressed by concomitant exposure to MPP(+), suggesting that caspase activity is inhibited through activation of calpain by this neurotoxin. These results indicate that calpain can act as a key negative regulator of caspases during neurotoxicity.
The present study provides data identifying Bcl-2 as a novel calpain substrate during MPP(+)-induced neurotoxicity in CGNs. Bcl-2 is a mitochondrial outer membrane protein which has been shown to regulate a pool of the critical antioxidant, GSH, at mitochondria (Zimmermann et al. 2007). This antioxidant function of Bcl-2 at mitochondria is in addition to its negative regulation of Bax-induced apoptosis through sequestration of BH3-only proteins like Bid (Cheng et al. 2001). Interestingly, Bcl-2 is also capable of inhibiting cell cycle progression by arresting cells in G0 (Janumyan et al. 2003). The putative calpain-dependent proteolysis of Bcl-2 is predicted to occur between G142 and R143 within the BH3 groove (see Fig. 4e). This site on rat Bcl-2 is homologous to the known calpain cleavage site on human Bid, and processing of Bcl-2 at this site would yield the observed 16 kDa cleavage fragment of this pro-survival protein. The BH3 groove is critical for Bcl-2 binding to and neutralizing pro-apoptotic Bid and Bax, as well as its potential to bind GSH and regulate the mitochondrial pool of this antioxidant (Zimmermann et al. 2007). Thus, proteolysis of Bcl-2 by calpain may contribute to enhanced pro-death activities of Bid and Bax, increased mitochondrial oxidative stress, and cell cycle re-entry during MPP(+) toxicity.
In addition to Bcl-2 proteolysis, we also observed calpain-dependent cleavage of Bax to an 18-kDa form known to be more active at mitochondria (Cao et al. 2003). Moreover, Bid was processed to tBid in MPP(+)-treated CGNs suggesting that this BH3-only protein was activated by calpain proteolysis as well. Collectively, these data indicate that calpain cleavage of Bcl-2, Bid, and Bax likely contributes to MPP(+)-induced neurotoxicity in CGNs. Cleavage of Bid and Bax may enhance the neurotoxic effects of MPP(+) in CGNs while cleavage of Bcl-2 may repress its ability to either sequester pro-death Bid and Bax or regulate the redox state at mitochondria through modulation of GSH pools.
Finally, our data suggest a key role for calpain in triggering cell cycle re-entry in MPP(+)-treated CGNs. A study by Libertini et al. (2005) significantly demonstrated—in MCF7 metastatic breast cancer cells—the ability of cyclin E to transactivate the gene for calpain 2, enhancing the cell cycle while simultaneously stimulating calpain activation. In response to MPP(+), calpain-dependent proteolysis of cyclin E to active LMWFs and reciprocal calpain activation may be acting in a feed forward manner to force post-mitotic CGNs into the cell cycle, ultimately resulting in cell death.
In summary, our data suggest the multi-faceted role of calpain in facilitating MPP(+)-induced neurotoxicity. While the data leave questions regarding the specific timing and localization of calpain activation, they strongly suggest the central role of calpain in CGN death induced by MPP(+) (Fig. 7). As proteolysis of cyclin E could be completely prevented by a calpain inhibitor alone in lieu of the fodrin proteolysis data showing that calpain inhibitors could not completely block processing, a hypothesis is reinforced that multiple isoforms of calpain may be activated at different time points and within distinct cellular compartments during MPP(+)-induced neurotoxicity. Additionally, cross-talk between members of the calpain and caspase families may be occurring which prevents one class of inhibitor alone from completely blocking the toxic effects of MPP(+). Finally, part of this calpain activation may be dependent on the degree and timing of the calcium influx into the neuron as Neumar et al. (2003) previously suggested. Therefore, calcium-mediated excitotoxicity due to MPP(+) may utilize distinct members of the calpain family to coordinate different stages of neurotoxicity. To date, at least 16 mammalian members of the calpain family have been identified and several of these are alternatively spliced into multiple isoforms (Zatz and Starling 2005; Saez et al. 2006). Nine of these calpain family members appear to demonstrate a ubiquitous expression pattern while the others show tissue-specific expression. In addition, at least 50 distinct endogenous or exogenous inhibitors of calpain have been discovered and many of these demonstrate broad selectivity for multiple family members (Saez et al. 2006). These facts make it a difficult task to definitively identify which calpain family members and/or isoforms may be involved in a given pathological state. Nonetheless, in the MPP(+) model of neurotoxicity in CGNs, our findings suggest a major role of calpain activation in triggering multiple cell death signals through proteolysis of critical substrates including pro-caspases, Bcl-2 family members, and cell cycle regulatory proteins. It is the complex interplay of these diverse death signals that ultimately triggers neuronal death in response to this neurotoxin.
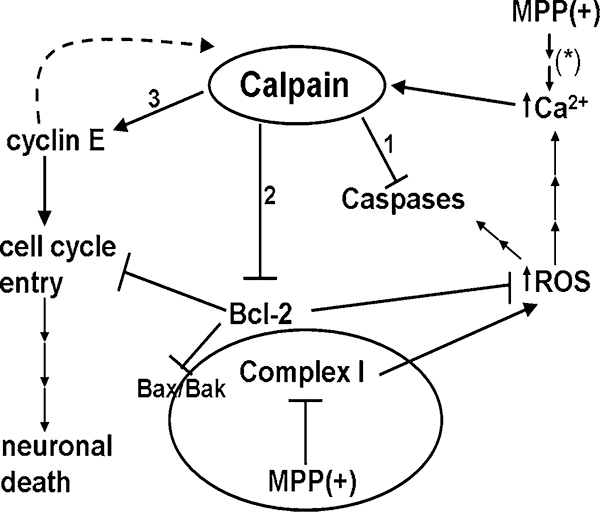
Fig. 7.
Calpain plays a central role in MPP(+)-induced neurotoxicity. The neurotoxin, MPP(+) causes neuronal death principally through inhibition of Complex I of the mitochondrial respiratory chain. Inhibition of Complex I leads to the formation of reactive oxygen species (ROS) and an influx of calcium (Ca2+) into the neuron. MPP(+) may cause increased intracellular Ca2+ via the production of ROS or by alternative pathways (*), e.g., via NMDA receptor sensitization. An increased concentration of intracellular Ca2+ results in the activation of calpain, a Ca2+-dependent cysteine protease. Activation of calpain in CGNs results in several important downstream effects detailed in this manuscript: (1) Calpain degrades initiator and executioner pro-caspases, −9 and −3, respectively. (2) The pro-survival protein, Bcl-2, localized to the mitochondrial outer membrane, is putatively cleaved by calpain. Cleavage of Bcl-2 likely disrupts its anti-apoptotic, antioxidant, and cell cycle inhibitory functions. (3) Cyclin E undergoes calpain-dependent proteolysis forming active low molecular weight fragments. Active cyclin E has been shown to transactivate the gene for calpain 2 which in turn, would lead to additional proteolysis of cyclin E. Constitutively active cyclin E/Cdk 2 complexes resulting from the production of active low molecular weight fragments of cyclin E induce entry into the cell cycle of terminally differentiated neurons, ultimately causing cell death
Contributor Information
Richard A. Harbison, Department of Biological Sciences and Eleanor Roosevelt Institute, University of Denver, Seeley G. Mudd Science Bldg., Rm.130, 2101 E. Wesley Ave, Denver 80208, CO, USA
Kristen R. Ryan, Toxicology Program, Department of Pharmaceutical Sciences, University of Colorado Denver, Aurora, CO, USA
Heather M. Wilkins, Department of Biological Sciences and Eleanor Roosevelt Institute, University of Denver, Seeley G. Mudd Science Bldg., Rm.130, 2101 E. Wesley Ave, Denver 80208, CO, USA
Emily K. Schroeder, Research Service, Veterans Affairs Medical Center, Denver, CO, USA
F. Alexandra Loucks, Neuroscience Program, University of California San Francisco, San Francisco, CA, USA.
Ron J. Bouchard, Research Service, Veterans Affairs Medical Center, Denver, CO, USA
Daniel A. Linseman, Department of Biological Sciences and Eleanor Roosevelt Institute, University of Denver, Seeley G. Mudd Science Bldg., Rm.130, 2101 E. Wesley Ave, Denver 80208, CO, USA Research Service, Veterans Affairs Medical Center, Denver, CO, USA; Division of Clinical Pharmacology and Toxicology, Department of Medicine, University of Colorado Denver, Aurora, CO, USA.
References
- Alvira D, Tajes M, Verdaguer E, de Arriba SG, Allgaier C, Matute C, Trullas R, Jiménez A, Pallàs M, Camins A (2007) Inhibition of cyclin-dependent kinases is neuroprotective in 1-methyl-4-phenylpyridinium-induced apoptosis in neurons. Neuroscience 146:350–365 [DOI] [PubMed] [Google Scholar]
- Anantharam V, Kaul S, Song C, Kanthasamy A, Kanthasamy AG (2007) Pharmacological inhibition of neuronal NADPH oxidase protects against 1-methyl-4-phenylpyridinium (MPP +)-induced oxidative stress and apoptosis in mesencephalic dopaminergic neuronal cells. Neurotoxicology 28:988–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizat N, Hermel JM, Humbert S, Jacquard C, Créminon C, Escartin C, Saudou F, Krajewski S, Hantraye P, Brouillet E (2003) In vivo calpain/caspase cross-talk during 3-nitropropionic acid-induced striatal degeneration: implication of a calpain-mediated cleavage of active caspase-3. J Biol Chem 278:43245–43253 [DOI] [PubMed] [Google Scholar]
- Blomgren K, Zhu C, Wang X, Karlsson JO, Leverin AL, Bahr BA, Mallard C, Hagberg H (2001) Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia. J Biol Chem 276:10191–10198 [DOI] [PubMed] [Google Scholar]
- Bo J, Ming BY, Gang LZ, Lei C, Jia AL (2005) Protection by puerarin against MPP+-induced neurotoxicity in PC12 cells mediated by inhibiting mitochondrial dysfunction and caspase-3-like activation. Neurosci Res 53:183–188 [DOI] [PubMed] [Google Scholar]
- Cao X, Deng X, May WS (2003) Cleavage of Bax to p18 Bax accelerates stress-induced apoptosis, and a cathepsin-like protease may rapidly degrade p18 Bax. Blood 102:2605–2614 [DOI] [PubMed] [Google Scholar]
- Chen M, He H, Zhan S, Krajewski S, Reed JC, Gottlieb RA (2001) Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J Biol Chem 276: 30724–30728 [DOI] [PubMed] [Google Scholar]
- Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ (2001) BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell 8:705–711 [DOI] [PubMed] [Google Scholar]
- Cheng YF, Zhu GQ, Wang M, Cheng H, Zhou A, Wang N, Fang N, Wang XC, Xiao XQ, Chen ZW, Li QL (2009) Involvement of ubiquitin proteasome system in protective mechanisms of puerarin to MPP(+)-elicited apoptosis. Neurosci Res 63:52–58 [DOI] [PubMed] [Google Scholar]
- Choi WS, Lee EH, Chung CW, Jung YK, Jin BK, Kim SU, Oh TH, Saido TC, Oh YJ (2001) Cleavage of Bax is mediated by caspase-dependent or–independent calpain activation in dopaminergic neuronal cells: protective role of Bcl-2. J Neurochem 77:1531–1541 [DOI] [PubMed] [Google Scholar]
- Choi WS, Lee E, Lim J, Oh YJ (2008) Calbindin-D28K prevents drug-induced dopaminergic neuronal death by inhibiting caspase and calpain activity. Biochem Biophys Res Commun 371:127–131 [DOI] [PubMed] [Google Scholar]
- Chu CT, Zhu JH, Cao G, Signore A, Wang S, Chen J (2005) Apoptosis inducing factor mediates caspase-independent 1-methyl-4-phenylpyridinium toxicity in dopaminergic cells. J Neurochem 94:1685–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua BT, Guo K, Li P (2000) Direct cleavage by the calcium-activated protease calpain can lead to inactivation of caspases. J Biol Chem 275:5131–5135 [DOI] [PubMed] [Google Scholar]
- D’Mello SR, Galli C, Ciotti T, Calissano P (1993) Induction of apoptosis in cerebellar granule neurons by low potassium: inhibition of death by insulin-like growth factor I and cAMP. Proc Natl Acad Sci USA 90:10989–10993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Ni HM, DiFrancesca D, Stolz DB, Yin XM (2004) Bid-dependent generation of oxygen radicals promotes death receptor activation-induced apoptosis in murine hepatocytes. Hepatology 40:403–413 [DOI] [PubMed] [Google Scholar]
- Domingues AF, Esteves AR, Swerdlow RH, Oliveira CR, Cardoso SM (2008) Calpain-mediated MPP+ toxicity in mitochondrial DNA depleted cells. Neurotox Res 13:31–38 [DOI] [PubMed] [Google Scholar]
- Du YS, Dodel RC, Bales KR, Jemmerson R, Hamilton-Byrd E, Paul SM (1997) Involvement of a caspase-3-like cysteine protease in 1-methyl-4-phenypyridinium-mediated apoptosis of cultured cerebellar granule neurons. J Neurochem 69:1382–1388 [DOI] [PubMed] [Google Scholar]
- Ekshyyan O, Aw TY (2004) Apoptosis: a key in neurodegenerative disorders. Curr Neurovasc Res 1:355–371 [DOI] [PubMed] [Google Scholar]
- Gao G, Dou QP (2000) N-terminal cleavage of bax by calpain generates a potent proapoptotic 18-kDa fragment that promotes bcl-2-independent cytochrome c release and apoptotic cell death. J Cell Biochem 80:53–72 [DOI] [PubMed] [Google Scholar]
- González-Polo RA, Mora A, Clemente N, Sabio G, Centeno F, Soler G, Fuentes JM (2001) Mechanisms of MPP(+) incorporation into cerebellar granule cells. Brain Res Bull 56:119–123 [DOI] [PubMed] [Google Scholar]
- González-Polo RA, Soler G, Alonso JC, Rodríguez-Martín A, Fuentes JM (2003a) MPP(+) causes inhibition of cellular energy supply in cerebellar granule cells. Neurotoxicology 24:219–225 [DOI] [PubMed] [Google Scholar]
- González-Polo RA, Soler G, Alvarez A, Fabregat I, Fuentes JM (2003b) Vitamin E blocks early events induced by 1-methyl-4-phenylpyridinium (MPP +) in cerebellar granule cells. J Neurochem 84:305–315 [DOI] [PubMed] [Google Scholar]
- González-Polo RA, Soler G, Fuentes JM (2004) MPP+: mechanism for its toxicity in cerebellar granule cells. Mol Neurobiol 30:253–264 [DOI] [PubMed] [Google Scholar]
- Han BS, Hong HS, Choi WK, Markelonis GJ, Oh TH, Oh YJ (2003) Caspase-dependent and-independent cell death pathways in primary cultures of mesencephalic dopaminergic neurons after neurotoxin treatment. J Neurosci 23:5069–5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwell RM, Porter DC, Danes C, Keyomarsi K (2000) Processing of cyclin E differs between normal and tumor breast cells. Cancer Res 60:481–489 [PubMed] [Google Scholar]
- Itoh T, Itoh H, Pleasure D (2003) Bcl-2-related protein family gene expression during oligodendroglial differentiation. J Neurochem 85:1500–1512 [DOI] [PubMed] [Google Scholar]
- Janumyan YM, Sansam CG, Chattopadhyay A, Cheng N, Soucie EL, Penn LZ, Andrews D, Knudson CM, Yang E (2003) Bcl-xL/Bcl-2 coordinately regulates apoptosis, cell cycle arrest and cell cycle entry. EMBO J 22:5459–5470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan-Sciutto KL, Malaiyandi LM, Bowser R (2002) Altered distribution of cell cycle transcriptional regulators during Alzheimer disease. J Neuropathol Exp Neurol 61:358–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdi H, Hamo L, Oka T, Seegan A, Baudry M (2009) BDNF mediates the neuroprotective effects of positive AMPA receptor modulators against MPP(+)-induced toxicity in cultured hippocampal and mesencephalic slices. Neuropharmacology 56: 876–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyomarsi K, Herliczek TW (1997) The role of cyclin E in cell proliferation, development and cancer. Prog Cell Cycle Res 3:171–191 [DOI] [PubMed] [Google Scholar]
- Keyomarsi K, Pardee AB (1993) Redundant cyclin overexpression and gene amplification in breast cancer cells. Proc Natl Acad Sci USA 90:1112–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyomarsi K, Conte D Jr, Toyofuku W, Fox MP (1995) Deregulation of cyclin E in breast cancer. Oncogene 11:941–950 [PubMed] [Google Scholar]
- Kim NK, Choi BH, Huang X, Snyder BJ, Bukhari S, Kong TH, Park H, Park HC, Park SR, Ha Y (2009) Granulocyte-macrophage colony-stimulating factor promotes survival of dopaminergic neurons in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced murine Parkinson’s disease model. Eur J Neurosci 29:891–900 [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzi L, Vandenabeele P, Abrams J, Alneri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nuñez G, Peter ME, Tscopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G (2009) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 16:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist M, Volbracht C, Fava E, Nicotera P (1998) 1-Methyl-4-phenylpyridinium induces autocrine excitotoxicity, protease activation, and neuronal apoptosis. Mol Pharmacol 54:789–801 [DOI] [PubMed] [Google Scholar]
- Liang LP, Patel M (2004) Mitochondrial oxidative stress and increased seizure susceptibility in Sod2−/+ mice. Free Radic Biol Med 36:542–554 [DOI] [PubMed] [Google Scholar]
- Libertini SJ, Robinson BS, Dhillon NK, Glick D, George M, Dandekar S, Gregg JP, Sawai E, Mudryj M (2005) Cyclin E both regulates and is regulated by calpain 2, a protease associated with metastatic breast cancer phenotype. Cancer Res 65:10700–10708 [DOI] [PubMed] [Google Scholar]
- Linseman DA, Phelps RA, Bouchard RJ, Le SS, Laessig TA, McClure ML, Heidenreich KA (2002) Insulin-like growth factor-I blocks Bcl-2 interacting mediator of cell death (Bim) induction and intrinsic death signaling in cerebellar granule neurons. J Neurosci 22:9287–9297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linseman DA, Butts BD, Precht TA, Phelps RA, Le SS, Laessig TA, Bouchard RJ, Florez-McClure ML, Heidenreich KA (2004) Glycogen synthase kinase-3beta phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J Neurosci 24:9993–10002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucks FA, Le SS, Zimmermann AK, Ryan KR, Barth H, Aktories K, Linseman DA (2006) Rho family GTPase inhibition reveals opposing effects of mitogen-activated protein kinase kinase/extracellular signal-regulated kinase and Janus kinase/signal transducer and activator of transcription signaling cascades on neuronal survival. J Neurochem 97:957–967 [DOI] [PubMed] [Google Scholar]
- Loucks FA, Schroeder EK, Zommer AE, Hilger S, Kelsey NA, Bouchard RJ, Blackstone C, Brewster JL, Linseman DA (2009) Caspases indirectly regulate cleavage of the mitochondrial fusion GTPase OPA1 in neurons undergoing apoptosis. Brain Res 1250:63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandic A, Viktorsson K, Strandberg L, Heiden T, Hansson J, Linder S, Shoshan MC (2002) Calpain-mediated Bid cleavage and calpain-independent Bak modulation: two separate pathways in cisplatin-induced apoptosis. Mol Cell Biol 22:3003–3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Green DR, Cotter TG (1994) Dicing with death: dissecting the components of the apoptosis machinery. Trends Biochem Sci 19:26–30 [DOI] [PubMed] [Google Scholar]
- McGinnis KM, Gnegy ME, Park YH, Mukerjee N, Wang KK (1999) Procaspase-3 and poly(ADP)ribose polymerase (PARP) are calpain substrates. Biochem Biophys Res Commun 263:94–99 [DOI] [PubMed] [Google Scholar]
- Nath R, Raser KJ, Stafford D, Hajimohammadreza I, Posner A, Allen H, Talanian RV, Yuen P, Gilbertsen RB, Wang KK (1996) Non-erythroid alpha-spectrin breakdown by calpain and interleukin 1 beta-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem J 319(Pt 3):683–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumar RW, Xu YA, Gada H, Guttmann RP, Siman R (2003) Cross-talk between calpain and caspase proteolytic systems during neuronal apoptosis. J Biol Chem 278:14162–14167 [DOI] [PubMed] [Google Scholar]
- O’Brien MA, Moravec RA, Riss TL (2001) Poly (ADP-ribose) polymerase cleavage monitored in situ in apoptotic cells. Biotechniques 30:886–891 [DOI] [PubMed] [Google Scholar]
- Okouchi M, Ekshyyan O, Maracine M, Aw TY (2007) Neuronal apoptosis in neurodegeneration. Antioxid Redox Signal 9: 1059–1096 [DOI] [PubMed] [Google Scholar]
- Rubin LL, Gatchalian CL, Rimon G, Brooks SF (1994) The molecular mechanisms of neuronal apoptosis. Curr Opin Neurobiol 4: 696–702 [DOI] [PubMed] [Google Scholar]
- Saez ME, Ramirez-Lorca R, Moron FJ, Ruiz A (2006) The therapeutic potential of the calpain family: new aspects. Drug Discov Today 11:917–923 [DOI] [PubMed] [Google Scholar]
- Shang T, Uihlein AV, Van Asten J, Kalyanaraman B, Hillard CJ (2003) 1-Methyl-4-phenylpyridinium accumulates in cerebellar granule neurons via organic cation transporter 3. J Neurochem 85:358–367 [DOI] [PubMed] [Google Scholar]
- Starkov AA, Polster BM, Fiskum G (2002) Regulation of hydrogen peroxide production by brain mitochondria by calcium and Bax. J Neurochem 83:220–228 [DOI] [PubMed] [Google Scholar]
- Tompa P, Buzder-Lantos P, Tantos A, Farkas A, Szilágyi A, Bánóczi Z, Hudecz F, Friedrich P (2004) On the sequential determinants of calpain cleavage. J Biol Chem 279:20775–20785 [DOI] [PubMed] [Google Scholar]
- Wales SQ, Laing JM, Chen L, Aurelian L (2008) ICP10PK inhibits calpain-dependent release of apoptosis-inducing factor and programmed cell death in response to the toxin MPP+. Gene Ther 15:1397–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Xu JX (2005) Salvianic acid A protects human neuroblastoma SH-SY5Y cells against MPP+-induced cytotoxicity. Neurosci Res 51:129–138 [DOI] [PubMed] [Google Scholar]
- Wang XD, Rosales JL, Magliocco A, Gnanakumar R, Lee KY (2003) Cyclin E in breast tumors is cleaved into its low molecular weight forms by calpain. Oncogene 22:769–774 [DOI] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesierska-Gadek J, Gueorguieva M, Wojciechowski J, Tudzarova-Trajkovska S (2004) In vivo activated caspase-3 cleaves PARP-1 in rat liver after administration of the hepatocarcinogen N-nitrosomomorpholine (NNM) generating the 85 kDa fragment. J Cell Biochem 93:774–787 [DOI] [PubMed] [Google Scholar]
- Willis SN, Adams JM (2005) Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol 17:617–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DE, Newcomb EW (2000) Cleavage of Bax enhances its cell death function. Exp Cell Res 256:375–382 [DOI] [PubMed] [Google Scholar]
- Yang Y, Geldmacher DS, Herrup K (2001) DNA replication precedes neuronal cell death in Alzheimer’s disease. J Neurosci 21: 2661–2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Mufson EJ, Herrup K (2003) Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer’s disease. J Neurosci 23:2557–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhao K, Calingasan NY, Luo G, Szeto HH, Beal F (2009) Mitochondria targeted peptides protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Antioxid Redox Signal. Epub 2009 Feb 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatz M, Starling A (2005) Calpains and disease. N Engl J Med 352:2413–2423 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Larner SF, Liu MC, Zheng W, Hayes RL, Wang KKW (2009) Multiple alphaII-spectrin breakdown products distinguish calpain and caspase dominated necrotic and apoptotic cell death pathways. Apoptosis 14:1289–1298 [DOI] [PubMed] [Google Scholar]
- Zimmermann AK, Loucks FA, Schroeder EK, Bouchard RJ, Tyler KL, Linseman DA (2007) Glutathione binding to the Bcl-2 homology-3 domain groove: a molecular basis for Bcl-2 antioxidant function at mitochondria. J Biol Chem 282:29296–29304 [DOI] [PMC free article] [PubMed] [Google Scholar]